Abstract
Activated fibroblasts are key players in the injury response, tumorigenesis, fibrosis, and inflammation. Dichotomous outcomes in response to varied stroma-targeted therapies in cancer emphasize the need to disentangle the roles of heterogeneous fibroblast subsets in physiological and pathophysiological settings. In wound healing, fibrosis, and myriad tumor types, fibroblast activation protein (FAP) and alpha-smooth muscle actin (αSMA) identify distinct, yet overlapping, activated fibroblast subsets. Prior studies established that FAPHi reactive fibroblasts and αSMAHi myofibroblasts can exert opposing influences in tumorigenesis. However, the factors that drive this phenotypic heterogeneity and the unique functional roles of these subsets have not been defined. We demonstrate that a convergence of ECM composition, elasticity, and transforming growth factor beta (TGF-β) signaling governs activated fibroblast phenotypic heterogeneity. Furthermore, FAPHi reactive fibroblasts and αSMAHi myofibroblasts exhibited distinct gene expression signatures and functionality in vitro, illuminating potentially unique roles of activated fibroblast subsets in tissue remodeling. These insights into activated fibroblast heterogeneity will inform the rational design of stroma-targeted therapies for cancer and fibrosis.
Keywords: Activated fibroblasts, Fibroblast activation protein, Alpha-smooth muscle actin, Fibroblast heterogeneity
1.1 Introduction
In development and homeostasis, fibroblasts synthesize extracellular matrix (ECM), which regulates cell differentiation and behavior. As sentinel cells, fibroblasts respond to injury and neoplasia by differentiating to highly secretory, proliferative, or contractile phenotypes. This heterogeneous activated fibroblast population orchestrates injury resolution. However, in fibrosis and cancer, the aberrant accumulation of activated fibroblasts and ECM disrupts organ function [1–5].
Fibroblast activation protein (FAP) is a marker of activated fibroblasts in the injury response[6], fibrotic and inflammatory conditions [7–9], and myriad tumor types [10–14]. In most epithelial-derived tumors, FAP and alpha smooth muscle actin (αSMA; the canonical myofibroblast marker) identify distinct, yet overlapping, fibroblast subsets [11,12,15–17]. Importantly, FAPHi fibroblasts and αSMAHi myofibroblasts diverge in their impact on tumorigenesis. FAPHi fibroblasts accelerate the growth of multiple tumor types by promoting intratumoral desmoplasia, angiogenesis, and immunosuppression [11,18–20]. In contrast, αSMAHi myofibroblasts reportedly can curb pancreatic cancer aggressiveness by restraining epithelial-mesenchymal transition, cancer stem cell generation, and immunosuppression [15]. FAP and αSMA similarly mark distinct fibroblast subsets in fibrotic conditions, including idiopathic pulmonary fibrosis and liver cirrhosis [7,8]. However, the conditions regulating this phenotypic heterogeneity and the unique functional roles of these phenotypically distinct subsets are not yet known. Disentangling these complexities will clarify the roles of heterogeneous fibroblast subsets in physiological and pathophysiological settings.
ECM composition and mechanics exhibit spatiotemporal heterogeneity in acute injury, fibrosis, and cancer [21–26]. Notably, both granulation tissue and intratumoral desmoplasia evolve from elastic, fibronectin (FN)-rich to stiff, collagen I (COL 1)-rich ECM [25,27–30]. Therefore, we hypothesized that ECM properties directly regulate fibroblast heterogeneity. To test this hypothesis, we assessed fibroblast activation on substrata of defined stiffness and composition. Gene expression analysis revealed distinct signatures, illuminating potentially unique functional roles of FAPHi fibroblasts and αSMAHi myofibroblasts in tissue remodeling, consistent with the distinct functionalities we observed in vitro. These insights will help foster the rational design and clinical application of stromal cell-targeted therapies in cancer and fibrosis.
1.2 Results
1.2.1 Substratum directs activated fibroblast phenotypic heterogeneity
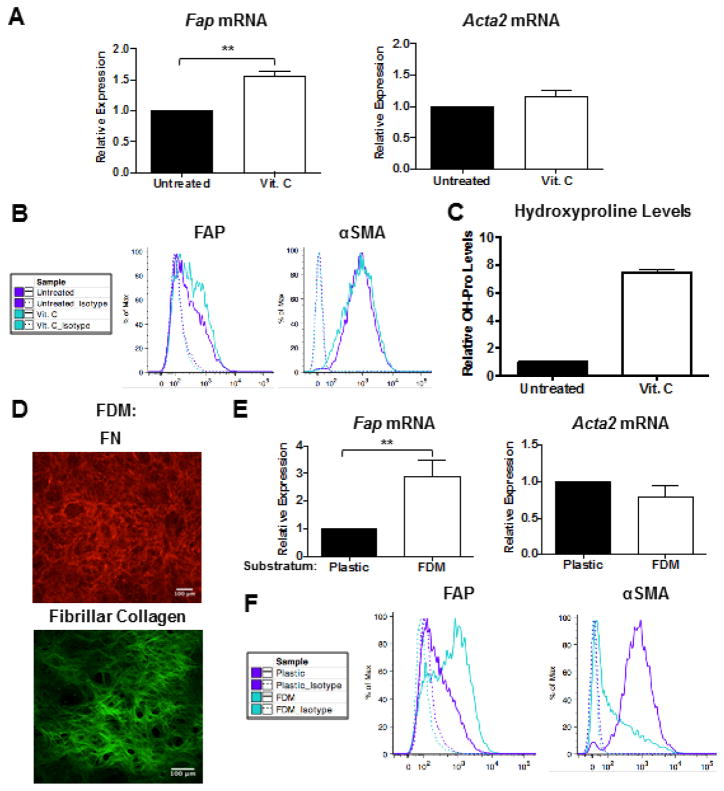
Primary murine adult pulmonary fibroblasts were cultured on tissue culture-treated plastic for 2 passages, which generated a heterogeneous population of activated fibroblasts with variable levels of FAP and αSMA (data not shown). These fibroblasts were reseeded on plastic and the impact of soluble mediators thought to promote fibroblast activation [31–33] on Fap and Acta2 (αSMA) gene expression was assessed. Consistent with prior studies [5], treatment with a variety of fibrotic and inflammatory mediators (in 1% serum to minimize exogenous growth factors) modulated Acta2 expression (Table S1). Unexpectedly, none of these mediators modulated Fap expression, except ascorbic acid, which promoted Fap expression at both the mRNA (Table S1 and Fig. 1A) and protein level (Fig. 1B).
Figure 1. Substratum directs activated fibroblast phenotypic heterogeneity.
QRT-PCR (A) and representative flow cytometric analysis (B) of FAP and αSMA expression in fibroblasts cultured in 10% serum on tissue culture-treated plastic in the presence or absence of 75 μg/ml ascorbic acid (Vit. C) for 4 days. Data were compiled from 4 independent experiments and bar graphs depict the mean +/− SEM. (C) Collagen levels (as measured via hydroxyproline content) in FDMs deposited by fibroblasts in the presence or absence of 75 μg/ml Vit. C. Data were compiled from 2 independent experiments and bar graphs depict the mean +/− SEM. (D) Representative IF staining of FN and two-photon second harmonic generation imaging of fibrillar collagen in lung FDMs. QRT-PCR (E) and representative flow cytometric analysis (F) of FAP and αSMA expression in fibroblasts in 10% serum on tissue culture-treated plastic or FDM for 4 days. Data were compiled from 4 independent experiments and bar graphs depict the mean +/− SEM.
Ascorbic acid (Vitamin C), an essential cofactor for lysyl and prolyl hydroxylation, promotes stable deposition of collagen (Fig. 1C) by ensuring proper folding of its triple helical structure [34]. Thus, we hypothesized that ascorbic acid regulates FAP expression by promoting ECM deposition. To test this hypothesis, fibroblasts were cultured on FN- and fibrillar collagen-rich fibroblast-derived matrices (FDMs), which had a mean elasticity of 1.5 kPa (Figs. 1D and S1). Interestingly, relative to culture on plastic, fibroblasts cultured on FDMs markedly up-regulated Fap gene expression (Fig. 1E). Flow cytometric analysis further demonstrated that culture on FDMs versus plastic enriched for FAPHi fibroblasts (Fig. 1F). Moreover, a concomitant reduction in αSMAHi fibroblasts was observed on FDMs versus plastic (Fig. 1F). These data demonstrate that varying substrata can enrich for phenotypically distinct subsets of activated fibroblasts.
