Abstract
Background
Coeliac disease is a substantially underdiagnosed disorder, with clinical testing currently guided by case finding.
Aim
To determine the presence of indications for diagnostic testing and frequency of clinical testing in undiagnosed coeliac disease.
Methods
This was a case-control study of adults without prior diagnosis of coeliac disease. Undiagnosed cases were identified through sequential serology, and unaffected age- and gender-matched controls were selected. Medical records were systematically reviewed for indications for and evidence of clinical testing.
Results
Of 47,557 adults, 408 cases of undiagnosed coeliac disease were identified. 408 serology negative matched controls were selected. Eight matched pairs were excluded, leading to 800 included individuals (61% female; median age 44.2 years). The odds of any indication for clinical testing were similar among undiagnosed coeliac disease and controls (odds ratio (OR) 1.18; 95% confidence interval (CI): 0.85-1.63, p-value=0.32). Most individual indications were not associated with serologic status. Exceptions to this include hypothyroidism, which was more likely in cases of undiagnosed coeliac disease, and dyspepsia and chronic diarrhea, which were less likely. Cases of undiagnosed coeliac disease were more likely to develop osteoporosis (p-value = 0.005), dermatitis herpetiformis (p-value=0.006), chronic fatigue (p-value=0.033), thyroiditis (p-value=0.003), autoimmune diseases (p-value=0.008), and have a family member diagnosed with coeliac disease (p-value=0.001).
Conclusion
This study strongly suggests that current case finding is not effective in detecting undiagnosed coeliac disease. Individuals with undiagnosed coeliac disease were more likely than controls to develop indications for testing overtime. A more effective method for detection of coeliac disease is needed.
Keywords: Targeted testing, Screening, Under-diagnosis, Clinical testing, Coeliac Disease, Natural History
INTRODUCTION
Coeliac Disease is common, currently affecting approximately 1% of North America and Western Europe1, and the prevalence is stable or rising2–4. However despite this, one study estimates that 83% of cases in the United States remain undiagnosed5. Explanations for this include the non-specific symptoms and signs as well as a lack of awareness of coeliac disease among care providers. Additionally, current strategies for clinical detection may be suboptimal in clinical practice.
Identification of coeliac disease in clinical practice is driven by testing symptomatic patients or by case finding. Case finding is defined as the practice of testing individuals who are at an increased risk for the disease, due primarily the presence of symptoms or conditions associated with coeliac disease6–8. Case finding has been reported to increase coeliac disease diagnosis by 32-43 fold in the primary care setting8, however there is increasing suspicion that case finding may not be effective, and that indications to test, such as gastrointestinal symptoms, have poor diagnostic accuracy in identifying coeliac disease9–12. The United States Preventive Services Task Force recently released a recommendation statement that there is insufficient evidence to support “targeted screening” of asymptomatic persons13, 14.
Determining the association between indications to test and having undiagnosed coeliac disease and the frequency of testing in the general population is crucial to assess the efficacy of current strategies to improve detection rates. While a recent study on the morbidity of undiagnosed cases of coeliac disease between the ages of 18 to 50 in Olmsted County reported on the lack of typical symptoms in this cohort, the study did not report on many of the non-classical symptoms recommended as indications to test by our current guidelines6. To fully evaluate the potential efficacy of case finding, we therefore aimed to look at the adult population of undiagnosed coeliac disease, including those over 50, and age- and gender-matched controls, in order to assess the relationship of serologic status and indications to test prior to serology testing, including both classical and non-classical symptoms, as well as the frequency of testing for coeliac disease in the community. Additionally, to address the paucity of information on the natural history of undiagnosed coeliac disease, we evaluated how often individuals with undiagnosed coeliac disease developed clinically apparent indicators for coeliac disease.
METHODS
Study Setting and Subjects
Serum samples were collected between 1995 and 2009 from individuals receiving primary or secondary care at Mayo Clinic and residing in Olmsted County, Minnesota at the time of collection. Subjects with general research authorization and without a known coeliac disease diagnosis prior or 2 weeks after serum collection were included. 47,557 samples were screened for coeliac disease serology.
Identification of Undiagnosed Coeliac Disease Cases and Matched Controls
Stored serum samples were first tested for immunoglobulin A tissue transglutaminase antibody levels using an enzyme-linked immunosorbent assay (Inova Diagnostics, San Diego, CA) 15. Samples with a positive or indeterminate tissue transglutaminase antibody level, defined as ≥2.0 U/mL, were then tested for endomysial antibodies using an immunofluorescence assay (Inova Diagnostics). Results were linked to a study ID. Undiagnosed coeliac disease was defined when immunoglobulin A tissue transglutaminase antibody was ≥2.0 U/mL and endomysial antibodies was positive2, 12, 15. 408 undiagnosed coeliac disease cases were identified. 408 gender, age, year of serum collection, and Mayo Clinic registration year- matched controls were selected from the remaining 47,056 with a negative composite serology. Those 84 individuals with equivocal serology (tissue transglutaminase antibody level ≥ 4 U/mL with negative endomysial antibodies) were excluded. Cases and controls were combined into one list and reviewed by investigators blinded to serological results.
