Abstract
Introduction
In mechanically ventilated patients, non-volitional assessment of quadriceps weakness using femoral-nerve stimulation (twitch force) while the leg rests on a right-angle trapezoid or dangles from the bed edge is impractical. Accordingly, we developed a knee-support apparatus for use in ventilated patients.
Methods
90 subjects (12 ventilated patients, 28 ambulatory patients and 50 healthy subjects) were enrolled. Twitches with leg-dangling, trapezoid, and knee-support setups were compared.
Results
Knee-support twitches were similar to trapezoid twitches but smaller than leg-dangling twitches (p<0.0001). Inter-operator and intra-operator agreement of knee-support twitches at one week and one month was high. In ventilated patients, knee-support twitches were smaller than in healthy subjects and ambulatory patients (p<0.004).
Discussion
The new knee-support apparatus allows accurate recording of quadriceps twitches. The ease of use in ventilated patients, and excellent inter-operator and intra-operator agreement suggest that this technique is suitable for cross-sectional and longitudinal studies in critically-ill patients.
Keywords: Quadriceps, magnetic stimulation, non-volitional strength, mechanical ventilation, ICU, femoral nerve, strength, weakness
INTRODUCTION
Mechanically ventilated patients commonly develop muscle wasting and physical disability that persists long beyond resolution of an acute illness.1–4 During the acute illness, many such patients are unable to follow commands, making it important to employ non-volitional methods when assessing muscle function.5
The standard non-volitional method to evaluate muscle function consists of measuring the force elicited by transcutaneous electrical or magnetic stimulation of a peripheral nerve.6–10 For instance, the force of thumb adduction elicited by ulnar-nerve stimulation has been used to quantify upper extremity weakness in critically ill patients.6, 9 The force of knee extension elicited by femoral-nerve stimulation (quadriceps twitch force) has been used to quantify weakness and fatigue of the quadriceps muscle in different patient groups.11, 12 Quantification of quadriceps function in critically ill patients is of particular interest because biopsies of this muscle can be easily obtained.13–15
Two setups are used to measure quadriceps twitch force: dangling the leg at the edge of a modified gurney16 or resting the leg on a right-angle trapezoid support (Figure 1).17 In ambulatory patients, recordings of twitch force with the dangling and the trapezoid setups have high inter- and intra-operator agreement,18–20 but both setups are highly problematic in critically ill patients. The dangling setup requires transfer to a specially designed gurney. The trapezoid setup has little flexibility in adapting to legs of different size and it elicits smaller twitches because gravity acts against twitch force.11, 17
Figure 1. Usual setups used to measure quadriceps twitch force elicited by magnetic stimulation of the femoral nerve.
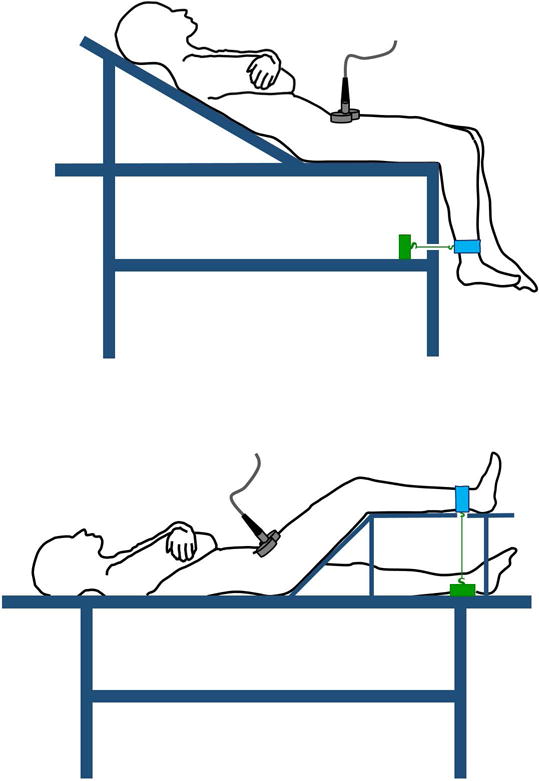
Upper panel, the leg dangles at the edge of a specially designed gurney. Lower panel, the leg rests on a right-angle trapezoid support. With both setups, a “branding iron” figure-of-eight coil is placed over the femoral triangle. When activated, the coil generates a broad magnetic field that causes depolarization of the femoral nerve eliciting quadriceps muscle contractions. The resulting force is transmitted to a strain amplifier/signal conditioner (green box) connected to an ankle strap (blue) using an inextensible cable.
To overcome these limitations, we developed a knee-support apparatus designed to be light and portable, which adapts to legs of different size and minimizes the effect of gravity on twitch force. Our objective was to determine whether this apparatus could be used to record quadriceps twitches in mechanically ventilated patients.
METHODS
Participants
Ninety subjects volunteered for the study. Pregnant women, leg amputees and patients with implanted electronic devices (such as cardiac pacemakers, defibrillators and intrathecal pumps) were excluded. The study was approved by the local human studies subcommittee. Written informed consent was obtained from all participants. In critically ill who were unable to consent to the study, permission was obtained from the patient’s authorized representative.
Experimental setup
Three setups, dangling, right-angle trapezoid and knee-support, were used. Each setup was equipped with a calibrated load cell (Model LCCA; Omega, Stanford) connected to an inextensible strap placed just proximal to the malleolus. In all setups, the force vector resulting from quadriceps contraction was perpendicular to the tibia and in series with the load cell.