1.2.2 ECM composition and elasticity govern activated fibroblast phenotypic heterogeneity
Compared to plastic, FDMs constitute a more physiologically relevant substratum with respect to multiple parameters, including ECM compliance, architecture, and composition [35]. To delineate the roles of ECM elasticity and composition in driving activated fibroblast heterogeneity, we employed polyacrylamide hydrogels (where ECM ligand and elasticity can be independently controlled [36]). We primarily utilized 2 and 20 kilopascal (kPa) hydrogels, which encompasses the range of stiffness found in pathophysiological conditions, including tumors and lung fibrosis [23,24]. Hydrogels were coated with FN or COL I to simulate early versus late stages, respectively, of wound repair, fibrosis, and tumorigenesis [27–29,37].
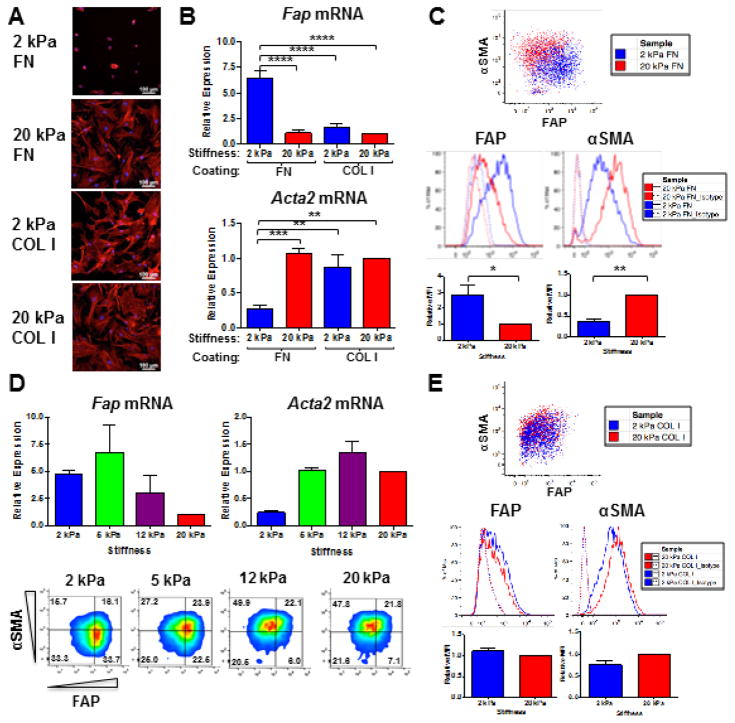
The elasticity of FN-coated hydrogels impacted fibroblast morphology, with reduced cell spreading and cytoskeletal organization after 72 hours of culture on 2 versus 20 kPa FN-coated hydrogels (Fig. 2A), consistent with previous reports [38,39]. Compared to 20 kPa FN-coated hydrogels, 2 kPa FN-coated hydrogels promoted higher FAP and lower αSMA expression, at the mRNA (Fig. 2B) and protein (Fig. 2C) level. Across the pathophysiological stiffness range, Fap gene expression inversely correlated, while Acta2 gene expression directly correlated with the stiffness of FN-coated hydrogels (Fig. 2D, top panel). The full spectrum of activated fibroblast phenotypic differentiation (FAPHiαSMALow, FAPHiαSMAHi, and FAPLowαSMAHi subsets) was observed on 2, 5, 12, and 20 kPa FN-coated hydrogels, as evidenced by flow cytometric analysis at the single cell level (Fig. 2D, bottom panel). However, our data clearly illustrate a shift in prevalence from the FAPHiαSMALow reactive fibroblast phenotype to the FAPLowαSMAHi myofibroblast phenotype with increasing stiffness (Fig. 2D, bottom panel).
Figure 2. ECM composition and elasticity govern activated fibroblast phenotypic heterogeneity.
Representative phalloidin staining of the actin cytoskeleton (A) and Fap and Acta2 gene expression (B) in fibroblasts cultured in 10% serum on 2 versus 20 kPa FN- or COL I-coated hydrogels for 72 hours. Data were compiled from 4 independent experiments and bar graphs depict the mean +/− SEM. (C) Representative flow cytometric analysis, including quantification of relative median fluorescent intensities (MFI) for FAP and αSMA expression in fibroblasts cultured in 10% serum on 2 kPa (blue) versus 20 kPa (red) FN-coated hydrogels for 72 hours. Data were compiled from 3 independent experiments and bar graphs depict the mean +/− SEM. (D) QRT-PCR (top) and flow cytometric analysis (bottom) of FAP and αSMA expression in fibroblasts cultured in 10% serum on 2, 5, 12, and 20 kPa FN-coated hydrogels for 72 hours. Data were compiled from 2 independent experiments and bar graphs depict the mean +/− SEM. (E) Representative flow cytometric analysis, including quantification of relative MFI for FAP and αSMA expression in lung fibroblasts cultured in 10% serum on 2 kPa (blue) versus 20 kPa (red) COL I-coated hydrogels for 72 hours. Data were compiled from 3 independent experiments and bar graphs depict the mean +/− SEM.
Unlike FN-coated hydrogels, both 2 and 20 kPa COL I-coated hydrogels promoted cell spreading and actin stress fiber formation after 72 hours of culture (Fig. 2A). Moreover, both 2 and 20 kPa COL I-coated hydrogels promoted low Fap and high Acta2 gene expression (Fig. 2B), thereby enriching for FAPLowαSMAHi myofibroblasts (Fig. 2E). Taken together, these data indicate that an integrated response to ECM composition and elasticity governs activated fibroblast heterogeneity.
1.2.3 FAPHi and αSMAHi fibroblasts exhibit morphologic and phenotypic plasticity
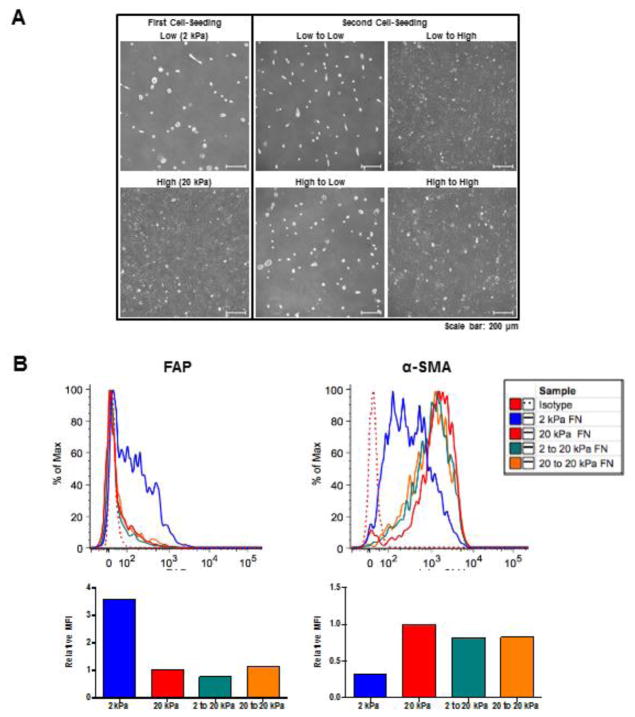
To assess the potential plasticity of these morphologic and phenotypic fibroblast subsets, we performed serial passage experiments. Specifically, fibroblasts were first cultured on either 2 or 20 kPa FN-coated hydrogels for 72 hours. These cells were recovered and then cultured a second time on either 2 or 20 kPa FN-coated hydrogels for an additional 72 hours.
By phase contrast microscopy, we found that the cell morphology associated with the first culture on 2 versus 20 kPa hydrogels (rounded versus spread cells, respectively) was retained when cultured on hydrogels of the same stiffness for a second time (Fig. 3A). In contrast, cells from 2 kPa hydrogels that were re-cultured on 20 kPa hydrogels displayed a morphology resembling that of cells initially cultured on 20 kPa hydrogels. Similarly, cells cultured on 20 kPa hydrogels that were re-cultured on 2 kPa hydrogels displayed a morphology resembling that of cells initially cultured on 2 kPa hydrogels. Combined, these data suggest that fibroblasts, with respect to their cell morphology, possess the ability to switch phenotypes when changing from soft to stiff substrata, and vice versa. This was further supported by flow cytometric analysis (Fig. 3B): cell populations re-cultured on stiff hydrogels (2 to 20 kPa FN, or 20 to 20 kPa FN) were enriched for αSMA (αSMAHI), showing comparable levels of αSMA to those cells seeded on stiff hydrogels in the first culture (20 kPA FN). Furthermore, the cell populations re-cultured on stiff hydrogels were not enriched for FAP, even when originating from a FAPHi-enriched population (2 to 20 kPa FN).