Indications for Clinical Testing
The indications for clinical testing included were consistent with current guidelines and prior research6,8,16, 17. These indications include “classic” coeliac disease symptoms, such as malabsorption, diarrhea, and weight loss, and “non-classic” coeliac disease symptoms, such as osteoporosis, anemia, unexplained elevation of liver enzymes, associated disorders such as autoimmune diseases, and a positive family history18 (Table 1).
Table 1.
Indications for Clinical Testing
| Indication for Clinical Testing |
|---|
| Symptomatic malabsorption |
| Diarrhea with weight loss |
| Chronic diarrhea |
| Recurrent abdominal pain |
| Iron deficiency |
| Anemia |
| Metabolic bone disease |
| Postprandial bloating and gaseousness |
| Unexplained weight loss |
| Abnormal elevated liver enzymes |
| Incidental discovery of villous atrophy endoscopically or histologically |
| Dermatitis herpetiformis |
| Idiopathic peripheral neuropathy |
| Oral aphthous ulcers |
| Growth failure |
| Discolored teeth or developmentally synchronous enamel loss |
| Thyroid disease |
| Irritable bowel syndrome |
| Down syndrome |
| Turner’s syndrome |
| Pulmonary hemosiderosis |
| Unexplained male or female infertility |
| Dyspepsia |
| Amenorrhea |
| Chronic fatigue |
| Apparent malabsorption of thyroid replacement medication |
| Epilepsy/ataxia |
| Constipation |
| Family history of coeliac disease |
| Autoimmune disorders |
Data Collection
The medical record of cases and controls, which included inpatient, outpatient, and emergency department documentation, was systematically reviewed by a physician (I.H.) blinded to serologic status. The investigator reviewed the charts for indications for clinical testing as well as evidence of testing in the community. Coeliac disease testing in the community was defined by a provider obtaining either coeliac serology or endoscopic duodenal biopsy6, 16–19 regardless of their reasons for testing. Testing in the community therefore encompassed both case finding and instances where a clinician had a high suspicion for coeliac disease.
A second blinded physician (A.S.) reviewed a randomly selected subset of 10% of cases and controls to validate the reliability of the initial reviewer. An electronic standardized data extraction template was used (Supplemental Table 1), and the data were stored as a limited data set in a secure encrypted archive.
Indications for clinical testing, except for ‘incidental discovery of villous atrophy’ and ‘family history of coeliac disease’, were found by reviewing the problem list from each clinical note in the medical record. These lists were generated by compiling the problems and diagnoses detailed in the care provider’s listed impression and plan. Incidental discovery of villous atrophy was identified through reviewing the medical record’s endoscopy and pathology sections, and was defined as incidental if the indication for endoscopy was not one of the listed indications to test for coeliac disease. Family history of coeliac disease was identified through reviewing the patient filled-out family medical history form and the family history section of the clinical note from that date, or the next chronologic clinical note. The date that an indication for testing appeared in the medical record was recorded. If there were no clinical notes, data on indications to test were labeled as missing, and these individuals along with their matched pair were excluded from analysis.
Serologic and pathologic data in the medical record were reviewed for evidence that coeliac disease testing had been performed. The medical record’s laboratory section was reviewed for measurement of immunoglobulin A anti-tissue transglutaminase antibody, immunoglobulin A antiendomysial antibodies, immunoglobulin A deamidated gliadin peptides, immunoglobulin G deamidated gliadin peptides, and immunoglobulin G anti-tissue transglutaminase antibodies, as well as human leukocyte antigen DQ2 and DQ8. The medical record’s pathology section was reviewed for evidence of a duodenal biopsy having been performed. If serology or a biopsy were performed, the date and result were recorded. The specific indication for serology or endoscopy was identified through reviewing the indication listed in the associated medical order.
The data on clinical testing and indications was combined with serologic status using the study ID and analysis was performed in a limited data set to preserve patient anonymity. Participants were not specifically identified or contacted.
Ethical Issues
This study was approved by the Mayo Foundation Institutional Review Board. The study was performed on a limited data set that preserved subject anonymity by blinding the investigators to the identity of cases. Only subjects who provided research authorization were included. No subject contacts were permitted by the Institutional Review Board.