Dangling technique
Participants rested in the semi-recumbent position (hip flexion at 30°) on a custom-made gurney while keeping the arms folded and the legs dangling at the gurney edge (knee angle flexed at 90°)21–23 (Figure 1, upper panel).
Right-angle trapezoid technique
Participants rested in the supine position (hip flexion at 45°) and the leg being tested was placed on the right-angle trapezoid (knee flexed at 45°; Figure 1, lower panel).17
Knee-support apparatus
With participants resting in the supine position, the popliteal fossa of the tested leg was supported by the apparatus cross bar. The height of the cross bar was set to attain hip flexion of about 45°. In turn, while avoiding undue pressure on the calcaneus, the apparatus footrest was adjusted to ensure knee flexion of 90° (Figure 2).
Figure 2. Portable knee-support apparatus used in a supine patient/subject.
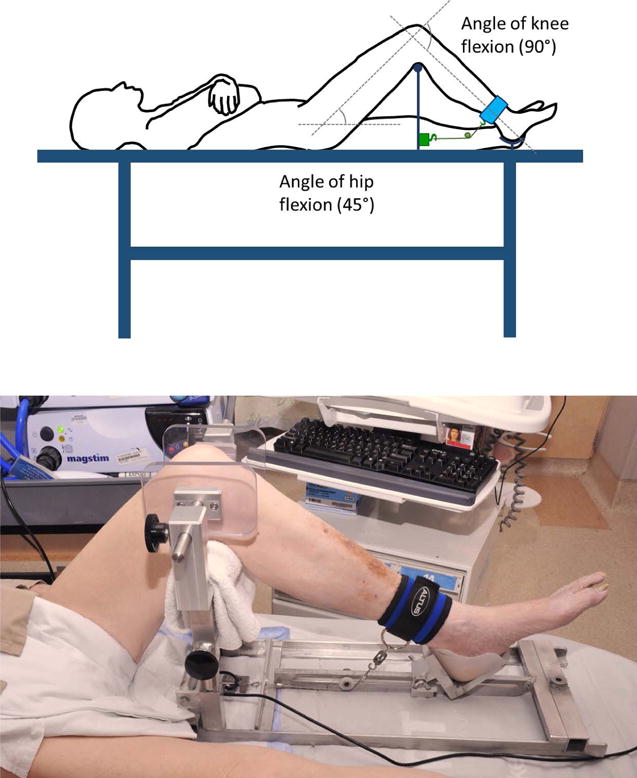
Upper panel, the popliteal fossa of the tested leg is supported by a cross bar. The height of the cross bar is adjusted to achieve hip flexion of about 45° and the footrest is concurrently adjusted to ensure knee flexion of 90°. An ankle strap (blue) is connected to a strain amplifier/signal conditioner (green box) using an inextensible cable. The cable is maintained perpendicular to the leg by a movable pulley; this arrangement is achieved by controlling the distance between the pulley and the strain amplifier/signal conditioner. Lower panel, knee-support apparatus used in a representative mechanically ventilated patient. The apparatus allows recording of quadriceps twitches in supine subjects of different body sizes without having to transfer the subjects to specialized beds.
Femoral-nerve stimulation
Femoral-nerve stimulations were performed using a 70-mm “branding iron” figure-of-eight coil (Jali Medical, Woburn, MA)23–27 powered by two magnetic stimulators (Magstim, Jali Medical, Woburn, MA).28 The coil was placed initially over the femoral triangle just lateral to the femoral artery and repositioned systematically to determine the best location for subsequent stimulations.16 The position that resulted in the largest twitch force was marked and used for the reminder of the study. This procedure was repeated with each setup.
To optimize femoral-nerve depolarization, the investigator pressed the stimulating coil against the femoral triangle using two vectors of force (Figure E1 on-line supplementary material). One vector was directed perpendicular to the shaft of the femur. The second vector was directed towards the inguinal ligament.
Once the best location for stimulation was located, participants rested at least 20 minutes.16, 29 Then, to determine whether femoral-nerve stimulation was supramaximal, a stimulus output force curve was obtained with the various setups. These stimulus output-force curves were obtained delivering single stimuli ranging from 70 to 100% of power output (2.2 Tesla)30 (Figure E2 on-line supplementary material). Thereafter, stimulations were delivered at 100% of stimulator output.23, 29 To preclude knee-joint movements, all stimulations were started at a minimum preload of 0.3 kg (right-trapezoid and knee support) or 0.8 kg (dangling). Before data collection, the preload was adjusted to achieve greatest twitch force, which was obtained by controlling the length of the inextensible cable joining the ankle strap and the strain amplifier/signal conditioner.
In all experiments, twitch force was assessed by measuring the force of knee extension elicited using single and paired stimulations (two stimuli delivered 10 ms apart, i.e., 100 Hz). The rationale for using paired stimulations in addition to more conventional single stimulation was based on the fact that paired stimulation elicits a larger twitch force than single stimulation.11 Moreover, critically ill patients, the population for which the knee holder was developed, commonly exhibit profound muscle weakness.1–4
Experiments
Six experiments were carried out (Table 1. Also see Supplementary Methods, available on-line). In experiment #1, we tested the agreement between twitch forces obtained while using the knee holder and the right-angle trapezoid setup. In experiment #2, we tested the agreement between twitch forces obtained while using the knee holder and the dangling setup. In experiment #3, we tested the intra-operator agreement between twitch force when using the knee holder during a given experimental session (experiment #3a), and one week (experiment #3b) and one month (experiment #3c) later. In experiment #4, we tested the inter-operator agreement between twitch forces recorded by two operators (FL and NK) when using the knee holder during a given experimental session. In experiment #5, we tested the impact of contraction history on twitch force. Specifically we determined whether it was possible to induce post-activation potentiation (a transient increase in twitch force following a forceful voluntary contraction)31 and whether it was possible induce potentiation by paired stimulations (a transient increase in twitch force after a muscle has been externally stimulated twice in quick succession (interstimulus interval of less than 125 ms for muscles with fast-twitch fibers and less than 250 ms for muscles with slow-twitch fibers).32 In experiment #6, we tested the feasibility of using the knee holder device to record twitch force elicited by femoral-nerve stimulations in mechanically ventilated patients.
