Abstract
Objectives
Interbirth intervals (IBIs) are a key metric of female reproductive success; understanding how they are regulated by environmental, social, and demographic factors can provide insight into sources of variance in female fitness.
Materials and Methods
Using 36 years of reproductive data on 490 IBIs for 160 wild female baboons, we identified sources of variance in the duration of IBIs and of their component phases: postpartum amenorrhea (PPA), sexual cycling, and pregnancy. We also examined how body fat and fecal hormone concentrations varied during female IBIs.
Results
We found that IBIs tended to be shorter (reproduction was accelerated) when female traits and environmental variables promoted energy acquisition, but with different specific effects for different component phases of the IBI. We also found that females lost a substantial amount of body fat during PPA, indicating that PPA imposes accumulating energetic costs as it progresses. Prior to cycle resumption females began to regain body fat; body fat was stable across the cycling phase and increased throughout most of pregnancy. However, body fat scores per se were not associated with the duration of any of the component phases. Finally, we found that fecal glucocorticoid concentrations decreased as PPA progressed, suggesting a decline in energetic stress over this phase. Fecal progestogen and estrogen concentrations changed over time during sexual cycling; the direction of these changes depended on the phase of the sexual cycle (luteal versus early or late follicular phases).
Discussion
Our study lends insight into the energetic constraints on female primate reproduction, revealing how female environments, changes in body fat, and steroid hormone concentrations relate to IBI duration and to reproductive readiness.
Keywords: postpartum amenorrhea, gestation, sexual cycling, body fat, steroid hormones
INTRODUCTION
After giving birth, most female mammals delay their next reproduction in order to invest in their current offspring. In seasonal breeders, females resume reproduction after a relatively fixed interval, triggered by seasonal cues such as day length, plant secondary compounds, or temperature (e.g., Bronson, 1985). In non-seasonal breeders, females must make a physiological ‘decision’ about when to resume reproduction in the absence of specific seasonal cues. The timing of this physiological decision reflects the tradeoff between three energetic demands: investment in the current offspring, investment in the next offspring, and investment in maternal somatic maintenance and growth. The resolution of this tradeoff is crucial for fitness: reproductive rate is a major component of female reproductive success, and mistakes in timing may be costly. If a female resumes reproduction before she is physiologically ready, she may endanger herself, her current offspring (who may still need energy investment from her), or her next offspring (see Stearns, 1989, 1992 for a discussion of such trade-offs). If she waits longer to resume than optimal, her delay will reduce her fitness relative to females that optimize their reproductive timing.
The interval between one birth and the next typically consists of three phases in primates: postpartum amenorrhea (or lactational amenorrhea), sexual cycling, and pregnancy. The most extensive research on the transitions between these three phases has occurred in humans. Early literature focused on energy status (the amount of energy stored in the body) as a key predictor of the ability of women to begin cycling and to conceive (e.g., Frisch, 1984; Frisch & McArthur, 1974). However, more recent evidence has failed to support a critical role for energy status in these transitions, pointing instead to energy balance (energy consumed minus energy expended) as a major determinant of a woman’s ability to resume cycling after postpartum amenorrhea and to conceive after resuming sexual cycling (Ellison, 2003, 2017; Lager & Ellison, 1990; Lipson & Ellison, 1996; Valeggia & Ellison, 2004, 2009; see also Thompson, 2013a for chimpanzees). Specifically, within a wide range of values for energy status, it is the presence of a surplus of energy that allows women to transition from amenorrhea to cycling, and from cycling to pregnancy (Ellison, 2003, 2017). In other words, women are evolved to initiate reproduction when they have surplus energy in their metabolic budget, independent of how much energy they have stored in the form of fat.
Variation in the duration of pregnancy, like the other phases of the IBI, is sensitive to energy availability in humans, but in the opposite direction to that seen for postpartum amenorrhea and cycling: low energy availability is associated with shorter pregnancies (e.g., Kline, Stein, & Susser, 1989). Pregnancy is by far the least variable phase of the IBI, but pre-term births have been associated with poor nutrition, poor body condition, low weight gain during pregnancy, and low socio-economic status, all likely indicators of energy restriction (reviewed in Wood, 1994). These results reflect the fact that, in humans, labor and birth are initiated when the fetus obtains insufficient energy to sustain growth (reviewed in Ellison, 2003).
In nonhuman primates, studies of energy balance per se are relatively rare. Rosetta and colleagues (Rosetta, Lee, & Garcia, 2011) noted that negative energy balance during postpartum amenorrhea (measured via doubly-labeled water) may contribute to reproductive delays in low-ranking captive female baboons. Thompson and colleagues (Thompson, Muller, & Wrangham, 2012) showed that wild female chimpanzees resumed cycling only after sustained periods of energy gain (measured via urinary C-peptides). However, while direct studies of energy balance are rare, a number of studies have examined predictors of the duration of the interbirth interval (IBI) or one of its three constituent phases (postpartum amenorrhea, cycling, and pregnancy) to gain indirect insight into the role of energetics in primate female reproduction. For instance, studies have shown that high dominance rank (typically associated with priority of access to resources) is associated with shorter postpartum amenorrhea (PPA) phases in baboons (Johnson, 2003; Packer, Collins, Sindimwo, & Goodall, 1995; Wasser, Norton, Kleindorfer, & Rhine, 2004; Wasser, Norton, Rhine, Klein, & Kleindorfer, 1998), and with shorter periods of cycling prior to conception in both baboons and mandrills (Setchell & Wickings, 2004; Smuts & Nicolson, 1989). Similarly, the greater food availability experienced by provisioned compared to wild-feeding animals leads to shorter interbirth intervals in many primate species (e.g., Lee, Brennan, Else, & Altmann, 1986; Rawlins & Kessler, 1986; Sugiyama & Ohsawa, 1982; reviewed in Asquith, 1989). Finally, young female baboons, mandrills, and macaques who are still investing in their own growth (i.e., who have never conceived or have conceived for the first time) often experience more cycles prior to conception, longer periods of PPA, and longer overall interbirth intervals, than multiparous females (Hinde, 2009; Koyama, Takahata, Huffman, Norikoshi, & Suzuki, 1992; Setchell, Lee, Wickings, & Dixson, 2002; Setchell & Wickings, 2004; Wasser et al., 1998).
Variation in the duration of pregnancy has been relatively little studied in nonhuman primates, perhaps because it is the least variable phase of the IBI (Kiltie, 1982; Martin, 2007), and because the onset of pregnancy can be difficult to detect in non-clinical settings. Gestation length in semi-free ranging provisioned mandrills does not appear to be predicted by rank, parity or offspring sex (Setchell et al., 2002), although gestation length in captive olive baboons is predicted by dominance rank, with low-ranking females having shorter gestation than high-ranking females (Garcia, Lee, & Rosetta, 2006). In other settings, gestation may be longer when nutritional conditions are poor (Riopelle & Hale, 1975 for rhesus macaques; Borries, Koenig, & Winkler, 2001 for hanuman langurs; Bronson, 1989; Racey, 1981; Sadlier, 1969 in other mammals). In wild female baboons in Amboseli, Kenya, pregnancies were shorter when they ended in the wet season than when they ended in the dry season, and females who spent progressively less time feeding over the course of pregnancy had longer gestations than those whose time spent progressively more time feeding (Silk, 1986). This result again supports the idea that decreased energy intake leads to longer gestations. This result contradicts observations in humans, in which poor nutrition results in shorter pregnancies, suggesting that humans may be different from other primates in this regard.
Studies that examine all three component phases of the IBI in a single mammalian study system are limited, to our knowledge, to two studies, both in baboons (Garcia et al., 2006; Smuts & Nicolson, 1989). Baboons are relatively non-seasonally breeding primates that have visual indicators of the transition from PPA to sexual cycling. These indicators include highly visible sexual swellings that increase in size during the follicular phase of the sexual cycle and decrease in size during the luteal phase (Dixson, 1983; Gesquiere, Wango, Alberts, & Altmann, 2007; Gillman, 1942; Wildt, Doyle, Stone, & Harrison, 1977), and a pregnancy sign that, along with the cessation of sexual cycles, allows observers to pinpoint the transition from cycling to pregnant (the paracallosal skin turns pink in pregnant female baboons: Altmann, 1973; Beehner, Nguyen, Wango, Alberts, & Altmann, 2006). These external indicators of reproductive state make it possible to precisely measure the duration of each IBI phase in baboons, and make them an ideal model for studying the duration of the three component phases of the IBI.
Here, we seek to understand how female reproductive rates in wild baboons living in the Amboseli ecosystem in southern Kenya are constrained or enhanced by environmental factors and individual traits that affect energy intake and expenditure. Our analysis has three parts. First, we analyze the duration of the IBI and its component phases, testing the predictive power of seven variables thought to be linked to female reproductive rates (see Table 1 for a description of these variables).
Table 1.