Statistical analysis
The prevalence and 95% exact confidence interval for undiagnosed coeliac disease among our sampled community was estimated with the Clopper-Pearson method. The cumulative rate of subsequent clinical coeliac disease diagnosis was estimated using the Kaplan-Meier product limit.
To estimate the efficacy of case finding, we assessed the frequency of indications for clinical testing prior to the date the stored serum was drawn in both cases of undiagnosed coeliac disease and their age- and gender- matched controls. Conditional logistic regression was used to generate odds ratios with corresponding 95% confidence intervals.
To estimate the natural history of undiagnosed coeliac disease, the rate of development of indications to test after collection of the blood sample was calculated. For each indication to test, the total number of individuals presenting with that indication after serum collection as well as the total number of years from serum collection to symptom development was determined. Individuals who had the indication prior to serum collection were excluded for that indication’s rate calculation, although they were included for other indications. Hazard ratios and non-parametric log-rank tests were calculated as appropriate.
All analyses were done using SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina). An alpha level of 0.05 was used to define statistical significance without correction for multiple comparisons. Agreement between the two investigators was quantified using the pooled estimator of the kappa statistic.
RESULTS
Of the 47,557 serum samples, 408 cases of undiagnosed coeliac disease were identified, with a prevalence of undiagnosed coeliac disease in this community of 0.88% (95% confidence interval (CI), 0.80-0.96%). 408 gender- and age- matched controls were then selected from the remaining 47,056. Eight matched pairs were excluded from analysis due to lack of clinical data, leaving 400 cases and 400 controls. The mean age of the two groups at the time the serum sample was drawn was 44.2 years, with a range of 18.1 to 87.7 years old (61% female).
Ninety-two of the 400 cases of undiagnosed coeliac disease (23%) were ultimately diagnosed with coeliac disease, with a median time to diagnosis of 5.69 years (interquartile range: 3.43 – 9.38), while zero of the 400 controls were clinically diagnosed.
Efficacy of Case finding
Roughly 40% of all individuals (n=306; 38.3%) had at least one indication for clinical testing prior to serum collection. Serologic status was not associated with having an indication for testing, 159 (40%) cases and 147 (37%) controls had any indication (odds ratio (OR) 1.18; 95% CI: 0.85-1.63, p-value = 0.32). The number of indications for testing per individual was also similar between the two groups (Figure 1). In undiagnosed coeliac disease, having multiple indications (n=67; 17%) for testing was less frequent than having one indication (n=92; 23%) (p-value = 0.047), while among controls having multiple indications (n=75; 19%) was as common as having one (n=72; 18%) (p-value = 0.81).
Figure 1.
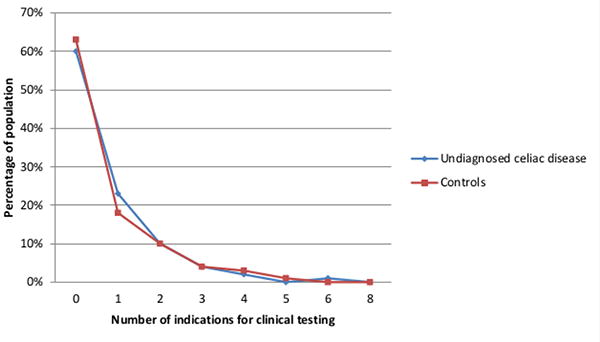
Comparison of the percentage of cases of undiagnosed coeliac disease and controls with varying number of indications for clinical testing.
Similarly, for the majority of indications for clinical testing, there was no statistical difference between cases and controls (Figure 2). Hypothyroidism was significantly more likely in those with undiagnosed coeliac disease (OR=2.07; 95% CI, 1.12-3.83, p-value = 0.02). Undiagnosed coeliac disease cases were less likely to have dyspepsia (OR=0.42; 95% CI 0.18-0.96, p-value = 0.04) and chronic diarrhea (OR=0.23; l 95% CI 0.07-0.81, p-value = 0.02).
Figure 2.
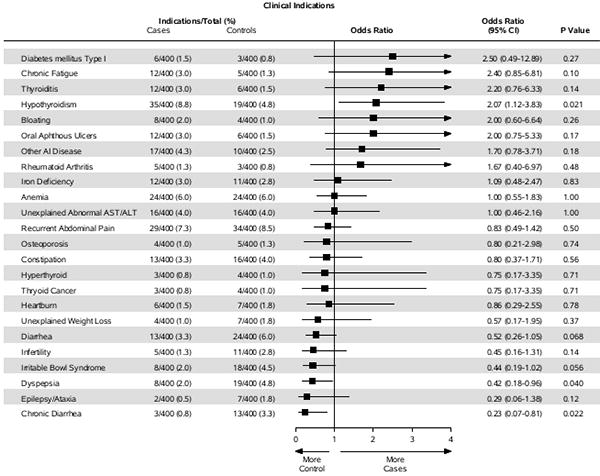
Forest plot of the odds ratio of having individual indications for clinical testing in cases of undiagnosed coeliac disease as compared to controls.