Table 1.
Experimental protocols
| Experiment #1: Knee holder vs. trapezoid box setup | Experiment #2: Knee holder vs. dangling setup | Experiment #3: Intra-operator agreement with knee holder setup | Experiment #4: Inter-operator agreement with knee holder setup | Experiment #5: Twitch potentiation with knee holder setup | Experiment #6: Feasibility in mechanically ventilated patients | ||||
|---|---|---|---|---|---|---|---|---|---|
| Exp #3a | Exp #3b | Exp #3c | Exp #5a (Post-activation potentiation) | Exp #5b (Potentiation elicited by paired stimulation) | |||||
| Purpose | To determine agreement in twitch force between right-angle trapezoid and knee holder setups | To determine agreement in twitch force between dangling and knee holder setups | To determine intra-operator agreement of twitch force recorded with knee holder setup during a given experimental session | To determine intra-operator agreement of twitch forces recorded with knee holder setup one week apart | To determine intra-operator agreement of twitch forces recorded with knee holder setup one month apart | To determine inter-operator agreement of twitch forces recorded with knee holder setup by two operators | To determine possibility to induce post-activation potentiation of twitch forces recorded with knee holder setup | To determine possibility to induce potentiation using paired stimuli delivered 10 ms apart | To determine feasibility of using knee holder setup in mechanically ventilated patients |
| Participants | 7 healthy, 7 COPD | 46 healthy, 22 COPD | 50 healthy, 28 COPD, 12 ventilated patients | 33 healthy, 29 COPD | 23 healthy, 17 COPD | 36 healthy, 15 COPD | 11 healthy, 4 COPD | 6 healthy, 2 COPD | 12 ventilated patients |
| Legs tested | 28 (both legs) | 68 (dominant leg only) | 90 (dominant leg only) | 62 (dominant leg only) | 40 (dominant leg only) | 51 (dominant leg only) | 15 (dominant leg only) | 8 (dominant leg only) | 12 (dominant leg only) |
| Number of single stimulations | At least 5 with each setup | At least 5 with each setup | At least 5 (knee holder only) | At least 5 (knee holder only) | At least 5 (knee holder only) | At least 5 by each investigator (knee holder only) | At least 5 before and after voluntary knee extension of 25–30 kg | At least 5 before and immediately after the set of paired stimulations | At least 5 (knee holder only, 12 patients) |
| Number of paired stimulations | At least 5 with each setup | At least 5 with each setup | At least 5 (knee holder only) | At least 5 (knee holder only) | At least 5 (knee holder only) | At least 5 by each investigator (knee holder only) | None | 5–7 (knee holder only) | At least 5 (knee holder only, 7 patients) |
Data analysis and statistics
The force signal was digitized at 2000 Hz (Experiments #1 to #5) and 200 Hz (Experiment #6) with an AD converter (DI-158U, DATAQ Instruments, Akron, Ohio) connected to a computer (Dell, Inc). Digitized data were processed using WINDAQ software (DATAQ Instruments, Akron, OH). Quadriceps twitch force was measured as the difference between maximal force displacement elicited by femoral-nerve stimulation and baseline of the force signal immediately preceding stimulation. The average of three strongest twitch force values during a given experimental session was used for statistical analysis.17 The Shapiro-Wilk test was used to assess distribution of data and all of the data had a normal distribution.
The agreement of twitch force between the knee holder and right-angle trapezoid setups and between the knee holder and dangling setups was estimated using the intraclass correlation coefficient.33 The same computational strategies were used to estimate the intra-operator agreement and the inter-operator agreement of twitch force. Student t-test was used to compare the measures obtained with different setups and different operators. Statistical tests were two-tailed. A Bonferroni adjusted p value of 0.004 (i.e., 0.05/14) was used to determine significance. All data are reported as mean (± standard error) values.
RESULTS
Fifty healthy men, twenty-eight ambulatory men with COPD and twelve critically ill mechanically ventilated patients volunteered for the study (Tables 2 and 3).
Table 2.
Characteristics of healthy subjects, ambulatory patients with COPD and mechanically ventilated patients
| Characteristic |
Healthy subjects (n = 50) |
Patients with COPD (n = 28) |
Mechanically ventilated patients (n = 12) |
|---|---|---|---|
| Age, years | 48 ± 3 | 68 ± 1 | 64 ± 4 |
| BMI, kg/m2 | 25.0 ± 0.4 | 25.4 ± 0.7 | 27.4 ± 1.8 |
| FEV1, L (% predicted) |
3.8 ± 0.1 (105 ± 2) |
1.1 ± 0.1 (37 ± 3) |
— |
| FVC, L (% predicted) |
4.8 ± 0.1 (103 ± 2) |
3.1 ± 0.1 (73 ± 3) |
— |
| FEV1/FVC, % | 80 ± 1 | 37 ± 2 | — |
| Total lung capacity, L (% predicted) |
— | 8.5 ± 0.3 (122 ± 4) |
— |
| Residual volume, L (% predicted) |
— | 5.2 ± 0.3 (202± 14) |
— |
| DL/VA, ml/min/mm Hg/L (% predicted) | — | 2.1 ± 0.2 (54 ± 4) |
— |
| pH | — | 7.43 ± 0.01 | — |
| PaCO2, mmHg | — | 41 ± 1 | — |
| PaO2, mmHg | — | 72 ± 2 | — |
Definition of abbreviations: BMI = body mass index, FEV1 = forced expired volume in one second, FVC = forced vital capacity, DL/VA = specific diffusing capacity, PaCO2 = partial pressure of arterial carbon dioxide, PaO2 = partial pressure of arterial oxygen. Values are means ± SE.
