Abstract
The colony‐stimulating factor 1 (CSF1) regulates the differentiation and function of tissue macrophages and determines the outcome of the immune response. The molecular mechanisms behind CSF1‐mediated macrophage development remain to be elucidated. Here we demonstrate that neutrophil‐derived CSF1 controls macrophage polarization and proliferation, which is necessary for the induction of tolerance. Inhibiting neutrophil production of CSF1 or preventing macrophage proliferation, using targeted nanoparticles loaded with the cell cycle inhibitor simvastatin, abrogates the induction of tolerance. These results provide new mechanistic insights into the developmental requirements of tolerogenic macrophages and identify CSF1 producing neutrophils as critical regulators of the immunological response.
Keywords: basic (laboratory) research/science, immunobiology, macrophage/monocyte biology: differentiation/maturation, tolerance: mechanisms
Short abstract
In this article, Ochando and colleagues demonstrate that graft‐infiltrating neutrophils produce CSF1 that promotes macrophage polarization and mediates transplantation tolerance in recipient mice treated with costimulation blockade.
Abbreviations
- CSF1
colony‐stimulating factor 1
- FACS
fluorescence‐activated cell sorting
- G‐MDSC
granulocytic myeloid‐derived suppressor cell
- HDL
high‐density lipoprotein
- MDSC
myeloid‐derived suppressor cell
- M‐MDSC
monocytic myeloid‐derived suppressor cell
- S‐HDL
simvastatin high‐density lipoprotein
1. INTRODUCTION
Macrophage accumulation in the transplanted organ has long been recognized as a feature of allograft rejection.1 Early after transplantation, macrophage precursors infiltrate the allograft and represent the major cell subset during antibody and T‐cell mediated rejection.2, 3 These inflammatory macrophages are characterized phenotypically by their high expression of Ly6C (Ly6Chi or M1).4 Recent evidence suggests that macrophages are also important during the induction of transplantation tolerance.5 Presence of graft infiltrating macrophages has been described in long‐term surviving transplant recipients and these immunosuppressive macrophages are associated with unresponsiveness to the transplanted organ.6 These suppressive macrophages are characterized phenotypically by their low expression of Ly6C (Ly6Clo or M2).5 This suggests that graft‐infiltrating monocytes differentiate into either immunogenic (Ly6Chi) or tolerogenic (Ly6Clo) macrophages, which determines the outcome of the immunological response. While the phenotype and function of macrophages that mediate the induction of transplantation tolerance has recently been reported,5 their developmental requirements remain poorly understood.
Monocytes differentiate into classically (Ly6Chi/M1) or alternatively (Ly6Clo/M2) activated macrophages according to the local environment.7, 8 Inflammatory Ly6Chi monocytes are rapidly recruited to inflamed tissues and become alternatively activated Ly6Clo macrophages once the inciting inflammatory stimulus has been resolved.9 In organ transplantation, inflammatory Ly6Chi monocytes infiltrate the allograft early after transplantation and differentiate into suppressive Ly6Clo macrophages following costimulatory blockade.5 Therefore, the signals that dictate macrophage polarization towards alternatively activated Ly6Clo/M2 control immunological tolerance.10, 11
Macrophage polarization into suppressive Ly6Clo macrophages is mediated by cytokines and growth factors.12, 13 In this respect, the colony‐stimulating factor 1 (CSF1) has been demonstrated to control macrophage polarization.14 CSF1 also controls the function of macrophages and several reports have documented suppressive function of CSF1 differentiated macrophages in mixed lymphocyte reactions.15, 16, 17 This suggests that the local production of CSF1 controls both the differentiation and immune regulatory function macrophages.
Here, we investigated the mechanistic insights of CSF1 on macrophage differentiation and function and we demonstrate that CSF1 producing neutrophils mediate immunological tolerance by promoting the development of proliferating Ly6Clo macrophages with suppressive function.
2. EXPERIMENTAL PROCEDURES
2.1. Mice
BALB/c and C57BL/6 mice eight weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). The C57BL/6 mKO2‐hCdt1(30/120) and mAG‐hGem(1/110) transgenic mice were from D. Atsushi Miyawaki (RIKEN, Brain Science Institute, Hirosawa, Saitama, Japan).18 The CSF1flox mice have been previously described.19 All experiments were performed with age‐ and sex‐matched mice in accordance with Institutional Animal Care and Utilization Committee‐approved protocols.
2.2. Vascularized heart transplantation
BALB/c hearts were transplanted as fully vascularized heterotopic grafts into C57BL/6 mice as previously described.20 Recipient mice were treated with 250 μg anti‐CD40L mAb (clone MR1, BioXcell, West Lebanon, NH) for tolerance induction on days 0, 2, and 4 as previously described.21 Graft function was monitored every other day by abdominal palpation. Untreated control mice received hamster IgG in PBS. Rejection was defined as complete cessation of a palpable beat and confirmed by direct visualization at laparotomy.
2.3. Isolation of graft infiltrating leukocytes (GIL)
Mouse hearts were rinsed in situ with HBSS with 1% heparin. Explanted hearts were cut into small pieces and digested for 40 minutes at 37°C with 400 U/ml collagenase IV (Sigma‐Aldrich), 10 mM HEPES (Corning Cellgro, Manassas, VA), and 0.01% DNase I (MP Biomedicals) in HBSS (Cellgro). Digested suspensions were passed through a nylon mesh and centrifuged, and the cell pellet was resuspended in 5 ml 45.5% Nycodenz solution (Sigma‐Aldrich). Complete DMEM (3 ml) was added to the top of the Nycodenz, and gradient centrifugation was performed (1700 g for 15 minutes at 4°C). The cells at the interface were recovered, washed with complete DMEM, stained, and analyzed by flow cytometry (BD LSR‐II; BD Biosciences, San Jose, CA).
2.4. Flow cytometry and cell sorting
Fluorochrome‐conjugated mAbs specific to mouse CSF‐1R (clone AFS98), CD11b (clone M1/70), CD11c (clone N418), I‐A/I‐E clone (clone M5/114.15.2), CD45 (clone 30‐F11), CD90.2 (clone 53‐2.1), CD8 (clone 53‐6.7), CD4 (clone GK1.5), Foxp3 (clone JFK 16s), CD44 (clone IM7), CD62L (clone MEL‐14), corresponding isotype controls, and secondary reagents (PE‐conjugated streptavidin) were purchased from eBioscience. Fluorochrome‐conjugated anti‐Ly6G (Clone 1A8) mAb was purchased from Biolegend. Cell washes and Ab dilutions were performed in PBS plus 1% BSA at 4°C. Flow cytometric analysis was performed on LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). Results are expressed as percentage of cells staining above background, and mAbs were tittered at regular intervals during the course of these studies to ensure that saturating concentrations were used. To purify graft infiltrating myeloid cells, donor heart single cell suspensions were sorted with an FACS Aria cell sorter (BD) to achieve >96% purity at the Flow Cytometry Shared Resource Facility at Icahn School of Medicine at Mount Sinai.
2.5. Immunofluorescence microscopy
Transplanted hearts were harvested, subdivided, frozen directly in OCT (Fisher, Waltham, MA), and stored at −80°C in preparation for immunological studies. Sections of 8 μm were cut using a Leica 1900CM cryomicrotome, fixed, and mounted with Gel/Mount (Biomeda, Foster City, CA) on polylysine‐coated slides. Anti‐Ki67 (AbD02531) and CD169 (clone 3D6.112) were purchased from AbD Serotec (Hercules, CA). All slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA) to preserve fluorescence. Images were acquired with a Leica DMRA2 fluorescence microscope (Wetzlar, Buffalo Grove, IL) and a digital Hamamatsu charge‐coupled device camera. Separate green, red, and blue images were collected and analyzed with Openlab software (Improvision, Coventry, England).
2.6. Microarray
Graft infiltrating recipient CD45+CD11b+Ly6ChiLy6G−, CD45+CD11b+ Ly6CloLy6G−, and CD45+CD11b+Ly6CintLy6G+ myeloid cells sorted from anti‐CD40L mAb treated and untreated recipients at day 5 after transplantation. Cells were sorted twice with a FACS Aria II sorter (BD Biosciences) to achieve >98% purity. A total of nine Affymetrix Mouse Exon GeneChip arrays were run in triplicate with the samples of interest. Raw CEL file data from Affymetrix Expression Console were background corrected, normalized, and summarized using Robust Multichip Average. The summary expression scores were computed at the transcript meta‐probeset level using annotation files supplied by the manufacturer. The data was imported into R. Since these arrays were run in different batches, the gene expression was batch corrected with Combat. Gene expression was filtered based on IQR (0.25) filter using genefilter package. The log2 normalized and filtered data (adjusted P < .05) was used for further analysis (GEO accession number GSE68648).
2.7. Quantitative RT‐PCR
Total RNA was extracted from purified cells with RNeasy Plus Micro Kit (Qiagen, Hilden, Germany). Reverse transcription was carried out using the Omniscript reverse‐transcription system (Qiagen) and random primers. Quantitative PCR was performed with the LightCycler system (Roche, Basel, Switzerland) and the SYBR Green PCR kit (Qiagen). All experiments were done in triplicate at least three separate times, and expression of specific genes was normalized and expressed as percentage relative to housekeeping genes or RNA fold expression according to the ddCT method.
2.8. Bone marrow–derived monocyte cultures
Bone marrow Ly6Chi monocytes were FACS sorted from the mice femur and plated in 96‐U‐botton well plate at 5 × 104 cell/well in RPMI‐1640 w/L‐Glutamine medium (Corning Cellgro) containing 10% heat‐inactivated FCS (Biochrom, Berlin, Germany), 1% penicillin/streptavidin (Corning Cellgro). Bone marrow Ly6Chi monocytes were then cultured for 72 hours in the presence of recombinant murine CSF1 at 10 ng/ml (Peprotech, Rocky Hill, NJ) or simvastatin loaded HDL nanoparticles (S‐HDL) at 10 μM.
2.9. In vitro suppression assay
Spleens of C57BL/6 mice were gently dissociated into single‐cell suspensions, and red blood cells were removed using hypotonic ACK lysis buffer. Splenocytes were either stained with anti‐CD8 mAb, followed by CFSE at 5 μM concentration (Molecular probes, Invitrogen, Waltham, MA) Responder CFSE+CD8+ T cells were sorted using FACS Aria II sorter (BD Biosciences) with a purity >98%. Spleens of BALB/c (H‐2d) mice were gently dissociated into single‐cell suspensions, and red blood cells were removed using hypotonic ACK lysis buffer. Splenocytes were enriched for CD11c+ cells using the EasySep Mouse CD11c Positive Selection Kit (StemCell, Cambridge, MA). Enriched CD11c+ splenocytes were stained with anti‐mouse CD11c mAb for 30 minutes on ice. CD11c+ cells were sorted using FACS Aria II sorter (BD Biosciences) and were used together with anti‐CD3/CD28 mAb (1 μg/ml) as stimulators. Responder CFSE+CD8+ T cells were stimulated allogeneic CD11c plus anti‐CD3/CD28 mAb in U‐bottom 96‐well plates (Corning). Graft infiltrating CD11b+ Ly6CloLy6G− sorted macrophages from anti‐CD40L mAb treated recipients at day 5 after transplantation were added to the cultures in a final volume of 250 μl complete medium (RPMI + 10% FCS + l‐Glutamine + sodium pyruvate + NEAA + Pen/Strep + β‐mercaptoethanol). Cells were cultured for four days at 37°C in a 5% CO2 incubator. T cell proliferation was measured by flow cytometric analysis of CFSE dilution on CD8+ T cells.
2.10. In vivo treatment
Human recombinant CSF1 (Peprotech) wan injected i.v. at 2 × 105 U/mice on days 0‐5 relative to transplantation.
2.11. Nanoparticles synthesis
Our targeted approach delivers the drug simvastatin using a synthetic high‐density lipoprotein (HDL) nanoparticle. These nanoparticles were synthesized using a lipid film hydration method. Phospholipids and simvastatin were dissolved in methanol/chloroform. After evaporating the solvents, human ApoA1 in PBS was added to hydrate the lipid film. This resulting solution was sonicated to form small simvastatin loaded HDL nanoparticles (S‐HDL). The animals received 3 intravenous tail injections of S‐HDL at 60 mg/kg on the day of transplantation as well as days 2 and 5 post‐transplantation.
2.12. Statistics
Differences between graft survival rates were assessed by Kaplan‐Meier survival analysis with Prism software. Unpaired Mann‐Whitney test was used when comparing two groups. Kruskal‐Wallis with Dunn's multiple comparison test was used for comparisons among multiple groups. Statistical significance is expressed as follows: *P ≤ .05, **P ≤ .01, NS not significant.
3. RESULTS
3.1. Neutrophil derived CSF1 mediates macrophage polarization
Suppressive Ly6Clo macrophages expressing the macrophage colony‐stimulating factor 1 receptor (CSF‐1R, MCSFR, or CD115) mediate the induction of indefinite allograft survival.5, 22 Induction of transplantation tolerance is controlled by local expression of CSF1, as in vivo blockade of CSF‐1 prevents the conversion of non‐suppressive Ly6Chi macrophages into suppressive Ly6Clo Mreg in the allograft and abrogates tolerance.5 To identify the cells that produce CSF1 BALB/c hearts were transplanted into anti‐CD40L mAb‐treated C57BL6 recipients for tolerance induction. Allografts were harvested at day 5 post‐transplantation, single cells were generated by collagenase treatment and distinct cells subsets were isolated by fluorescence activated cells sorting (FACS), according to the gating strategy in Figure 1A, for CSF1 expression by real‐time PCR. Our results indicate that graft‐infiltrating neutrophils express the highest levels of CSF1 among the CD45+ hematopoietic (Figure 1B). Within the hematopoietic CD45+ cells, myeloid cells express the highest CSF1 levels, and within the myeloid subsets, we found that neutrophils express the highest levels of CSF1. Further gene array analysis of different myeloid subsets indicated that, while neutrophils from tolerized recipient allografts express high levels of CSF1, neutrophils from untreated rejecting mice express low levels of CSF1 (Figure S1). This suggests a potential role of CSF1 producing neutrophils in the development of Ly6Clo macrophages during the induction of tolerance.
Figure 1.
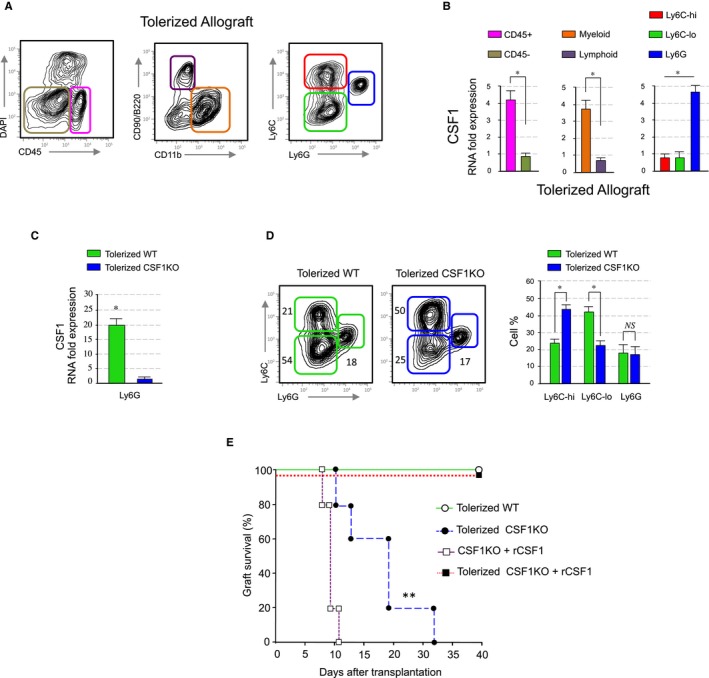
Neutrophil derived CSF1 mediates macrophage polarization. (A and B) CSF1 expression in the allografts. Heart allografts of CD40L mAb treated mice were isolated and single cells suspensions were made. CSF1 expression in distinct flow sorted cell subsets was investigated by qPCR. Results represent mean ± SEM of three independent experiments (unpaired Mann‐Whitney and Kruskal‐Walis with Dunn's multiple comparison test; *P ≤ .5). (C) CSF1 expression in the allografts of S100A8Cre CSF1 fl deficient recipient mice. Heart allografts of wt and S100A8Cre CSF1 fl recipients treated with CD40L mAb were analyzed for CSF1 expression by qPCR. Results represent mean ± SEM of three independent experiments (unpaired Mann‐Whitney test; *P ≤ .5). (D) Representative and quantitative flow cytometry results of graft infiltrating myeloid subsets from anti‐CD40L mAb treated wt and S100A8Cre CSF1 fl recipients at day 5 post‐transplantation. Results represent mean ± SEM (n = 4 mice per group of three independent experiments; unpaired Mann‐Whitney test; *P ≤ .5). (E) Graft survival of tolerized wt versus S100A8Cre CSF1 fl recipients. Tolerized S100A8Cre CSF1 fl recipient mice rejected their allografts despite anti‐CD40L mAb treatment. A third group of tolerized S100A8Cre CSF1 fl recipients received 2 × 105 U of recombinant CSF1 i.v. on the day of transplantation and on days 1‐5 post‐transplantation. Non‐tolerized S100A8Cre CSF1 fl recipients treated with 2 × 105 U of recombinant CSF1 were used as controls. Graft survival was assessed with Kaplan‐Meier analysis (MST 18 ± 8 days; ** P ≤ .01; n = 5 mice per group)
To determine whether CSF1 secreting neutrophils favor the development suppressive Ly6Clo macrophages in vivo, Balb/c (H‐2d) heart grafts were transplanted into fully allogeneic C57/BL6 (H‐2b) transplant recipients that are deficient for CSF1 in neutrophils. To generate these recipients, we crossed CSF1 floxed19 with the neutrophil specific S100A8‐Cre mice.23 Real‐time PCR analysis of the CSF1 expression in graft‐infiltrating neutrophils reveals that the expression of CSF1 is significantly decreased in CSF1 fl/fl neutrophils in contrast to wild type controls, despite tolerogenic regimen (Figure 1C).
To test for a mechanistic link between CSF1 and the development of suppressive Ly6Clo macrophages, we transplanted BALB/c hearts into fully allogeneic wild type or neutrophil specific CSF1 fl/fl recipients and treated them with tolerizing anti‐CD40L mAb regimen. Our results indicate that interfering with in vivo neutrophil CSF1 production significantly decreases intra‐graft accumulation of suppressive Ly6Clo macrophages (Figure 1D). Remarkably, while neutrophil CSF1 deficiency abrogated the induction of transplantation tolerance despite anti‐CD40L mAb treatment, peritransplant administration of recombinant CSF1 restored prolonged allograft survival in tolerized CSF1 fl/fl recipients (Figure 1E). This demonstrates that CSF1 producing neutrophils mediate the development of suppressive Ly6Clo macrophages that promote the induction of indefinite allograft survival.
3.2. Suppressive function of polarized macrophages depends on cell proliferation
Based on the CSF1 requirement for tolerance induction and previous work by others linking CSF1 to cell cycle progression,24, 25 we tested relationship between cell cycle progression of suppressive Ly6Clo macrophages and induction of tolerance. Microarray data analysis of Ly6Chi, Ly6Clo, Ly6G myeloid subsets revealed upregulated expression of genes associated with cell cycle progression in graft infiltrating Ly6Clo macrophages, including the cell proliferation associated gene Mki67 (Figure 2A). Fluorescent immunohistochemistry and flow cytometry analysis confirmed Ki67 protein expression in graft infiltrating Ly6Clo (CD169+) macrophages (Figures 2B and C).
Figure 2.
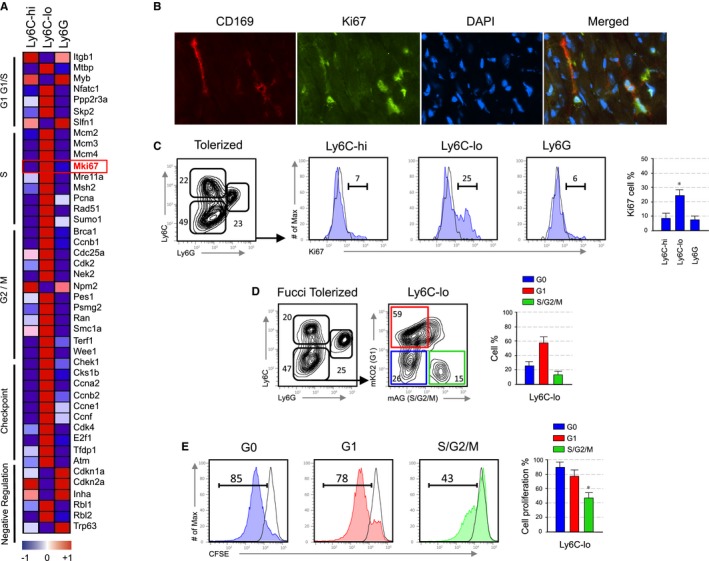
Suppressive function of polarized macrophages depends on cell proliferation. (A) Heatmap of cell cycle transcripts derived from microarray data with a P‐value P < .05 in the myeloid subsets obtained from the allografts of anti‐CD40L mAb treated recipients at day 5 post‐transplantation. Shown is an average of n = 3. (B) Representative immunofluorescent images of allograft tissue stained for CD169, Ki67, DAPI and a merge image depicting overlap obtained from an anti‐CD40L mAb treated recipients at day 5 post‐transplantation (magnification ×40). (C) Representative and quantitative flow cytometry results for Ki67 expression on myeloid subsets from the allografts of anti‐CD40L mAb treated recipients at day 5 post‐transplantation. Data are representative of three independent experiments. Results represent mean ± SEM (n = 3 mice per group of three independent experiments; Kruskal‐Walis with Dunn's multiple comparison test; *P ≤ .5). (D) Representative and quantitative flow cytometry results of myeloid subsets from the allografts of anti‐CD40L mAb treated Fucci recipients at day 5 post‐transplantation. Further evaluation of cell cycle fluorescent probes indicated that the majority of Ly6Clo macrophages from anti‐CD40L mAb‐treated Fucci recipients are in G1/S/G2/M phase. Results represent mean ± SEM (n = 3 mice per group of three independent experiments). (E) Suppressive function of Ly6Clo macrophages that are either proliferating (G1S/G2/M) or non‐proliferating (G0). Representative and quantitative flow cytometry results of CSFE + CD8+ T proliferation after 72 hours of culture. Results represent mean ± SEM (n = 3 mice per group of three independent experiments; Kruskal‐Walis with Dunn's multiple comparison test; *P ≤ .5)
To investigate the relationship between cell cycle progression and suppressive function of Ly6Clo macrophages we employed Fucci transgenic mice. Using Fucci mice as transplant recipients, graft‐infiltrating macrophages can be FACS sorted based on their cell cycle stage, as these mice are genetically encoded for fluorescent probes that effectively label the G1 phase nuclei in red (mKO2‐hCdt1 30/120) and the S/G2/M phases in green (mAG‐hGem 1/110).18 We transplanted BALB/c hearts into anti‐CD40L mAb‐treated C57BL6 Fucci transgenic (mKO2/mAG) recipient mice, harvested the allografts at day 5 post‐transplantation, and analyzed graft infiltrating myeloid subsets by flow cytometry. We found that >50% of Ly6Clo macrophages from anti‐CD40L mAb‐treated Fucci recipients are in G1, while >10% are in S/G2/M phase (Figure 2D). We next sorted Ly6Clo macrophages from manti‐CD40L mAb‐treated Fucci recipients into those in G0, G1, and S/G2/M and tested their immunosuppressive capacity in vitro (Figure 2E). The suppression assay demonstrated that the in vitro inhibitory function of graft infiltrating Ly6Clo macrophages is confined to the proliferating S/G2/M subset. This is consistent with our previous collaborative finding, which demonstrated that suppressive monocytic‐derived cells from tumor bearing mice are highly proliferative.26
3.3. Preventing macrophage cell cycle progression abrogates tolerance
To demonstrate that cell proliferation of Ly6Clo macrophages is necessary for the induction of tolerance, we incorporated simvastatin in a high‐density lipoprotein (HDL) nanoparticle to generate simvastatin‐HDL (S‐HDL) nanoparticles, as we recently described.27 We cultured bone marrow Ly6Chi monocytes with CSF1 to induce their polarization towards suppressive Ly6Clo monocyte‐derived cells (Figure 3A). As expected, addition of S‐HDL prevented the polarization of Ly6Chi into Ly6Clo monocyte‐derived cells in vitro. Next, we tested their ability to suppress T CD8+ T cell proliferation and our results indicate that S‐HDL treatment prevents the suppressive function of Ly6Clo monocyte‐derived cells. This was associated with a S‐HDL‐mediated cell cycle arrest in G1. This indicates that preventing cell cycle progression interferes with monocyte‐derived cell polarization and inhibits their suppressive function. To evaluate the effects of S‐HDL in vivo, we next incorporated simvastatin at a concentration of 60 mg/kg in S‐HDL nanoparticles to inhibit macrophages proliferation.28 A conservative S‐HDL regimen that included three i.v. injections on days 0, 2, and 5 after transplantation affects the accumulation of Ly6Clo macrophages in the tolerized allografts and promotes graft infiltrating macrophage cell cycle arrest at G1 (Figure 3B). Remarkably, in vivo inhibition of macrophage cell cycle progression abrogated the induction of transplantation tolerance despite tolerogenic regimen (Figure 3C), which demonstrates that proliferation of Ly6Clo macrophages, is required to induce immunological tolerance in the context of organ transplantation.
Figure 3.
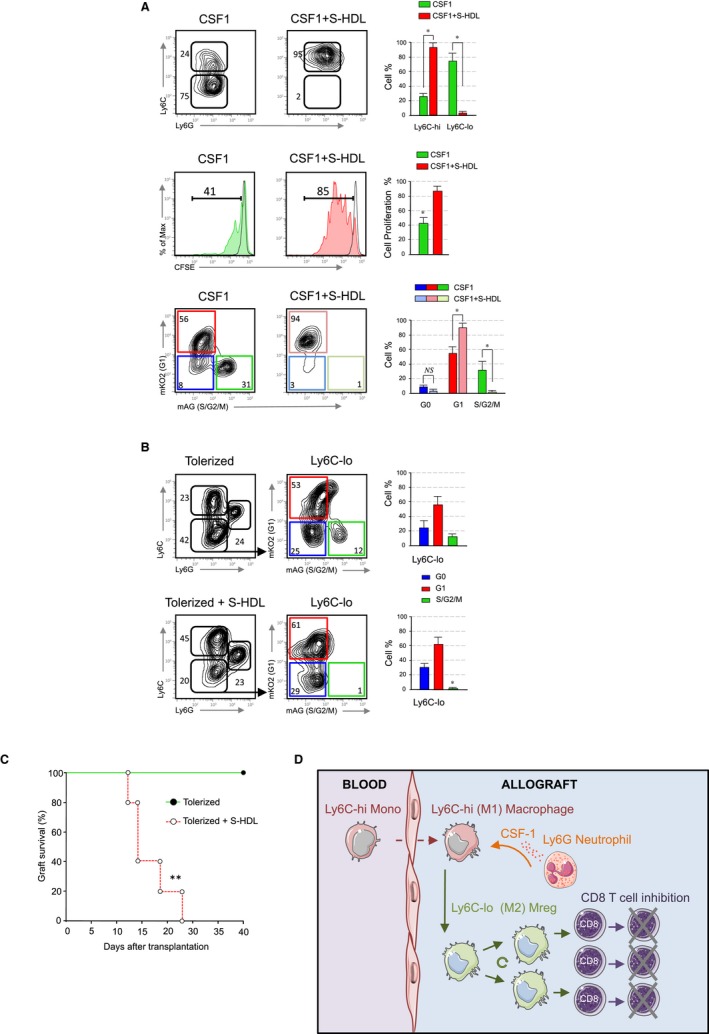
Preventing macrophage cell cycle progression abrogates tolerance. (A) Top panel; representative and quantitative flow cytometry results of in vitro cultured bone marrow Ly6Chi monocytes with either CSF1 (10 ng/ml) or CSF1 plus simvastatin loaded HDL nanoparticles (S‐HDL) at 10 μM for 72 hours. Middle panel; suppressive function of bone marrow derived Ly6Chi monocytes after treatment with wither CSF1 or CSF1 + S‐HDL. Representative and quantitative flow cytometry results of CSFE + CD8+ T proliferation after 72 hours of culture. Bottom panel; representative and quantitative flow cytometry results of bone marrow derived Ly6Chi monocytes after treatment with wither CSF1 (10 ng/ml) or CSF1 + S‐HDL at 10 μM indicating cell cycle progression after 72 h of culture. Results represent mean ± SEM (n = 3 independent experiments; unpaired Mann‐Whitney test; *P ≤ .5). (B) Representative and quantitative flow cytometry results of myeloid subsets from the allografts of anti‐CD40L mAb treated Fucci recipients at day 5 post‐transplantation treated with S‐HDL (60 mg/kg). Further evaluation of cell cycle fluorescent probes indicated that the majority of Ly6Clo macrophages from anti‐CD40L mAb + S‐HDL treated Fucci recipients are arrested in G1. Results represent mean ± SEM (n = 3 mice per group of three independent experiments; Kruskal‐Walis with Dunn's multiple comparison test; *P ≤ .5). (C) Graft survival of tolerized recipients treated with or without S‐HDL (60 mg/kg) on days 0, 2, and 5 post‐transplantation. Tolerized S‐HDL recipient mice rejected their allografts despite anti‐CD40L mAb treatment. Graft survival was assessed with Kaplan‐Meier analysis (MST 20 ± 9 days; ** P ≤ .01; n = 5 mice per group). (D) Working hypothesis showing that neutrophil derived CSF1 controls polarization, proliferation, and suppressive function of tolerogenic macrophages that mediate transplantation tolerance
4. DISCUSSION
We demonstrate here that graft‐infiltrating neutrophils produce CSF1 that mediates polarization of Ly6Clo suppressive macrophages and promotes transplantation tolerance in the context of costimulatory blockade (Figure 3D). This study provides novel understandings about how distinct myeloid cell subsets are interconnected in the tissue and highlights the critical contribution of neutrophils during the induction of indefinite allograft survival.
Neutrophils are the most abundant myeloid cell subset in circulation and rapidly infiltrate the inflamed tissue. As a result, neutrophils have been have been historically viewed as pro‐inflammatory cells that protect against intracellular pathogens though the release of extracellular traps.29 In organ transplantation, the role of neutrophils is commonly associated to antibody mediated and chronic rejection or ischemia reperfusion injury and resolution of inflammation.30 However, murine neutrophils also release anti‐inflammatory cytokines,31 and recent evidence suggests that neutrophils are able to negatively regulate T cell mediated immune responses.32 Here, we show that neutrophils favor tolerance by mediating macrophage polarization in the transplanted organ uncovering a previously unrecognized function of neutrophils in the context of organ transplantation.
Data from tumor models also suggest a close relationship between neutrophilic and monocytic‐derived suppressor cells. Myeloid‐derived suppressor cells (MDSC) are comprised of two groups of immunosuppressive cells with monocytic (M‐MDSC) and granulocytic (G‐MDSC) morphology. G‐MDSC express high levels of CSF1,33 which represents a growth factor involved in the generation of M‐MDSC that prolongs allograft survival upon adoptive transfer.34 Therefore, CSF1‐dependent macrophage development represents a novel approach for therapeutic intervention either by inhibiting (ie, cancer), or by promoting (ie, transplantation) macrophage polarization. In this respect, the development of monoclonal antibodies that target macrophage polarization through CSF1 receptor signaling has been shown to interfere with macrophage differentiation/proliferation and to prevent tumor progression.35, 36
We conclude that neutrophils secrete CSF1 that promotes macrophage polarization, progression through the cell cycle, and suppressive function of graft infiltrating macrophages. Future experiments are aimed at elucidating the mechanisms by with anti‐CD40L mAb promote “tolerogenic” neutrophils. In this respect, we hypothesize neutrophils scanning for platelets37 may receive a “tolerogenic” signal following CD40L blockade in activated platelets38 that may lead to CSF1 production and transplantation tolerance under sterile inflammatory conditions.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
We thank the technical contributions of the flow cytometry and microsurgery cores at Mount Sinai. We also acknowledge Marcy Kuenzel and Sridar Chittur at the University of Albany Center for Functional Genomics microarray core facility for their assistance in generating the microarray data. This work was supported by the Cancer Center Grant P30 CA196521; the National Institute of Health grants AG045040 to S.A.W. and R01 HL118440, R01 HL125703, R01 CA155432 to W.J.M.M; and the Ministerio de Economia y Competitividad SAF2016‐80031‐R to J.O. This work was also supported by the COST Action BM1305: Action to Focus and Accelerate Cell Tolerogenic Therapies (A FACTT), the COST action BM1404: European Network of Investigators Triggering Exploratory Research on Myeloid Regulatory Cells (Mye‐EUNITER), and the Mount Sinai Recanati/Miller Transplantation Institute career development funds.
Braza MS, Conde P, Garcia M, et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am J Transplant. 2018;18:1247‐1255. https://doi.org/10.1111/ajt.14645
REFERENCES
- 1. Poulter LW, Bradley NJ, Turk JL. The role of macrophages in skin allograft rejection. I. Histochemical studies during first‐set rejection. Transplantation. 1971;12(1):40‐44. [DOI] [PubMed] [Google Scholar]
- 2. Bergler T, Jung B, Bourier F, et al. Infiltration of macrophages correlates with severity of allograft rejection and outcome in human kidney transplantation. PLoS ONE. 2016;11(6):e0156900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation. 2005;80(12):1641‐1647. [DOI] [PubMed] [Google Scholar]
- 4. Swirski FK, Wildgruber M, Ueno T, et al. Myeloperoxidase‐rich Ly‐6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120(7):2627‐2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conde P, Rodriguez M, van der Touw W, et al. DC‐SIGN(+) macrophages control the induction of transplantation tolerance. Immunity. 2015;42(6):1143‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ochando J, Conde P, Bronte V. Monocyte‐derived suppressor cells in transplantation. Curr Transplant Rep. 2015;2(2):176‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953‐964. [DOI] [PubMed] [Google Scholar]
- 8. Okabe Y, Medzhitov R. Tissue‐specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549‐555. [DOI] [PubMed] [Google Scholar]
- 11. Porta C, Rimoldi M, Raes G, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106(35):14978‐14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T‐cell proliferation by Stat6‐dependent expression of PD‐L2. Blood. 2010;116(17):3311‐3320. [DOI] [PubMed] [Google Scholar]
- 13. Oishi S, Takano R, Tamura S, et al. M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T‐cell proliferation. Immunology. 2016;149(3):320‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte‐to‐macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303‐7311. [DOI] [PubMed] [Google Scholar]
- 15. Munn DH, Pressey J, Beall AC, Hudes R, Alderson MR. Selective activation‐induced apoptosis of peripheral T cells imposed by macrophages. A potential mechanism of antigen‐specific peripheral lymphocyte deletion. J Immunol. 1996;156(2):523‐532. [PubMed] [Google Scholar]
- 16. Sakurai T, Yamada M, Simamura S, Motoyoshi K. Recombinant human macrophage‐colony stimulating factor suppresses the mouse mixed lymphocyte reaction. Cell Immunol. 1996;171(1):87‐94. [DOI] [PubMed] [Google Scholar]
- 17. Wing EJ, Magee DM, Pearson AC, Waheed A, Shadduck RK. Peritoneal macrophages exposed to purified macrophage colony‐stimulating factor (M‐CSF) suppress mitogen‐ and antigen‐stimulated lymphocyte proliferation. J Immunol. 1986;137(9):2768‐2773. [PubMed] [Google Scholar]
- 18. Sakaue‐Sawano A, Kurokawa H, Morimura T, et al. Visualizing spatiotemporal dynamics of multicellular cell‐cycle progression. Cell. 2008;132(3):487‐498. [DOI] [PubMed] [Google Scholar]
- 19. Harris SE, MacDougall M, Horn D, et al. Meox2Cre‐mediated disruption of CSF‐1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50(1):42‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H‐2D, H‐2K, and non‐H‐2 antigens in rejection. Transplantation. 1973;16(4):343‐350. [DOI] [PubMed] [Google Scholar]
- 21. Jiang X, Sun W, Guo D, et al. Cardiac allograft acceptance induced by blockade of CD40‐CD40L costimulation is dependent on CD4 + CD25 + regulatory T cells. Surgery. 2011;149(3):336‐346. [DOI] [PubMed] [Google Scholar]
- 22. Garcia MR, Ledgerwood L, Yang Y, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120(7):2486‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38(3):489‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH. CSF‐1–a mononuclear phagocyte lineage‐specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151‐159. [DOI] [PubMed] [Google Scholar]
- 25. Roussel MF. Regulation of cell cycle entry and G1 progression by CSF‐1. Mol Reprod Dev. 1997;46(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 26. Ugel S, Peranzoni E, Desantis G, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2(3):628‐639. [DOI] [PubMed] [Google Scholar]
- 27. Duivenvoorden R, Tang J, Cormode DP, et al. A statin‐loaded reconstituted high‐density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang J, Lobatto ME, Hassing L, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv 2015;1(3): pii: e1400223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicolas‐Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46(1):15‐28. [DOI] [PubMed] [Google Scholar]
- 30. Scozzi D, Ibrahim M, Menna C, Krupnick AS, Kreisel D, Gelman AE. The role of neutrophils in transplanted organs. Am J Transplant. 2017;17(2):328‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL‐10‐secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11(11):1039‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac‐1. J Clin Invest. 2012;122(1):327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Condamine T, Dominguez GA, Youn JI, et al. Lectin‐type oxidized LDL receptor‐1 distinguishes population of human polymorphonuclear myeloid‐derived suppressor cells in cancer patients. Sci Immunol 2016;1(2):pii: aaf8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang F, Li Y, Wu T, et al. TNFalpha‐induced M‐MDSCs promote transplant immune tolerance via nitric oxide. J Mol Med (Berl). 2016;94(8):911‐920. [DOI] [PubMed] [Google Scholar]
- 35. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF‐1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Overmeire E, Stijlemans B, Heymann F, et al. M‐CSF and GM‐CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res. 2016;76(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 37. Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346(6214):1234‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591‐594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