There were four cases of Down syndrome and two cases of family history of coeliac disease in those with undiagnosed coeliac disease and zero cases of either in controls.
Frequency of Testing and Coeliac Disease Diagnosis in the Community
Less than 5% (n=35; 4.4%) were tested for coeliac disease by a provider in the community prior to our serologic testing. Testing was less in undiagnosed coeliac disease, with 9 (2.3%) tested as opposed to 26 (6.5%) controls (OR=0.32; 95% CI, 0.14-0.71, p-value = 0.005). Serologic testing was similar between the two groups (OR=0.583, 95% CI 0.23-1.48, p-value=0.26), however small bowel biopsies were significantly more likely in controls (OR=0.06, 95% CI 0.01-0.42, p-value =0.005) (Figure 3). Symptoms and signs that were associated with receiving a diagnosis of coeliac disease in the community included: vitamin deficiency (p-value = 0.01), anemia (p-value=0.001), iron deficiency (p-value=0.001), diarrhea (p-value=0.001), chronic diarrhea (0.001), recurrent abdominal pain (p-value = 0.02), bloating (p-value = 0.001), incidental villous atrophy (p-value = 0.001), dermatitis herpetiformis (p-value = 0.002), hypothyroidism (p-value = 0.003), infertility (p-value = 0.001), chronic fatigue (p-value = 0.03), family history of coeliac disease (p-value = 0.001), type I diabetes mellitus (p-value = 0.004), and vitiligo (p-value = 0.01).
Figure 3.
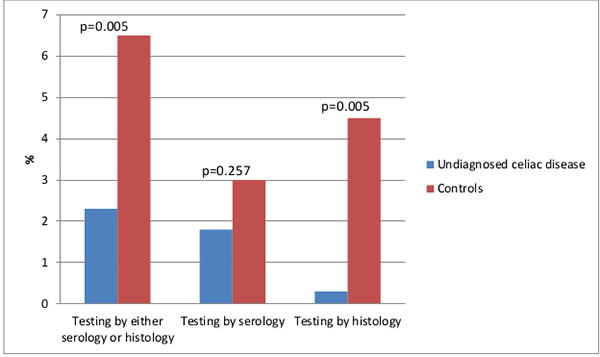
Comparison of the frequency of clinical testing for coeliac disease with serology or histology between cases of undiagnosed coeliac disease and controls.
Natural History of Undiagnosed Coeliac Disease
Individuals with undiagnosed coeliac disease were more likely to develop osteoporosis (19.08 versus 11.20 diagnoses/1,000 person years, p-value=0.005), dermatitis herpetiformis (0.93 versus 1.92 diagnoses/1,000 person years, p-value=0.006), chronic fatigue (9.48 versus 5.17 diagnoses/1,000 person years, p-value=0.03), thyroiditis (4.27 versus 0.79 diagnoses/1,000 person years, p-value=0.003), and autoimmune disease (8.36 versus 3.57 diagnoses/1,000 person years, p-value=0.008). They were more likely to be incidentally found to have villous atrophy (1.63 versus 0 diagnoses/1,000 person years, p-value=0.01), and more likely to have a family member diagnosed with coeliac disease (5.59 versus 0.79 diagnoses/1,000 person years, p-value<0.001). (Table 2).
Table 2.
Development of indications to test in cases of undiagnosed coeliac disease and controls
| Indication to Test following serology test | Total Count/follow-up years (rate over 1000 person years) | Cases Count/follow-up years (rate over 1000 person years) | Controls Count/follow-up years (rate over 1000 person years) | Hazard Ratio (95% CI) | P-value (Log-Rank)† |
|---|---|---|---|---|---|
| Malabsorption | 1/7,537 (0.13) | 1/3,699 (0.27) | 0/3,838 (0.0) | - | 0.31 |
| Vitamin Deficiency | 55/7,290 (7.54) | 31/3,575 (8.67) | 24/3,715 (6.46) | 1.37 (0.8-2.34) | |
| Anemia | 131/6,251 (20.96) | 70/2,958 (23.66) | 61/3,292 (18.53) | 1.29 (0.92-1.83) | |
| Iron Deficiency | 73/6,872 (10.62) | 41/3,301 (12.42) | 32/3,571 (8.98) | 1.38 (0.87-2.20) | |
| Diarrhea | 93/6,715 (13.85) | 44/3,392 (12.97) | 49/3,323 (14.75) | 0.88 (0.59- 1.32) | |
| Chronic Diarrhea | 52/7,073 (7.35) | 25/3,537 (7.07) | 27/3,536 (7.64) | 0.93 (0.54- 1.60) | |
| Recurrent Abdominal Pain | 114/6,281 (18.15) | 51/3,160 (16.14) | 63/3,121 (20.19) | 0.80 (0.55- 1.16) | |
| Bloating | 38/7,242 (5.25) | 16/3,550 (4.51) | 22/3,692 (5.96) | 0.76 (0.40- 1.44) | |
| Osteoporosis | 99/6,627 (14.94) | 60/3,145 (19.08) | 39/3,481 (11.20) | 1.77 (1.18- 2.65) | |
| Premature Osteoporosis | 1/7,534 (0.13) | 1/3,704 (0.27) | 0/3,829 (0.000) | - | 0.32 |
| Osteomalacia | 2/7,526 (0.27) | 2/3,687 (0.54) | 0/3,838 (0.000) | - | 0.15 |
| Gaseousness/Flatulence | 11/7,411 (1.48) | 6/3,640 (1.65) | 5/3,771 (1.33) | 1.22 (0.37- 4.00) | |
| Unexplained Weight Loss | 49/7,148 (6.86) | 28/3,486 (8.03) | 21/3,662 (5.74) | 1.43 (0.81- 2.52) | |
| Unexplained AST/ALT Elevation | 46/6,927 (6.64) | 22/3,422 (6.43) | 24/3,505 (6.85) | 0.93 (0.52- 1.66) | |
| Incidental Discovery of Villous Atrophy | 6/7,510 (0.80) | 6/3,672 (1.63) | 0/3,838 (0.00) | – | 0.01 |
| Diagnosis of Dermatitis Herpetiformis | 7/7,490 (0.93) | 7/3,651 (1.92) | 0/3,838 (0.00) | – | 0.006 |
| Peripheral Neuropathy | 22/7,394 (2.98) | 11/3,642 (3.02) | 11/3,752 (2.93) | 1.05 (0.45- 2.41) | |
| Oral Apthous Ulcers | 17/7,315 (2.32) | 5/3,598 (1.39) | 12/3,717 (3.23) | 0.43(0.15- 1.23) | |
| Hyperthyroidism | 6/7,462 (0.80) | 4/3,655 (1.09) | 2/3,807 (0.53) | 2.08 (0.38- 11.36) | |
| Hypothyroidism | 52/6,684 (7.78) | 30/3,165 (9.48) | 22/3,519 (6.25) | 1.53 (0.88- 2.65) | |
| Thyroid Cancer | 3/7,459 (0.40) | 2/3,669 (0.55) | 1/3,790 (0.26) | 2.17(0.20- 23.92) | |
| Irritable Bowel Syndrome | 27/7,073 (3.82) | 11/3,574 (3.08) | 16/3,500 (4.57) | 0.67 (0.31- 1.44) | |
| Infertility | 20/7,331 (2.73) | 6/3,638 (1.65) | 14/3,693 (3.79) | 0.42 (0.16- 1.10) | |
| Dyspepsia | 47/6,885 (6.83) | 21/3,493 (6.01) | 26/3,392 (7.67) | 0.79 (0.44- 1.40) | |
| Chronic Fatigue | 52/7,159 (7.26) | 33/3,483 (9.48) | 19/3,677 (5.17) | 1.83 (1.04- 3.23) | |
| Family History of Coeliac Disease | 23/7,391 (3.11) | 20/3,577 (5.59) | 3/3,814 (0.79) | 7.03 (2.09- 23.65) | |
| Constipation | 91/6,739 (13.5) | 41/3,373 (12.16) | 50/3,366 (14.85) | 0.82 (0.54- 1.24) | |
| Epilepsy/Ataxia | 6/7,417 (0.81) | 4/3,652 (1.10) | 2/3,765 (0.53) | 2.07 (0.38- 11.31) | |
| Heartburn | 20/7,311 (2.74) | 6/3,621 (1.66) | 14/3,690 (3.80) | 0.44(0.17- 1.14) | |
| Metabolic Bone Disease | 1/6,717 (0.15) | 1/3,191 (0.31) | 0/3,526 (0.0) | – | 0.27 |
| Type I Diabetes | 3/7,465 (0.40) | 2/3,643 (0.55) | 1/3,822 (0.26) | 2.20 (0.20- 24.28) | |
| Thyroiditis | 18/7,296 (2.47) | 15/3,511 (4.27) | 3/3,786 (0.79) | 5.33 (1.54- 18.42) | |
| Rheumatoid Arthritis | 10/7,435 (1.35) | 4/3,642 (1.10) | 6/3,793 (1.58) | 0.69 (0.20- 2.46) | |
| Connective Tissue Disease | 4/7,525 (0.53) | 3/3,696 (0.81) | 1/3,828 (0.26) | 3.12 (0.33- 30.03) | |
| Vitiligo | 6/7,507 (0.80) | 5/3,679 (1.36) | 1/3,828 (0.26) | 5.24 (0.61- 44.87) | |
| Sjogrens Syndrome | 6/7,497 (0.80) | 3/3,689 (0.81) | 3/3,809 (0.79) | 1.04 (0.21- 5.15) | |
| Other Autoimmune disease | 41/6,992 (5.86) | 28/3,350 (8.36) | 13/3,642 (3.57) | 2.37 (1.23- 4.58) |
Magnitude of the difference in incidence between cases and controls was estimated using proportional hazards model when appropriate. When no events occurred in at least one group, the non-parametric log-rank test was used.
Validity of Data Collection
The pooled kappa statistic for agreement between the two investigators on collected variables was 0.966, indicating near perfect agreement.
DISCUSSION
This study evaluated the utility of case finding to detect coeliac disease in a North American community as well as the development of indications for testing for coeliac disease. The major findings of this study are 1) the majority of currently used indications to test for coeliac disease do not predict undiagnosed coeliac disease 2) the rate of testing for coeliac disease was low in both groups but was more likely in controls, and 3) individuals with undiagnosed coeliac disease develop more indications for testing, specifically osteoporosis, dermatitis herpetiformis, chronic fatigue, thyroiditis, and autoimmune disease over time, presenting a later opportunity for diagnosis.
The frequency of indications to test before serologic testing was similar in those with undiagnosed coeliac disease and controls, suggesting that case finding based on the presence of these accepted indications would be unable to discriminate among those with and without the disease. Given the estimated frequency of having any indication to screen, if case finding were applied to our sample population of 47,557, roughly 17,569 would have indications to test (Figure 4). These 17,569 would have to be tested to identify the 159 cases of undiagnosed coeliac disease who had indications to test. Thus, case finding will increase the rate of clinical diagnosis but expose 17,410 individuals without coeliac disease to the potential risk or even harm of screening. Additionally, 60% of cases of coeliac disease would have no indication to test and would remain undiagnosed. This is consistent with prior observations20,9.
Figure 4.
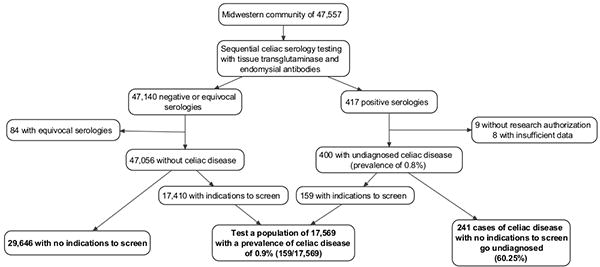
Flow chart detailing the application of case finding strategies to the study population. Using the prevalence of indications to test as determined in this study (40% in cases of undiagnosed coeliac disease and 37% in controls), the application of case finding to this population would mean 17,569 individuals out of 47,557 would have to be tested to identify 159 cases of coeliac disease. 60.2% of cases would remain undiagnosed.
Few relationships were statistically significant between serologic status and indications for clinical testing, even without controlling for multiple comparisons. Hypothyroidism was more likely among undiagnosed coeliac disease, which is congruent with prior reports suggesting it is one of the most common autoimmune diseases associated with coeliac disease18. Other autoimmune diseases were more likely in coeliac disease but were not statistically significant. Dyspepsia and chronic diarrhea were less likely among those with undiagnosed coeliac disease. Irritable bowel was also less likely, but not significantly so. These findings are compatible with prior studies on the poor performance of abdominal symptoms in identifying coeliac disease10,12.
The low frequency of testing in the community is consistent with prior observations19. Testing was significantly more likely among controls, which may be explained by the higher frequency of dyspepsia and chronic diarrhea in this group than in those with hidden coeliac disease. With the high prevalence of indications to test in those with and without undiagnosed coeliac disease, and the low prevalence of testing, it is unclear whether testing in Olmsted County was driven by case finding or usual practice.
Our study suggests that case finding is likely an ineffective strategy to identify most cases of undiagnosed coeliac disease in the general population, raising the question of how to detect coeliac disease. While there are proponents for mass screening, this strategy remains controversial. There is currently insufficient evidence on the cost-effectiveness and benefit of identifying and treating asymptomatic coeliac disease19; it is likely that mass screening will identify both symptomatic or asymptomatic coeliac disease11, 21. Current strategies based on case finding will not. Along with the unclear benefits of identifying asymptomatic cases of coeliac disease, there is also the possibility of harm such as over-diagnosis.
Alternatives to mass screening and case finding should be actively investigated. The challenge is to increase detection of undiagnosed symptomatic coeliac disease but also decrease the number of individuals without coeliac disease exposed to the risks of screening. Methods to explore as alternatives to improve identification of who to test may include the use of natural language processing software of the electronic medical record or the systematic acquisition of symptoms and family history of coeliac disease at clinical encounters22. Other potential alternatives include testing those with a certain combinations of symptoms or indications to test or perhaps identifying new indications for clinical testing by evidence-based methodology.
Our study has several potential limitations. Undiagnosed coeliac disease was identified through sequential serology, and duodenal biopsies were not available due to study limitations that did not permit contact with subjects. However, prior studies have shown sequential serology with immunoglobulin A tissue transglutaminase and endomysial antibodies to be effective at identifying coeliac disease in large populations12. The Kalixanda study found that almost all individuals with positive sequential serologic testing had histology consistent with coeliac disease12. This technique has been used in a number of prior studies5, 15. Additionally, serology was tested at a random point of time in the subject’s medical history, and there has been evidence of regression of seropositivity in the pediatric population23. Another potential limitation is that we did not identify immunoglobulin A deficiency, which can lead to a false negative immunoglobulin A tissue transglutaminase antibody. Based on a 1 in 400 prevalence of immunoglobulin A deficiency in non-Hispanic whites, and a 10% prevalence of coeliac disease in this group, we may have misclassified around 9 individuals with undiagnosed coeliac disease as controls out of 47,557. Another possible cause for false negative immunoglobulin A anti-tissue transglutaminase antibody was we did not identify those on a gluten free diet. A prior study estimated that 0.63% of Americans were on a gluten-free diet, suggesting a possible prevalence of 300 individuals on this diet in our population of 47,5775. However, during review of the medical record, there was no report of gluten-free diets. Additionally, the collection years for the serologic samples predates the gluten avoidance trend24.
Family history was gathered from Patient Family History forms which did not specifically ask about coeliac disease. Because of this, we were likely unable to accurately assess the frequency of family history, which has previously been found to be highly indicative of being at risk for coeliac disease1. Thus, information on testing family members of individuals with coeliac disease should be interpreted cautiously. By basing our data on the medical record, we selected for individuals who utilized medical care; this limitation may be mitigated by the large community-based sample. Additionally, we gathered data by reviewing the care-provider’s listed problems which may have incompletely captured those symptoms raised by the patient but not documented, but may have increased the accuracy of the diagnosis of indications to test. This may have underestimated the frequency of indications to test, as possibly evidenced in the low frequency of irritable bowel syndrome in cases and controls (2% and 4.5% respectively), which is significantly below the 12% prevalence in North America25. However, our study method reflects the indications recognized during a healthcare visit, and therefore reflects case finding taking place in the studied community. Finally, a single investigator collected the entire data set; however, the reliability of this method was validated by the near perfect agreement with the subset of data collected by the second investigator.
In conclusion, this study found that case finding was ineffective at discriminating between those with and without undiagnosed coeliac disease. Individuals with undiagnosed coeliac disease were more likely to develop indications to test such as osteoporosis and autoimmune conditions. If further studies confirm the ineffectiveness of case finding, alternative methods of detecting symptomatic coeliac disease are needed.
Supplementary Material
Acknowledgments
Financial support: The study was funded in part by NIH, RO1-DK57892 (JAM).
Footnotes
- Guarantor: Alberto Rubio-Tapia
-
Specific author contributions:IH: drafted manuscript, data acquisition, interpretation of dataCVD: identified cases of coeliac diseaseTB: expert of serology testingJL, KK: statistical analysis, contributed to manuscript preparationAS: data acquisition, contributed to manuscript preparationJAM: Study design, input on content and analysis, contributed to manuscript preparation, financial supportART: concept, study design, contributed to manuscript preparation, interpretation of data
- All authors have approved the final version of the manuscript.