Figure 3. FAPHi and αSMAHi fibroblasts exhibit morphologic and phenotypic plasticity.
Fibroblasts were serially cultured (in 10% FCS DMEM) first on either 2 or 20 kPa FN-coated hydrogels for 72 hours, and then cells recovered by trypsinization from each of these groups were cultured for a second time on either 2 or 20 kPa FN-coated hydrogels for an additional 72 hours. At both rounds 1 and 2, cell monolayers were analyzed by phase contrast microscopy and cell suspensions were analyzed by flow cytometry. Representative phase contrast images (A) and flow cytometric analysis (B), including quantification of relative MFI for FAP and αSMA expression, are shown.
1.2.4 Fibroblast differentiation in response to transforming growth factor beta (TGF-β) is governed by ECM composition and elasticity
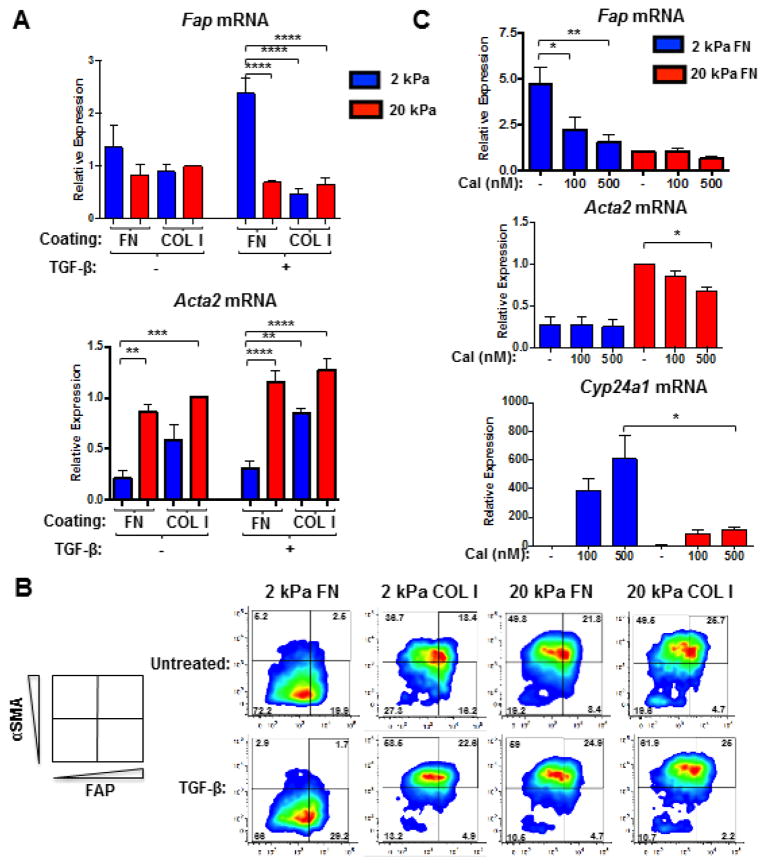
To dissect the role of growth factors in regulating fibroblast heterogeneity, fibroblasts were cultured in low (1%) serum (to minimize exogenous growth factors) on FN- or COL I-coated hydrogels (2 or 20 kPa) for 72 hours. ECM stiffness regulated Acta2 expression, even in 1% serum (Fig. 4A). In contrast, Fap expression was comparably low in fibroblasts on all substrata in 1% serum (Fig. 4A), unlike in 10% serum (Fig. 2). Serum contains appreciable levels of TGF-β, a pro-fibrotic cytokine thought to promote fibroblast activation. Therefore, we investigated the role of TGF-β-mediated signaling in ECM-dependent regulation of FAP expression by using both gain- and loss-of-function approaches. Addition of recombinant, active TGF-β to 1% serum was sufficient to promote the FAPHiαSMALow reactive fibroblast phenotype on 2 kPa FN-coated hydrogels. Conversely, TGF-β promoted the FAPLowαSMAHi myofibroblast phenotype on 2 kPa COL I-coated hydrogels and 20 kPa hydrogels coated with either FN or COL I (Fig. 4A–B).
Figure 4. Fibroblast differentiation in response to TGF-β is governed by ECM composition and elasticity.
QRT-PCR (A) and flow cytometric analysis (B) of FAP and αSMA expression in fibroblasts that were seeded in 1% serum on 2 versus 20 kPa COL I- or FN-coated hydrogels, allowed to adhere overnight, and subsequently treated with 2 ng/ml TGF-β for 48 (A) or 72 hours (B). Data were compiled from 4 independent experiments and bar graphs depict the mean +/− SEM. (C) QRT-PCR analysis of Fap, Acta2, and Cyp24a1 gene expression in fibroblasts that were seeded in 10% serum on FN-coated hydrogels (2 or 20 kPa), allowed to adhere overnight, and subsequently treated with calcipotriol (Cal; 100 or 500 nM) or vehicle control for 48 hours. Data were compiled from 3 independent experiments and bar graphs depict the mean +/− SEM.
To determine whether generation of either activated fibroblast phenotype could be attenuated, fibroblasts in 10% serum were treated with calcipotriol, a vitamin D receptor (VDR) agonist that inhibits TGF-β/SMAD signaling via genomic competition and reverts activated stromal cells to more quiescent phenotypes [40]. The expression of Cyp24a1, a VDR target gene, was measured as a positive control for calcipotriol efficacy. Interestingly, calcipotriol induced higher Cyp24a1 expression in fibroblasts cultured on 2 versus 20 kPa FN-coated hydrogels (Fig. 4C), indicating that high ECM stiffness can confer resistance to calcipotriol. Furthermore, calcipotriol attenuated high Fap and Acta2 gene expression in fibroblasts on 2 and 20 kPa FN-coated hydrogels, respectively (Fig. 4C). These findings demonstrate that VDR agonists can curtail both activated fibroblast phenotypes.
1.2.5 Neither ROCK nor FAK activity are required to induce the FAPHi reactive fibroblast phenotype
Both Rho kinase (ROCK) and focal adhesion kinase (FAK) activity can promote αSMAHi myofibroblast differentiation. ROCK activity facilitates the actin cytoskeletal reorganization necessary for myofibroblast differentiation in response to ECM stiffening [41]. FAK activity facilitates TGF-β-induced myofibroblast differentiation [42]. Furthermore, FN-mediated FAK activation depends on stiffness, since exposure of the cryptic FN synergy site requires mechanical tension [43]. Therefore, we assessed whether ROCK or FAK activity similarly regulate FAPHi reactive fibroblast differentiation. FAPHi fibroblasts (on 2 kPa FN) and αSMAHi myofibroblasts (on 20 kPa FN) were treated with a ROCK (Y-27632) or FAK (PF573228) inhibitor. As expected, ROCK inhibition attenuated actin stress fiber formation (Fig. S2A) and Acta2 expression (Fig. S2B) on 20 kPa FN-coated hydrogels. In contrast, ROCK inhibition had no effect on Fap expression on 2 or 20 kPa FN-coated hydrogels (Fig. S2B). Treatment with PF573228 diminished FAK activity, as indicated by reduced phospo-FAKTyr397 levels (Fig. S2C). However, FAK inhibition did not revert either the FAPHi or αSMAHi phenotype on 2 or 20 kPa FN, respectively (Fig. S2D). Thus, FAK activity does not appear to be required for the induction of either activated fibroblast phenotype in response to changes in the elasticity of FN-coated hydrogels.
1.2.6 Gene expression profiling indicates that FAPHi reactive fibroblasts predominantly synthesize and proteolyze ECM, while αSMAHi myofibroblasts mediate contraction
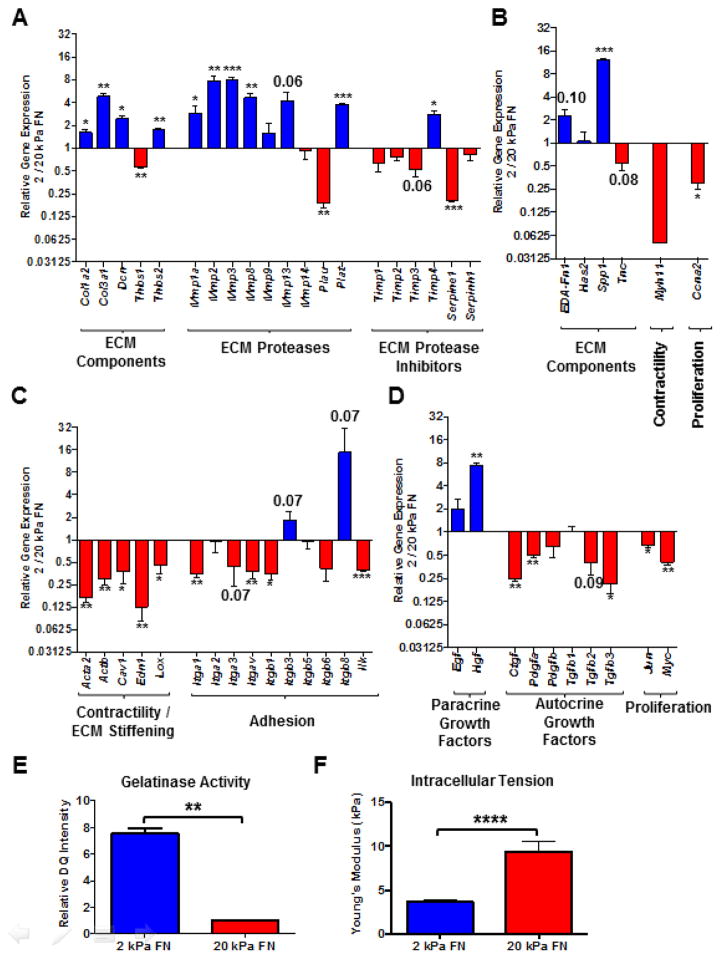
To identify potentially unique functional roles of fibroblast subsets in tissue remodeling, we compared gene expression profiles of fibroblasts cultured in 10% serum on 2 (enriched for FAPHi reactive fibroblasts) versus 20 (enriched for αSMAHi myofibroblasts) kPa FN-coated hydrogels. FAPHi fibroblasts exhibited a more ECM proteolytic phenotype, with higher gene expression of most matrix metalloproteinases (Mmps) and tissue plasminogen activator (Plat; Fig. 5A). Meanwhile, gene expression of most tissue inhibitors of metalloproteinases (Timps) was comparable or reduced in FAPHi compared to αSMAHi fibroblasts (Fig. 5A). FAPHi fibroblasts also exhibited higher gene expression of many ECM and matricellular constituents, including collagens I (Col1a1) and III (Col3a1), decorin (Dcn), extra domain A FN (EDA-Fn1), thrombospondin 2 (Thbs2), and osteopontin (Spp1; Fig. 5A–B). Notably, αSMAHi myofibroblasts on 20 kPa (FN- or COL1-coated) and 2 kPa COL 1-coated hydrogels exhibited comparably low gene expression of ECM proteases and components (Fig. S3). Thus, regardless of the stiffness and/or composition of the underlying substratum, αSMAHi myofibroblasts seem to consistently exhibit a tissue remodeling gene expression signature distinct from that of FAPHi fibroblasts. Collectively, these results implicate FAPHi fibroblasts in the dynamic ECM deposition and turnover characteristic of desmoplastic tissue.
Figure 5. Gene expression profiling and functional tests indicate that FAPHi reactive fibroblasts predominantly synthesize and proteolyze ECM, while αSMAHi myofibroblasts mediate contraction.
Comparison of gene expression profiles, assessed via Qiagen Fibrosis Gene Expression Array (A, C, and D) and independent qRT-PCR analyses (B), of fibroblasts cultured in 10% serum on 2 (FAPHi fibroblasts) or 20 kPa (αSMAHi fibroblasts) FN-coated hydrogels for 72 hours. Relative gene expression (FAPHi/αSMAHi fibroblasts) of ECM proteases (A), ECM protease inhibitors (A), ECM and matricellular components (A and B), contractile mediators (B and C), integrin subunits (C), growth factors (D), and proliferative mediators (B and D). Data were compiled from 3 independent experiments and bar graphs depict the mean +/− SEM. (E) Quantification of relative DQ intensity of fibroblasts that were cultured on 2 or 20 kPa FN-coated hydrogels for 48 hours, and then incubated with DQ gelatin for an additional 24 hours. Data were compiled from 3 independent experiments and bar graphs depict the mean +/− SEM. (F) Atomic force microscopy (AFM) measurements of intracellular tension in fibroblasts cultured in 10% serum on 2 or 20 kPa FN-coated hydrogels for 72 hours. Bar graph depicts the mean +/− SEM (n=10 cells).
On the other hand, FAPHi fibroblasts showed lower gene expression of constituents of the actomyosin contractile apparatus, such as smooth muscle myosin heavy chain (Myh11; Fig. 5B) and beta actin (Actb; Fig. 5C). Furthermore, the gene expression of mediators known to promote fibroblast contractility, including caveolin-1 (Cav1) and endothelin (Edn1), was attenuated in FAPHi compared to αSMAHi fibroblasts (Fig. 5C). The reduced gene expression of most integrin subunits in FAPHi fibroblasts (Fig. 5C) also indicates reduced contractile capacity, considering the necessity of integrins for fibroblasts to exert contractile force on ECM [25]. The gene expression of lysyl oxidase (Lox), an enzyme that crosslinks collagen, was also reduced in FAPHi fibroblasts (Fig. 5C). Taken together, these results suggest that FAPHi reactive fibroblasts have a diminished capacity to contract and crosslink ECM compared to αSMAHi myofibroblasts.
FAPHi reactive fibroblasts and αSMAHi myofibroblasts also diverged with respect to their gene expression of growth factors, inflammatory mediators, and regulators of cell cycle progression. Compared to αSMAHi myofibroblasts, FAPHi fibroblasts expressed lower mRNA levels of autocrine growth factors that can promote myofibroblast differentiation and expansion, including connective tissue growth factor (Ctgf), platelet derived growth factor α (Pdgfa), Tgfb2, and Tgfb3 (Fig. 5D). Conversely, FAPHi fibroblasts expressed higher mRNA levels of hepatocyte growth factor (Hgf), a paracrine growth factor known to promote epithelial proliferation and survival (Fig. 5D). FAPHi fibroblasts also expressed higher mRNA levels of most inflammatory mediators assessed in our fibrosis array, however, they were expressed at low levels, exhibited relatively high variability, and did not achieve statistical significance at a confidence level of 95% (Table S2). Lastly, FAPHi fibroblasts exhibited lower gene expression of pro-proliferative transcription factors, such as jun oncogene (Jun; Fig. 5D) and myelocytomatosis oncogene (Myc; Fig. 5D), and mediators of cell cycle progression, such as cyclin A2 (Ccna2; Fig. 5B), suggestive of a less proliferative phenotype.
We then performed functional assays to corroborate the unique functionality of αSMAHi and FAPHi fibroblasts indicated by gene expression profiling. Assessment of cellular gelatinase activity using DQ gelatin demonstrated that FAPHi fibroblasts exhibited higher gelatinase activity than αSMAHi myofibroblasts (Figs. 5E and S4). Furthermore, atomic force microscopy demonstrated that FAPHi fibroblasts exhibit lower intracellular tension than αSMAHi myofibroblasts (Fig. 5F). Taken together with our gene expression data, these results further suggest that FAPHi fibroblasts display a more highly ECM proteolytic phenotype than the more highly contractile αSMAHi myofibroblasts.
1.3 Discussion
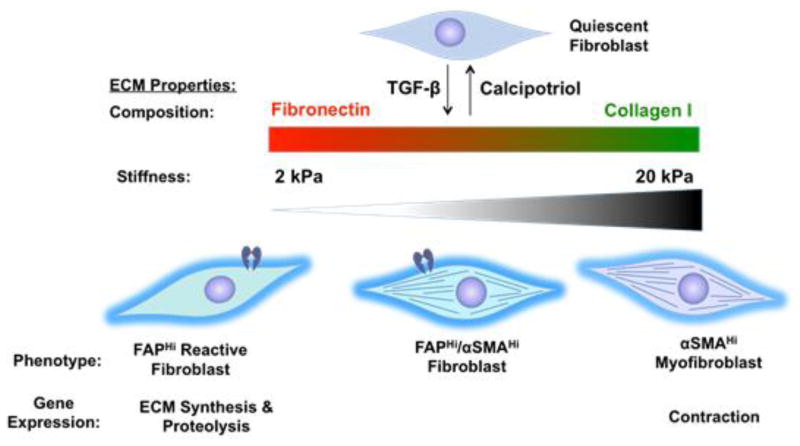
In summary, we delineated features of remodeling tissues that direct activated fibroblast heterogeneity. In our proposed model (Fig. 6), a convergence of ECM composition, elasticity, and TGF-β signaling governs FAPHiαSMALow, FAPHiαSMAHi, and FAPLowαSMAHi fibroblast phenotypic heterogeneity. These data help explain the activated fibroblast heterogeneity observed in vivo, where FAP and αSMA identify distinct, yet overlapping, fibroblast subsets. This heterogeneity likely stems from the spatiotemporally dynamic variation in ECM composition and elasticity in injury, fibrosis, and cancer [21–26,28]. Furthermore, our data from gene expression profiling and functional tests indicate potentially unique functionality of these fibroblast subsets in tissue remodeling. FAPHi reactive fibroblasts exhibited an ECM synthetic and proteolytic phenotype, while αSMAHi myofibroblasts exhibited a contractile and proliferative phenotype.