Table 3.
Characteristics of mechanically ventilated patients
| Patient No. | Age (yr) | Sex | Diagnosis | Days of ventilator support before stimulation | Single twitches (kg) | Paired Twitches (kg) |
|---|---|---|---|---|---|---|
| 1 | 63 | M | GI bleeding, respiratory failure NSTEMI | 22 | 2.37±0.06† | — |
| 2 | 86 | M | Perforated colon, sepsis | 21* | 2.59±0.06 | — |
| 3 | 66 | M | Pneumonia in COPD, peripheral vascular disease (homebound on admission) | 19 | 0.37±0.01 | 0.62±0.02 |
| 4 | 60 | M | Pneumonia, Multiple Myeloma | 15 | 1.06±0.01 | — |
| 5 | 60 | M | COPD exacerbation | 14 | 1.08±0.01 | — |
| 6 | 52 | M | Severe pneumonia | 14 | 1.36±0.01 | — |
| 7 | 82 | F | Septic shock, NSTEMI on vasopressors | 6 | 0.13±0.01 | 0.42±0.01 |
| 8 | 52 | M | Alcohol withdrawal, pneumonia, pulmonary edema | 2 | 4.70±0.03 | 11.68±0.12 |
| 9 | 52 | M | Submandibular abscess, HIV (intubated airway protection) | 2 | 5.68±0.05 | 17.14±0.06 |
| 10 | 79 | M | Pneumonia in COPD | 2 | 1.78±0.03 | 6.59±0.06 |
| Patient No. | Age (yr) | Sex | Diagnosis | Days of ventilator support before stimulation | Single twitches (kg) | Paired Twitches (kg) |
| 11 | 67 | M | Idiopathic pulmonary fibrosis, heart failure | 1 | 3.89±0.06 | 12.73±0.32 |
| 12 | 47 | M | Alcohol withdrawal | 1 | 8.60±0.06 | 22.05±0.05 |
Definition of abbreviations: M = male, F = female, NSTEMI = non-ST elevation myocardial infarction, COPD = chronic obstructive pulmonary disease, HIV = infection caused by human immunodeficiency virus,
non-supramaximal stimulation of the femoral nerve when using 100% stimulator’s output,
femoral nerve stimulations performed four days after discontinuation of mechanical ventilation. Values of single and paired twitch force are means ± SE.
Experiment #1: Agreement of twitch force obtained using knee holder and right-angle trapezoid
Stimulations delivered to the right and the left femoral nerve were supramaximal in all seven healthy subjects and all seven patients with COPD. In one healthy subject, stimulations while the left leg rested on the right-angle trapezoid were not supramaximal (data not shown).
Mean twitch forces elicited by single and paired stimulations while the leg rested on the knee holder were 10.1 ± 0.8 and 22.1 ± 1.1 kg in healthy subjects and 6.2 ± 0.6 and 18.0 ± 1.1 kg in patients with COPD. Corresponding values recorded with the right-angle trapezoid were 10.5 ± 0.9 and 23.9 ± 1.0 kg in healthy subjects and 4.2 ± 0.4 kg and 17.3 ± 0.8 kg in COPD.
Twitch force elicited by single and paired stimulations recorded with the two setups in the two groups of participants were not significantly different. Intraclass correlation coefficients of single and paired twitch forces with the two setups were greater than 0.77 (Table 4).
Table 4.
Intraclass correlation coefficient of twitch force recorded with different setups
| Comparison | Single stimulations ICC (95% CI) |
Paired stimulations ICC (95% CI) |
|---|---|---|
| Knee-support vs. Trapezoid setup | 0.794 (0.600, 0.899) |
0.779 (0.569, 0.894) |
| Knee-support vs. Dangling setup | 0.864 (0.447, 0.947) |
0.821 (0.196, 0.937) |
Definition of abbreviations: ICC = Intraclass correlation coefficient; 95% CI = 95% confidence interval
Experiment #2: Agreement of twitch force obtained using knee holder and dangling setup
Stimulations were supramaximal in 44 of 46 healthy subjects and 21 of 22 patients with COPD (data not show). In three healthy subjects, stimulations with the dangling setup were not supramaximal.
The mean twitch force elicited by single and paired stimulations with the knee holder were 8.9 ± 0.5 and 20.5 ± 0.7 kg in healthy subjects and 5.4 ± 0.5 and 14.2 ± 1.0 kg in patients with COPD. The corresponding values recorded with the leg dangling were 9.7 ± 0.5 and 22.7 ± 0.8 kg in healthy subjects and 7.5 ± 0.4 and 17.6 ± 1.0 kg in patients with COPD.
The force of single and paired twitches with the knee holder were less than with the dangling setup both in healthy subjects and patients with COPD (p < 0.0001 in all instances). Intraclass correlation coefficients of single and paired twitch force with the two setups were greater than 0.80. The corresponding 95% CI intraclass correlation coefficients were wide (Table 4).
Experiment #3: Intra-operator agreement of twitch force when using knee holder
During a given session and at one week and one month, the intra-operator intraclass correlation coefficients for single and paired twitch force were equal or greater than 0.96. The corresponding 95% CI intraclass correlation coefficients were narrow (Table 5).
Table 5.
Intra-observer and inter-observer intraclass correlation coefficients of twitch force recorded with the knee-holder device
| Comparison | Single stimulations ICC (95% CI) |
Paired stimulations ICC (95% CI) |
|
|---|---|---|---|
| Intra-observer | Same day | 0.998 (0.998, 0.999) |
0.998 (0.998, 0.999) |
| One week apart | 0.982 (0.971, 0.989) |
0.981 (0.968, 0.989) |
|
| One month apart | 0.960 (0.925, 0.978) |
0.964 (0.933, 0.981) | |
| Inter-observer | Same day | 0.993 (0.988, 0.996) |
0.992 (0.986, 0.995) |
Definition of abbreviations: ICC = Intraclass correlation coefficient; 95% CI = 95% confidence interval
Experiment #4: Inter-operator agreement of twitch force when using knee holder
The inter-operator intraclass correlation coefficients for single and paired twitch force using the knee holder device were greater than 0.99. The corresponding 95% CI for the intraclass correlation coefficients were narrow (Table 5).
Experiment#5: Impact of contraction history on twitch force – Potentiation
In 11 healthy subjects and four patients with COPD, twitch force elicited by single stimulations increased from 7.4 ± 0.8 kg at baseline to 8.3 ± 0.9 kg immediately after a set of 5–7 paired stimulations (potentiation due to paired stimulation; p < 0.0001). In six healthy subjects and two patients with COPD, twitch force elicited by single stimulations increased from 8.9 ± 0.8 kg at baseline to 15.6 ± 1.0 kg immediately after a knee extension effort of 25–30 kg (post-activation potentiation; p = 0.0001).
Experiment #6: Feasibility of using of the knee holder device in mechanically ventilated patients
Mechanically ventilated patients tolerated the procedure without untoward effects. Single and paired stimulations elicited an identifiable twitch force in all patients (Table 3). As expected, twitch force elicited by paired stimulations (10.2±3.1 kg) was greater than the twitch force elicited by single stimulations (2.8±0.7 kg; p < 0.0001). Twitch force elicited by single stimulations in ventilated patients were less than the corresponding values recorded in healthy subjects and in patients with COPD (p < 0.004). Twitch force elicited by paired stimulations in ventilated patients were less than the corresponding values recorded in healthy subjects (p < 0.004) and tended to be less than in patients with COPD (p = 0.008).
DISCUSSION
This study has three major findings. Recordings of quadriceps twitch force while the leg rested on a knee-support apparatus compared favorably with twitch force recorded with conventional dangling and trapezoid setups. Inter-operator and intra-operator agreement over time of twitch force measurements using the knee-support were high. The knee-support apparatus was successfully used in mechanically ventilated patients.
Inter-setup comparisons
Several factors modulate the force of knee extension during an isometric contraction. These include leg inertia, gravitational force, hip and knee flexion, and extent of femoral-nerve recruitment (Figure 3).34–37 At the time of stimulation, leg inertia, knee flexion and femoral nerve recruitment were the same with the knee support and with dangling setup. Accordingly, none of these factors could have caused the smaller twitch forces with the knee-support apparatus than with the dangling setup.
Figure 3. Schematic representation of factors that modulate the force of knee extension during magnetic stimulation of the femoral nerve.
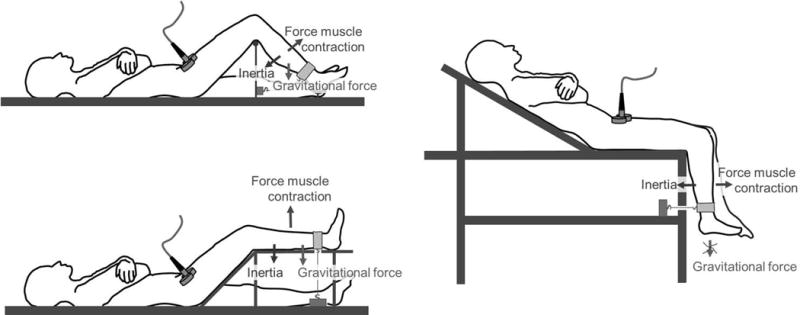
Left upper panel. With the knee-support apparatus, the angle of knee flexion is 90° and the angle of hip flexion is about 45° (also, see Figure 2). The force output of the contracting quadriceps muscle depends on the angles of knee flexion and hip flexion. Major resisting forces to the contracting quadriceps are the inertia of the calf and foot and the gravitational force exerted on them.
Left lower panel. With the trapezoid setup, the angle of hip flexion is nearly the same as with the knee-support apparatus while the angle of knee flexion is different (also, see Figure 2). The force of knee extension with these different angles should have been equivalent. (See text for details.) Gravitational force opposing the contraction is maximal. Inertia remains unchanged.
Right panel. With the dangling setup, the angle of knee flexion is the same as with the knee support apparatus while the angle of hip flexion differs only slightly. Accordingly, the force output of the quadriceps is equivalent to the force output of the muscle when using the knee support apparatus. Gravitational force opposing the contraction of the quadriceps is zero. Inertia remains unchanged. (See text for details.)