DR ISABEL A HUJOEL (Orcid ID : 0000-0002-4065-539X)
DR AYUSH SHARMA (Orcid ID : 0000-0003-0700-2909)
DR JOSEPH A MURRAY (Orcid ID : 0000-0003-1941-9090)
Statements of Interests:
Potential competing interests: None
References
- 1.Dubé C, Rostom A, Sy R, et al. The Prevalence of Celiac Disease in Average-Risk and At-Risk Western European Populations: A Systematic Review. Gastroenterology. 2005;128:S57–S67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased Prevalence and Mortality in Undiagnosed Celiac Disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray JA, van Dyke C, Plevak MF, et al. Trends in the Identification and Clinical Features of Celiac Disease in a North American Community, 1950-2001. Clinical Gastroenterology and Hepatology. 2003;1:19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 4.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing Prevalence of coeliac disease over time. Alimentary Pharmacology and Therapeutics. 2007;26:1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The Prevalence of Celiac Disease in the United States. The American Journal of Gastroenterology. 2012;107:1538–1544. doi: 10.1038/ajg.2012.219. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Tapia A, Hill ID, Kelly CP, et al. American College of Gastroenterology Clinical Guideline: Diagnosis and Management of Celiac Disease. American Journal of Gastroenterology. 2013;108:656–677. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal S, Lebwohl B, Green PH. Screening for celiac disease in average-risk and high-risk populations. Therapeutic Advances in Gastroenterology. 2012;5:37–47. doi: 10.1177/1756283X11417038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of Celiac Disease in Primary Care: A Multicenter Case-Finding Study in North America. American Journal of Gastroenterology. 2007;102:1454–1460. doi: 10.1111/j.1572-0241.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosén A, Sandström O, Carlsson A, et al. Usefulness of Symptoms to Screen for Celiac Disease. Pediatrics. 2014;133:211–218. doi: 10.1542/peds.2012-3765. [DOI] [PubMed] [Google Scholar]
- 10.van der Windt DAWM, Jellema P, Mulder CJ, et al. Diagnostic Testing for Celiac Disease Among Patients with Abdominal Symptoms: A Systematic Review. JAMA. 2010;303:1738–1746. doi: 10.1001/jama.2010.549. [DOI] [PubMed] [Google Scholar]
- 11.Katz KD, Rashtak S, Lahr BD, et al. Screening for Celiac Disease in a North American Population: Sequential Serology and Gastrointestinal Symptoms. American Journal of Gastroenterology. 2011;106:1333–1339. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker MM, Murray JA, Ronkainen J, et al. Detection of Celiac Disease and Lymphocytic Enteropathy by Parallel Serology and Histopathology in a Population-Based Study. Gastroenterology. 2010;139:112–119. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibbins-Domingo K, Grossman D, Curry S, et al. Screening for Celiac Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:1252–1257. doi: 10.1001/jama.2017.1462. [DOI] [PubMed] [Google Scholar]
- 14.Choung R, Murray JA. The US Preventive Services Task Force Recommendation on Screening for Asymptomatic Celiac Disease: a Dearth of Evidence. JAMA. 2017;317:1221–1223. doi: 10.1001/jama.2017.1105. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and Mortality among Older Individuals with Undiagnosed Celiac Disease. Gastroenterology. 2010;139:763–769. doi: 10.1053/j.gastro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–1228. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai JC, Fried M, Corazza G, et al. World Gastroenterology Organisation global guidleines on celiac disease. J Clin Gastroenterol. 2013;47:121–6. doi: 10.1097/MCG.0b013e31827a6f83. [DOI] [PubMed] [Google Scholar]
- 18.Kelly CP, Bai J, Liu E, et al. Advances in Diagnosis and Management of Celiac Disease. Gastroenterology. 2015;148:1175–1186. doi: 10.1053/j.gastro.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebwohl B, Rubio-Tapia A, Guandalini S, et al. Diagnosis of Celiac Disease. Gastrointestinal Endoscopy Clinics of North America. 2012;22:661–677. doi: 10.1016/j.giec.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of Celiac Disease in At-Risk and Not-At-Risk Groups in the United States. Archives of Internal Medicine. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 21.Mariné M, Fernández-Banares F, Alsina M, et al. Impact of mass screening for gluten-sensitive enteropathy in working population. World Journal of Gastroenterology. 2009;15:1331–1338. doi: 10.3748/wjg.15.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Pathak J, Murphy S, et al. Use of computerized algorithm to identify individuals in need of testing for celiac disease. Journal of American Medical Informatics Association. 2013;20:e306–10. doi: 10.1136/amiajnl-2013-001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simell S, Hoppu S, Hekkala A, et al. Fate of Five Celiac Disease-Associated Antibodies During Normal Diet in Genetically At-Risk Children Observed from Birth in a Natural History Study. The American Journal of Gastroenterology. 2007;102:2026–2035. doi: 10.1111/j.1572-0241.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Patel K, Orosz E, et al. Time Trends in the Prevalence of Celiac Disease and Gluten-Free Diet in the US Population: Results from the National Health and Nutrition Examination Surveys 2009–2014. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.5254. [DOI] [PubMed] [Google Scholar]
- 25.Lovell R, Ford A. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clinical Gastroenterology and Hepatology. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


