Abstract
We have previously shown that 12 days of high-dose calcineurin inhibition induced tolerance in MHC inbred miniature swine receiving MHC-mismatched lung, kidney, or co-transplanted heart/kidney allografts. However, if lung grafts were procured from brain dead donors, and transplanted alone, they were rejected within 19–45 days. Here, we investigated whether donor brain death with or without allograft ischemia would also prevent tolerance induction in kidney or heart/kidney recipients. Four kidney recipients treated with 12 days of calcineurin inhibition received organs from donors rendered brain dead for 4 hours. Six heart/kidney recipients also treated with calcineurin inhibition received organs from donors rendered brain dead for 4 hours, 8 hours or 4 hours with 4 additional hours of cold storage. In contrast to lung allograft recipients, all isolated kidney or heart/kidney recipients that received organs from brain dead donors achieved long term survival (> 100 days) without histologic evidence of rejection. Pro-inflammatory cytokine gene expression was upregulated in lungs and hearts, but not kidney allografts after brain death. These data suggest that the deleterious effects of brain death and ischemia on tolerance induction are organ-specific, which has implications in the application of tolerance to clinical transplantation.
Introduction
Clinical studies have demonstrated that long-term tolerance can be achieved in human recipients of kidney allografts (1). However, kidney allografts transplanted into these patients were procured from living-related donors and transplanted with short ischemic times. Thus, the donor organs had not been exposed to the highly inflammatory milieu consequent to brain death, nor had they been exposed to prolonged ischemia. Given the deleterious effects of brain death and ischemia on organ function and allograft survival (2), it is necessary to understand how brain death and ischemia affects tolerance induction before extending tolerance protocols proven successful in recipients of living-donor organs to recipients of cadaveric-donor organs.
Our laboratory has previously studied the effects of brain death in a lung transplantation model using MHC-inbred miniature swine. We showed that a 12-day course of high-dose tacrolimus induced long-term tolerance in recipients of fully MHC-mismatched lung allografts procured from healthy, non-brain-dead donors. However, when lung allografts were procured from donors rendered brain dead and mechanically ventilated for four hours prior to organ procurement, they were all rejected within 45 days (3). These data confirm, in a preclinical model, that the deleterious effects of brain death in the donor have the potential to render an otherwise successful tolerance protocol ineffective.
In separate tolerance studies using miniature swine, it has been shown that a 12-day course of high-dose tacrolimus could also induce tolerance in recipients of fully MHC-mismatched kidney allografts (4), but not of heart allografts. In fact, when isolated heart allografts were transplanted across the same full MHC barrier with the same 12-day course of tacrolimus, they were all rejected within 40 days (5). However, when heart and kidney allografts from the same MHC-mismatched donor were co-transplanted under a 12-day course of tacrolimus, recipients uniformly became tolerant of both organs (5). These studies demonstrate that the ability of a particular tolerance protocol to induce long-term unresponsiveness is organ-specific (6).
Given the striking organ-specific differences we have observed in the ability of tolerance induction protocols to achieve immune unresponsiveness, we asked whether the effects of brain death and ischemia on tolerance induction would also differ depending on the organ transplanted. Here we show that in contrast to lung allograft recipients, donor brain death and prolonged organ ischemia did not prevent tolerance induction in isolated kidney or heart plus kidney allograft recipients.
Material and Methods
Animals
Transplant donors and recipients were selected from our herd of MHC inbred miniature swine (age, 3–6 months; weight, 15–50 kg) (7). Swine leukocyte antigen (SLA)gg (MHC class Ic/IId) donor organs were transplanted into SLAdd (MHC class Id/IId) recipients to achieve a 2-haplotype, class I MHC mismatch, while SLAdd (MHC class Id/IId) donor organs were transplanted into SLAcc (MHC class Ic/IIc) recipients to achieve a 2-haplotype, full MHC mismatch. All animal care and procedures were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and conducted in compliance with the Guide for the Care and Use of Laboratory Animals.
Experimental groups
Eight miniature swine received combined heart and kidney allografts from the same fully MHC disparate donors and were treated with a 12-day course of tacrolimus. Organs in Group 1 recipients were procured from donors rendered brain dead for 4 hours (n=2). Organs in Group 2 were procured from donors rendered brain dead for 8 hours (n=2). Organs in Group 3 were procured from donors rendered brain dead for 4 hours then stored for 4 additional hours in University of Wisconsin solution on ice (n=2). Group 4 animals received a combined heart/kidney transplant without brain death, but were taken back to the OR on POD 2 to undergo renal artery clamping for 80 minutes so as to induce a period of warm ischemic injury in the kidney graft. Group 5 animals underwent bilateral native nephrectomies then received isolated MHC class I-disparate kidney allografts from donors rendered brain dead for 4 hours and a 12-day course of cyclosporine (n=4). Recipients were followed for over 3 months and regarded tolerant if, at POD 100, heart allografts showed strong contractions with no signs of rejection on biopsy and recipients had normal creatinine levels with no rejection on kidney biopsy.
Operative procedures
Donor brain death and cold ischemia
Under general anesthesia, brain death was induced by intracranial inflation of a 30 cc Foley catheter over a period of one minute (3) which elicited a Cushing response. All donors were mechanically ventilated and similarly supported with crystalloid fluids and dopamine to maintain an adequate blood pressure for 4 or 8-hours following brain death. Organs in Group 1, 2, 4, and 5 were transplanted immediately after organ procurement. Organs in Group 3 were stored in University of Wisconsin solution on ice to prolong the cold ischemic times; 3 hours for kidneys, 4 hours for hearts (as hearts were implanted after the kidneys).
Heart and kidney transplantation
Recipients underwent bilateral nephrectomy. The aorta and inferior vena cava were used for end-to-side arterial and venous anastomoses for both the heart and kidney, with the heart placed on the right and the kidney on the left in cases of heart/kidney co-transplantation. A vesicoureteral anastomosis was performed as part of the kidney implantation (8).
Renal artery clamping
The laparotomy was re-opened and, after heparin administration (200 units/kg), the renal artery was clamped for 80 minutes with a bulldog clamp. Twenty-four hours after clamping, a kidney biopsy was performed to evaluate the degree of ischemic injury. Kidney-injury molecule-1 (KIM-1), a transmembrane protein that is specifically up-regulated in injured proximal tubular epithelial cells was used to assess the degree of ischemic renal injury (9).
Skin grafting
Split-thickness skin grafts were placed on the dorsum of long-term tolerant recipients in Group 4. Animals received fresh (self: SLAcc) or frozen (donor: SLAdd, third party: SLAll) skin grafts.
Immunosuppression and rejection monitoring
Tacrolimus (Haorui Pharma-Chem Inc., Irvine, CA) was administered as a continuous infusion at a dose of 0.08–0.20 mg/kg (target trough level of 30–50 ng/ml) for 12 consecutive days, starting on the day of transplantation. Cyclosporine was given as a daily intravenous infusion over 1 hour (13 to 16 mg/kg/day with target levels 400 to 800 ng/mL) for 12 consecutive days, starting on the day of transplantation. Cardiac allograft rejection was defined by either loss of a ventricular impulse on palpation, and/or the lack of ventricular contraction on echocardiography. Renal allograft rejection was defined as sustained rise in serum creatinine to >10 mg/dL and/or uremia.
Histopathological examination
Scoring of acute rejection on cardiac allograft biopsies was based on the International Society for Heart and Lung Transplantation System (10). Acute rejection in kidney allograft biopsies was scored according to the Banff classification (11). Some kidney samples were also stained with an anti-mouse/rat FoxP3 antibody (clone: FJK-16s, eBioscience, Inc., San Diego, CA).
Cell-mediated lympholysis (CML) assay
Effector cells were incubated with target cells at effector/target ratios of 100:1, 50:1, 25:1, and 12.5:1. Two target cells were tested in each assay: (1) PBLs SLA matched to the donor (SLAdd: class Idd and class IIdd), and (2) third-party PBLs. 51Cr release was determined on a gamma counter (Micromedics, Huntsville, AL). The results were expressed as a percentage of specific lysis and calculated as follows: Percentage of specific lysis = ((Experimental release [cpm] − Spontaneous release [cpm]) / (Maximum release [cpm] − Spontaneous release [cpm])) × 100 (5).
Mixed lymphocyte reaction (MLR) assay
Cultures containing 4 × 106 responder and 4 × 106 irradiated (2500 cGy) stimulator PBMCs were incubated for 5 days after which 1 uCi of [3H]-thymidine was added to each well. [3H]-thymidine incorporation was determined in triplicate samples by beta-scintillation counting. Absolute counts were compensated for background and then expressed as stimulation indices (SI), calculated as SI = average cpm for a responder− stimulator pair per cpm of the same responder stimulated by an autologous stimulator (5).
Flow cytometry
The presence of anti-donor immunoglobulin (IgM and IgG) in the serum of experimental swine was examined by flow cytometry using a Becton Dickinson FACScalibur (Sunnyvale, CA) as previously described (5).
Immunofluorescence staining for KIM-1
Tissue from a kidney biopsy performed 24 hours after renal artery clamping was stained with the anti-pig-KIM-1 antibody as described previously (12).
Quantitative PCR
Kidney, lung, and heart biopsy specimens obtained before and after brain death were submerged in RNAlater (Qiagen, Valencia, CA, USA) and snap-frozen in liquid nitrogen. RNA was isolated from tissue (Qiagen RNeasy Mini Kit) and used to derive first-strand cDNA (Invitrogen cDNA Synthesis Kit). Fold change difference was calculated using the double delta Ct method, and all samples were normalized to GAPDH. qPCR primers were synthesized based on previously published reports of swine-specific assays for IL-1, IL-6, TNF-α, IFN-γ and GAPDH (13).
Results
Donor brain death did not prevent tolerance induction in recipients of co-transplanted heart and kidney allografts or isolated kidney allografts
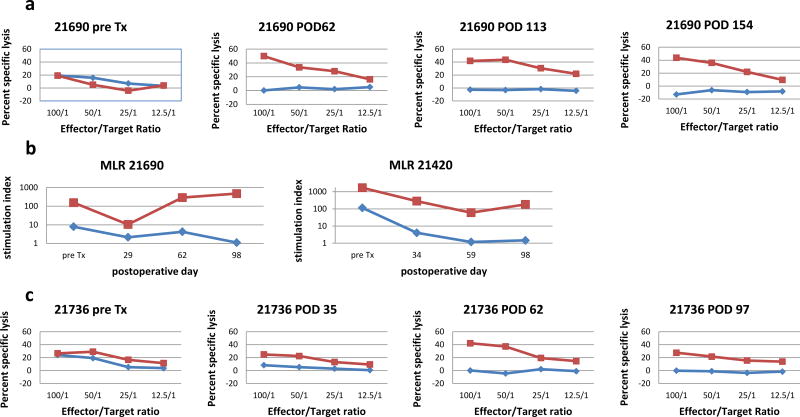
We have previously demonstrated that MHC-mismatched recipients of co-transplanted heart/kidney allografts from the same healthy, non-brain dead donors all became tolerant of their allografts after a 12-day course of tacrolimus and survived long term (5). Table I shows that long-term, stable tolerance was still observed in uncomplicated recipients which received fully MHC-disparate heart and kidney allografts from donors rendered brain dead for 4 hours (Group 1) or for 8 hours (Group 2) before organ procurement. Allografts in these tolerant recipients survived over 100 days without any histologic evidence of rejection on serial cardiac biopsies (Fig S1a). Serial CML assays (Fig 1a) and MLR (Fig 1b) assays demonstrated the loss of host anti-donor T cells responses by PODs 30–60, while serial FACS analysis revealed the absence of significant alloantibody levels at any time point (Fig S2). One animal (#21505) died on POD 16 from pulmonary embolism. Another animal (#22029) developed recurrent pneumonias with septicemia (Gram+ cocci and Gram− rods), requiring intravenous antibiotic (vancomycin, piperacillin/tazobactam) treatment for several weeks. However, despite this prolonged inflammatory state, recipient #22029 continued to exhibit donor-specific unresponsiveness in in vitro assays with only minimal changes on cardiac biopsies (Table I).
Table 1.
Graft survival, histology of heart-kidney and isolated kidney recipients
| Group | Organ | Protocol | Animal # | Graft survival |
Histology at 3 months |
|---|---|---|---|---|---|
| 1 | Heart & Kidney | Brain death × 4hrs | 21690 | >100 days | Heart: ISHLT 0R Kidney: i1t2+TOLS |
| 21505 | 16 days (died of PE) | n/a (i0t1+TOLS at 16 days) | |||
| 2 | Heart & Kidney | Brain death × 8hrs | 22025 | >100 days | Heart: ISHLT 0R Kidney: i1t3+TOLS |
| 22029 | >100 days | Heart: ISHLT 1R Kidney: i0t2+TOLS | |||
| 3 | Heart & Kidney | Brain death × 4hrs Cold ischemia × 4hrs | 21740 | >100 days | Heart: ISHLT 0R Kidney: i1t2+TOLS |
| 22026 | >100 days | Heart: ISHLT 0R Kidney: i1t2 no TOLS | |||
| 4 | Heart & Kidney | Warm renal ischemia × 80mins | 21420 | >100 days | Heart: ISHLT 0R Kidney: i1t2+TOLS |
| 21736 | >100 days | Heart: ISHLT 0R Kidney: i1t2+TOLS | |||
| 5 | Kidney | Brain death × 4hrs | 18055 | >100 days | i1t2+TOLS |
| 18225 | >100 days | i0t3+TOLS | |||
| 18226 | >100 days | i1t2+TOLS | |||
| 18353 | >100 days | i0t2+TOLS |
POD, postoperative day; ISHLT, International Society for heart and lung transplantation; ACR, acute cellular rejection; PE, pulmonary embolism; TOLS, Treg-rich Organized Lymphoid Structure; i, t Banff lesion scores for interstitial inflammation and tubulitis, respectively.
Figure 1.
a) Serial cell-mediated lympholysis (CML) assays using responder cells from animal #21690. Pre-transplant anti-donor responses (blue) were lost by POD 62 but strong responses against 3rd party antigen (Yorkshire PBMC) (red) persisted. b) Representative mixed-lymphocyte reaction (MLR) assays. In animal #21690 and animal #21420 pre-transplant anti-donor responses were lost by POD 30 (blue) but strong response against 3rd party antigen (Yorkshire PBMC) (red) persisted. c) Serial CML assays using responder cells from animal #21736. Pre-transplant anti-donor responses were lost by POD35 (blue) but strong responses against 3rd party antigen (Yorkshire PBMC) (red) persisted.
The kidney allografts in these recipients all maintained normal function as evidence by serial creatinine levels despite the finding of a variable mononuclear cellular infiltrate classified as Banff borderline or TCMR I (Figure S1b). The infiltrates often formed organized aggregates around arterioles and small arteries rich in FoxP3+ cells (Fig S1c) similar to the Treg-rich organized lymphoid structures (TOLS) that we have observed in spontaneously accepted murine kidney allografts (14). TOLS were seen as early as 16 days (#21505) and were present in 10/11 allograft kidneys at 3 months (Table 1) but were not observed in the heart allografts.
Donor brain death had no effect on recipients of isolated kidney allografts, as long-term stable tolerance was also observed in all cyclosporine-treated recipients receiving class I disparate kidney allografts from donors rendered brain dead for 4 hours (Table I, Group 5). Although the protocol used in Group 5 varied from the Groups 1–4, previous studies in MHC-inbred miniature swine indicate that the rejection response and survival of class I-disparate kidney (15) or heart allografts (8) transplanted under the cover of cyclosporine mimic that of fully MHC-disparate kidneys (4) or hearts (5) transplanted under the cover of tacrolimus. Isolated cardiac allografts were not tested in this brain death study as hearts from non-brain dead donors were all rejected within 40 days (5).
Comparison of donor brain death effects in recipients of MHC disparate heart/kidney versus lung allografts
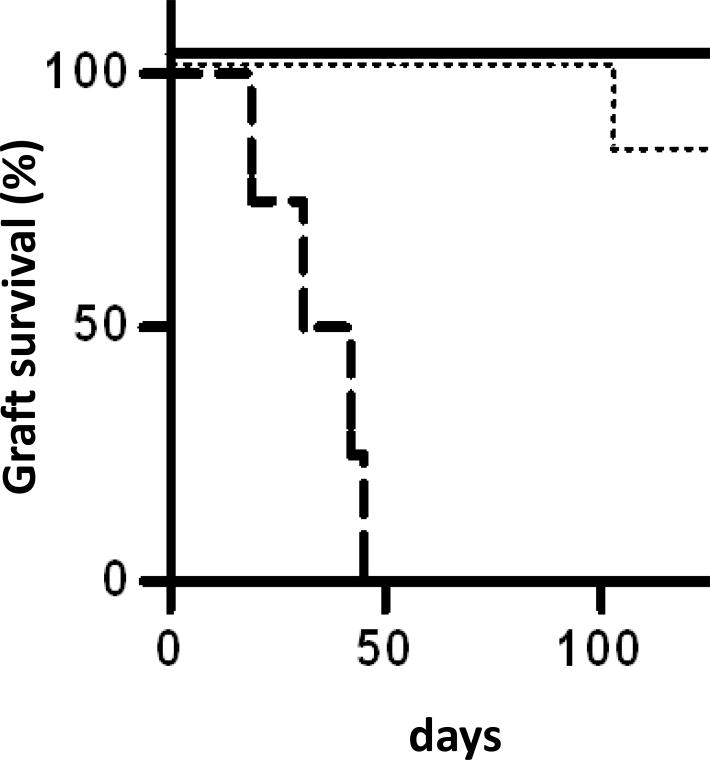
To demonstrate the organ-specific effects of brain death on tolerance induction, we compared the survival times of recipients co-transplanted with heart/kidney allografts from brain dead donors to recipients of brain-dead lung allografts (data published previously (3)). In this comparison, all brain-dead allografts were retrieved four hours after intracerebral injury and all allografts were transplanted across a full MHC barrier with the same 12-day course of tacrolimus. Figure 2 shows the clear organ-specific differences in the effects of brain death on tolerance; recipients of brain-dead lungs all rejected by postoperative day 45, whereas recipients of brain dead kidneys or heart/kidney allografts survived long term.
Figure 2.
Donor brain death effects in recipients of heart/kidney versus lung allografts. Survival times of brain dead heart and kidney allograft recipients (solid line) are compared to the survival times of non-brain dead lung allograft recipients (dotted line) and brain dead lung allograft recipients (dashed line). The lung allograft data was published previously (3).
Organ-specific changes in tissue cytokine gene expression after brain death
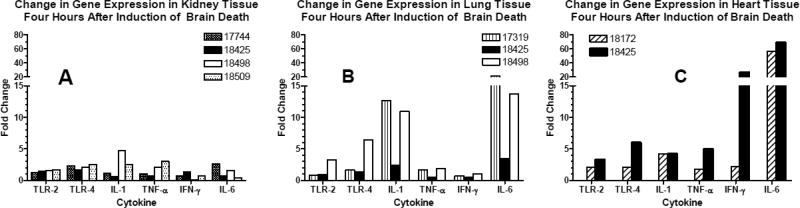
To determine the inflammatory effects of brain death, we measured the change in cytokine gene expression of TLR2, TLR4, IL-1, IL-6, TNF α, and IFNγ in donor organs. Kidney, lung, and heart tissue was collected from animals (n=4 for kidney, n=3 for lung, n=2 for heart) before and 4 hours after induction of brain death. Quantitative PCR was performed and fold change in gene expression was calculated before normalizing to GAPDH. There was little change in RNA levels of cytokine gene expression in the kidneys (Fig 3a). In contrast, substantial increases in RNA for IL-1 and IL-6 were observed in 2/3 lungs and in 2/2 hearts after brain death (Fig 3b and c).
Figure 3.
Change in tissue gene expression after brain death. qPCR was performed on RNA isolated from kidney (A), lung (B), and heart (C) tissue collected from animals before and 4 hours after induction of brain death. Fold change difference was calculated using the double delta Ct method, and all samples were normalized to GAPDH.
Cold ischemia in addition to brain death did not prevent the induction of tolerance of heart and kidney allografts
When the heart and kidneys were exposed to a 3–4 hour period of cold ischemia in addition to the four hour period of brain death, long-term stable tolerance was still achieved (Table I, Group 3). Serial heart biopsies showed no rejection and in vitro assays revealed no anti-donor T or B cell responses after 3 months (Table I). The only difference noted in this group was the cardiac allograft’s greater susceptibility to ventricular arrhythmia after reperfusion.
Warm ischemic renal injury induced by renal artery clamping did not prevent the induction of tolerance
Two additional heart/kidney transplants were performed using organs from healthy, non-brain dead donors that were intentionally subjected to warm ischemia by returning the recipients to the operating room on POD 2 to undergo in situ donor renal artery clamping for 80 minutes (Table I, Group 4). This resulted in a sharp increase in serum creatinine in both recipients with levels peaking between 4 and 6 mg/dL 72 hours after the ischemic insult. However, creatinine levels gradually decreased to baseline levels over a period of two weeks. Kidney biopsies performed 24 hours after clamping and stained for KIM-1 confirmed that the renal tubule epithelium had suffered an ischemic injury (compare Fig S1d and e). Despite this extended period of warm ischemia with documented tubular injury, both recipients accepted the heart and kidney allografts long term and showed no anti-donor T cell (Fig 1c and 1b) or B cells responses. Serial biopsies showed no signs of cellular rejection in the heart (Fig S1f) and minimal infiltrate in the kidney (Table I).
Donor-specific (SLAdd) and third-party (SLAll) skin grafts were placed on the Group 4 recipients on PODs 97 (#21736) and 98 (#21420). The third-party skin grafts were rejected within 10 days; however, the donor-specific skin grafts survived to days 35 (#21420) and 62 (#21736). Of note, serial biopsies of the heart and kidney allografts in both recipients showed no evidence of rejection well after rejection of donor skin (over 60 days). This finding demonstrates that even after an ischemic insult the tolerogenic kidney is able to induce and maintain a robust state of unresponsiveness.
Discussion
The recent successes achieved in inducing tolerance in human recipients of living-donor kidney allografts has prompted investigators to consider applying the same protocols to recipients of organs procured from brain-dead donors1. However, there is little experimental literature to guide this new initiative, as most small- and large-animal tolerance studies have used organs from healthy, non-brain-dead donors transplanted under optimal circumstances with very short ischemic times. This concern is heightened by our previous report demonstrating that donor brain death can render ineffective a tolerance protocol that was otherwise successful in recipients receiving lungs from healthy, non-brain-dead donors (Fig 2)(3).
Previously documented organ-specific differences in tolerance induction (4, 5) led us to consider whether the effects of brain death and ischemia on tolerance induction would also differ depending on the organ transplanted. Here we demonstrate that, in contrast to lung allograft recipients, brain death and ischemia (cold and warm) did not prevent tolerance induction in isolated kidney or heart plus kidney recipients. These findings suggest that, like tolerance, the effects of donor brain death and ischemia vary with the organ transplanted.
The effect of donor brain death and organ ischemia on organ quality has been studied extensively; however, investigations into the effect of brain death and ischemia on tolerance induction are limited. Francuski et al (16) showed that donor brain death affected the long term function and histology of rat kidneys transplanted into recipients treated with 10 days of CyA and anti-CD4 mAb therapy, but it did not have an effect on overall graft survival. The same group demonstrated that extended cold ischemia did not interfere with the induction of rat kidney allograft tolerance using anti-CD4 mAb treatment (17). These findings are in line with our results in porcine recipients of isolated kidney allograft. However, to our knowledge, comparable studies evaluating the effects of brain death on tolerance in heart or lung allograft recipients have not been performed.
Our finding that isolated kidney recipients and heart/kidney recipients were spared from the deleterious effects of brain death and ischemia (in contrast to lung allografts) suggests an organ-specific mechanism which has not been previously described. These results extend our earlier studies using non-brain-dead donors by showing that not only are kidney allografts able to conferring tolerance on otherwise tolerance-resistant hearts (5), but also that they possess an intrinsic ability to counter the deleterious effects of brain death and ischemia on tolerance induction.
To investigate the mechanisms underlying these organ-specific differences, we measured the changes in gene expression of TLR2, TLR4, IL-1, IL-6, TNF α, and IFNγ which occurred in kidney, heart and lung tissue after 4 hours of brain death. Little change in RNA levels of cytokine gene expression was observed in kidney tissue but substantial increases in RNA for IL-1 and IL-6 were observed in lung and heart tissue after brain death. These organ-specific differences in gene expression following brain death are consistent with previous reports in the literature(18, 19)
These findings suggest that the differential effects observed after brain death observed in our study results from organ-specific differences in the upregulation of pro-inflammatory genes like IL-6 versus protective genes such as HO-1. This theory posits that following brain death, the balance of protective gene expression versus inflammatory gene expression in kidney allografts favors a protective milieu, allowing for the induction of tolerance; whereas in lung allografts, that balance is shifted towards a more pro-inflammatory state which tilts the overall immune response from tolerance toward rejection. Recently, Zheng et al. (20) demonstrated a population of donor-derived, nonclassical monocytes retained in donor lung grafts that may explain the pro-inflammatory nature of these allografts.
In summary, our results show that the deleterious effects of brain death and ischemia on tolerance induction differ depending on the organ transplanted, with kidney being more resistant to the effects of brain death and ischemia than lungs. Moreover, kidney allografts from brain dead donors are capable of extending their protective effects to co-transplanted brain dead heart allografts. These findings suggest that attempts to apply tolerance induction protocols to recipients of extra-renal, brain dead organs may require separating in time the organ implantation and the initiation of a tolerance protocol. Indeed, we have shown that delaying the induction of tolerance until the inflammatory state associated with brain death and I/R injury dissipates is both feasible and effective (21).
Supplementary Material
Figure S1: Histology of heart and kidney allografts. (a) H&E staining of heart tissue biopsied from animal #21690 on POD 113 and graded as ISHLT 0. (b) PAS staining and (c) FoxP3 staining (brown) of kidney tissue biopsied from animal #21690 on POD 113. Focally dense infiltrates contain abundant Foxp3+ cells (resembling Treg-rich organized lymphoid structures (TOLS) (14). KIM-1 immunofluorescence staining of renal tubular epithelial cells in animal #21420 before (d) and 24 hours after (e) renal artery clamping (nuclei staining blue, KIM-1 staining green). Negative controls were incubated with the secondary antibody only and did not show significant staining (data not shown). (f) H&E staining of heart tissue biopsied from animal #21736 on POD 97 and graded as ISHLT 0.
Figure S2: Serum alloantibody levels in heart/kidney recipients. Serum levels of anti-SLAdd IgM and IgG antibodies were measured by flow cytometry. Data were normalized to the mean fluorescence intensity (MFI) of negative control values to plot normalized MFI as a function of postoperative day. Dotted line represents the average normalized MFI of positive control serum.
Acknowledgments
We acknowledge C06RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine and are indebted to Mr J. Scott Arn for herd management and quality control typing. We thank Drs. Takahari Ichimura and Joseph V. Bonventre for providing the anti-pig-KIM-1 antibody and Wiebke Sommer for careful review of the manuscript.
Dr. Michel is a recipient of an ASTS-Novartis Scientist Scholarship Grant. Dr. Madariaga is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital and a recipient of a fellowship from the International Society for Heart and Lung Transplantation and a National Research Service Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health (F32HL117540).
Abbreviations
- ACR
acute cellular rejection
- CAV
cardiac allograft vasculopathy
- CML
cell mediated lympholysis
- MHC
major histocompatibility complex
- MLR
mixed-lymphocyte reaction
- POD
post-operative day
- PBL
peripheral blood leukocytes
- PSL
percent specific lysis
- SLA
swine lymphocyte antigen
- Tregs
T regulatory cells
Footnotes
Request for Proposals from the Immune Tolerance Network (ITN) entitled “Clinical Trials of Immune Tolerance in Transplantation using Deceased Donor Organs” at http://www.immunetolerance.org/sites/files/2014%20RFP%20Deceased%20Donor%20Transplantation.pdf.
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Floerchinger B, Oberhuber R, Tullius SG. Effects of brain death on organ quality and transplant outcome. Transplant Rev (Orlando) 2012;26(2):54–9. doi: 10.1016/j.trre.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer AJ, Veillette GR, Aoyama A, Kim KM, Cochrane ME, Wain JC, et al. Donor Brain Death Inhibits Tolerance Induction in Miniature Swine Recipients of Fully MHC-Disparate Pulmonary Allografts. Am J Transplant. 2012;12(5):1290–5. doi: 10.1111/j.1600-6143.2011.03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utsugi R, Barth RN, Lee RS, Kitamura H, LaMattina JC, Ambroz J, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71(10):1368–79. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 5.Madariaga ML, Michel SG, Tasaki M, Villani V, La Muraglia GM, Sihag S, et al. Induction of cardiac allograft tolerance across a full MHC barrier in miniature swine by donor kidney cotransplantation. Am J Transplant. 2013;13(10):2558–66. doi: 10.1111/ajt.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madariaga ML, Kreisel D, Madsen JC. Organ-specific differences in achieving tolerance. Curr Opin Organ Transplant. 2015;20(4):392–9. doi: 10.1097/MOT.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–67. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Madsen JC, Yamada K, Allan JS, Choo JK, Erhorn AE, Pins MR, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65(3):304–13. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 12.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–F63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 13.Duvigneau JC, Hartl RT, Groiss S, Gemeiner M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J Immunol Methods. 2005;306(1–2):16–27. doi: 10.1016/j.jim.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della PP, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178(4):1635–45. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianello PR, Sachs DH. Effect of major histocompatibility complex matching on the development of tolerance to primarily vascularized renal allografts: A study in miniature swine. Human Immunology. 1996;50:1–10. doi: 10.1016/0198-8859(96)00059-6. [DOI] [PubMed] [Google Scholar]
- 16.Francuski M, Reutzel-Selke A, Weiss S, Pascher A, Jurisch A, Ulrich F, et al. Donor brain death significantly interferes with tolerance induction protocols. Transpl Int. 2009;22(4):482–93. doi: 10.1111/j.1432-2277.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 17.Reutzel-Selke A, Hartmann J, Brandenburg P, Jurisch A, Francuski M, Ulrich F, et al. Cold ischemia does not interfere with tolerance induction. Transplantation. 2009;87(8):1116–24. doi: 10.1097/TP.0b013e31819dfb29. [DOI] [PubMed] [Google Scholar]
- 18.Stiegler P, Sereinigg M, Puntschart A, Bradatsch A, Seifert-Held T, Wiederstein-Grasser I, et al. Oxidative stress and apoptosis in a pig model of brain death (BD) and living donation (LD) Journal of translational medicine. 2013;11:244. doi: 10.1186/1479-5876-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritschl PV, Ashraf MI, Oberhuber R, Mellitzer V, Fabritius C, Resch T, et al. Donor brain death leads to differential immune activation in solid organs but does not accelerate ischaemia-reperfusion injury. J Pathol. 2016;239(1):84–96. doi: 10.1002/path.4704. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Z, Chiu S, Akbarpour M, Sun H, Reyfman PA, Anekalla KR, et al. Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci Transl Med. 2017;9(394) doi: 10.1126/scitranslmed.aal4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12(2):330–40. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Histology of heart and kidney allografts. (a) H&E staining of heart tissue biopsied from animal #21690 on POD 113 and graded as ISHLT 0. (b) PAS staining and (c) FoxP3 staining (brown) of kidney tissue biopsied from animal #21690 on POD 113. Focally dense infiltrates contain abundant Foxp3+ cells (resembling Treg-rich organized lymphoid structures (TOLS) (14). KIM-1 immunofluorescence staining of renal tubular epithelial cells in animal #21420 before (d) and 24 hours after (e) renal artery clamping (nuclei staining blue, KIM-1 staining green). Negative controls were incubated with the secondary antibody only and did not show significant staining (data not shown). (f) H&E staining of heart tissue biopsied from animal #21736 on POD 97 and graded as ISHLT 0.
Figure S2: Serum alloantibody levels in heart/kidney recipients. Serum levels of anti-SLAdd IgM and IgG antibodies were measured by flow cytometry. Data were normalized to the mean fluorescence intensity (MFI) of negative control values to plot normalized MFI as a function of postoperative day. Dotted line represents the average normalized MFI of positive control serum.