Hip flexion was greater with the knee support than with the dangling setup: 45° vs. 30°. Accordingly, we would expect quadriceps-force output to be greater with the knee-support apparatus than with the dangling setup.36, 38 Our expectation is based on the greater force of knee extension when sitting (hip flexion of 50° to 80°)36 than when supine (hip flexion of 0°).38 The smaller twitch forces with the knee support suggests that a factor other than hip flexion was at play. This factor could have been the different relationship between the vector of gravitational force on the leg and the vector generated by quadriceps contraction with the two setups. While dangling, the two vectors were perpendicular to each other and thus had no effect on each other. In contrast, with the knee-support apparatus, a fraction of the force of gravity imposed a force which was in the opposite direction to the force resulting from quadriceps contraction (Figure 3) and thus curtailed the force vector resulting from quadriceps contraction.
Force elicited by single and paired twitches in healthy subjects and in patients with COPD was equivalent with the knee support and trapezoid setups. The similar angle of hip flexion and leg inertia and the supramaximal femoral nerve recruitment in most participants likely contributed to this result. Another factor contributing to this result is the angle of knee flexion: 90° with knee support and 45° with the trapezoid. Maximum torque of knee extension occurs when the knee is flexed between 50° and 70°.34, 37, 39, 40 As the angle of knee flexion departs from 50° and 70°, the torque of knee extension decreases and, relevant to our investigation, the torque of knee extension when the angle is about 90° happens to be equivalent to the torque when the angle is about 45° (see Figures 1 and 2 in Knapik et al).35 The effect of gravity was less with knee support – a condition that should have increased twitch force. That twitch force was equivalent with the two setups indicates that gravity had little effect on force output in these circumstances.
Intra-operator and inter-operator comparisons
The investigators performing femoral-nerve stimulations in the study always made sure to press the stimulating coil against the femoral triangle using two vectors of force. One vector was directed perpendicular to the shaft of the femur. The second vector was directed towards the inguinal ligament. We developed this strategy during the course of preliminary investigations to optimize femoral-nerve depolarization. That femoral-nerve depolarization was optimized is underscored by the high intraclass correlation coefficients for single and paired twitches during a given experimental session. Comparison of twitch forces at one week and one month revealed high repeatability of measurements over time – equivalent to the results obtained with the dangling setup.19, 20 In obtaining these results, we took great care to avoid twitch potentiation.31 Care in avoiding potentiation and in optimizing femoral-nerve depolarization were also responsible for the excellent inter-operator agreement of twitch forces using the knee support. Given the excellent intra- and inter-operator agreements, we propose that quadriceps twitches may be used to accurately follow force of knee extension in patients (see section on future directions).
Use of the knee support in mechanically ventilated patients
Recordings of quadriceps twitch force while the leg rested on the knee-support apparatus in ventilated patients was well tolerated. The procedure caused minimal disruption to position or treatment for two main reasons. Magnetic stimulation produces a wide field of stimulation.41, 42 Hence, the area in which to position the stimulating probe to achieve maximal nerve depolarization is relatively large.6, 41, 42 In addition, the knee-support apparatus allows recording of quadriceps twitches in supine individuals of different body sizes without having to transfer them to specialized beds.
Although the purpose of studying ventilated patients was to test the feasibility of recording quadriceps twitches in these patients, our data support the view that severe muscle weakness is common in the ICU.1–4 Because of this severe weakness, force signals elicited by paired stimulations had a more favorable signal-to-noise ratio than single stimulations. Accordingly, paired stimulations should be considered the technique of choice to assess the force of knee extension independent of patient cooperation in the ICU. Moreover, twitch potentiation following voluntary contractions is considerably less for paired than for single twitches.43 Accordingly, limited post contraction potentiation makes the interpretation of changes in twitch force over time easier when using paired stimulations than single stimulations. Paired stimulations with different interstimulus intervals could be used to construct a surrogate force frequency curve28, 44 to discriminate between low- and high-frequency fatigue.44
Future directions
Diagnosis of muscle dysfunction in critically ill patients is difficult.45 Clinical evaluation underestimates the true incidence of neuromuscular impairment.45 Recordings of maximal voluntary isometric contractions or Medical Research Council scale are not applicable when consciousness is impaired.5 In addition, they lack of sensitivity to detect change over time in muscle strength when applied to stronger muscle groups.46 Electrophysiological tests have poor discriminatory value in identifying patients with clinically significant muscle dysfunction.9, 45, 47
The above limitations can be overcome by recording the force of contraction elicited by stimulation of peripheral nerves.6, 8, 9 Early identification of weakness in the ICU should facilitate the design and implementation of interventions intended to preserve or restore muscle function. Such measurements, combined with muscle biopsies,13–15 may yield new insight into the mechanism(s) of neuromuscular abnormalities in critically ill patients.
In conclusion, the new knee-support apparatus allows recording of quadriceps twitches in supine individuals. The ease with which the apparatus can be used in the ICU, and the excellent inter operator and intra-operator agreement of twitch force suggest that the technique is suitable for cross-sectional and longitudinal studies in critically ill patients.
Supplementary Material
Acknowledgments
The authors gratefully thank Mr. Chad Tarr (Engineering Service, Edward Hines Jr. VA Hospital) for his help in assembling the knee holder, Dr. Veeranna Maddipati (Division of Pulmonary and Critical Care, Brody School of Medicine at East Carolina University. Greenville, NC) for his suggestions on positioning the tension transducer in the knee holder, Dr. James M. Sinacore (Department of Preventive Medicine & Epidemiology, Loyola University, Maywood, IL) for his statistical advice, and all the veterans who enthusiastically took part to this project.
Source of Funding: Grants from the Veterans Administration Research Service
Dr. Laghi has received research grants from the National Institutes of Health, VA Research Service, Liberate Medical LLC, and the National Science Foundation. Dr. Shaikh has received research grants from the National Science Foundation. Dr. Collins has received research grants from the VA Research Service. Dr. Jubran has received research grants from the National Institutes of Health. Dr. Tobin receives royalties from McGraw-Hill for two books published on critical care medicine.
ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- DL/VA
specific diffusing capacity
- F
female
- FEV1
forced expired volume in one second
- FL
Franco Laghi
- FVC
forced vital capacity
- HIV
infection caused by human immunodeficiency virus
- ICC
intraclass correlation coefficient
- ICU
intensive care unit
- M
male
- NK
Najeeb Khan
- NSTEMI
non-ST elevation myocardial infarction
- PaCO2
partial pressure of arterial carbon dioxide
- PaO2
partial pressure of arterial oxygen
- SE
standard error
Footnotes
Part of the material in this manuscript was presented in abstract form at the 2014 and 2015 International Conference of the American Thoracic Society held in San Diego (May 2014) and Denver (May, 2015)
Ethical publication statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of conflict of interests
The remaining authors have no conflicts of interest.
Reference List
- 1.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen JB, Rose MH, Jensen BR, Moller K, Perner A. Biomechanical and nonfunctional assessment of physical capacity in male ICU survivors. Crit Care Med. 2013;41(1):93–101. doi: 10.1097/CCM.0b013e31826a3f9e. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Hermans G, Van MH, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 5.Puthucheary Z, Montgomery HE, Moxham J, Harridge SD, Hart N. Structure to function: muscle failure in critically ill patients. J Physiol. 2010;588(23):4641–4648. doi: 10.1113/jphysiol.2010.197632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris ML, Luo YM, Watson AC, et al. Adductor pollicis twitch tension assessed by magnetic stimulation of the ulnar nerve. Am J Respir Crit Care Med. 2000;162(1):240–245. doi: 10.1164/ajrccm.162.1.9902073. [DOI] [PubMed] [Google Scholar]
- 7.Verges S, Maffiuletti NA, Kerherve H, Decorte N, Wuyam B, Millet GY. Comparison of electrical and magnetic stimulations to assess quadriceps muscle function. J Appl Physiol. 2009;106(2):701–710. doi: 10.1152/japplphysiol.01051.2007. [DOI] [PubMed] [Google Scholar]
- 8.Ginz HF, Iaizzo PA, Urwyler A, Pargger H. Use of non-invasive-stimulated muscle force assessment in long-term critically ill patients: a future standard in the intensive care unit? Acta Anaesthesiol Scand. 2008;52(1):20–27. doi: 10.1111/j.1399-6576.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 9.Eikermann M, Koch G, Gerwig M, et al. Muscle force and fatigue in patients with sepsis and multiorgan failure. Intensive Care Med. 2006;32(2):251–259. doi: 10.1007/s00134-005-0029-x. [DOI] [PubMed] [Google Scholar]
- 10.Laghi F, Shaikh HS, Morales D, Sinderby C, Jubran A, Tobin MJ. Diaphragmatic neuromechanical coupling and mechanisms of hypercapnia during inspiratory loading. Respir Physiol Neurobiol. 2014;198:32–41. doi: 10.1016/j.resp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Mador MJ, Kufel TJ, Pineda LA, et al. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am J Respir Crit Care Med. 2001;163(4):930–935. doi: 10.1164/ajrccm.163.4.2006125. [DOI] [PubMed] [Google Scholar]
- 12.Hopkinson NS, Dayer MJ, Antoine-Jonville S, et al. Central and peripheral quadriceps fatigue in congestive heart failure. Int J Cardiol. 2013;167(6):2594–2599. doi: 10.1016/j.ijcard.2012.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan D, Lewis A, Patel MS, et al. Using laser capture microdissection to study fiber specific signaling in locomotor muscle in COPD: A pilot study. Muscle Nerve. 2017;55(6):902–912. doi: 10.1002/mus.25423. [DOI] [PubMed] [Google Scholar]
- 14.Bloch SA, Lee JY, Syburra T, et al. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70(3):219–228. doi: 10.1136/thoraxjnl-2014-206225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puthucheary ZA, McNelly AS, Rawal J, et al. Rectus Femoris Cross-Sectional Area and Muscle Layer Thickness: Comparative Markers of Muscle Wasting and Weakness. Am J Respir Crit Care Med. 2017;195(1):136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve. 1996;19(5):549–555. doi: 10.1002/(SICI)1097-4598(199605)19:5<549::AID-MUS1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Saey D, Debigare R, LeBlanc P, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(4):425–430. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 18.Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25(3):438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- 19.Schonhofer B, Zimmermann C, Abramek P, Suchi S, Kohler D, Polkey MI. Non-invasive mechanical ventilation improves walking distance but not quadriceps strength in chronic respiratory failure. Respir Med. 2003;97(7):818–824. doi: 10.1016/s0954-6111(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 20.Harris ML, Polkey MI, Bath PM, Moxham J. Quadriceps muscle weakness following acute hemiplegic stroke. Clin Rehabil. 2001;15(3):274–281. doi: 10.1191/026921501669958740. [DOI] [PubMed] [Google Scholar]
- 21.Burtin C, Saey D, Saglam M, et al. Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J. 2012;40(2):338–344. doi: 10.1183/09031936.00111811. [DOI] [PubMed] [Google Scholar]
- 22.Hammond K, Mampilly J, Laghi FA, et al. Validity and reliability of rectus femoris ultrasound measurements: Comparison of curved-array and linear-array transducers. J Rehabil Res Dev. 2014;51(7):1155–1164. doi: 10.1682/JRRD.2013.08.0187. [DOI] [PubMed] [Google Scholar]
- 23.Amann M, Regan MS, Kobitary M, et al. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R314–R324. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swallow EB, Gosker HR, Ward KA, et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol. 2007;103(3):739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- 25.Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64(5):418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 26.Hamnegard CH, Sedler M, Polkey MI, Bake B. Quadriceps strength assessed by magnetic stimulation of the femoral nerve in normal subjects. Clin Physiol Funct Imaging. 2004;24(5):276–280. doi: 10.1111/j.1475-097X.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 27.Seymour JM, Ward K, Raffique A, et al. Quadriceps and ankle dorsiflexor strength in chronic obstructive pulmonary disease. Muscle Nerve. 2012;46(4):548–554. doi: 10.1002/mus.23353. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery MM, Kufel TJ, Pineda LA. Quadriceps and diaphragmatic function after exhaustive cycle exercise in the healthy elderly. Am J Respir Crit Care Med. 2000;162(5):1760–1766. doi: 10.1164/ajrccm.162.5.2001005. [DOI] [PubMed] [Google Scholar]
- 29.Mador MJ, Kufel TJ, Pineda LA. Quadriceps fatigue after cycle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(2):447–453. doi: 10.1164/ajrccm.161.2.9904092. [DOI] [PubMed] [Google Scholar]
- 30.Jalinous R. Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8(1):10–25. doi: 10.1097/00004691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Sale DG. Postactivation potentiation: role in human performance. Exerc Sport Sci Rev. 2002;30(3):138–143. doi: 10.1097/00003677-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Parmiggiani F, Stein RB. Nonlinear summation of contractions in cat muscles. II. Later facilitation and stiffness changes. J Gen Physiol. 1981;78(3):295–311. doi: 10.1085/jgp.78.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Haffajee D, Moritz U, Svantesson G. Isometric knee extension strength as a function of joint angle, muscle length and motor unit activity. Acta Orthop Scand. 1972;43(2):138–147. doi: 10.3109/17453677208991252. [DOI] [PubMed] [Google Scholar]
- 35.Knapik JJ, Wright JE, Mawdsley RH, Braun J. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther. 1983;63(6):938–947. doi: 10.1093/ptj/63.6.938. [DOI] [PubMed] [Google Scholar]
- 36.Currier DP. Positioning for knee strengthening exercises. Phys Ther. 1977;57(2):148–152. doi: 10.1093/ptj/57.2.148. [DOI] [PubMed] [Google Scholar]
- 37.Anderson DE, Madigan ML, Nussbaum MA. Maximum voluntary joint torque as a function of joint angle and angular velocity: model development and application to the lower limb. J Biomech. 2007;40(14):3105–3113. doi: 10.1016/j.jbiomech.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herzog W. Individual muscle force prediction in athletic movements. University of Iowa; 1985 [Google Scholar]
- 39.Clarke HH, Elkins EC. Relationship between body position and the application of muscle power to movements of the joints. Arch Phys Med Rehabil. 1950;31(2):81–99. [PubMed] [Google Scholar]
- 40.Houtz SJ, Lebow MJ, Beyer FR. Effect of posture on strength of the knee flexor and extensor muscles. J Appl Physiol. 1957;11(3):475–480. doi: 10.1152/jappl.1957.11.3.475. [DOI] [PubMed] [Google Scholar]
- 41.Laghi F, D’Alfonso N, Tobin MJ. A paper on the pace of recovery from diaphragmatic fatigue and its unexpected dividends. Intensive Care Med. 2014;40(9):1220–1226. doi: 10.1007/s00134-014-3340-6. [DOI] [PubMed] [Google Scholar]
- 42.Laghi F, Harrison MJ, Tobin MJ. Comparison of magnetic and electrical phrenic nerve stimulation in assessment of diaphragmatic contractility. J Appl Physiol (1985) 1996;80(5):1731–1742. doi: 10.1152/jappl.1996.80.5.1731. [DOI] [PubMed] [Google Scholar]
- 43.Froyd C, Beltrami FG, Jensen J, Millet GY, Noakes TD. Potentiation and electrical stimulus frequency during self-paced exercise and recovery. J Hum Kinet. 2014;42:91–101. doi: 10.2478/hukin-2014-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan S, Gauthier AP, Similowski T, Faltus R, Macklem PT, Bellemare F. Force-frequency relationships of in vivo human and in vitro rat diaphragm using paired stimuli. Eur Respir J. 1993;6(2):211–218. [PubMed] [Google Scholar]
- 45.Deem S, Lee CM, Curtis JR. Acquired neuromuscular disorders in the intensive care unit. Am J Respir Crit Care Med. 2003;168(7):735–739. doi: 10.1164/rccm.200302-191UP. [DOI] [PubMed] [Google Scholar]
- 46.Vanpee G, Hermans G, Segers J, Gosselink R. Assessment of limb muscle strength in critically ill patients: a systematic review. Crit Care Med. 2014;42(3):701–711. doi: 10.1097/CCM.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 47.Morris C, Trinder JT. Electrophysiology adds little to clinical signs in critical illness polyneuropathy and myopathy. Crit Care Med. 2002;30(11):2612. doi: 10.1097/00003246-200211000-00048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