Figure 6. A convergence of ECM composition, elasticity, and TGF-β signaling governs activated fibroblast phenotypic heterogeneity.
In FN-rich ECMs of low stiffness, TGF-β promotes the FAPHiαSMALow reactive fibroblast phenotype. Conversely, TGF-β promotes the FAPLowαSMAHi myofibroblast phenotype in the context of stiff and/or COL I-rich ECMs. Gene expression profiling indicates unique functionality of these divergent fibroblast subsets in tissue remodeling, with FAPHi reactive fibroblasts exhibiting an ECM synthetic and proteolytic phenotype, and αSMAHi myofibroblasts exhibiting a contractile and proliferative phenotype.
Extrapolating our model to the well-characterized progression of acute injury resolution [22] illuminates ECM-dependent regulation and diverse functionality of fibroblast subsets in tissue remodeling. In early wounds, the highly elastic, FN-rich environment likely promotes FAPHi fibroblast differentiation. The gene expression and functional profile of FAPHi fibroblasts suggests that they orchestrate early phases of wound resolution by secreting: (1) ECM proteases to facilitate cell migration into the wound, (2) paracrine growth factors to support re-epithelialization, and (3) ECM and matricellular components to replace provisional ECM with COL 1-rich connective tissue. Thereafter, the stiff, COL 1-rich environment of late granulation tissue likely promotes αSMAHi myofibroblast differentiation. The gene expression and functional profile of αSMAHi myofibroblasts suggests that they orchestrate late phases of wound resolution, including fibro-proliferation (via secretion of autocrine growth factors) and wound closure (via contraction). We are now conducting studies utilizing murine models of tissue injury to assess this proposed model of temporal regulation and diverse functionality of FAPHi and αSMAHi fibroblasts in wound resolution.
Comparable regulation and functionality of FAPHi and αSMAHi fibroblast subsets likely occur in tumors, considering the parallels between the wound healing response and tumorigenesis [27–29]. Previous studies have characterized diverse mechanisms by which carcinoma-associated fibroblasts promote tumor growth. However, the diverse roles of fibroblast subsets in tumorigenesis remained unclear. Our gene expression and functional analysis suggests that FAPHi and αSMAHi fibroblasts diverge in their tumor-promoting functions. For example, αSMAHi myofibroblasts express higher mRNA levels of Lox, an enzyme that stiffens ECM by crosslinking collagen, which promotes focal adhesions, PI3K signaling, and consequently, tumor invasion [44]. αSMAHi myofibroblasts also exhibit higher gene expression of Cav1, which promotes Rho-dependent contraction, ECM alignment and stiffening, and consequently, tumor invasion [45]. Meanwhile, FAPHi fibroblasts exhibit higher gene expression of many tumor-promoting ECM proteases, including Fap itself and most Mmps. Extensive research has previously demonstrated fundamental roles of these stromal proteases in tumor invasion [46], angiogenesis [47,48], proliferation [47,49,50], and epithelial-mesenchymal transition [51,52]. Moreover, compared to αSMAHi myofibroblasts, FAPHi fibroblasts exhibit higher gene expression of Hgf, a previously described mechanism by which stromal fibroblasts promote oncogenesis and resistance to targeted cancer therapies [53,54].
Importantly, our study addresses the ongoing controversy regarding intratumoral fibroblasts as therapeutic targets [55,56]. A number of laboratories have employed diverse approaches to deplete FAPHi fibroblasts in murine tumor models [11,20,57–62]. Virtually all these studies illustrated that depletion of FAPHi fibroblasts disrupts desmoplastic stroma and impedes tumor progression, corroborating the data presented herein and the correlation between high FAP expression and poor prognosis in myriad solid tumor types [63–79]. On the contrary, the net effect of αSMAHi myofibroblasts in tumor progression remains obfuscated due to conflicting reports. Although many studies have demonstrated a pro-tumorigenic role for αSMAHi myofibroblasts[80,81], one recent study utilizing αSMA-thymidine kinase transgenic mice demonstrated that depletion of proliferating αSMAHi myofibroblasts (via systemic ganciclovir administration) accelerated pancreatic ductal adenocarcinoma aggressiveness [15]. Importantly, αSMAHi myofibroblast depletion did not diminish the prevalence of FAPHi fibroblasts [15], a population that possesses unique tumor-promoting capabilities. In contrast, FAPHi fibroblast depletion also eliminated most intratumoral αSMAHi myofibroblasts [11]. Thus, FAPHi fibroblast depletion may have demonstrated superior anti-tumor efficacy than αSMAHi myofibroblast depletion because it eliminated heterogeneous, tumor-promoting populations of activated fibroblasts. Alternately, the net benefit of FAPHi fibroblast depletion may have overcome the potential detrimental effect of αSMAHi myofibroblast depletion. Our results from serial passage experiments suggest that FAPHi and αSMAHi fibroblasts exhibit morphologic and phenotypic plasticity. However, future studies should further investigate the lineage, interdependence, and plasticity of these activated fibroblast subsets in vivo. FAPHi fibroblasts may facilitate the generation and/or recruitment of αSMAHi myofibroblasts, or myofibroblast precursors may express FAP.
Unraveling the complex regulation of fibroblast heterogeneity will hasten the successful application of therapies that target fibroblasts. In fact, our findings may help explain recent failures in clinical trials of a few molecularly targeted approaches. For example, simtuzumab (an antibody that blocks LOXL2 enzymatic activity) failed in clinical trials for colorectal adenocarcinoma [82], metastatic pancreatic adenocarcinoma[83], and idiopathic pulmonary fibrosis [84,85]. The clinical use of simtuzumab aimed to curb tumor and fibrosis progression by reducing ECM stiffness and myofibroblast generation. Our finding that FAPHi fibroblasts prevail at low stiffness might help explain the inefficacy of simtuzumab, considering the tumor- and fibrosis-promoting propensities of FAPHi fibroblasts. The attenuation of myofibroblast generation via inhibitors of sonic hedgehog signaling may have failed in clinical trials for similar reasons [86–88]. Intriguingly, calcipotriol-mediated blockade of TGF-β/SMAD signaling (currently being evaluated in clinical trials) has shown promise in pre-clinical models of both pancreatic ductal adenocarcinoma and liver cirrhosis [40,89]. We have now demonstrated that calcipotriol can attenuate fibroblast activation to both the FAPHi and αSMAHi phenotype, which helps explain the efficacy of calcipotriol in treating both fibrosis and cancer. Overall, our findings underscore the importance of comprehensive monitoring of heterogenous fibroblast populations in response to stroma-targeted therapies to identify optimal treatment modalities.
1.4 Methods
1.4.1 Isolation of murine lung fibroblasts
Lungs were harvested from 10- to 16-week-old male or female C57BL/6 (Charles River) mice. Lungs were finely minced, then digested in a cocktail of collagenases I, II, and IV (Worthington; each at 250 μg/ml in DMEM) at 37°C for 2 hours. The collagenase solution was quenched with an equivalent volume of 10% heat-inactivated fetal calf serum (FCS) DMEM. The digested tissue was sequentially strained through 70 and 40 μm cell strainers then spun at 1200 r.p.m. for 10 minutes at 4°C. The cell pellet was re-suspended in 10% FCS DMEM (containing HEPES, L-glutamine, penicillin-streptomycin, fungizone, and gentamicin) and plated on tissue culture-treated plastic (polystyrene). Cells were maintained at 37°C with 5% CO2 in a humidified incubator. The next day, cell monolayers were washed several times with PBS to remove non-adherent cells. Cells were maintained in 10% FCS DMEM on tissue culture-treated plastic for two passages. Second passage fibroblasts were used for all experiments.
1.4.2 Fibroblast-derived matrices (FDMs)
Decellularized FDMs were generated as described previously [90], using murine lung fibroblasts cultured at confluence in the presence of 75 μg/ml ascorbic acid for 8 days.
1.4.3 Hydrogels
Polyacrylamide hydrogels of varying stiffness were prepared and coated overnight at 4°C with bovine FN (EMD Millipore; 5 μg/ml) or COL I (PureCol; Advanced BioMatrix; 10 μg/ml) as described previously [36]. Hydrogels were rinsed twice with PBS and unreacted N-hydroxysuccinimide was blocked with 1 mg/ml heat-inactivated bovine serum albumin in serum-free DMEM for 30 minutes at 37°C. Hydrogels were rinsed twice with PBS before cell seeding.
1.4.4. RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Cells were rinsed with PBS, lysed in 1 ml Trizol (Invitrogen), and processed for RNA extraction according to manufacturer’s instructions. After quality assessment by agarose gel electrophoresis, reverse transcription was performed following the standard protocol for the TaqMan Reverse Transcription kit (Applied Biosystems). Transcript levels were assayed via the StepOnePlus Real-Time PCR System (Applied Biosystems) using SYBR Green (Invitrogen). mRNA levels for all genes of interest were normalized to Hprt1 mRNA levels. Primer sequences: Fap-F, 5’-cacctgatcggcaatttgtg; Fap-R, 5’-cccattctgaaggtcgtagatgt; Acta2-F, 5’-ccagagcaagagagggatcct; Acta2-R, 5’-tgtcgtcccagttggtgatg; Hprt1-F, 5’-tgacactggtaaaacaatgca; Hprt1-R, 5’-ggtccttttcaccagcaagct; Tnc-F, 5’-ggacttacgggtgtctgaaacc; Tnc-R, 5’-tgaggcggtaacgatcaaact; Cyp24a1-F, 5’-ccagcggctagagatcaaac; Cyp24a1-R, 5’-cacgggcttcatgagtttct; Spp1-F, 5’-ccctcgatgtcatccctgtt; Spp1-R, 5’-tgccctttccgttgttgtc; Myh11-F, 5’-gacaactcctctcgctttgg; Myh11-R, 5’-gctctccaaaagcaggtcac; Ccna2-F, 5’-tggatggcagttttgaatcacc; Ccna2-R, 5’-ccctaaggtacgtgtgaatgtc; EDA-Fn1-F, 5’-cgagccctgaggatggaat; EDA-Fn1-R, 5’-agctctgcagtgtcgtcttcac; Has2-F, 5’-cggtcgtctcaaattcatctg; Has2-R, 5’-acaatgcatcttgttcagctc; Col1a1-F, 5’-cccaaggaaaagaagcacgtc; Col1a1-R, 5’-acattaggcgcaggaaggtca; Col3a1-F, 5’-gaaagagtggtgacagaggag; Col3a1-R, 5’-tgatgccattagagccacg; Mmp2-F, 5’-cccatgaagccttgtttacca; Mmp2-R, 5’-tggaagcggaacgggaact.
1.4.5 Fibrosis gene expression array
RNA was extracted as described above. RNA purification was performed following the standard protocol for the RNeasy Mini Kit with on-column DNase digestion (Qiagen). After quality assessment by agarose gel electrophoresis, cDNA was synthesized (using 1 μg RNA per sample) with the RT2 First Strand Kit (Qiagen). Both RNA and cDNA quality were confirmed using the Murine RT² Profiler Quality Control Array (Qiagen). Transcript levels were assessed with the RT² Profiler Mouse Fibrosis PCR Array (Qiagen) using RT2 Sybr Green ROX qPCR Mastermix (Qiagen). RNA levels for all genes of interest were normalized to β2 microglobulin (β2M) mRNA levels.
1.4.6 Flow cytometry
For hydrogel (Figs. 2–4) and Vit. C (Fig 1B) experiments, cells were trypsinized to obtain a single cell suspension. For FDM experiments (Fig. 1F), cells (on plastic or FDM) were trypsinized, and then incubated with 500 μg/ml collagenase I (Worthington) for 30 minutes at 37°C to obtain a single cell suspension. Flow cytometric analyses were performed on an LSR-Fortessa using FACSDiva software (BD Bioscience) and data were analyzed using FlowJo (Tree Star).
1.4.7 Antibodies used for flow cytometry
Biotinylated anti-FAP (Clone 73-3) antibody was generated in-house, and detected using Streptavidin-APC (BioLegend). The specificity of the anti-FAP antibody was verified based on reactivity with cells from wild-type but not FAP-null mice. Dead cells were excluded by using the Live/Dead Fixable Violet Dead Cell Stain Kit (Molecular Probes). Staining with anti-αSMA antibody (Sigma, clone1A4, FITC) was performed after permeabilizing cell suspensions with the Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences), as per the manufacturer’s instructions.
1.4.8 Treatment with inhibitors or soluble factors
For indicated experiments, cells were treated with recombinant human active TGF-β (R&D), PF573228 (Tocris), Y-27632 (Sigma), calcipotriol (Tocris), L-ascorbic acid (Vit. C; Fisher), basic fibroblast growth factor (bFGF; Peprotech), interleukin (IL)-1β (Peprotech), IL-3 (Peprotech), interferon gamma (IFNγ; R&D), IL-6 (Peprotech), tumor necrosis factor alpha (TNFα; Peprotech), high molecular weight hyaluronan (HMW HA; Healon), low molecular weight hyaluronan (LMW HA; ICN Biochemicals), periostin/OSF-2 (R&D), buthionine-sulfoximine (BSO; Sigma), tiron (Sigma), trichostatin A (Sigma), pleiotrophin (R&D), angiotensin II (Sigma), and platelet-derived growth factor-bb (PDGFbb; Calbiochem).
1.4.9 Immunoblot
Total cell lysates were prepared from fibroblasts cultured on hydrogels by placing coverslips face down on 4X NuPAGE LDS Sample Buffer (Invitrogen; supplemented with 10 mM 2-mercaptoethanol) for 2 minutes. Cell lysates were then heated at 70°C for 10 minutes. Equal amounts of extracted protein were resolved on NuPAGE 4–12% Bis-Tris gels (Invitrogen) and the fractioned proteins were transferred onto PVDF membranes. Membranes were probed with antibodies to FAK (Cell Signaling, 3285) and phospo-FAKTyr397 (Cell Signaling, 3283), followed by HRP-conjugated goat anti-rabbit secondary antibody (Sigma, A0545).
1.4.10 Microscopy
Imaging of fibrillar collagen in decellularized fibroblast-derived matrices was based on second harmonic generation utilizing a Leica SP5 confocal/multiphoton microscope (Leica Microsystems) at 20X magnification, as described previously[91]. Immunofluorescent (IF) and phase contrast images were obtained with a Nikon Eclipse Ti-E inverted microscope at 10X (FN, phase contrast) or 20X (phalloidin; DQ gelatin, phase contrast) magnification.
1.4.11 Hydroxyproline assay
Collagen content in FDMs produced by lung fibroblasts (in the presence or absence of 75 μg/ml ascorbic acid) was quantified by measuring hydroxyproline levels (normalized to cell number as measured by calcein AM levels), as previously described[92].
1.4.12 IF
For FN IF, FDMs produced by lung fibroblasts were incubated with rabbit anti-FN antibody (Sigma, Clone F3648), followed by Alexa Fluor 658 goat anti-rabbit antibody (Invitrogen). For visualization of the actin cytoskeleton, lung fibroblasts on 2 and 20 kPa hydrogels were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X-100, then stained with Alexa Fluor 594-conjugated phalloidin (Molecular Probes) and DAPI.
1.4.13 Atomic Force Microscopy
The elastic moduli of cells, FDMs, and hydrogels were measured using a Catalyst Bioscope AFM mounted on a Nikon Eclipse TE 200 microscope. Samples were indented with a standard silicon nitride cantilever (spring constant =0.07 N/m) with a 1 μm spherical tip using a maximum force of 7 nN. AFM force curves were analyzed by fitting the sample indentation to the Hertz model for a sphere making contact with a homogeneous elastic half space. Three measurements of intracellular stiffness were collected at regions of the cell between the nucleus and the periphery, and ten cells were measured per condition. AFM force curves were analyzed and converted to Young’s modulus using NanoScope Analysis software from Bruker.