Variables used as predictors in our models of female baboon interbirth intervals
| Predictor | Predictor Description | Predictor type; mean±SE, range | Predicted effect | Example citations* | |
|---|---|---|---|---|---|
|
Female traits |
Dominance rank | The ordinal dominance rank of the female at the birth of the infant that initiated the IBI. | Fixed effect; ordinal (1 for highest ranking female, and so on). Mean±SE 9.32±0.28; range 1-28 | Higher ranking females → priority of access to food → accelerated reproduction |
(Barton & Whiten, 1993; Cheney et al., 2004; C. H. Janson, 1988; Johnson, 2003; Packer et al., 1995; Smuts & Nicolson, 1989; Wasser et al., 1998) |
| Primiparity | Whether the female was primiparous at the birth of the infant that initiated the IBI. | Fixed effect; binary. Primiparous (parity of 1), multiparous (parity of at least 2). | Multiparous females → no longer investing in their own growth →accelerated reproduction |
(J. Altmann & Alberts, 2005; Hinde, 2009; Smuts & Nicolson, 1989) | |
| Age | The age of the female at the birth of the infant that initiated the IBI. | Fixed effect; continuous. Modeled as both a linear and a quadratic term: previous analyses indicate highest reproductive rates in middle ages. Mean±SE 10.38±0.16 years; range 5.03-20.25 years. | Middle-aged multiparous females → accelerated reproduction |
(Beehner, Onderdonk, et al., 2006; Smuts & Nicolson, 1989; Strum & Western, 1982) see reviews in (Anderson, 1986; Stewart, Harcourt, & Watts, 1988) | |
| Environmental variables | Group size | Number of females in the group at the birth of the infant that initiated the IBI. | Fixed effect; categorical. small (4-14 adult females), intermediate (15-20) and large (21-31). | Smaller groups →less intragroup competition for food → accelerated reproduction |
(C. H. Janson, 1988; van Schaik, van Noordwijk, Deboer, & Dentonkelaar, 1983) |
| Rainfall | Average daily rainfall over the duration of the IBI and its components phases. Calculated as total rainfall divided by the number of days that the female was in that phase. | Fixed effect; continuous. Mean±SE for annual rainfall is 340±22mm; range141-757 mm | Increase in rainfall → increase in food → accelerated reproduction |
(Barton & Whiten, 1993; Fedigan, Carnegie, & Jack, 2008) | |
| Habitat quality | Whether the female’s group was living in a relatively low- or high-quality habitat (ssee Methods and Altmann and Alberts 2003a). | Fixed effect; binary (lower- or higher-quality habitat). | Higher habitat quality → increase in food → accelerated reproduction |
(J. Altmann & Alberts, 2003; Lee et al., 1986) | |
| Infant trait | Infant sex | The sex of the infant that initiated the IBI. | Fixed effect; binary (M or F). | Female offspring → less energetically costly →accelerated reproduction |
(Bercovitch and Berard, 1993; Berman, 1988; Boesch, 1997; McFarland Symington, 1987); but see reviews in (Brown, 2001) |
Second, we analyze estimates of female body fat that were collected during all phases of female IBIs, to examine changes in body fat during the three component phases of the IBI. Persistent decreases in body fat over time indicate a consistent state of negative energy balance, while increases over time indicate consistent positive energy balance. Thus, we expect that body fat scores will decrease as postpartum amenorrhea (PPA) progresses, as a consequence of the energetic cost of lactation and offspring transportation, and that body fat scores will increase with the transition to cycling. We also predict an increase in fat scores during pregnancy: pregnancy is associated with mass gain (including fetal mass) in humans and a number of other mammals (Institute of Medicine, 2009; Robinson, 1986), and also with increased resistance to leptin and insulin (i.e., with increased appetite) in a range of mammals (reviewed in Henson & Castracane, 2006). We further predict that, because energy status per se (amount of energy stored in the body) is expected to have a weaker influence on reproductive readiness than energy balance (Ellison, 2003), a female’s absolute level of body fat will be a poor predictor of her phase durations.
Third, we examine data on concentrations of steroid hormone metabolites in fecal samples. Specifically, for the subset of IBIs for which we have accompanying hormone data, we investigate whether changes in fecal glucocorticoid (fGC), fecal estrogens (fE), or fecal progestogen (fP) concentrations precede cycling resumption and conception (steroid hormone profiles during pregnancy are described in Altmann, Lynch, Nguyen, Alberts, & Gesquiere, 2004; Beehner, Nguyen, et al., 2006). We predict that as females approach both the transition from PPA to cycling, and the transition from cycling to pregnancy, fGC concentrations will decrease, indicating the females’ more positive energetic balance. We further predict that their fE concentrations will increase as the females approach cycling resumption, an indication that follicular development has resumed (McNeilly, 2001). Finally, we predict that both fE and fP will increase over the course of cycling as hormone levels become adequate to sustain pregnancy (McNeilly, 2001).
MATERIALS AND METHODS
Study subjects and sample sizes
The subjects for all analyses were adult females that belonged to multiple social groups of wild-feeding baboons in the Amboseli ecosystem, in southern Kenya. This population consists of yellow baboons (Papio cynocephalus) that experience some natural admixture with neighboring populations of olive baboons (P. anubis) (Alberts and Altmann, 2001; Charpentier et al., 2012; Tung, Charpentier, Garfield, Altmann, Alberts, 2008). These two species are similar enough that they are sometimes considered subspecies rather than species (e.g., Jolly, 1993); they show little evidence of ecological differentiation (Wango et al., 2017; Winder, 2015) and produce viable and fertile offspring with little or no evidence of hybrid dysgenesis (Ackermann, Rogers, & Cheverud, 2006, Tung et al., 2008). The majority of the population shows no admixture, and all individuals (regardless of admixture) are included in this analysis (Tung et al., 2008).
Individuals in this population are monitored on a near-daily basis; reproductive, demographic, and behavioral data have been collected for over four decades (Alberts & Altmann, 2012). All data collection procedures adhered to the regulations of the Institutional Animal Care and Use Committee of Duke, Princeton, and Notre Dame Universities, and the laws of Kenya.
We conducted three separate analyses. In the first we examined the duration of IBIs and their component phases. For this analysis we used 36 years of data (collected between 1977 and 2012) on reproductive states, demographic events, dominance rank, and rainfall for 160 wild-feeding females. Specifically, we had a total of 490 IBIs for 160 females that fit our analysis criteria (see Data Analysis), with each female contributing an average of 3 IBIs to the dataset (range: 1-10).
In the second analysis we examined body fat scores collected between 2008 and 2014. We had 345 body fat scores for 105 females during 260 IBIs. Scores were collected during July, August, or November of each year, and the timing of the score within the IBI varied across females. 183 fat scores were assessed during PPA, 70 during cycling, and 92 during pregnancy. For any given phase of a given IBI, females were usually assessed once, but in a few cases females were assessed twice during a given PPA (n=11). For these 11 cases an average body fat score was calculated so that each female contributed only one score for each PPA.
In our third analysis, we examined fecal glucocorticoids (fGC), fecal estrogens (fE), and fecal progestogens (fP) during PPA and sexual cycling. For fGC and fE we had a total of 5,470 hormone samples collected between 2000 and 2014 for 138 females during 549 IBIs. The number of IBIs included in this analysis is larger than in our first analysis because the IBIs in this third analysis were not subject to the same filtering criteria as those in the first analysis (see Data Analysis). Of these samples, 4,150 fecal samples were collected during PPA, and 1,320 were collected during cycling. Each female contributed fecal samples from an average of 4 IBIs (range: 1-11) with an average of 10 hormone samples per IBI (range: 1-55) and an average total number of 40 samples per female (range: 1-145). For fP, we had a smaller sample size of 4,095 hormone samples collected between 2003 and 2014 for 130 females during 456 IBIs; 2,922 were collected during PPA and 1,173 during cycling. Each female contributed samples from an average of 3.5 IBIs (range 1-8), with an average of 9 (range 1-47) samples per IBI, for an average total number of 32 (range 1-141) samples per female. Note that the number of IBIs analyzed for hormone analysis is larger than the number analyzed for the duration analysis because fecal samples from incomplete IBIs (but not incomplete phases of an IBI) were included in the hormone analysis but not in the duration analysis.
Female reproductive states and the phases of the interbirth interval
Baboons are one of 20 primate species that exhibit regular sexual skin swellings and externally visible menstruation that closely reflect hormonal variation during the sexual cycle (Dixson, 1983). During the follicular phase of the cycle, the sexual skin of the anogenital area begins to swell as estrogen concentrations increase. The sexual skin reaches maximum turgescence at ovulation when estrogen concentrations peak (Gesquiere et al., 2007; Shaikh, Celaya, Gomexz, & Shaikh, 1982; Wildt et al., 1977). Then, as estrogen decreases and progesterone increases during the luteal phase of the cycle, the female’s sexual skin progressively deturgesces until becoming completely flat, and menstruation occurs in the absence of conception. Pregnancy is relatively easy to detect in baboons: sexual cycling ceases and the paracallosal skin begins to turn pink within a few weeks. Female baboons, unlike some other primate species, do not experience sexual swellings during pregnancy (Altmann, 1973; Beehner, Nguyen, et al., 2006; Gilbert & Gillman, 1951).
We defined the interbirth interval (IBI) as the number of days between two successive live births. An IBI is composed of three distinct phases. (1) The duration of postpartum amenorrhea (PPA) is defined as the number of days from an infant’s birth to the onset of swelling in the first sexual cycle following the infant’s birth; postpartum amenorrhea is often referred to as lactational amenorrhea. (2) The duration of sexual cycling is defined as the number of days from the onset of swelling in the first cycle after PPA to the onset of deturgescence during the conceptive cycle. (3) The duration of pregnancy is defined as the number of days from the first day of deturgescence during the conceptive cycle to the birth of the infant. Thus, the transition from postpartum amenorrhea to sexual cycling is marked by the onset of regular sexual swellings, the transition from sexual cycling to pregnancy is signaled by the cessation of sexual swellings and the gradual pinkening of the paracallosal skin, and the transition from pregnancy to postpartum amenorrhea is signaled by the appearance of a dependent infant.
During each observation day, observers recorded the reproductive state of every female in the social group (each social group was observed 1 to 5 times per week during the 36-year period of observation). Specifically, observers recorded the following measures for each female: (i) the sexual skin state (turgescent, deturgescent, or flat) and size (on a scale of 0 (skin is flat) to 10 (very large swelling)); (ii) the presence or absence of external menstrual blood; and (iii) the color of the paracallosal skin, which turns pink 3-4 weeks after conception (Alberts, Altmann, Archie, & Tung, 2016; Altmann, 1973). Pregnancy date was determined a posteriori as the first day of deturgescence of the last sexual cycle before the birth of an infant. Together, these reproductive data allowed us to assign a date each time a female gave birth, resumed cycling after the birth, and conceived again.
Female dominance rank, female age, parity, group size, and infant sex
Birthdates for 132 of 160 subjects are known to within a few days, and birthdates for the remaining 28 females are known to within a year; birthdates of all offspring are known to within a few days. Infants are sexed by visual inspection, usually within a few days of birth. These procedures allow us to calculate female and offspring age precisely (mean±SE for female age in this study: 10.38±.16 years; range is 5.03-20.25 years). We are also able to assign a maternal parity to each birth, and a sex to virtually all live born infants. For this analysis, female parity is scored as one (primiparous) or more than one (multiparous). Primiparous mothers have not yet achieved full body size or the skills required for efficient foraging during infant care. Consequently, in addition to primiparous mothers recovering more slowly than multiparous mothers from the energetic demands of pregnancy, first-born infants in several species of primates experience higher mortality than later-born infants, and first-borns in Amboseli experience slower growth than later-borns (Altmann & Alberts, 2005; Altmann, Altmann, & Hausfater, 1988; Smuts & Nicolson, 1989). These effects attenuate quickly after the first birth, so that the strongest effect of parity is generally seen when comparing primiparous to multiparous mothers (Altmann & Alberts, 2005). Dominance ranks are assigned monthly for each female by using observation of wins and losses in dyadic agonistic encounters between females. A dominance matrix is created based on these win/loss outcomes, and rank orderings are then assigned to minimize entries below the diagonal (Hausfater, 1975). This method of rank assignment has been extensively described in previous papers (Hausfater, Altmann, & Altmann, 1982; Onyango, Gesquiere, Wango, Alberts, & Altmann, 2008). The mean±SE for dominance rank in this study was 9.32±0.28 with a range of 1-28.
A census is conducted every time an observer is with the group, allowing us to determine the number and identity of all animals in the group on each observation day; we used the number of adult females in the group as a proxy for group size, as this measure is both correlated with total group size and appears the most salient for female reproductive rates (Altmann & Alberts, 2003). For the purposes of these analyses, we categorized groups as small (11.30±0.21 adult females; range 4-14 adult females), intermediate (17.94±0.13; range 15-20), or large (24.52±0.25; range 21-31). These divisions reflect the bottom, middle, and upper third of the distribution of group sizes, respectively.
Rainfall
Rainfall data are collected daily with a rain gauge at the research field camp, which is located 2-17 km away from the baboon study groups (Altmann, Alberts, Altmann, & Roy, 2002; Gesquiere et al., 2008). The Amboseli basin is a semiarid tropical habitat that is characterized by low and highly seasonal rainfall. A predictable 5-month long dry season from June through October is devoid of rain, and availability of food and drinking water progressively declines during this season (Alberts et al., 2005; Gesquiere et al., 2008). The long dry season is followed by a seven-month wetter period in which rainfall is highly variable and unpredictable from month to month. Rainfall is also highly variable across years (mean±SE annual rainfall 340±22 mm; range 141-757 mm; Alberts et al., 2005; Altmann et al., 2002), and drought conditions occur primarily when the normal dry season is unpredictably extended at either end by failure of rains. For each interbirth interval of each female, we calculated average daily rainfall during each phase of the IBI.
Habitat quality
In the late 1980’s and early 1990’s the two original study groups, Alto’s and Hook’s, initiated a home-range shift as the fever trees (Acacia xanthophloea), an important refuge and food source for baboons, died off in the central portions of their original home ranges. Both groups moved 5-6km west of their original home ranges, into a habitat that contained a higher density of fever trees as well as a diversity of other food sources. These home range shifts resulted in significant behavioral changes for animals in our study groups; after the move, the animals spent more time resting and socializing, less time feeding, and exhibited shorter daily travel distances (Alberts et al., 2005; Bronikowski & Altmann, 1996). Females also experienced higher reproductive success after these home range shifts, including higher infant survival, earlier maturation, and shorter IBIs (Altmann & Alberts, 2003). To control for these marked behavioral and reproductive changes in our analysis of variance in IBIs, we identified IBIs as occurring in low-quality habitat if they occurred before the home range shifts (offspring born in or before 1987 in Alto’s Group, in or before 1991 in Hook’s Group), and as occurring in high-quality habitat if they occurred after those shifts. All current study groups are considered to reside in high quality habitat; no baboon groups have returned to the original home range areas.
Body fat scores
Female body fat scores have been assessed once yearly by a single observer (S.C.A.) starting in 2008 by visual scoring of the degree of protrusion of the ischial tuberosities (the sitz bones) and the iliac crests (the edges of the ilium). Our approach is based on methods that correlate well with actual measures of body weight and body fat in primates (e.g., Berman & Schwartz, 1988) and ungulates (e.g., Pollock, 1980; Riney, 1960); general approach reviewed in Stevenson & Woods (2006). We score each adult on the degree of protrusion of the ischial tuberosities and the Illiac crests, using binoculars from 5-7 m distance, using a 5-point scale: 5 = no protrusion, bones not visible under the skin; 4 = slight protrusion with bone outlines just visible; 3 = marked protrusion with contours visible for at least one part of the bone; 2 = excessive protrusion, with visible bone contours and ridges; 1 = emaciated. We calculated an average ‘fat score’ by averaging the ilium and the ischium scores.
Collection, preparation, and hormone assay of fecal samples
Fecal sample collection, storage, and extraction were done as described previously (Khan, Altmann, Isani, & Yu, 2002; Lynch, Khan, Altmann, Njahira, & Rubenstein, 2003). In brief, immediately after collection of freshly deposited fecal samples from known individuals, samples were mixed and placed in 95% ethanol, and kept refrigerated until shipped to University of Nairobi (every two weeks). In Nairobi, samples were freeze-dried and sifted to remove plant matter. Samples were then stored at −20°C until they were shipped to Princeton University, where 0.2 g of fecal powder was extracted into 2 ml 90% methanol using a multi-pulse vortexer for 30 minutes. Following extraction, samples were further purified using a HLB Oasis cartridge (Waters, Milford, MA) and stored at −20°C.
The samples were then assayed for glucocorticoids (fGC), estrogen (fE) and progestogens (fP) by radioimmunoassays (full laboratory protocols available in Gesquiere et al., 2017). All samples were run in duplicate, and the results are expressed as ng/g dry fecal matter. fGC concentrations were determined using the Corticosterone kit (MP Biomedicals, Costa Mesa, CA); the assay was previously validated in yellow baboons (Khan et al., 2002; Lynch et al., 2003; Wasser, Monfort, Southers, & Wildt, 1994) for parallelism, accuracy, and precision of the assay (see also Wasser et al., 2000, for physiological validation of the assay). The primary antibody cross-reacts with major cortisol metabolites present in baboon feces (Wasser et al., 2000). fE concentrations were assayed using the Total Estrogen kit (MP Biomedicals, Costa Mesa, CA), a radioimmunoassay previously validated with our population (Khan et al., 2002; Lynch et al., 2003; Wasser et al., 1994 for parallelism, accuracy, and precision of the assay; see also Altmann et al., 2004; Beehner, Nguyen, et al., 2006; Gesquiere et al., 2005 for physiological validation of the assay). According to the manufacturer, the primary antibody cross-reacts 100% with E1 and E2, 9% with estriol, 7% with estradiol-17α, and 2.5% with equilin. fP concentrations were assayed using the monoclonal antibody (Quidel clone no. 425) produced by C. Munro against 4-pregnen-11-ol-3, 20-dione hemisuccinate:bovine serum albumin (BSA). This antibody has been used in a variety of animal species to monitor luteal function and pregnancy in the field, notably in baboons (Wasser, Risler, & Steiner, 1988; reviewed in Graham, Schwarzenberger, Mostl, Galama, & Savage, 2001). The primary antibody cross-reacts with numerous progesterone metabolites (Graham et al., 2001). See Supplementary Material and Fig. S1 for a complete description of the fP assay, including validation.
Data analysis
Predictors of the duration of the IBI and its component phases
In our first analyses, we only included IBIs for which we had complete data on all three component phases (PPA, sexual cycling and pregnancy), only IBIs that began and ended with live births, and only IBIs that opened with the birth of an infant that survived to at least one year of age (females usually conceive within one to three months after the death of their infant (Altmann & Alberts, 2003). We restricted our analysis to live births because fetal loss results in shortened pregnancies, shortened PPA phases, and fewer cycles to the next conception.
We constructed four Linear Mixed Models (LMMs) using IBM SPSS Statistics 22: one for the complete IBI and one for each of its component phases (PPA, sexual cycling and pregnancy). In each LMM, we modeled the duration of the reproductive phase in question as a function of seven predictor variables (Table 1). In each model, year (opening the IBI), and female identity nested within social group were entered as random variables. These random variables were included to control for variation in habitat quality and other environmental factors not accounted for by our fixed effects, as well as to account for the fact that different females contributed different numbers of IBIs to the sample (range = 1 to 10; see Study Subjects and Sample Sizes). Of our four response variables, only PPA was normally distributed. To approach normality for the other three response variables, we carried out a log transformation for IBI, a square-root transformation for the duration of sexual cycling, and a square-root transformation of the reverse score for pregnancy duration. However, careful comparison of models using transformed and untransformed data revealed that transformation had no effect on the direction, relative magnitude, or statistical significance levels for any predictor variable in any of the LMMs, with one exception. This exception was the effect of infant sex on pregnancy duration, which showed a trend towards significance in the non-transformed model (p=0.097) and became marginally statistically significantly (p=0.048; Table S1) when pregnancy duration was transformed: females had slightly longer pregnancy when they were gestating female infants. Because of the almost complete correspondence between the analyses using transformed and untransformed variables, we present the results of the models using untransformed data here in the main text. This allows us to interpret effects more clearly by reporting results in a standard unit of duration (number of days) for all effects in all models. We present all the results of the models using transformed variables in the Supplemental Material (Table S1).
We measured the duration of sexual cycling in two ways. In our primary analyses we measured it as the number of days from cycling resumption to conception (see ‘Female reproductive states and the phases of the interbirth interval’ above). However, for completeness we also ran a second model that defined cycling duration as the number of cycles to conception; we did not transform this variable, and included age as a quadratic term. This model yielded qualitatively similar results to the model of cycling duration measured in number of days; thus, we retained the analysis of number of days here, for better comparison with the other phases (See Supplemental Table S2 for results on the number of cycles to conception).
Finally, primiparity (a binary variable) is dependent upon age (a continuous variable), because primiparous females are invariably young (age range 5.0 to 8.7 years, compared to an age range of 5.5 to 20.3 years for multiparous females). Consequently, we interpret any effect of age in our model, after controlling for primiparity, as reflecting an effect of age in multiparous females.
Body fat scores during IBI component phases
To examine changes in body fat, we included 260 IBIs between 2008 and 2014 for which we had at least one body fat score assessed at some point in the IBI. We assessed body fat in two ways with these data. In the first, we examined how female body fat varied over the three phases of the IBI, regardless of the duration of a given IBI. Specifically, for each fat score, we retrospectively determined the reproductive phase of the female and the number of days she had been in that phase when the fat score was assessed. We then determined the proportion of her time-in-phase that had elapsed when the fat score was assessed, by dividing the number of days the female had been in that phase by the total duration of that phase. For example, if a female had been in PPA for 100 days on the day the fat score was assessed, and her PPA lasted a total duration of 200 days, then the proportion of time-in-phase for that fat score was 0.5. In this analysis we included only fat scores collected during pregnancies that produced live births, and fat scores collected during PPAs for which the offspring survived to at least one year.
In our second analysis of body fat, we explicitly asked how the duration of the IBI related to the fatness of the female. We used a LMM with female identity as a random factor to control for the fact that each female contributed a variable number of points (number of IBIs per female ranged from 1-7, mean=3). Specifically, we expressed our data as residuals of locally weighted regressions of fat score against time-in-phase (for description of LOWESS regression see Cleveland & Devlin, 1988; for similar analyses see Altmann & Alberts, 2005; Beehner, Nguyen, et al., 2006; Gesquiere et al., 2005). A negative residual indicated that a female was relatively thin for her time-in-phase, while a positive value indicated that a female was relatively fat for her time-in-phase. Eleven females had two fat score values for a given PPA of a given IBI, and an average residual value was calculated so that each female contributed only once to a given phase of an IBI. The female fatness for time-in-phase was used as fixed effect in our LMM to predict the duration of a given phase of her IBI.
Hormone profiles
For our third analysis, we included the subsets of IBIs between 2000 and 2014 for which we had fGC, fE, and fP data; we included only pregnancies that produced live births, and PPAs for which the offspring survived to at least one year. In this analysis we focused on the duration of PPA and cycling (see Beehner, Nguyen, et al., 2006 for a detailed analysis of hormone profiles during pregnancy). In these analyses, we did not exclude fecal samples collected during IBIs that were incomplete; however, in all cases, the phase of the IBI in which the sample was collected was complete at the time of analysis, even if the entire IBI was still in progress. We included only fecal samples from parous females to exclude adolescent females from the analysis. For the PPA analysis, we included only fecal samples collected after the 1st week of PPA, as hormone levels usually still reflect pregnancy in the 1st week following birth (see Altmann et al., 2004).
To examine how fGC, fE, and fP concentrations changed during PPA, we used LMMs with the log-transformed hormone concentrations as response variables, and months relative to cycle resumption as the predictor variable. To examine how hormone concentrations changed during cycling, we broke down each sexual cycle into the luteal phase (from the onset of deturgescence until the onset of menses), the early follicular phase (first day of swelling until five days prior to the onset of deturgescence) and late follicular phase (the five-day window prior to the onset of deturgescence, corresponding to the most probable time of ovulation). We constructed mixed linear models with the log-transformed hormone concentrations as response variables, and number of cycles until conception as the predictor variable. In all models, we entered female ID and a unique code for the specific IBI as random variables in the model, because each female usually contributed multiple samples during IBIs and multiple IBIs to the data set (see Study Subjects and Sample Sizes).
RESULTS
Duration of the IBI and its component phases
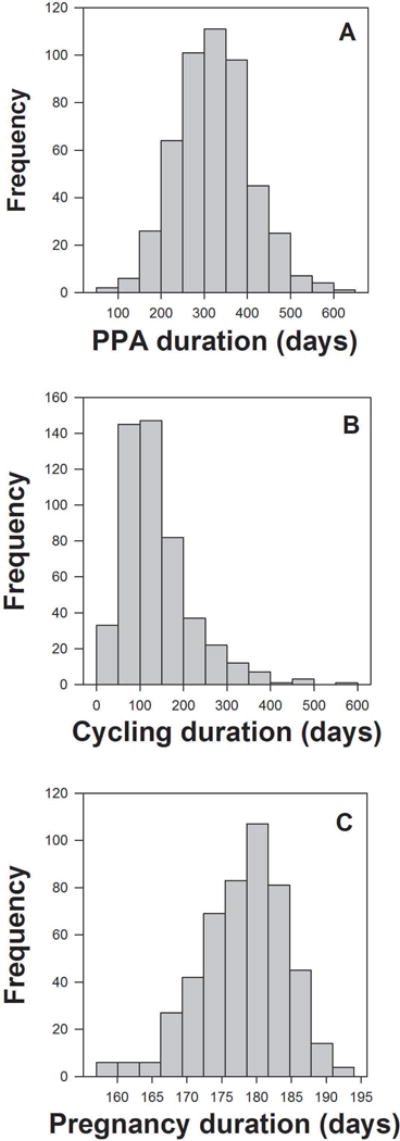
The duration of interbirth intervals (IBI) following the birth of an infant that survived to one year varied more than three-fold, from just under one year to almost three years (Fig. 1). Specifically, the mean IBI following the birth of a surviving infant was 638 ± 116 days (range 333-1,084 days). Postpartum amenorrhea (PPA) after the birth of a surviving infant was also highly variable, ranging from 71 to 635 days, with a mean of 322 days and a coefficient of variation of 27% (Figs. 1, 2A). The duration of sexual cycling was the most variable of the three phases of the IBI, ranging from 18 to 590 days, with a mean duration of 138 days and a coefficient of variation of 60%. However, the distribution of sexual cycling had a long right tail: 90% of the variation in sexual cycling duration fell between 18 and 244 days, indicating that a relatively small fraction of cycling durations drives the high variation in this distribution (Figs. 1, 2B). Pregnancy duration was by far the least variable of the phases, with a mean duration of 178 days (5.9 months), a range of 157 to 194 days, and a coefficient of variation of 3% (Figs. 1, 2C).
Fig. 1.
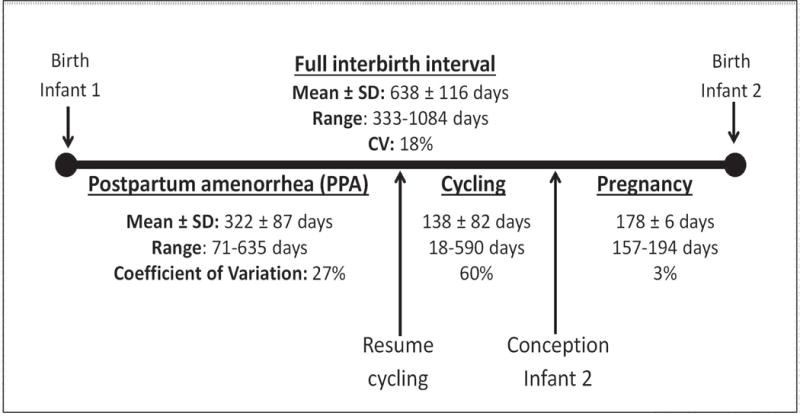
Fig. 2.
As a rule, interbirth intervals were shorter (reproduction was accelerated) when female traits and environmental variables promoted the ability of females to devote more energy to reproduction (Tables 1, 2). However, the details of which effects significantly predicted interval lengths differed among the various phases of the IBI.
Table 2.
Results of the LMMs showing the factors affecting duration of the IBI and its component phases. (A) Models with age a linear term; (B) models with age as a quadratic term. *
| A. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Duration of: | IBI | PPA | Cycling | Pregnancy | ||||
| FIXED FACTORS | b | Sig. | b | Sig. | b | Sig. | b | Sig. |
| Dominance rank | 4.146 | <0.001 | 3.371 | <0.001 | 0.606 | 0.414 | 0.003 | 0.952 |
| Primiparity (Yes/No) | −58.785 | <0.001 | −18.815 | 0.035 | −41.670 | <0.001 | 1.323 | 0.085 |
| Age | −0.675 | 0.676 | 0.966 | 0.398 | −1.574 | 0.212 | −0.039 | 0.673 |
| Group size** | 0.004 | 0.003 | 0.405 | 0.615 | ||||
| Sm vs. Int | 43.194 | 0.001 | 31.736 | 0.001 | 6.283 | 0.527 | 0.731 | 0.331 |
| Sm vs. Lg | 40.871 | 0.009 | 22.938 | 0.048 | 14.633 | 0.184 | 0.429 | 0.627 |
| Rainfall | −29.525 | 0.144 | −26.397 | <0.001 | 0.482 | 0.884 | −1.169 | 0.002 |
| Habitat quality (higher vs. lower) | −45.116 | 0.025 | −30.056 | 0.070 | −12.391 | 0.350 | 2.864 | 0.012 |
| Infant Sex (female vs. male) | −14.311 | 0.092 | −10.883 | 0.054 | −4.283 | 0.540 | 0.810 | 0.097 |
| RANDOM FACTORS | Variance | Sig. | Variance | Sig. | Variance | Sig. | Variance | Sig. |
| Year | 491.62 | 0.238 | 717.73 | 0.025 | 33.56 | 0.731 | 1.80 | 0.064 |
| Sname (group) | 4370.91 | <0.001 | 3050.93 | <0.001 | 1464.93 | 0.001 | 12.54 | <0.001 |
| Residual | 6536.76 | 2655.82 | 4981.33 | 21.95 | ||||
| B. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Duration of: | IBI | PPA | Cycling | Pregnancy | ||||
| FIXED FACTORS | b | Sig. | b | Sig. | b | Sig. | b | Sig. |
| Dominance rank | 4.322 | <0.001 | 3.410 | <0.001 | 0.773 | 0.305 | −0.010 | 0.855 |
| Primiparity (Yes/No) | −28.305 | 0.086 | −9.792 | 0.379 | −17.273 | 0.204 | −0.962 | 0.308 |
| Age | −27.211 | 0.002 | −6.940 | 0.249 | −22.894 | 0.002 | 1.986 | <0.001 |
| Age2 | 1.087 | 0.003 | 0.324 | 0.181 | 0.871 | 0.004 | −0.083 | <0.001 |
| Group size** | 0.007 | 0.004 | 0.388 | 0.522 | ||||
| Sm vs. Int | 41.056 | 0.002 | 30.817 | 0.001 | 5.561 | 0.572 | 0.847 | 0.255 |
| Sm vs. Lg | 38.073 | 0.015 | 21.670 | 0.062 | 14.672 | 0.180 | 0.589 | 0.503 |
| Rainfall | −31.611 | 0.116 | −26.678 | <0.001 | 0.705 | 0.827 | −1.144 | 0.002 |
| Habitat quality (higher vs. lower) | −44.746 | 0.028 | −29.827 | 0.074 | −12.419 | 0.351 | 2.874 | 0.012 |
| Infant Sex (female vs. male) | −16.133 | 0.054 | −11.351 | 0.044 | −5.758 | 0.405 | 0.991 | 0.039 |
| RANDOM FACTORS | Variance | Sig. | Variance | Sig. | Variance | Sig. | Variance | Sig. |
| Year | 524.18 | 0.206 | 723.49 | 0.025 | 13.37 | 0.880 | 1.93 | 0.057 |
| Sname (group) | 1841.07 | <0.001 | 3143.55 | <0.001 | 1657.12 | <0.001 | 13.11 | <0.001 |
| Residual | 6189.20 | 2618.17 | 4797.14 | 20.77 | ||||
Cells with gray shading indicate statistically significant effects; underlined data indicate marginally significant data.
Significance values are shown for the overall effect of group size, followed by effect size and significance values for small versus intermediate groups and small versus large group
Postpartum amenorrhea
The duration of PPA was predicted (significantly or near-significantly) by 6 of the 7 variables in our model, all in the expected direction (Table 2). High-ranking females had shorter PPA periods than low-ranking females (F1,273=19.678, p<0.001); a difference of 10 ranks corresponded to a 33-day difference in PPA duration. Females in smaller groups had shorter PPAs than females in medium- or large-size groups (F2,407=5.849, p=0.003). Females who experienced better habitat quality or higher rainfall experienced shorter PPAs than females who experienced worse habitat quality or lower rainfall, although the effect of habitat quality only trended towards significance when year was entered in the model as a random factor (F1,85=3.358, p=0.070 and F1,375=14.556, p<0.001, for habitat quality and rainfall respectively). An increase of 30 mm in the average monthly rainfall shortened PPA duration by 26 days. Finally, primiparity affected PPA duration when age was entered as a simple linear term, with females experiencing longer PPAs following the birth of their first infant than after subsequent infants (F1,392=4.487, p=0.035; Table 2A). However, when age was entered as a quadratic term, the effect of primiparity disappeared (F1,390=0.777, p=0.379; Table 2B). Finally, mothers who had a female infant had marginally shorter PPAs than mothers with a male infant (F1,372=3.739, p=0.054 when maternal age was entered as a linear term in the model, Table 2A; F1,365=4.092, p=0.044 when maternal age was entered as a quadratic term, Table 2B).
Sexual cycling
The duration of sexual cycling was insensitive to all the predictors in our model with the exception of age and/or primiparity. Specifically, when age was entered as a simple linear term in the model (Table 2A, Table S1A), primiparity was the only significant predictor of the duration of sexual cycling: females that had just experienced their first birth had longer periods of sexual cycling prior to conception than multiparous females (F1,449=14.392, p<0.001). However, the model of sexual cycling with age as a quadratic term was an equally robust model (Table 2B, Table S1B), and indicated that both the youngest and oldest females experienced longer periods of sexual cycling prior to conception than females in their middle years; no other predictors were significant.
Pregnancy
Rainfall, habitat quality, female age (when modeled as a quadratic term) and infant sex all affected the duration of pregnancy (Table 2). Pregnancies were shorter for the youngest and the oldest females in our dataset. Mothers with female infants had slightly longer pregnancies than mothers with male infants, this effect was not significant when age was modeled as a linear term, but reached significance when age was entered as a quadratic term (F1,417=4.288, p=0.039) (Table 2). Females who experienced higher average daily rainfall experienced shorter pregnancies (F1,407=10.157, p=0.002), such that an increase of 30mm in the average monthly rainfall shortened gestation by one day. The effects of habitat quality on pregnancy were opposite to the effects of rainfall: better habitat quality was associated with slightly longer pregnancies; F1,126=6.549, p=0.012).
Total interbirth interval
As with PPA, high ranking females had shorter IBIs than low ranking females (F1,240=16.309, p<0.001): a difference of 10 ranks corresponded to a 41 days difference in the total IBI duration. In addition, females in smaller groups had shorter IBIs than females in medium- or large-size groups (F2,292=5.670, p=0.004), and females who experienced better habitat quality had shorter IBIs than females who experienced poorer habitat quality (F1,87=5.213, p=0.025). Finally, as with the duration of sexual cycling, primiparity affected IBI duration when age was entered as a simple linear term, with females experiencing longer IBIs following the birth of their first infant than after later infants (F1,419=19.460, p<0.001; Table 2A). However, when age was entered as a quadratic term, the model indicated that both the youngest and oldest females experienced longer IBIs than females in their middle years, and the effect of primiparity declined to the level of a trend (F1,416=2.966, p=0.086; Table 2B). Mothers with female infants had a tendency to have shorter IBI than mothers with male infants, but this effect did not quite reach statistical significance (Tables 2 and S1).
Female body fat scores
Females had relatively high fat scores immediately after giving birth, at the start of PPA (mean fat score=3.3; Fig. 3). As PPA progressed, fat scores progressively decreased to their lowest mean value of 2.1, presumably because of the costs of lactation. In the month prior to cycling resumption, female body fat started to increase, implicating positive energy balance as key for this transition. Fat scores increased just before the onset of cycling and then remained constant during most of the cycling phase, for a mean fat score of 2.8 at conception. During pregnancy, females continued to accumulate fat slowly, reaching a maximum average fat score of 3.5. A slight decrease was observed immediately before the infant’s birth (Fig. 3).
Fig. 3.
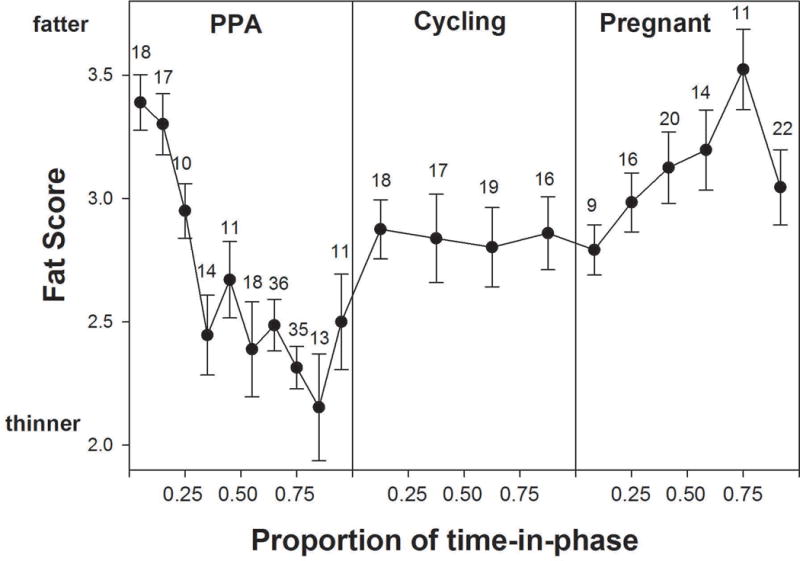
As we predicted, female fat scores per se did not predict the duration of PPA, sexual cycling or pregnancy, even after the fat score values were corrected for time-in-phase (PPA: t=−0.644, p=0.520, N=172; cycling: t=−0.199, p=0.842, N=70; pregnancy: t=0.069, p=0.945, N=92).
Physiological changes during PPA and sexual cycling: glucocorticoids, estrogens, and progestogens
We observed marked changes over time in fecal steroid hormone concentrations during PPA (Table 3, Fig. 4). As females approached the resumption of sexual cycling, fGC and fP concentrations progressively declined (F1,4141=15.984, p<0.001, and F1,2913=44.356, p<0.001, respectively). In contrast, fE did not change across the PPA phase (F1,4116=0.376, p=0.540).
Table 3.
Changes in concentrations of fecal glucocorticoid (fGC), fecal estrogen (fE) and fecal progestogens (fP) as a function of months prior to cycle resumption during PPA, and number of cycles to conception during sexual cycling.
| Hormones | During PPA: Months to resumption of cycling | During sexual cycling: Number of cycles to conception | ||||||
|---|---|---|---|---|---|---|---|---|
| Early Follicular | Late Follicular | Luteal | ||||||
| b | Sig. | b | Sig. | b | Sig. | b | Sig. | |
| fGC | −3.12e-3 | <0.001 | 3.10e-4 | 0.937 | 4.41 e-3 | 0.475 | 7.22e-3 | 0.228 |
| fE | 6.43e-4 | 0.540 | 6.55e-3 | 0.159 | −1.19e-3 | 0.892 | 2.08e-2 | 0.005 |
| fP | −6.54e-3 | <0.001 | −1.27e-2 | 0.009 | −2.26e-2 | 0.004 | 1.77e-2 | 0.023 |
Fig. 4.
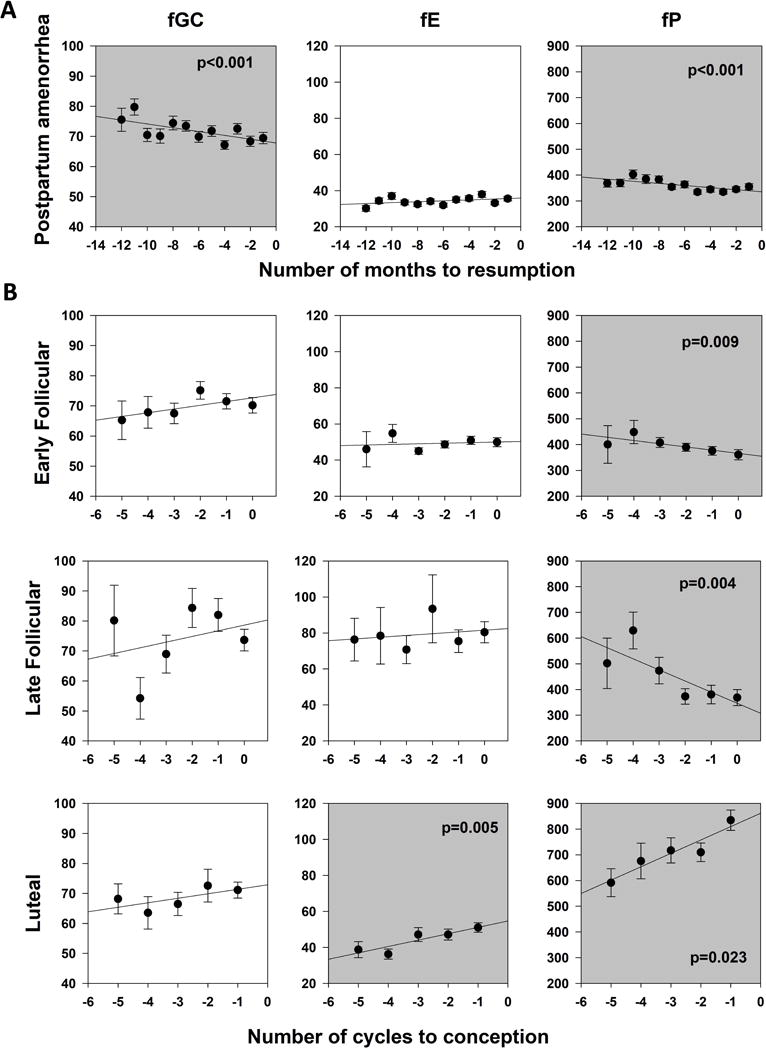
Concentrations of both of the sex steroids (fP and fE) changed over the course of sexual cycling (Table 3, Fig. 4). As females approached the transition from sexual cycling to pregnancy (i.e., as they approached conception) fP increased in the luteal phase, and decreased in both the early and late follicular phases (luteal phase: F1,344=5.216, p=0.023; early follicular: F1,589=6.799, p=0.009; late follicular: F1,203=8.456, p=0.004). fE increased significantly in the months prior to conception during the luteal phase, but not during the follicular phase (luteal phase: F1,391=7.980, p=0.005; Early Follicular: F1,455=1.986, p=0.159; late follicular: F1,150=0.019, p=0.892). We observed no changes in fGC during any phase of the sexual cycle as females approached the transition from cycling to pregnancy (luteal phase: F1,391=1.455, p=0.228; early follicular: F1,645=0.006, p=0.937; late follicular: F1,211=0.513, p=0.475; Table 3, Fig. 4).
DISCUSSION
Female baboons show highly dynamic patterns in interbirth interval durations. In doing so, they take advantage of their ability as non-seasonal breeders to flexibly hasten or slow their reproductive rates in response to current environmental conditions. Interbirth intervals vary enormously over a continuous range in our dataset, from less than a year to nearly three years, with a mean of 1.75 years (Fig. 1). The biological significance of this ability to hasten or slow reproduction may lie partly in that it allows females to slowly accumulate small accelerations over many years, but it is more likely that its significance is linked to the ability of females to achieve occasional large gains by taking advantage of particularly good conditions (see also the discussion of female body fat scores, below).
Importantly, the flexibility to continuously adjust interbirth intervals is not available to seasonal breeders, including many primates (Janson & Verdolin, 2005). Rather than responding directly to energy balance, as baboons appear to do, seasonal breeders must time their reproductive events relatively precisely so that energetically costly phases of the reproductive cycle fall during the period of food abundance, or they suffer the cost of reproductive failure (Brockman & van Schaik 2005). This limits the extent to which they can adjust reproductive rates, a limitation that baboons and other non-seasonal breeders (including humans) have escaped. Interestingly, baboons achieve non-seasonal breeding even in challenging environments such as Amboseli, which is typified by very low and highly variable rainfall, and highly seasonal plant productivity (Alberts et al., 2005; Altmann et al., 2002). However, not all phases of the IBI are equally adjustable, and only postpartum amenorrhea is highly responsive to environmental conditions. Below we discuss the extent to which each IBI phase is flexible, and the variables that affect the duration of each.
Postpartum amenorrhea is the IBI phase that is most sensitive to the environment
Postpartum amenorrhea (PPA) was both highly variable and highly responsive to the environment. The shortest PPA period following the birth of a surviving infant was 71 days, and the longest was more than 9 times that, at 635 days (Fig 1). The duration of PPA was sensitive not only to environmental variables but also to female and infant traits in our models, in the expected directions. Specifically, the duration of PPA was shorter when groups were smaller, when rainfall was higher, and when females were higher ranking or had a female infant. Known patterns of fecal glucocorticoid concentrations (fGC) strongly support the idea that at least some of these effects are mediated by energy availability. For instance, not only do females experience shorter PPA phases with higher rainfall and higher dominance rank, they also experience lower fGC concentrations in these contexts (Gesquiere et al., 2008 and unpublished data; see also review in Beehner & Bergman, 2017). In addition, female fGC concentrations are elevated in large groups (Markham, Gesquiere, Alberts, & Altmann, 2015), consistent with our result that females have longer PPAs and longer IBIs in large groups: females in larger groups are subject to more intense food competition within their own group than females in smaller groups. However, our results are not entirely consistent with the recent observation in Amboseli that females in small groups also have elevated fGC concentrations relative to females in mid-sized group (Markham et al., 2015). This surprising result suggests a pattern of reduced energy availability in the smallest as well as the largest groups, which is not borne out by our finding that females had shorter PPAs and IBIs at small group sizes than at both intermediate and large group sizes (see also Altmann & Alberts, 2003; Bulger & Hamilton, 1987; Packer et al., 1995). A possible explanation for these discrepancies is that the high GC concentrations found in small groups are not the consequence of a decrease in energy availability but result from psychological stress - caused by predation threat or intergroup competition from larger groups. In support of this hypothesis, Markham et al. (2015) found that females in the smallest groups spent less time spend foraging, an indication that food was available at higher rates than for larger groups.
In general, these results are consistent with what has been reported in other studies. For example, the duration of PPA is considerably lengthened in years of low rainfall for olive baboons in Gombe (Packer et al., 1995). In addition, low ranking mothers have longer PPAs than high ranking mothers in several baboon populations (Cheney et al., 2004; Johnson, 2003; Packer et al., 1995) but not in semi-provisioned mandrills (Setchell & Wickings, 2004). Finally, several other primate studies have reported the same pattern we observe, in which mothers have longer IBIs after producing sons than daughters, and this is interpreted as a consequence of the higher energetic costs to produce sons who weigh more at birth and grow more rapidly than daughters (Bercovitch and Berard, 1993; Berman, 1988; Boesch, 1997; McFarland Symington, 1987). However, overall, primate species and populations appear highly variable in whether mothers exhibit sex-biased investment or longer IBIs with sons than daughters, and no clear pattern emerges overall with regards to the effects of infant sex on maternal IBI (reviewed in Brown, 2001).
Other primate studies have shown similar effects for one or two of these predictors at a time (Table 1). Ours is the first study, to our knowledge, to establish simultaneous independent effects of all these predictors on all phases of the IBI. The relatively large sample size in this study may contribute to our ability to detect all these effects simultaneously. Alternatively or in addition, each of these variables may predict food acquisition in a fairly direct way in Amboseli, while the relationships may be indirect, or may be swamped by other effects, in other populations. For example, Strum and Western (1982) found no effect of rainfall on birth rate in Gilgil baboons, primarily because patterns of land use by human livestock overrode the effects of rainfall on food availability. This effect may depend upon the intensity of grazing by livestock: Amboseli baboons experience competition with a full complement of wild ungulates and Maasai livestock, but perhaps involving less livestock than in the Gilgil study, because rainfall still produced a strong signal in our dataset. In addition, while Amboseli baboons rely heavily on grass blades, seeds, and corms for nourishment, they also employ a large range of other food types that ungulate grazers and browsers cannot access, even during dry seasons (Alberts et al., 2005; Altmann, 1998).
Cycling duration is highly variable with a long right tail
The duration of sexual cycling was the largest contributor to variation in IBIs, with a coefficient of variation of 60%: after females resumed cycling, some conceived again in as little as 18 days (i.e., on their first cycle) while others cycled for as long as 590 days (approximately 18 cycles) before conceiving. Importantly, however, the distribution of sexual cycles had a long right tail – 90% of the variation in sexual cycling duration fell between the lowest value (18 days) and 244 days, which is less than half the maximum. In other words, most females conceive within 6-8 cycles, and the high coefficient of variation in cycling duration is largely accounted for by the ~20% of IBIs in which the female waits between 8 and 18 cycles to conceive.
Intriguingly, this highly variable phase of the IBI was the least predicted by the seven variables in the model, and was apparently relatively insensitive to energy availability. Indeed, of the seven variables, only age (and possibly primiparity) predicted the duration of sexual cycling prior to conception. The long right tail of the distribution of cycling durations (Fig. 2) may be important in understanding this apparent lack of environmental predictors. Specifically, unusually long periods of cycling (the upper 20% of the distribution that encompasses waiting times of 8-18 cycles) may reflect specific constraints or conditions that, if discovered and controlled for, would allow us to identify environmental variation underlying the majority of IBIs. Such an analysis is currently planned with our dataset.
Congruent with previous analyses in this population, we found that females in middle age conceived the most rapidly, while the youngest and oldest females conceived after longer periods of cycling. Similarly, the probability that an adult female baboon will conceive, given that she is cycling, peaks at ~0.30, when she is between 11 and 18 years of age, after increasing from a low of ~0. 20 at age 6 years, and decreasing to a similar low in her early 20s (Beehner, Onderdonk, Alberts, & Altmann, 2006). Interestingly, the dependence of cycling duration on age, combined with the increase in fE and fP concentrations during the luteal phase as females approach conception, suggests that follicle quality may play a role in predicting conception. A ‘high quality’ follicle will produce sufficient E and P to sustain pregnancy, and follicle quality will vary with age, with younger and older females having more follicles of lower quality.
Beehner and colleagues (Beehner, Onderdonk et al., 2006) also identified an environmental variable, drought, that predicted the probability of conception in Amboseli, but only for female baboons living in large groups. Specifically, females in large groups were less likely to conceive on a given cycle when that cycle occurred after a dry period that was longer than the normal Amboseli 5-month dry season. In contrast, we saw no effect of rainfall on the duration of sexual cycling; in post-hoc analyses we found no interaction between group size and rainfall (data not shown). This difference between our studies may stem from both the exact variable we analyzed (i.e., we modeled the duration of the sexual cycling phase rather than the probability of conception given cycling) and the way we constructed our predictor variables (average daily rainfall during the IBI phase, rather than drought preceding the follicular phase of each cycle).
Primiparity was a significant predictor of the duration of sexual cycling only when age was entered as a linear rather than a quadratic term in the model: females cycled longer after the birth of their first infant than after subsequent infants. Similar results have been reported in chimpanzees (Deschner & Boesch, 2007; Robbins, Robbins, Gerald-Steklis, & Steklis, 2006; Setchell & Wickings, 2004), and such a result would presumably stem from the fact that younger, primiparous females are still investing in their own growth and as a consequence have reduced energy to put toward ovarian function (Robbins et al., 2006; Setchell & Wickings, 2004). However, the lack of an effect of primiparity when age was entered as a quadratic term in the model suggests that any lingering effects of the adolescent growth phase are gradually diminishing over several years, rather than marking a sharp delineation between the period after the first birth and the period after subsequent births, and that a similarly gradual increase in the period of sexual cycling occurs as females age.
Pregnancy is the least variable phase of the IBI and the least influenced by our predictor variables
The durations of pregnancies that produced live births varied little, with a mean of 178±6 days and a coefficient of variation of only 3%. Our mean values are similar to those reported for other studies of baboons (e.g., Albrecht, Aberdeen, & Pepe, 2000; Bentley-Condit & Smith, 1997). The range of recorded gestations that produced a live birth in our study was 157-194 days (5.2-6.4 months), and the middle 90% of gestations fell in the 20-day range between 168 and 187 days (5.5-6.2 months). This pattern of relatively low variation in the duration of pregnancies that produce live births is consistent across primate species, and indeed, across mammals. Furthermore, the coefficient of variation in the parameter, at 3%, is identical to that reported, on average, across a large number of mammal species (Jukic, Baird, Weinberg, McConnaughey, & Wilcox 2013; Kiltie, 1982; Martin, 2007). This small coefficient of variation implies a strong constraint on the duration of pregnancy, and is consistent with the observation that the length of gestation is an important predictor of infant survival, particularly in humans, for which median gestation lengths are best for infant survival (Gladstone, White, Kafulafula, Neilson, van den Broek, 2011; Hilder, Costeloe, Thilaganathan, 1998).
The duration of pregnancy was influenced by four variables in our models: rainfall, habitat quality, maternal age, and infant sex. Some of these effects were puzzling. For instance, shorter pregnancies for the oldest and youngest females in a sample have not been reported before to our knowledge, although longer pregnancies have been reported among older females in some primates and other large mammals (e.g., Mysterud, Roed, Holand, Yoccoz, & Nieminen, 2009; Silk, Short, Roberts, & Kusnitz, 1993; see also Clements, Clutton-Brock, Albon, Pemberton, & Kruuk, 2011 on red deer, in which no effect of maternal age is seen). The effects of rainfall and habitat quality were also somewhat puzzling: higher rainfall (signaling better nutritional conditions) was associated with shorter gestation, while better habitat quality (also signaling better nutritional conditions) was associated with longer gestation. The latter result is broadly consistent with the human literature, which reports that gestations are shorter during periods of energetic stress (reviewed in Ellison, 2003), while the former result is consistent with reports in other mammals, including primates, in which gestations are longer when nutritional intake is lower (Borries et al., 2001; Bronson, 1989; Racey, 1981; Riopelle & Hale, 1975; Silk, 1986).
These conflicting results may be explained by the hypothesis that parturition occurs when fetal energy demands surpass, or “cross over,” the mother’s ability to meet those demands (Ellison, 2001). Under this hypothesis, a longer gestation could indicate either that infant growth was slow (poor infant development) requiring additional gestation time, or that maternal condition was good, allowing the mother to sustain the cost of pregnancy for longer period of time. Our results are broadly consistent with this crossover hypothesis: if lower quality habitats are associated with generally poorer body condition, mothers might be unable to meet the demands of their fetus, and gestation would be shortened in lower quality habitats. If so, we would also expect to see lower infant survival in lower quality habitats, and this prediction is confirmed in Amboseli: the shift to better quality habitats in the late 1980’s and early 1990’s was associated with a 38% increase in infant survival (Altmann & Alberts, 2003). In contrast to persistent effects of habitat quality, a relatively temporary decrease in rainfall (and therefore food availability) during pregnancy may slow fetal development and therefore increase pregnancy duration. In addition, shorter pregnancies for females with male infants may result if males grow faster than females during fetal development, as they do during the infant and juvenile stages (Altmann & Alberts 2005), resulting in an earlier metabolic “crossover” for mother carrying males than females.
However, in general variation in gestation length was very small relative to variation in other phases of the IBI, suggesting that its functional significance for overall IBI length and hence reproductive rates may be quite limited. For instance, our models estimate that gestation would be shortened by just one day (0.5% of the average gestation length) if the female experienced a 30 mm increase in the average monthly rainfall (180 mm over the course of a 6-month gestation). A 30 mm change in rainfall is an extremely large difference that implies very high total rainfall in the context of average annual rainfall in Amboseli, which is 348 mm (Altmann et al. 2002). In general, as noted by Borries et al. (2001) for langurs, environmentally-driven variation in gestation length will have little effect on the overall interbirth interval, and hence on reproductive rate. Thus, variation in gestation length will be driven much more by its effects on infant and maternal survival and energy balance rather than by its effects on reproductive rates.
Female body fat scores
Our data on female body fat scores were strikingly consistent with the hypothesis that a change from negative to positive energy balance precedes the transition from PPA to sexual cycling. Females lost body fat throughout 90% of their postpartum amenorrhea phase, indicating negative energy balance during this phase. In the final 10% of the PPA, pooled across both short and long PPAs, females gained body fat, indicating a shift back into positive energy balance. This shift was followed by the onset of sexual cycling, during which female body fat remained steady. Our observation is in agreement with results in both humans and chimpanzee indicating that the resumption of cycling is preceded by a shift to positive energy, detectable in humans as a gain in BMI (e.g., Valeggia & Ellison, 2004, 2009 for humans; Thompson et al., 2012 for chimpanzees). Our observations of changes in body fat also lend strong support to our interpretation of IBI phase durations, namely that the duration of PPA is much more dependent upon energy availability than the durations of sexual cycling and of pregnancy.
Specifically, the transition from cycling to pregnancy was not preceded by changes in body fat scores (i.e., by marked changes in energy balance), suggesting that conception was relatively independent of a female’s energy balance. However, this is in contrast with results obtained in female olive baboons, showing that body weight for cycling females was inversely correlated with the duration of sexual cycling prior to conception (Bercovitch, 1987). After the onset of pregnancy, Amboseli females again began to gain body fat (Fig. 3). This observation is consistent with observations in many human populations, but is not universally observed in humans or other mammals, and depends upon both nutrient availability and species-specific strategies for maintaining pregnancies. For instance, a typical human pattern involves fat gain during pregnancy (reviewed in Institute of Medicine, 2009; Robinson, 1986; Wade & Schneider, 1992), but the opposite pattern has also been reported in extreme environmental conditions—Gambian women during the ‘hungry season’ lose body fat during pregnancy (Lawrence, Coward, Lawrence, Cole, & Whitehead, 1987). Fat gain during pregnancy is reported for a number of mammal species, but in others species mothers lose fat during pregnancy, as they transfer fat stores to the offspring (reviewed in Robinson, 1986; Wade & Schneider, 1992; Widdowson, 1976). Near the end of pregnancy, Amboseli females appear to experience a small dip in body condition: it is possible that this dip coincides with the increasing demands of pregnancy at the end of the 3rd trimester when the energetic requirements of fetal brain growth are very high (Ellison, 2003, see also Martin, 1996, 2007).
Our observation that female body fat scores per se did not predict the duration of any phase of the IBI is also consistent with the energy balance hypothesis, which posits that reproductive readiness in female primates is strongly affected by whether females can gain more energy than they are currently using, rather than by energy stored as body fat. This reproductive tactic of using an increase in available energy to commence reproduction, is linked to the concept of income breeding (making reproductive decisions based on the rate of energy accumulation) as distinct from capital breeding (making reproductive decisions based on the amount of energy stored; Drent & Daan, 1980; see also Jonsson, 1997; Stearns, 1992 for slightly different uses of these terms). However, baboons, like other large primates, fail to conform to either of these simple breeding strategies, and neither one is a useful description of their reproductive strategy, which relies on both income and capital (Brockman & van Schaik, 2005; Thompson, 2013b). That is, the very extended reproductive cycle of baboons, which often unfolds in a highly unpredictable environment, has apparently selected for both sensitivity to current energetic conditions and the ability to store energy for offspring needs (Thompson 2013b). The resumption of cycling with positive energy balance allows female baboons to respond quickly and flexibly to environmental windfalls, and poses some risks in an unpredictable environment like Amboseli, as female baboons cannot predict whether a positive energy balance is sustainable. If not, they risk a failed pregnancy as documented when females conceive during drought (Beehner, Onderdonk et al., 2006). However, reproductive risks in baboons appear concentrated in the early phases of a given reproductive event. During pregnancy, females start accumulating fat by reducing their basal metabolic rate, and increasing the time spent feeding as pregnancy progresses (Ellison, 2003; Silk, 1986). The resulting accumulation of fat is a highly risk-averse strategy which allows pregnant and lactating baboons to be extremely well-buffered against energy shortages and achieve high infant survival as shown for humans (Ellison, 2003, 2017). To support both the rapid and flexible initiation of breeding and the accumulation of fat during pregnancy, baboons pursue a highly successful generalist foraging pattern that combines omnivory with great feeding selectivity: baboons eat many different food types, and focus on particular plant species and parts that allow them to maximize nutrient intake (Alberts et al., 2005; Altmann, 1998; Byrne, Whiten, Henzi & McCulloch, 1993; Hamilton, Buskirk, Buskirk, 1978; Norton Rhine, Wynn, & Wynn, 1987; Whiten, Byrne, Barton, Waterman, & Henzi, 1991). This flexible feeding strategy may be crucial for reducing the risks of initiating breeding in an unpredictable environment, and for helping baboons to break free of the constraints of their highly seasonal environment. A highly flexible feeding strategy characterizes our own species as well. Indeed, the exigencies of the savannah environment, including unpredictable food resources, are often invoked as central selective pressures that shaped the hominid lineage (see reviews in Foley, 1987, 1993; Klein, 1999; Potts, 1996, 1998a,b).
Physiological changes during PPA and sexual cycling: glucocorticoids, estrogens, and progestogens
In periods of negative energy balance, when the body needs to mobilize its own energy reserves, glucocorticoids are secreted. As expected, fGC concentrations in Amboseli females were higher during the early phase of the PPA when lactation demands were high, and progressively declined in the months prior to the resumption of cycling, until they reached levels similar to those observed during the cycling phase. This result is highly congruent with our data on changes in body fat and with our observation that the duration of PPA is sensitive to energy availability. Taken as a whole, these results indicate that female baboons, like humans and chimpanzees (Thompson et al., 2012; Valeggia & Ellison, 2004, 2009) do not resume cycling until the rate at which they are mobilizing their stored energy reserves drops to a sufficiently low level. This transition depends on both maternal and infant nutrition; here we have focused on maternal conditions, but this dual dependency is a key feature of any mammalian system. For nonseasonal breeders in particular, maternal flexibility to resume cycling depends on the mother-infant pair achieving a sufficient aggregate energy intake to free the mother for investment in the next offspring (Lee, 1997).
We observed a marked decline in fP levels over the course of postpartum amenorrhea as females approached the resumption of cycling. The source of fP during PPA is not clear in our study; progesterone is usually produced by the corpus luteum during cycling, but during PPA, ovarian function has not yet resumed yet. The most likely source of the fP levels that we documented during PPA is the adrenal glands (Balfour, Comline & Short, 1957). Once females resumed cycling, fP levels in the early and late follicular phase of the menstrual cycle declined as females approach conception. These results are consistent with the known contraceptive role of progestogens: when progestogens are elevated, follicular development is prevented and ovulation is suppressed (Glasier, 2015; Mueck & Sitruk-Ware, 2011; Sitruk-Ware, 2006). Consequently, elevated progesterone concentrations have been associated with failure to conceive in humans (Baird et al., 1999). However, in contrast to patterns of fP concentrations during PPA and the follicular phase of the sexual cycle, we observed increases in both fE and fP during the luteal phase of the sexual cycle as female baboons approach conception. This observation is consistent with the necessity for sufficient levels of progesterone and estrogen in the luteal phase in order for females to sustain pregnancy: in humans, low levels of estrogen and progesterone are associated with several causes of infertility, including failure to ovulate, luteal insufficiency, and subfecundity (reviewed in Ellison, Panterbrick, Lipson, & Orourke, 1993; Vitzthum, Spielvogel, & Thornburg, 2004).
Conclusions
Like other non-seasonally breeding primates, wild female baboons are sensitive to and constrained by the energetic demands of reproduction, and can accelerate or delay the resumption of reproduction depending on environmental conditions. Specifically, reproduction is accelerated (interbirth intervals are shortened) when females have greater access to energy sources, and reproduction is slowed (interbirth intervals are lengthened) when females experience energy restriction. Postpartum amenorrhea is by far the most sensitive of the phases to environmental variation; in contrast, the duration of sexual cycling is surprisingly insensitive to environmental variation but can be quite variable and contributes significantly to overall variation in interbirth intervals; understanding the biology underlying the variation in sexual cycling duration is an important next step in explaining the evolution of primate reproduction. Variation in gestation length, while highly significant for infant survival, represents a negligible contribution to overall variation in reproductive rates. Changes in female body fat scores, particularly during postpartum amenorrhea, were strikingly consistent with the energy balance hypothesis, which posits that females are able to resume cycling only when they are able to acquire more energy than they expend. This observation points to baboons as able to take immediate advantage of increases in available energy to resume reproduction, but as subsequently dependent upon storing energy to support pregnancy and lactation. Our results from steroid hormone analyses support this interpretation: fGC concentrations were initially high during PPA when infant energy demands were high, and declined during PPA as female energetic condition improved. Notably, changes in fecal progestogens, when examined as a function of specific reproductive states, paint a clear picture of changes in female fecundability. fP declined during PPA, and also declined during sexual cycling when we examined data for the follicular phase, consistent with the known contraceptive effects of fP. However, fP increased during sexual cycling when we examined data for the luteal phase, consistent with the role of fP as an index of ovarian function. As a whole, our study contributes to a growing understanding of the ways in which variation in energy availability constrains and enhances female primate reproductive rates.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Science Foundation and the National Institute on Aging for support of the long-term research. In the past 15 years in particular we acknowledge support from NIA R01AG034513, NIA P01AG031719, NIA R21AG049936, NSF BCS 0323553, NSF BCS 0323596, NSF IBN 0322613, NSF IBN 0322781, NSF DEB 0846286, NSF DEB 0846532, NSF DEB 0846286, NSF DEB 0919200, NSF IOS 0919200, NSF IOS 1053461, and NSF IOS 1456832. For shorter-term support at various times over the years we thank the Princeton Center for the Demography of Aging (P30AG024361), the Chicago Zoological Society, the Max Planck Institute for Demographic Research, the L.S.B. Leakey Foundation, and the National Geographic Society. We are particularly grateful to an anonymous donor who, since 2014, has provided critical financial support for the long-term field site in Amboseli. For cooperation and assistance in Kenya, we thank the Kenya Wildlife Services, Institute of Primate Research, National Museums of Kenya, National Council for Science and Technology, members of the Amboseli-Longido pastoralist communities, Tortilis Camp, Ker & Downey Safaris, Air Kenya, and Safarilink. A number of people contributed to the long-term data collection over the years, and we are grateful to all of them for their dedication and contributions. Particular thanks go to the Amboseli Baboon Project long-term field team (R.S. Mututua, S. Sayialel, and J.K. Warutere), and to V. Somen and T. Wango for their assistance in Nairobi. We are grateful to Karl Pinc for his contributions to the development of Babase, the Baboon Project database. We also thank numerous database managers over the years, particularly D. Onderdonk, C. Markham, T. Fenn, L. Maryott, P. Onyango, N. Learn, and J. Gordon. This research was approved by the IACUC at Duke University, Princeton University, and University of Notre Dame and adhered to all the laws and guidelines of Kenya.
Grant sponsorship: NIA R01AG034513, NIA P01AG031719, NIA R21AG049936: NSF BCS 0323553, BCS 0323596, IBN 0322613, IBN 0322781, DEB-0846286, DEB-0846532, DEB 0846286, DEB-0919200, IOS-0919200, IOS 1053461, IOS 1456832.
LITERATURE CITED
- Ackermann RR, Rogers J, Cheverud JM. Identifying the morphological signatures of hybridization in primate and human evolution. Journal of Human Evolution. 2006;51(6):632–645. doi: 10.1016/j.jhevol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Altmann J. Immigration and hybridization patterns of yellow and anubis baboons in and around Amboseli, Kenya. American Journal of Primatology. 2001;53:139–154. doi: 10.1002/ajp.1. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Altmann J. The Amboseli baboon research project: Forty years of continuity and change. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Berlin: Springer; 2012. [Google Scholar]
- Alberts SC, Altmann J, Archie EA, Tung J. Monitoring guide for the Amboseli Baboon Research Project. 2016 Retrieved from https://amboselibaboons.nd.edu/assets/211484/abrp_monitoring_guide_sep2016.pdf.
- Alberts SC, Hollister-Smith J, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long-term change in a savannah environment. In: Brockman DK, Van Schaik CP, editors. Seasonality in primates: Studies of living and extinct human and non-human primates. Cambridge: Cambridge University Press; 2005. pp. 157–196. [Google Scholar]
- Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. American Journal of Obstetrics and Gynecology. 2000;182(2):432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- Altmann J, Alberts SC. Variability in reproductive success viewed from a life-history perspective in baboons. American Journal of Human Biology. 2003;15:401–409. doi: 10.1002/ajhb.10157. [DOI] [PubMed] [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: Ecological influences and maternal effects. Behavioral Ecology and Sociobiology. 2005;57:490–501. [Google Scholar]
- Altmann J, Alberts SC, Altmann SA, Roy SB. Dramatic change in local climate patterns in the Amboseli basin, Kenya. Afr J Ecol. 2002;40:248–251. [Google Scholar]
- Altmann J, Altmann S, Hausfater G. Determinants of reproductive success in savannah baboons (papio cynocephalus) In: Clutton-Brock TH, editor. Reproductive success. Chicago: University of Chicago Press; 1988. pp. 403–418. [Google Scholar]
- Altmann J, Lynch JW, Nguyen N, Alberts SC, Gesquiere LR. Life-history correlates of steroid concentrations in wild peripartum baboons. American Journal of Primatology. 2004;64(1):95–106. doi: 10.1002/ajp.20064. [DOI] [PubMed] [Google Scholar]
- Altmann S. The pregnancy sign in savannah baboons. The Journal of Zoo Animal Medicine. 1973;4:8–12. [Google Scholar]
- Altmann SA. Foraging for survival. Chicago: University of Chicago Press; 1998. [Google Scholar]
- Anderson CM. Female age : Male preference and reproductive success in primates. International Journal of Primatology. 1986;7(3):305–326. doi: 10.1007/bf02736394. [DOI] [Google Scholar]
- Asquith PJ. Provisioning and the study of free-ranging primates: History, effects, and prospects. Yearbook of Physical Anthropology. 1989;32:129–158. [Google Scholar]
- Baird DD, Weinberg CR, Zhou HB, Kamel F, McConnaughey DR, Kesner JS, Wilcox AJ. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertility and Sterility. 1999;71(1):40–49. doi: 10.1016/s0015-0282(98)00419-1. [DOI] [PubMed] [Google Scholar]
- Balfour WE, Comline RS, Short RV. Secretion of progesterone by the adrenal gland. Nature. 1957;180:1480–1481. doi: 10.1038/1801480a0. [DOI] [PubMed] [Google Scholar]
- Barton RA, Whiten A. Feeding competition among female olive baboons, Papio anubis. Animal Behaviour. 1993;46(4):777–789. doi: 10.1006/anbe.1993.1255. [DOI] [Google Scholar]
- Beehner JC, Bergman TJ. The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Hormones and Behavior. 2017;91:68–83. doi: 10.1016/j.yhbeh.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Nguyen N, Wango EO, Alberts SC, Altmann J. The endocrinology of pregnancy and fetal loss in wild baboons. Hormones and Behavior. 2006;49(5):688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy failure in wild baboons. Behavioral Ecology. 2006;17(5):741–750. doi: 10.1093/beheco/arl006. [DOI] [Google Scholar]
- Bentley-Condit VK, Smith EO. Female reproductive parameters of Tana river yellow baboons. International Journal of Primatology. 1997;18(4):581–596. doi: 10.1023/a:1026315323400. [DOI] [Google Scholar]
- Bercovitch FB. Female weight and reproductive condition in a population of olive baboons (Papio anubis) American Journal of Primatology. 1987;12(2):189–195. doi: 10.1002/ajp.1350120206. [DOI] [PubMed] [Google Scholar]
- Bercovitch FB, Berard JD. