Abstract
Liver ischemia-reperfusion injury (IRI) represents a major risk factor of early graft dysfunction and a key obstacle to expanding the donor pool in orthotopic liver transplantation (OLT). Although graft autophagy is essential for resistance against hepatic IRI, its significance in clinical OLT remains unknown. Despite recent data identifying heme oxygenase-1 (HO-1) as a putative autophagy inducer, its role in OLT and interactions with Sirtuin-1 (SIRT1), a key autophagy regulator, have not been studied. We aimed to examine HO-1 mediated autophagy induction in human OLT and in a murine OLT model with extended (20h) cold storage, as well as to analyze the requirement for SIRT1 in autophagy regulation by HO-1. Fifty-one hepatic biopsies from OLT patients were collected under an IRB protocol two hours after portal reperfusion, followed by Western blot analyses. High HO-1 levels correlated with well-preserved hepatocellular function and enhanced SIRT1/LC3B expression. In mice, HO-1 overexpression by genetically-modified HO-1 macrophage therapy, was accompanied by decreased OLT damage, increased SIRT1/LC3B expression, whereas adjunctive inhibition of SIRT1 signaling diminished HO-1 mediated hepatoprotection and autophagy induction. Our translational study confirms the clinical relevance of HO-1 cytoprotection and identifies SIRT1-mediated autophagy pathway as a new essential regulator of HO-1 function in IR-stressed OLT.
Introduction
Orthotopic liver transplantation (OLT) is the standard of care for patients with end-stage liver disease and those with hepatic malignancies, but liver graft shortage remains a major challenge. Liver ischemia-reperfusion injury (IRI), an innate immune-driven sterile inflammation response leading to hepatocellular death, is an inevitable consequence of multiple clinical conditions, including trauma, sepsis, hepatic tumor resection, and liver transplantation. Indeed, hepatic IRI has been recognized as a major risk factor for delayed early graft function, acute and chronic rejection as well as a key obstacle to expanding the donor organ pool. However, despite obvious clinical importance, mechanisms that account for liver IRI are only partially understood and no effective therapy is available to prevent or treat this condition in humans (1).
Autophagy is an evolutionarily conserved intracellular self-digesting pathway responsible for maintaining energy homeostasis and removing long-lived or damaged organelles and proteins (2). A growing body of evidence indicates a tissue protective function for autophagy in various pathologic states, such as aging, diabetes and neurodegenerative diseases (3) as well as in liver IRI (4-6). Although enhanced autophagy may represent a novel therapeutic target against IR-damage, its efficacy and mechanism in OLT settings remain to be assessed, while clinical relevance of autophagy pathway in liver transplant patients has not been studied before.
Heme oxygenase-1 (HO-1; Hmox1), a rate-limiting enzyme that catalyzes the conversion of heme into biliverdin, carbon monoxide, and free iron, exerts potent anti-oxidative, anti-inflammatory and cytoprotective functions (7). We and others have reported on beneficial function of HO-1 in hepatic IRI murine models using chemical HO-1 inducers (8-11), adenovirus (Ad) gene transfer (8, 12) and genetically-modified animals (13). As macrophages are key mediators of innate immune-driven inflammation and the primary source of HO-1 in IR-stressed liver (10, 14), we have established a novel molecular transfer approach using ex-vivo genetically-modified HO-1-overexpressing macrophages. This regimen effectively transferred the target molecule into 40-50% of hepatic cells and successfully alleviated warm IRI in mice (15, 16); its putative efficacy in clinically-relevant OLT models has not been tested. Although anti-inflammatory phenotype is central to HO-1 function in liver IRI (17), recent studies have identified HO-1 as a novel hepatic autophagy inducer (18, 19); its role in liver transplantation remains unknown and putative regulatory mechanisms need to be studied.
Sirtuin 1 (SIRT1), an NAD+-dependent type III histone/protein deacetylase involved in cellular senescence, inflammation and stress resistance (20), plays a key role in autophagy induction (21, 22). In addition to an anti-inflammatory role in pathogenesis of liver IRI (23), recent studies have demonstrated the significance of SIRT1-induced autophagy in hepatocyte resistance against IR-stress (24, 25). Molecular communication between SIRT1 and HO-1 in OLT has not been studied and the mechanism by which SIRT1 may regulate the HO-1 autophagy pathway remains to be elucidated.
To gain further insight into HO-1 mediated autophagy in liver transplantation, we used an ex-vivo genetically-modified HO-1 macrophage adoptive transfer approach in a clinically relevant murine OLT model and analyzed human OLT samples (n=51) in parallel. Our translational study confirms the clinical relevance of HO-1 hepatoprotection and identifies SIRT1-dependent autophagy as a novel and essential regulator of HO-1 function in OLT under IR-stress.
Materials and Methods
Clinical liver transplant study
Fifty-six consecutive adult primary OLT recipients were recruited under an IRB protocol (13-000143; May 2013 – August 2015). Routine standard of care and immunosuppressive therapy were administered as specified by UCLA liver transplant protocols. Study data were collected and managed using REDCap electronic data capture tools (26). Donor organs, procured using standardized techniques, were perfused with and stored in cold University of Wisconsin (UW) solution (ViaSpan; Bristol-Meyers Squibb Pharma). Cold ischemia time was defined as the time between the perfusion of the donor liver with UW solution and its removal from cold storage. Recipient venous blood was collected within the hour prior to the transplant and on post-operative days 1-14 (POD 1-14). Hepatocellular injury was evaluated by serum alanine aminotransferase (sALT) levels. Post-transplant biopsies (Bx) were obtained from the left liver lobe using a Tru-Cut biopsy needle approximately two hours after portal reperfusion (prior to the abdominal closure) and snap-frozen. Out of fifty-six cases, fifty-one were examined; four were excluded because the Bx samples were too small for Western blot analyses, and one was excluded due to unavailability of clinical data. Early allograft dysfunction (EAD) was defined by the presence of one or more of the following: total bilirubin ≥10 mg/dL (171 μmol/L) or INR ≥1.6 on POD 7, and ALT/AST >2,000 IU/L within the first 7 POD (27) (28). Post-transplant rejection was diagnosed by follow-up Bx done per clinical standard of care.
Animals
C57BL/6 mice at 6-8 weeks of age were used (Jackson Laboratory, Bar Harbor, ME). Animals were housed in the UCLA animal facility under specific pathogen-free conditions, received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985). All studies were reviewed and approved by the UCLA Animal Research Committee.
Generation of genetically modified bone marrow-derived macrophages (BMM)
L929 cells (ATCC, Rockville, MD) were cultured in RPMI-1640 medium supplemented with 2 mmol/l l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum. The conditioned media was collected from cells grown for 7 days. The BMM were generated according to standard procedures (15). In brief, bone marrow cells were removed from the femurs and tibias of C57BL/6 mice and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/l l-glutamine, with the addition of 15% L929-conditioned medium. Cells were cultured at 5×106 cells/well for 7 days with 5% CO2 and 95% air at 37 °C, and used for in vitro transfection.
Replication-defective recombinant adenovirus encoding HO-1 (AdHO-1) was generated as described (29). BMM (5×106/well) were incubated for 1h with AdHO-1 or Adβ-gal (at a MOI of 10). After medium change, cells were cultured for additional 48h prior to in vivo adoptive transfer. By 48h of transfection the X-Gal staining was >90%, compared with control BMM (15).
Mouse liver cold ischemia and transplantation model
We used a well-established mouse model of ex-vivo hepatic cold storage and orthotopic liver transplantation (OLT) as described (30). Donor livers were stored in UW solution at 4°C for 20h prior to transplantation into syngeneic mice. BMM (5×106) transfected with AdHO-1/Adβ-gal (2.5×109 pfu) or untreated (5×106) were injected via portal vein prior to liver reperfusion at the completion of transplant surgery. In some experiments, SIRT1 inhibitor (EX527, Sigma Aldrich, St. Louis, MO) was administered (10mg/kg i.p.) at 30 min prior to OLT surgery (15, 16). Liver and serum samples were collected 6h after reperfusion, the peak of hepatocellular damage in this model. The sham group underwent the same procedures except for OLT.
Serum biochemistry
Serum alanine transaminase (sALT) levels, an indicator of hepatocellular injury, were measured by IDEXX Laboratories (Westbrook, ME).
Liver histology and IRI grading
Formalin-fixed paraffin-embedded OLT sections (5μm) were stained with hematoxylin and eosin (HE). The severity of hepatic IRI was graded using Suzuki criteria (31).
Quantitative RT-PCR analysis
RNA was extracted from liver tissue samples using RNAse Mini Kit (Qiagen, Germantown, MD). A total of 5.0μg of RNA was reverse-transcribed into cDNA. Quantitative PCR was performed using DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA) (32). The primers sequences are listed (Table S1). The expression of the target gene was normalized to the housekeeping HPRT.
Western blot assay
Proteins were extracted from liver tissues and their concentration was measured (BCA Protein Assay Kit, Thermo Scientific). Equal amount of protein was electrophoresed, blotted, and incubated with primary Ab, secondary HRP-conjugated Ab, and developed. Primary Ab detecting HO-1 (Enzo Life Sciences, Farmingdale, NY), SIRT1, LC3B, β-actin (Cell Signaling Technology, Danvers, MA) were used. To compare target protein expression in multiple human OLT samples, densitometry quantification was conducted using a reference sample and normalization with β-actin as reported (23).
Immunohistochemistry
OLT-infiltrating neutrophils were detected using monoclonal rat anti-Ly6G Ab (BD Biosciences, San Jose, CA). Immunostaining signals were visualized with a labeled polymer in the EnVision + system horseradish peroxidase kit (Dako). Positively-stained cells were counted blindly (10 HPF/section). LC3B expression in OLT was detected using rabbit anti-LC3B Ab and signals were visualized with secondary Ab: Alexa Fluor 488 anti-rabbit IgG.
TdT-mediated dUTP nick end labeling (TUNEL) assay
Cell death in formalin-fixed paraffin-embedded liver sections (5μm) was detected by Apop Tag Plus Peroxidase in Situ Apoptosis Kit (Millipore, Temecula, CA). Results were scored semi-quantitatively by blindly counting the number of positive cells in 10 HPF/section.
Statistical Analysis
In mouse experiments, group comparisons were performed using a Student t-test. For human data, continuous values were analyzed by Mann-Whitney U test and categorical variables by Fisher’s exact test. Spearman’s correlation coefficient (r) was used to evaluate the strength of linear relationship between variables. The cumulative survival rate was analyzed by Kaplan-Meier method, and differences between groups were compared using a log-rank test. JMP for Windows 8.0 (SAS Institute, Cary, NC) was used for statistical analyses. A p-value of <0.05 was considered statistically significant.
Results
AdHO-1 transfected BMM alleviate IRI in OLT
We first aimed to validate the efficacy of HO-1 induction following the adoptive transfer of AdHO-1 BMM in a clinically relevant mouse IRI-OLT model. Cold-stored liver grafts were infused with BMM via portal vein immediately prior to reperfusion. At 6h after reperfusion, AdHO-1 BMM-treated OLT showed increased HO-1 expression as compared with naive or Adβ-gal transfected BMM-treated controls (Fig. 1A). AdHO-1 BMM-conditioned OLT displayed attenuated sinusoidal congestion, edema/vacuolization and hepatocellular necrosis as compared with control OLT infused with untreated or Adβ-gal transfected cells (Fig. 1B). These correlated with depressed Suzuki grading of histological liver damage (OLT: + AdHO-1 BMM = 1.7±0.6 vs. OLT: + BMM = 3.7±1.2 or IR + Adβ-gal = 3.3±0.6; n=4-6/group, p<0.05, Fig. 1C) and preserved liver function, evidenced by sALT levels (OLT: +AdHO-1 BMM = 3,900±2,642 vs. OLT: +BMM = 7,997±2,068 or IR + Adβ-gal = 7,726±2,129 IU/L; mean±SD, n=4-6/group, p<0.05, Fig. 1C) in AdHO-1 BMM-treated OLT. Thus, infusion of AdHO-1 BMM into the graft enhanced HO-1 expression and minimized the adverse effects of hepatic IRI in OLT.
Figure 1. Adoptive transfer of AdHO-1 BMM ameliorates IR-damage in OLT.
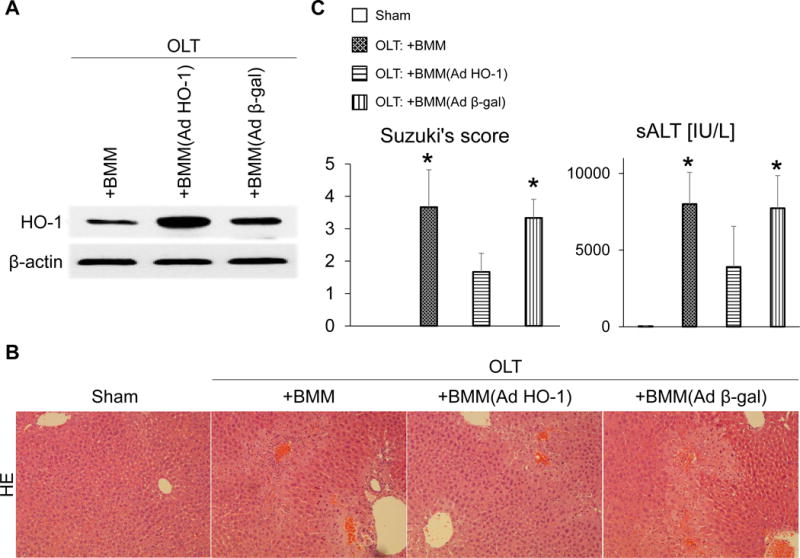
Mouse (C57BL/6) livers subjected to 20h of cold storage were transplanted to syngeneic mice. BMM (5×106) transfected with AdHO-1 or Adβ-gal (2.5×109 pfu) or untreated BMM were infused into liver grafts via portal vein immediately prior to reperfusion. Liver grafts and serum samples were analyzed at 6h post-OLT. (A) Representative (n=3) Western blot-assisted detection of HO-1; β-actin expression served as an internal control. (B) Representative H&E staining (n=3; original magnification, ×100). (C) Suzuki histological grading of liver IRI and sALT levels. (n=4-6/group); *p<0.05 vs. OLT: +BMM (AdHO-1). Data shown as mean±SD.
AdHO-1 gene transfer promotes anti-inflammatory signature in OLT
As anti-inflammatory function is one of the key HO-1 biological features (17), we examined whether AdHO-1 gene transfer alters the anti-/pro-inflammatory gene expression profile in IR-stressed OLT. Indeed, AdHO-1 BMM infused directly into the graft increased mRNA coding for anti-inflammatory IL10 and TGFβ, while decreasing pro-inflammatory TNFα, MCP1 and CXCL10 as compared with naïve/Adβ-gal BMM treated controls (Fig. 2A/B). This confirms HO-1 overexpression exerts an anti-inflammatory function and inhibits inflammatory cytokine-induced OLT injury. We also tested whether AdHO-1 gene transfer may affect cytokine-induced neutrophil OLT influx. As expected, HO-1 overexpression reduced neutrophil (Ly6G) numbers in IR-stressed OLT infused with AdHO-1 BMM (Fig. 3A/B), indicating HO-1 gene transfer enhanced the anti-inflammatory phenotype and diminished cytokine-induced inflammation.
Figure 2. Adoptive transfer of AdHO-1 BMM inhibits pro-inflammatory signature and enhances anti-inflammatory program in OLT.
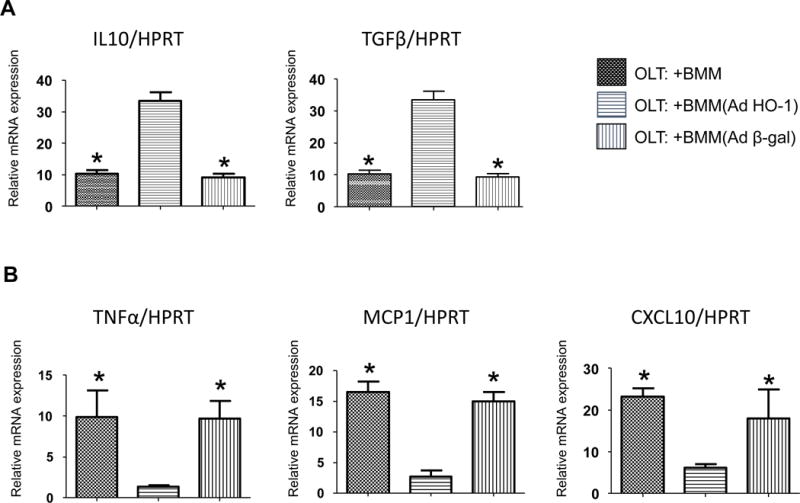
Mouse (C57BL/6) livers subjected to 20h of cold storage and transplanted to syngeneic mice were infused immediately before reperfusion via portal vein with BMM (naïve, AdHO-1 or Ad β-gal). Samples were analyzed at 6h post-OLT. qRT-PCR-assisted detection of mRNA coding for IL10 and TGFβ (A), TNFα, MCP1, CXCL10 (B). Data were normalized to HPRT gene expression (n=4/group) * p<0.05 vs. OLT: +BMM (AdHO-1). Data shown as mean±SD.
Figure 3. Adoptive transfer of AdHO-1 BMM suppresses neutrophil trafficking and mitigates hepatocellular death in OLT.
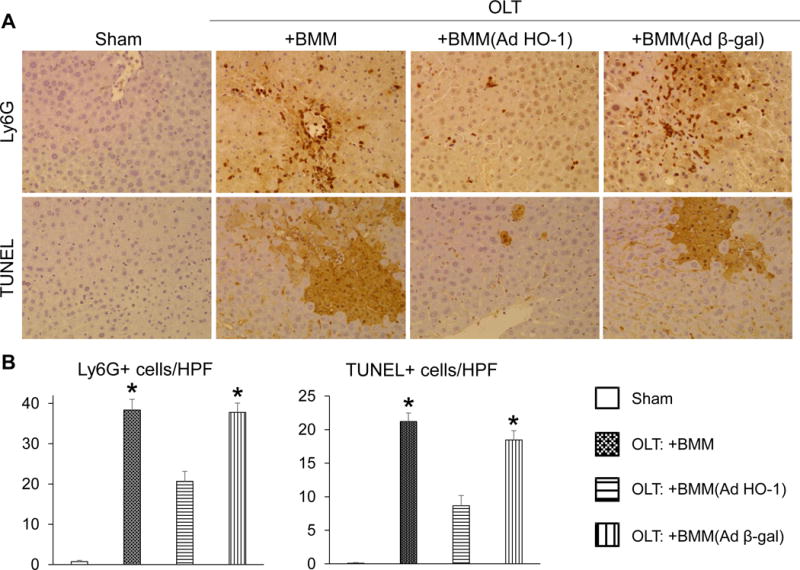
Mouse (C57BL/6) livers subjected to 20h of cold storage and transplanted to syngeneic mice were infused via portal vein with BMM (naïve, AdHO-1 or Ad β-gal). Samples were analyzed at 6h post-OLT. (A) Representative immunohistochemical Ly6G (upper panels) and TUNEL (lower panels) staining. (B) Quantification of Ly6G (left) and TUNEL (right) positive cells/HPF (n=4-6/group). * p<0.05 vs. OLT: +BMM (AdHO-1). Data shown as mean±SD.
AdHO-1 gene transfer attenuates hepatocellular death while enhancing SIRT1/LC3B expression in OLT
We next aimed to determine whether AdHO-1 gene transfer affects cell death and autophagic activity in IR-stressed OLT. As shown in Fig. 3A/B, a reduced frequency of TUNEL+ apoptotic/necrotic cell death was observed in AdHO-1 BMM-treated OLT as compared with naive BMM or Adβ-gal BMM controls. Moreover, we found that AdHO-1 BMM therapy not only upregulated hepatic SIRT1 (a key autophagy regulator) but also enhanced LC3B (an autophagosome marker) expression, as compared with controls (Fig. 4A-C), underscoring the importance of the HO-1 – SIRT1 axis in cell autophagy regulation. Taking together, these results are consistent with the idea that HO-1 overexpression exerts cytoprotective and autophagic functions in IR-stressed OLT.
Figure 4. Adoptive transfer of AdHO-1 BMM enhances SIRT1/LC3B expression in OLT.
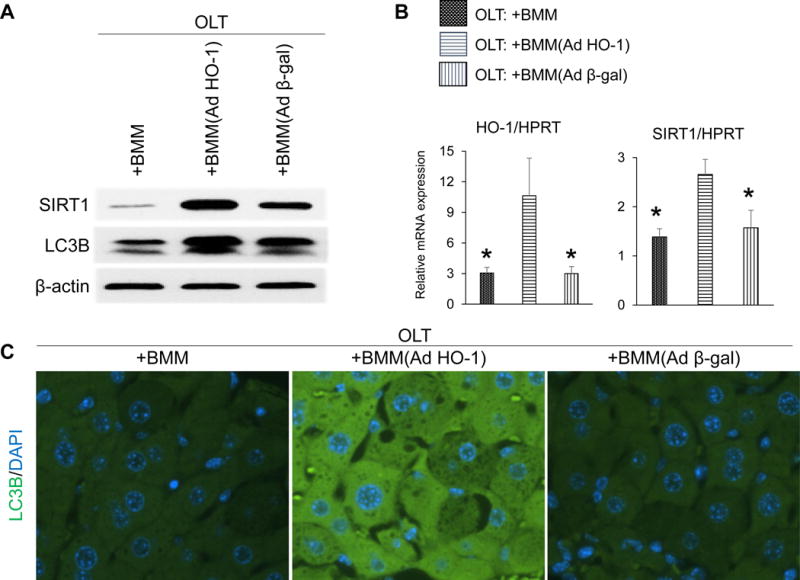
Mouse (C57BL/6) livers subjected to 20h of cold storage and transplanted to syngeneic mice were infused via portal vein with BMM (naïve, AdHO-1 or Ad β-gal). Samples were analyzed at 6h post-OLT. (A) Representative (n=3) Western blot-asisted detection of SIRT1 and LC3B; β-actin expression served as an internal control. (B) qRT-PCR-assisted detection of mRNA coding for HO-1 and SIRT1. Data were normalized to HPRT gene expression (n=4/group) * p<0.05 vs. OLT: +BMM (AdHO-1). Data shown as mean±SD. (C) Representative (n=3) immunohistochemical detection of LC3B in OLT at 6h post-reperfusion.
HO-1 expression correlates with IR-liver damage and SIRT1/LC3B signaling in human OLT
Having shown AdHO-1 BMM-facilitated hepatoprotection accompanied by increased SIRT1/LC3B levels in a murine OLT model, we next aimed to validate the relevance of these findings in human liver transplantation. Liver post-reperfusion biopsies (Bx) from fifty-one human OLTs were screened by Western blots for HO-1, SIRT1 and LC3B expression (Fig. 5A). Hepatic HO-1 expression profile exhibited weak negative correlation with sALT levels at POD1 (r=−0.3306, p=0.0178, Fig. 5B), indicating higher HO-1 was associated with milder IR-liver injury. In addition, graft HO-1 expression showed positive correlation with SIRT1 (r=0.4229, p=0.0020, Fig. 5C) and weak positive correlation with LC3B (r=0.3594, p=0.0096, Fig. 5D).
Figure 5. Post-transplant HO-1 expression negatively correlates with hepatocellular damage and positively with SIRT1/LC3B expression in human OLT.
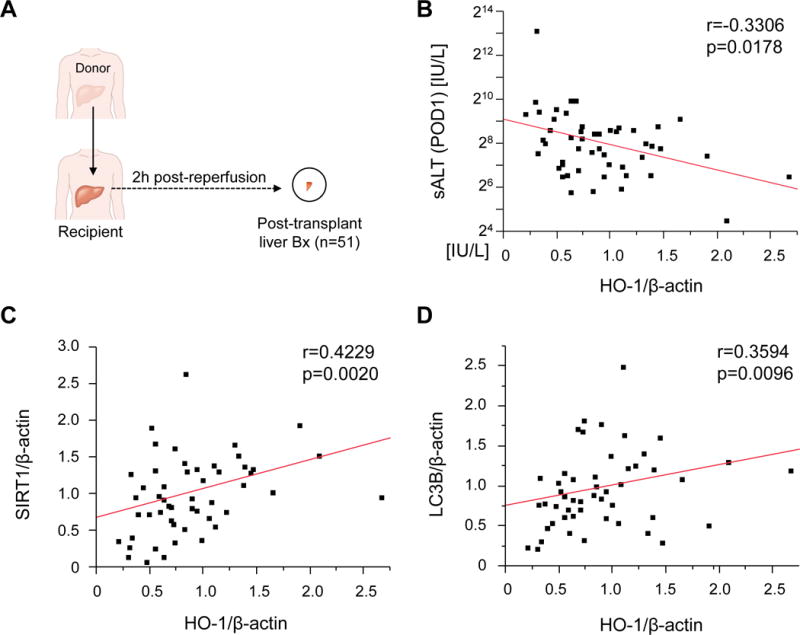
(A) Post-transplant liver biopsies (Bx; 2h post-reperfusion) were collected from fifty-one OLT patients. Bx samples were analyzed by Western blots with β-actin normalization. (B) Relationship between post-transplant HO-1 expression and sALT levels at postoperative day 1 (POD1). (C/D) Relationship between post-transplant HO-1 and SIRT1 (C) and LC3B (D) expression. r: Spearman’s correlation coefficient.
To evaluate the impact of hepatic HO-1 on clinical outcomes, fifty-one OLT samples were split evenly into low (n=26) and high (n=25) HO-1 expression groups (Fig. 6A). The patients’ demographic data and clinical parameters are shown (Table S2). There was no correlation between HO-1 expression on and donor background, including age, gender, weight, BMI or pre-procurement sALT level. We also found no correlation between HO-1 expression and recipient factors, including age, gender, weight, BMI, race, disease etiology, presence of HCC, ABO-compatibility, MELD score, pre-transplant sALT or dialysis. Furthermore, there was no correlation between HO-1 expression and cold ischemic time or intra-operative blood loss.
Figure 6. Increased post-transplant HO-1 levels correlate with attenuated hepatocellular damage and enhanced SIRT1 – LC3B pathway in human OLT.
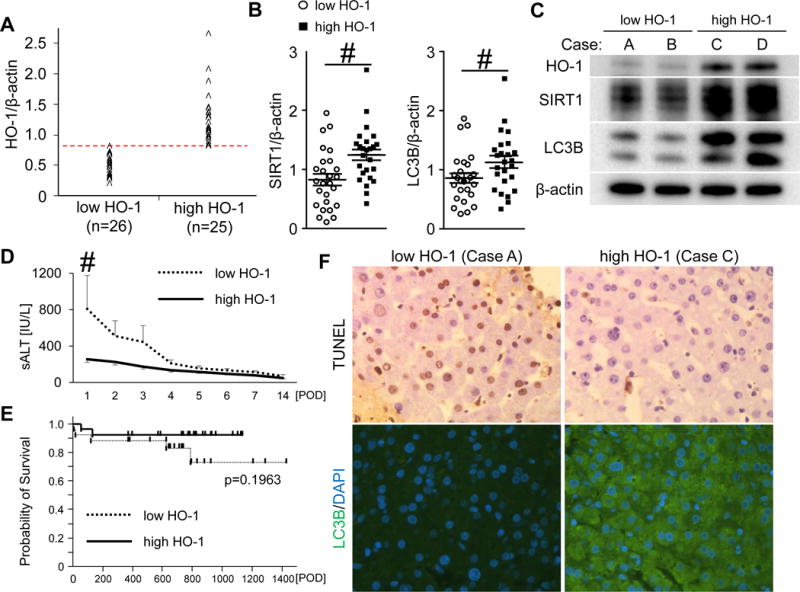
(A) Bx samples collected from the fifty-one OLT patients were classified into low HO-1 (n=26) and high HO-1 (n=25) groups. (B) Western blot-assisted expression of SIRT1 and LC3B. Data shown in dot plots and bars indicate mean±SEM. # p<0.05 (Mann-Whitney U test). (C) Representative Western-blots assisted detection of HO-1, SIRT1 and LC3B (Case A/B: low HO-1 group, Case C/D: high HO-1 group) (D) sALT levels at postoperative day 1-14 (POD1-14) shown as mean±SEM. Dotted line indicates low HO-1, while the solid line high HO-1. # p<0.05 (Mann-Whitney U test). (E) The cumulative probability of OLT survival (Kaplan-Meier method). Dotted line indicates low HO-1, while the solid line high HO-1 groups (log-rank test). (F) Representative TUNEL and LC3B staining pattern in OLT.
Consistent with Fig. 5C/D data, the high HO-1 group showed increased SIRT1 (low HO-1: 0.83±0.10 vs high HO-1: 1.25±0.10, mean±SEM, p<0.05, Fig. 6B left) and LC3B (low HO-1: 0.87± 0.08 vs high HO-1: 1.13±0.10, mean±SEM, p<0.05, Fig. 6B right) expression. Representative Western blots are shown (Fig. 6C). In addition, the high HO-1 group exhibited significantly lower sALT levels at POD1 (low HO-1: 809±367 vs high HO-1: 255±34 IU/L, mean±SEM, p<0.05, Fig. 6D). To examine the relationship between HO-1 expression and OLT outcomes, we analyzed cumulative post-transplant survival, with the median follow-up of 740 days (range, 4-1432 days). None of the patients underwent secondary liver transplantation. Despite consistent trends, the improved survival in the high HO-1 expression group failed to reach statistical significance when compared with the low HO-1 expression group (p=0.1963, Fig. 6E). The high HO-1 expression group was characterized by lower frequency of early graft dysfunction (3.8% vs 8.0%) and post-OLT rejection episodes (3.8% vs 16.0%). However, these differences failed to reach statistical significance (p=0.5150 and p=0.1871, respectively). Representative TUNEL and LC3B staining in low vs high HO-1 Bx groups are shown (Fig. 6F).
SIRT1 inhibition abrogates hepatoprotection and depresses autophagy despite HO-1 induction in mouse OLT
Based on the aforementioned preclinical and clinical data, preservation of hepatocellular function (sALT) and enhanced autophagy (LC3B) in HO-1 overexpressing OLT was accompanied by increased SIRT1 signaling, a putative regulator of autophagy and stress resistance against hepatic IR-damage (25). Hence, we hypothesized SIRT1 might be essential in cytoprotection after HO-1 induction in IR-stressed OLT. As shown in Fig. 7A, adjunctive SIRT1 inhibition prior to transplantation (EX527, 10mg/kg i.p.) recreated cardinal features of hepatic IRI in otherwise well-functioning AdHO-1 BMM-treated OLT, evidenced by increased sALT levels and Suzuki histological grading scores (Fig. 7B). Moreover, SIRT1 inhibition depressed LC3B expression in AdHO-1 BMM-treated liver grafts. These data indicate the SIRT1 – autophagy pathway is required for HO-1 mediated hepatoprotection against IRI in OLT.
Figure 7. SIRT1 inhibition depresses LC3B expression and abrogates hepatoprotection in AdHO-1 BMM-conditioned IR-OLT.
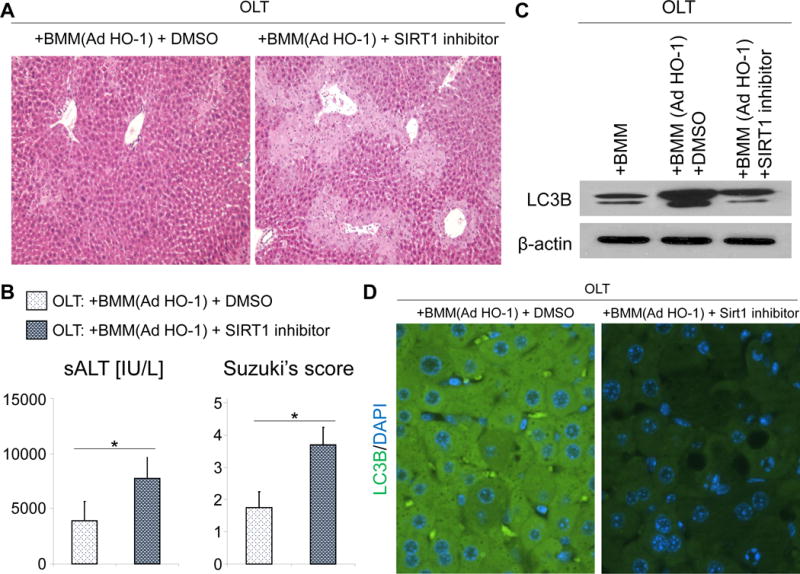
Mouse (C57BL/6) livers subjected to 20h of cold storage were transplanted to syngeneic mice and conditioned with AdHO-1 BMM with or without adjunctive SIRT1 inhibition (EX527, 10mg/kg i.p). Samples were analyzed at 6h post-OLT. (A) Representative H&E staining (original magnification, ×100). (B) sALT levels (left) and Suzuki histological grading of liver IRI (right). *p<0.05 vs. OLT: + AdHO-1 BMM + DMSO (n=3-4/group). Data shown as mean±SD. (C) Representative (n=3) Western blot-asisted detection of LC3B; β-actin expression served as an internal control. (D) Representative (n=3) immunhistochemical detection of LC3B.
Discussion
This study is the first to document HO-1 mediated autophagy enhancement in human liver transplant settings and in a clinically relevant mouse OLT model. Although anti-inflammatory signaling is critical for HO-1 function against IR-stress, our findings document autophagy regulation as a novel mechanistic component of its biological effects. Our clinical study showed positive correlation between HO-1 and SIRT1 levels in human OLT (Fig. 5C/D and Fig. 6B/C), while SIRT1 inhibition in parallel mouse studies diminished autophagy phenotype seen otherwise after HO-1 induction in IR-resistant OLT (Fig. 4A/B and Fig. 7C/D). These data are consistent with the notion that HO-1 regulates hepatic autophagy in a SIRT1 dependent manner. Although previous studies suggested the role of p38 MAPK in HO-1 induced liver autophagy (18, 33), it remains unknown as to how HO-1 may regulate hepatic p38 signaling. In the context of our present data, a recent finding of liver-specific SIRT1 increasing p38 translocation/phosphorylation via YAP/MKK3 dependent manner (34) implies SIRT1-p38 signaling may underlie the autophagy regulation by HO-1. These complex molecular mechanisms require further in-depth analyses.
It is well documented that hepatocyte autophagy is essential in liver stress resistance (4, 35, 36). Indeed, the loss of Atg4B increased sensitivity of aged mouse livers to warm IRI (5), whereas rapamycin increased autophagy and protected against IR-stress (6). In contrast, recent studies in myeloid-specific Atg5 knockout mice have shed a new light on macrophage autophagy regulation (37-39). While macrophage Atg5 deficiency was shown to limit acute toxic liver injury by downregulating IL1β (38), the impaired macrophage Atg5 promoted proinflammatory macrophage polarization in obese mice (37). Although macrophages are pivotal in innate-immune driven IR-damage, the significance of macrophage autophagy in liver IRI, using myeloid autophagy-deficient animals, awaits future research.
Despite numerous preclinical reports identifying HO-1 as a major cytoprotective molecule (10, 15, 16), Geuken reported high “pre-transplant” HO-1 expression in human donor livers paradoxically correlated with augmented post-transplant injury (40). As “post-transplant” HO-1 features specific to clinical liver transplantation have not been well defined, we have recently reported that high HO-1 expression in human OLT (n=21) at the time of reperfusion are associated with suppressed IR-liver damage (41). In agreement with the latter, our present data from human OLT recipients (n=51) consistently demonstrated negative correlation between HO-1 levels and post-transplant sALT levels (Fig. 5B and Fig. 6D), implying therapeutic benefit of HO-1 induction in clinical liver transplantation. Unlike hepatocytes under basal conditions, liver macrophages (infiltrating and tissue-resident) are the major HO-1 producers, both in human OLT and IR-stressed murine livers (10, 41).
Although aging and obesity are poor-prognostic factors influencing HO-1 expression, we found no correlation between post-transplant HO-1 levels and donor/recipient demographic parameters or surgical factors (Supp. Table 2). As HO-1 is not only a cytoprotective molecule but also a stress-inducible heat shock protein-32 (hsp32), its expression increases with the severity of IR-stress. Paradoxically, high HO-1 levels were associated with a more attenuated liver damage in the clinical arm of our study (Fig. 6D). We speculate that the divergent HO-1 expression in OLT might be caused by variable individual inductivity of HO-1 in response to IR-stress, resulting in variable susceptibility against IRI. Indeed, short guanine-thymine nucleotide repeats polymorphism in the promotor region on chromosome 22q13.1 of human HO-1 was shown to enhance HO-1 expression in response to oxidative stress (42), whereas a more recent study associated HO-1 polymorphism with post-OLT hepatocellular damage (43). As others failed to correlate HO-1 polymorphism with its expression profile (40), more studies on putative factors influencing human HO-1 responses are warranted.
We have also recently reported on the anti-inflammatory and hepatoprotective role of SIRT1 activation in a warm liver IRI mouse model alongside negative correlation between SIRT1 and T-bet/IL-1β levels in twenty-one clinical OLT cases (23). The positive SIRT1 regulation by HO-1 seen in a mouse IRI model was then confirmed in twenty-one OLT patient cohort (41). The current study documents the functional significance of HO-1 – SIRT1 axis in a clinically-relevant mouse hepatic cold ischemia/OLT model (Fig. 4A/B) and in fifty-one human OLT cases (Fig. 5C and 6B). Although in our previous clinical study (n=21), post-OLT “high” HO-1 expression was accompanied by a statistically significant improvement in OLT survival (41), we failed to detect a similar statistically significant survival differences in the current (n=51) liver transplant patient cohort (Fig, 6E, p=0.1963). As discussed earlier (41), the link between graft and recipient long-term outcomes and IRI severity remains controversial. The presence of early lesions on the time-zero biopsy in older grafts (n=16) did not translate into early graft dysfunction or graft loss (44), while histological IRI grading in post-reperfusion liver biopsies and peak transaminases (n=55) did not show any correlation with clinical outcomes in the first post-transplant month (45). Likewise, despite preferable trends, our present study (n=51) failed to detect significant differences in EAD, post-OLT rejection or overall survival. In marked contrast, severe IRI on the time-zero biopsy and peak of post-transplant ALT in 476 OLT cases were significantly associated with a 1-year graft survival in an earlier study (46). The correlation between graft/recipient outcomes and IRI severity may thus require considerably larger number of study subjects.
A chemical HO-1 inducer (COPP; cobalt protoporphyrin) was recently shown to increase SIRT1 expression in hepatocytes, while adjunctive competitive HO-1 inhibitor (SnMP; tin mesoporphyrin) abrogated SIRT1 upregulation, indicating HO-1 dependent SIRT1 induction by exogenous COPP (47, 48). Likewise, in our current study, transfer of genetically modified HO-1-overexpressing BMM consistently enhanced liver graft SIRT1 expression levels (Fig. 4A/B). The mechanisms accounting for HO-1 mediated SIRT1 upregulation remain largely unknown. As oxidative stress suppresses hepatic SIRT1 (23, 49), anti-oxidant properties may be the key for HO-1 to regulate SIRT1 expression.
Adenovirus is an efficient vector for in vivo gene therapy due to its ability to mediate transgene expression in various cell types. However, serious off-target effects, including activation of innate immune responses, thrombocytopenia, coagulopathy, and liver damage restrict systemic use of Ad vector in clinical practice (50). In our present and previous preclinical studies (15, 16), we used replication-defective recombinant Ad to generate HO-1-overexpressing BMM. Indeed, the ex-vivo gene modified BMM local infusion upregulated hepatic HO-1/LC3B expression and alleviated IRI in a mouse OLT model. This regimen makes Ad-related adverse effects negligible (51), while direct BMM infusion into the liver improves macrophage transfer efficacy as a cellular delivery system for HO-1 trafficking into the disease site (52). The present study using ex-vivo gene modified HO-1-overexpressing BMM in a clinically relevant mouse model is likely to validate and support future experiments in translational and clinical settings.
In our current study, the injection of BMM (Adβ-gal) to OLT increased hepatic HO-1 expression as compared with untreated BMM (no virus) treated graft (Fig. 1A). This result is consistent with our previous findings in a mouse model of liver warm IRI (16). As a foreign pathogen, virus infection itself can cause cellular stress to activate antioxidant system, accompanied by increased antioxidant gene expression, such as manganese superoxide dismutase, HO-1, indoleamine-2, 3-dioxygenase, and glutathione peroxidase (53). We speculate that increased HO-1 expression following Adβ-gal BMM injection was induced by virus-related BMM stress, which was accompanied by increased SIRT1 and slightly higher LC3B levels (Fig. 4A). However, Adβ-gal BMM failed to ameliorate liver damage in IR-stressed OLT (Fig. 1B/C) as “pure” viral-mediated HO-1 induction may not be proficient enough to protect the liver graft. In contrast, unlike the Adβ-gal BMM controls, infusion of Ad HO-1 BMM markedly enhanced local HO-1 (Fig. 1A) and SIRT1/LC3B (Fig. 4A) levels leading to attenuated hepatocellular injury in IR-stressed OLT (Fig. 1B/C).
In conclusion, our study with genetically modified HO-1-overexpressing BMM transfer in a mouse OLT model and parallel clinical examination of fifty-one human liver transplants not only confirmed the clinical relevance of HO-1 hepatoprotection but also identified a novel regulatory role of SIRT1 mediated autophagy as a part of cytoprotective function in transplant recipients. The present data, in agreement with our recent reports (15, 16), validate a novel investigative tool in which host macrophages can be transfected ex-vivo with cytoprotective HO-1 and then infused, if needed, into prospective recipients to mitigate IR-mediated inflammation during liver transplantation, resection or trauma. As decreasing donor organ quality represents one of the most challenging problems, our findings provide the rationale for a new and clinically attractive gene targeted strategy to “rejuvenate” extended criteria donor livers and improve their function via HO-1–SIRT1–autophagy pathway in transplant recipients.
Supplementary Material
Table S1: Primer sequences used for Real-Time Quantitative PCR.
Table S2: Donor/recipient demographics and surgical factors. Post-transplant liver biopsy samples were collected from fifty-one human liver transplant cases and analyzed by Western-bolts. Based on Western-blot assisted HO-1 expression, 51 cases were divided into low HO-1 (n=26) and high HO-1 (n=25) groups. Correlations between post-transplant HO-1 expression with donor/recipient demographic parameters and surgical factors were analyzed using Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous values. Values are expressed as median (range).
Acknowledgments
This work was supported by NIH grants: R01 DK102110; R01 DK107533; and R01 DK062357 (JWKW); NIH PO1 AI120944 and Keck Foundation Award 986722 (JWKW and EFR); and Ruth L. Kirschstein National Research Service Award T32CA009120 (RAS). We thank Ko Takanashi at UCLA-TPCL for immunohistochemical assistance.
Abbreviations
- Ad
adenovirus
- ALT
alanine aminotransferase
- BMM
bone marrow derived macrophages
- Bx
biopsy
- HE
hematoxylin and eosin
- HO-1
heme oxygenase-1
- IRI
ischemia-reperfusion injury
- OLT
orthotopic liver transplantation
- POD
post-operative day
- sALT
serum alanine aminotransferase
- SIRT1
sirtuin 1
- TUNEL
TdT-mediated dUTP nick end labeling
Footnotes
DR REBECCA A SOSA (Orcid ID : 0000-0002-9860-6830)
The authors declare that no conflict of interest exists.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
References
- 1.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation–from bench to bedside. Nature reviews Gastroenterology & hepatology. 2013;10(2):79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehrecke EH. Autophagy: dual roles in life and death? Nature reviews Molecular cell biology. 2005;6(6):505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 3.Czaja MJ, Ding WX, Donohue TM, Jr, Friedman SL, Kim JS, Komatsu M, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9(8):1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JS, Nitta T, Mohuczy D, O’Malley KA, Moldawer LL, Dunn WA, Jr, et al. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology (Baltimore, Md) 2008;47(5):1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JH, Ahn IS, Fischer TD, Byeon JI, Dunn WA, Jr, Behrns KE, et al. Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice. Gastroenterology. 2011;141(6):2188–2199.e2186. doi: 10.1053/j.gastro.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Lu T, Yue S, Shen X, Gao F, Busuttil RW, et al. Rapamycin protection of livers from ischemia and reperfusion injury is dependent on both autophagy induction and mammalian target of rapamycin complex 2-Akt activation. Transplantation. 2015;99(1):48–55. doi: 10.1097/TP.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends in immunology. 2003;24(8):449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 8.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. The Journal of clinical investigation. 1999;104(11):1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura T, Kondo T, Ogawa K, Fukunaga K, Ohkohchi N. Protective effect of heme oxygenase-1 on hepatic ischemia-reperfusion injury through inhibition of platelet adhesion to the sinusoids. Journal of gastroenterology and hepatology. 2013;28(4):700–706. doi: 10.1111/jgh.12075. [DOI] [PubMed] [Google Scholar]
- 10.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, et al. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2001;1(2):121–128. [PubMed] [Google Scholar]
- 11.Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation. 2007;83(12):1628–1634. doi: 10.1097/01.tp.0000266917.39958.47. [DOI] [PubMed] [Google Scholar]
- 12.Ke B, Buelow R, Shen XD, Melinek J, Amersi F, Gao F, et al. Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Human gene therapy. 2002;13(10):1189–1199. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. Journal of immunology (Baltimore, Md : 1950) 2006;177(7):4749–4757. doi: 10.4049/jimmunol.177.7.4749. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, van de Poll MC, Greve JW, Buurman WA, Fearon KC, McNally SJ, et al. Early stress protein gene expression in a human model of ischemic preconditioning. Transplantation. 2004;78(10):1479–1487. doi: 10.1097/01.tp.0000144182.27897.1e. [DOI] [PubMed] [Google Scholar]
- 15.Ke B, Shen XD, Gao F, Ji H, Qiao B, Zhai Y, et al. Adoptive transfer of ex vivo HO-1 modified bone marrow-derived macrophages prevents liver ischemia and reperfusion injury. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(5):1019–1025. doi: 10.1038/mt.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Shen XD, Yue S, Zhu J, Gao F, Zhai Y, et al. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Molecular medicine (Cambridge, Mass) 2014;20:448–455. doi: 10.2119/molmed.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. Journal of hepatology. 2012;56(2):359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology (Baltimore, Md) 2011;53(6):2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu A, Huang L, Guo E, Li R, Yang J, Li A, et al. Baicalein pretreatment reduces liver ischemia/reperfusion injury via induction of autophagy in rats. Scientific reports. 2016;6:25042. doi: 10.1038/srep25042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem cells (Dayton, Ohio) 2014;32(5):1183–1194. doi: 10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathyanarayan A, Mashek MT, Mashek DG. ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism. Cell reports. 2017;19(1):1–9. doi: 10.1016/j.celrep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Kageyama S, Ke B, Fujii T, Sosa RA, Reed EF, et al. Sirtuin 1 Attenuates Inflammation and Hepatocellular Damage in Liver Transplant Ischemia-Reperfusion: From Mouse-to-Human. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2017 doi: 10.1002/lt.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickenbacher A, Jang JH, Limani P, Ungethum U, Lehmann K, Oberkofler CE, et al. Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice. Journal of hepatology. 2014;61(2):301–308. doi: 10.1016/j.jhep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Biel TG, Lee S, Flores-Toro JA, Dean JW, Go KL, Lee MH, et al. Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell death and differentiation. 2016;23(2):279–290. doi: 10.1038/cdd.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deschenes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation. 1998;66(3):302–310. doi: 10.1097/00007890-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16(8):943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 29.Shibahara S, Muller R, Taguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(23):7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(10):1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55(6):1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Kamo N, Ke B, Ghaffari AA, Shen XD, Busuttil RW, Cheng G, et al. ASC/caspase-1/IL-1beta signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology (Baltimore, Md) 2013;58(1):351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, et al. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PloS one. 2014;9(6):e98834. doi: 10.1371/journal.pone.0098834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Cui R, Zhang X, Qiao Y, Liu X, Chang Y, et al. SIRT1 increases YAP- and MKK3-dependent p38 phosphorylation in mouse liver and human hepatocellular carcinoma. Oncotarget. 2016;7(10):11284–11298. doi: 10.18632/oncotarget.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139(5):1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology (Baltimore, Md) 2012;55(1):222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11(2):271–284. doi: 10.1080/15548627.2015.1009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilyas G, Zhao E, Liu K, Lin Y, Tesfa L, Tanaka KE, et al. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1beta. Journal of hepatology. 2016;64(1):118–127. doi: 10.1016/j.jhep.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodder J, Denaes T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM, et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11(8):1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, et al. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa R, et al. Macrophage HO-1-SIRT1-p53 Axis Regulates Sterile Inflammation in Liver Ischemia–Reperfusion Injury. Journal of hepatology. 2017 doi: 10.1016/j.jhep.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, et al. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102(5):1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 43.Zhang ZY, Guan J, Li H, Zhou ZQ, Zhou GW. Heme Oxygenase-1 Promoter Polymorphism Protects Liver Allograft. The Indian journal of surgery. 2016;78(1):14–19. doi: 10.1007/s12262-015-1309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deschenes M, Forbes C, Tchervenkov J, Barkun J, Metrakos P, Tector J, et al. Use of older donor livers is associated with more extensive ischemic damage on intraoperative biopsies during liver transplantation. Liver transplantation and surgery : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 1999;5(5):357–361. doi: 10.1002/lt.500050501. [DOI] [PubMed] [Google Scholar]
- 45.Berberat PO, Friess H, Schmied B, Kremer M, Gragert S, Flechtenmacher C, et al. Differentially expressed genes in postperfusion biopsies predict early graft dysfunction after liver transplantation. Transplantation. 2006;82(5):699–704. doi: 10.1097/01.tp.0000233377.14174.93. [DOI] [PubMed] [Google Scholar]
- 46.Ali JM, Davies SE, Brais RJ, Randle LV, Klinck JR, Allison ME, et al. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21(4):487–499. doi: 10.1002/lt.24072. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Gao Y, Li M, Geng C, Xu H, Yang Y, et al. Sirt1 mediates the effect of the heme oxygenase inducer, cobalt protoporphyrin, on ameliorating liver metabolic damage caused by a high-fat diet. Journal of hepatology. 2015;63(3):713–721. doi: 10.1016/j.jhep.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Sodhi K, Puri N, Favero G, Stevens S, Meadows C, Abraham NG, et al. Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet. PloS one. 2015;10(6):e0128648. doi: 10.1371/journal.pone.0128648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Archives of biochemistry and biophysics. 2010;501(1):79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appledorn DM, McBride A, Seregin S, Scott JM, Schuldt N, Kiang A, et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene therapy. 2008;15(24):1606–1617. doi: 10.1038/gt.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Froh M, Wheeler MD, Smutney O, Zhong Z, Bradford BU, Thurman RG. New method of delivering gene-altered Kupffer cells to rat liver: studies in an ischemia-reperfusion model. Gastroenterology. 2003;124(1):172–183. doi: 10.1053/gast.2003.50002. [DOI] [PubMed] [Google Scholar]
- 52.Burke B, Sumner S, Maitland N, Lewis CE. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. Journal of leukocyte biology. 2002;72(3):417–428. [PubMed] [Google Scholar]
- 53.Choi AM, Knobil K, Otterbein SL, Eastman DA, Jacoby DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. The American journal of physiology. 1996;271(3 Pt 1):L383–391. doi: 10.1152/ajplung.1996.271.3.L383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Primer sequences used for Real-Time Quantitative PCR.
Table S2: Donor/recipient demographics and surgical factors. Post-transplant liver biopsy samples were collected from fifty-one human liver transplant cases and analyzed by Western-bolts. Based on Western-blot assisted HO-1 expression, 51 cases were divided into low HO-1 (n=26) and high HO-1 (n=25) groups. Correlations between post-transplant HO-1 expression with donor/recipient demographic parameters and surgical factors were analyzed using Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous values. Values are expressed as median (range).