1.4.14 Gelatinolysis Assay
Cells were seeded onto 2 or 20 kPa FN-coated hydrogels and incubated for 48 hours. Growth medium was then removed and replaced with a 1:1 ratio of growth medium:reaction buffer (50 mM Tris, 150 mM NaCl, 5 mM CaCl2, 0.2 mM NaN3) containing 20 μg/ml DQ gelatin (D12054, Thermofisher). Cells were incubated for a further 24 hours before being fixed with 4% paraformaldehyde, and counter-stained with DAPI. Hydrogels were imaged in phase contrast / fluorescence at 20x magnification. DQ intensity levels were analyzed using NIS Elements software as follows: integrated fluorescent intensity levels for DQ gelatin were calculated per cell (150 cells per group; 2 groups per experiment; 3 independent experiments). Mean relative DQ intensities per cell area were then calculated for the 3 independent experiments.
1.4.15 Statistics
All results are reported as mean (of the indicated number of independent experiments) +/− standard error of the mean (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA) with the Tukey’s multiple comparison test or two-tailed Student’s t test (Prism 7.0, GraphPad Software). Asterisks denote statistical significance: **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05.
Highlights.
Fibroblast activation protein (FAP) and alpha-smooth muscle actin (αSMA) identify distinct, yet overlapping, activated fibroblast subsets in a variety of pathophysiological settings, including wound healing, fibrosis, and tumorigenesis.
A convergence of extracellular (ECM) composition, elasticity, and transforming growth factor beta (TGF-β) signaling governs activated fibroblast phenotypic heterogeneity.
Low stiffness, fibronectin-rich ECMs promote the FAPHiαSMALow reactive fibroblast phenotype.
Stiff and/or collagen I-rich ECMs promote the FAPLowαSMAHi myofibroblast phenotype.
Gene expression profiling indicates unique functionality of FAPHi and αSMAHi activated fibroblast subsets in tissue remodeling.
Acknowledgments
We thank the Biomechanics Core Facility of the Institute for Translational Medicine and Therapeutics (ITMAT) for providing hydrogels and performing atomic force microscopy, and the Shared Matrix Analysis Facility of the Penn Vet Cancer Center for technical support. We also thank Dr. Rebecca Wells and Dr. Sandra Ryeom for helpful discussions and assistance in preparing the manuscript. The authors declare no competing financial interests.
1.6 Funding
This work was supported by the National Institute of Health (grant numbers: R01CA141144, R21CA169741, and R01CA172921 (EP)).
Abbreviations
- AFM
atomic force microscopy
- αSMA
alpha-smooth muscle actin
- COL 1
collagen I
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- FAP
fibroblast activation protein
- FDM
fibroblast-derived matrix
- FN
fibronectin
- kPa
kilopascal
- ROCK
rho kinase
- TGF-β
transforming growth factor beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.7 References
- 1.Servais C, Erez N. From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J Pathol. 2013;229:198–207. doi: 10.1002/path.4103. [DOI] [PubMed] [Google Scholar]
- 2.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. http://dx.doi.org/10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat M-L, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 6.Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Acharya PS, Zukas A, Chandan V, Katzenstein ALA, Puré E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Pathol. 2006;37:352–360. doi: 10.1016/j.humpath.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Müller E, Rettig WJ, Gorrell MD. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29:1768–78. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 9.Bauer S, Jendro MC, Wadle A, Kleber S, Stenner F, Dinser R, Reich A, Faccin E, Gödde S, Dinges H, Müller-Ladner U, Renner C. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther. 2006;8:R171. doi: 10.1186/ar2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo A, Wang L-CS, Scholler J, Monslow J, Avery D, Newick K, O9Brien S, Evans RA, Bajor DJ, Clendenin C, Durham AC, Buza EL, Vonderheide RH, June CH, Albelda SM, Puré E. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. http://cancerres.aacrjournals.org/content/75/14/2800.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchou J, Zhang PJ, Bi Y, Satija C, Marjumdar R, Stephen TL, Lo A, Chen H, Mies C, June CH, Conejo-Garcia J, Puré E. Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer. Hum Pathol. 2013;44:2549–2557. doi: 10.1016/j.humpath.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 14.Busek P, Balaziova E, Matrasova I, Hilser M, Tomas R, Syrucek M, Zemanova Z, Krepela E, Belacek J, Sedo A. Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma. Tumor Biol. 2016;37:13961–13971. doi: 10.1007/s13277-016-5274-9. [DOI] [PubMed] [Google Scholar]
- 15.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, Hwang C-I, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–596. doi: 10.1084/jem.20162024. http://jem.rupress.org/content/early/2017/02/23/jem.20162024.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilvaer TK, Khanehkenari MR, Hellevik T, Al-Saad S, Paulsen EE, Bremnes RM, Busund LT, Donnem T, Martinez IZ. Cancer associated fibroblasts in stage I-IIIA NSCLC: prognostic impact and their correlations with tumor molecular markers. PLoS One. 2015;10:e0134965. doi: 10.1371/journal.pone.0134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, Dang Y, Chu Y, Fan J, He R. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3–CCL2 signaling. Cancer Res. 2016;76:4124 LP–4135. doi: 10.1158/0008-5472.CAN-15-2973. http://cancerres.aacrjournals.org/content/76/14/4124.abstract. [DOI] [PubMed] [Google Scholar]
- 19.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein–α. Science. 2010;330:827–830. doi: 10.1126/science.1195300. http://science.sciencemag.org/content/330/6005/827.abstract. [DOI] [PubMed] [Google Scholar]
- 20.Wang L-CS, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC, Solomides CC, June CH, Puré E, Albelda SM. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154 LP–166. doi: 10.1158/2326-6066.CIR-13-0027. http://cancerimmunolres.aacrjournals.org/content/2/2/154.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurme T, Kalimo H, Sandberg M, Lehto M, Vuorio E. Localization of type I and III collagen and fibronectin production in injured gastrocnemius muscle. Lab Invest. 1991;64:76–84. http://europepmc.org/abstract/MED/1703587. [PubMed] [Google Scholar]
- 22.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. http://jcb.rupress.org/content/190/4/693.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, LYH, Schoenenberger C-A. The nanomechanical signature of breast cancer. Nat Nano. 2012;7:757–765. doi: 10.1038/nnano.2012.167. http://dx.doi.org/10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun. 2015;6:8026. doi: 10.1038/ncomms9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvorak HF. Tumors: wounds that do not heal--Redux. Cancer Immunol Res. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: wounds that do not heal. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 29.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. http://dx.doi.org/10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 30.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–68. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. http://jem.rupress.org/content/211/8/1503.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. http://dx.doi.org/10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 33.Puré E, Lo A. Can targeting stroma pave the way to enhanced antitumor immunity and immunotherapy of solid tumors? Cancer Immunol Res. 2016;4:269–278. doi: 10.1158/2326-6066.CIR-16-0011. http://cancerimmunolres.aacrjournals.org/content/4/4/269.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinnell SR. Regulation of collagen biosynthesis by ascorbic acid: a review. Yale J Biol Med. 1985;58:553–559. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2589959/ [PMC free article] [PubMed] [Google Scholar]
- 35.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. http://science.sciencemag.org/content/294/5547/1708.abstract. [DOI] [PubMed] [Google Scholar]
- 36.Cretu A, Castagnino P, Assoian R. Studying the effects of matrix stiffness on cellular function using acrylamide-based hydrogels. J Vis Exp. 2010:2089. doi: 10.3791/2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybinski B, Franco-Barraza J, Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol Genomics. 2014;46:223–244. doi: 10.1152/physiolgenomics.00158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein EA, Castagnino P, Kothapalli D, Yin L, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–1518. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, Lau SL, Atkins AR, Barish GD, Gunton JE, Liddle C, Downes M, Evans RM. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 43.Seong J, Tajik A, Sun J, Guan JL, Humphries MJ, Craig SE, Shekaran A, García AJ, Lu S, Lin MZ, Wang N, Wang Y. Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc Natl Acad Sci. 2013;110:19372–19377. doi: 10.1073/pnas.1307405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibáñez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJP, Garcia R, Keely PJ, Sánchez-Mateos P, Cukierman E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal Caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. http://cancerres.aacrjournals.org/content/58/5/1048.abstract. [PubMed] [Google Scholar]
- 49.Cheng JD, Dunbrack RL, Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62:4767–4772. [PubMed] [Google Scholar]
- 50.Cheng JD, Valianou M, Canutescu AA, Jaffe EK, Lee HO, Wang H, Lai JH, Bachovchin WW, Weiner LM. Abrogation of fibroblast activation protein enzymatic activity attenuates tumor growth. Mol Cancer Ther. 2005;4:351–360. doi: 10.1158/1535-7163.MCT-05-0128. [DOI] [PubMed] [Google Scholar]
- 51.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier J-P, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2853255/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. http://science.sciencemag.org/content/303/5659/848.abstract. [DOI] [PubMed] [Google Scholar]
- 54.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. http://dx.doi.org/10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. http://dx.doi.org/10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 56.Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer Cell. 2014;25:711–712. doi: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M, Xiang R, Wen Y, Xu G, Wang C, Luo S, Yin T, Wei X, Shao B, Liu N, Guo F, Li M, Zhang S, Li M, Ren K, Wang Y, Wei Y. A whole-cell tumor vaccine modified to express fibroblast activation protein induces antitumor immunity against both tumor cells and cancer-associated fibroblasts. Sci Rep. 2015;5:14421. doi: 10.1038/srep14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. http://cancerres.aacrjournals.org/content/65/23/11156.abstract. [DOI] [PubMed] [Google Scholar]
- 59.Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang J, Xiao L, Joo KI, Liu Y, Zhang C, Liu S, Conti PS, Li Z, Wang P. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int J Cancer. 2016;138:1013–1023. doi: 10.1002/ijc.29831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LeBeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther. 2009;8:1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. http://mct.aacrjournals.org/content/8/5/1378.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu Z, Hao Z, Li Z, Liu L, Xie J. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 63.Wen XX. Fibroblast activation protein-α-positive fibroblasts promote gastric cancer progression and resistance to immune checkpoint blockade. Oncol Res. 2016;25:629–640. doi: 10.3727/096504016X14768383625385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasa S, Okada K, Chen W-T, Jin X, Yamane T, Ooi A, Mitsumata M. Increased expression of seprase, a membrane-type serine protease, is associated with lymph node metastasis in human colorectal cancer. Cancer Lett. 2005;227:229–236. doi: 10.1016/j.canlet.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Qiu X, Wang X, He J. FAP positive fibroblasts induce immune checkpoint blockade resistance in colorectal cancer via promoting immunosuppression. Biochem Biophys Res Commun. 2017;487:8–14. doi: 10.1016/j.bbrc.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 66.Henry LR, Lee H-O, Lee JS, Klein-Szanto A, Watts P, Ross EA, Chen W-T, Cheng JD. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. http://clincancerres.aacrjournals.org/content/13/6/1736.abstract. [DOI] [PubMed] [Google Scholar]
- 67.Wikberg ML, Edin S, Lundberg IV, Van Guelpen B, Dahlin AM, Rutegård J, Stenling R, Öberg Å, Palmqvist R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumor Biol. 2013;34:1013–1020. doi: 10.1007/s13277-012-0638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, Hoffman JP, Weiner LM, Cheng JD. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 69.Kawase T, Yasui Y, Nishina S, Hara Y, Yanatori I, Tomiyama Y, Nakashima Y, Yoshida K, Kishi F, Nakamura M, Hino K. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2015;15:109. doi: 10.1186/s12876-015-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patsouras D, Papaxoinis K, Kostakis A, Safioleas M, Lazaris A, Nicolopoulou-Stamati P. Fibroblast activation protein and its prognostic significance in correlation with vascular endothelial growth factor in pancreatic adenocarcinoma. Mol Med Rep. 2015;11:4585–90. doi: 10.3892/mmr.2015.3259. [DOI] [PubMed] [Google Scholar]
- 71.Errarte P, Guarch R, Pulido R, Blanco L, Nunes-Xavier CE, Beitia M, Gil J, Angulo JC, López JI, Larrinaga G. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. PLoS One. 2016;11:e0169105. doi: 10.1371/journal.pone.0169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López JI, Errarte P, Erramuzpe A, Guarch R, Cortés JM, Angulo JC, Pulido R, Irazusta J, Llarena R, Larrinaga G. Fibroblast activation protein predicts prognosis in clear cell renal cell carcinoma. Hum Pathol. 2016;54:100–105. doi: 10.1016/j.humpath.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y, Zhang Y, Hua S, Fu Q, Zhao M, Chen Y, Fang W, Lv X. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis. 2014;5:e1155. doi: 10.1038/cddis.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu H, Yang J, Li Y, Jiao S. The expression of fibroblast activation protein-α in primary breast cancer is associated with poor prognosis. [accessed April 30, 2017];Chinese J Cell Mol Immunol. 2015 31:370–4. http://www.ncbi.nlm.nih.gov/pubmed/25744843. [PubMed] [Google Scholar]
- 75.Jia J, Martin T, Ye L, Jiang W. FAP-α (Fibroblast activation protein-α) is involved in the control of human breast cancer cell line growth and motility via the FAK pathway. BMC Cell Biol. 2014;15:16. doi: 10.1186/1471-2121-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan D, Liu B, Liu K, Zhu G, Dai Z, Xie Y. Overexpression of fibroblast activation protein and its clinical implications in patients with osteosarcoma. J Surg Oncol. 2013;108:157–162. doi: 10.1002/jso.23368. [DOI] [PubMed] [Google Scholar]
- 77.Liao Y, Ni Y, He R, Liu W, Du J. Clinical implications of fibroblast activation protein-α in non-small cell lung cancer after curative resection: a new predictor for prognosis. J Cancer Res Clin Oncol. 2013;139:1523–1528. doi: 10.1007/s00432-013-1471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Fujikawa H, Matsusita K, Kawamura M, Inoue Y, Miki C, Kusunoki M. Cancer-associated fibroblasts correlate with poor prognosis in rectal cancer after chemoradiotherapy. Int J Oncol. 2011;38:655–63. doi: 10.3892/ijo.2011.906. [DOI] [PubMed] [Google Scholar]
- 79.Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, Zhou J, Li YW, Tang ZY. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131:498–510. doi: 10.1309/AJCP86PPBNGOHNNL. [DOI] [PubMed] [Google Scholar]
- 80.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 81.Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2:1139–1145. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hecht JR, Bendell JC, Vyushkov D, Bencardino K, Verma UN, Yang Y, Thai DL. A phase II, randomized, double-blinded, placebo-controlled study of simtuzumab or placebo in combination with FOLFIRI for the second line treatment of metastatic KRAS mutant colorectal adenocarcinoma. J Clin Oncol. 2015;33:243–e23. doi: 10.1200/jco.2015.33.15_suppl.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benson A, III, Bendell J, Wainberg ZA, Vyushkov D, Acs P, Kudrik F, Dong H, Thai D. 618PD A phase 2 randomized, double-blind, placebo controlled study of simtuzumab or placebo in combination with gemcitavine for the first line treatment of pancreatic adenocarcinoma. Ann Oncol. 2014;25:iv211–iv211. doi: 10.1634/theoncologist.2017-0024. http://dx.doi.org/10.1093/annonc/mdu334.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raghu G, Brown KK, Collard HR, Cottin V, Gibson KF, Kaner RJ, Lederer DJ, Martinez FJ, Noble PW, Song JW, Wells AU, Whelan TPM, Wuyts W, Moreau E, Patterson SD, Smith V, Bayly S, Chien JW, Gong Q, Zhang JJ, O’Riordan TG. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med. 2017;5:22–32. doi: 10.1016/S2213-2600(16)30421-0. [DOI] [PubMed] [Google Scholar]
- 85.Meyer KC. Great expectations for simtuzumab in IPF fall short. Lancet Respir Med. 2017;5:2–3. doi: 10.1016/S2213-2600(16)30420-9. [DOI] [PubMed] [Google Scholar]
- 86.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winter SP, Ireland H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, Nagle JM, Deshpande V, Boucher Y, Kato T, Chen JK, Willmann JK, Bardeesy N, Beachy PA. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O’Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beacham DA, Amatangelo MD, Cukierman E. Curr Protoc Cell Biol. John Wiley & Sons, Inc; 2001. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. [DOI] [PubMed] [Google Scholar]
- 91.Brisson BK, Mauldin EA, Lei W, Vogel LK, Power AM, Lo A, Dopkin D, Khanna C, Wells RG, Puré E, Volk SW. Type III collagen directs stromal organization and limits metastasis in a murine model of breast cancer. Am J Pathol. 2015;185:1471–1486. doi: 10.1016/j.ajpath.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. J Mol Biol. 2009;385:902–911. doi: 10.1016/j.jmb.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]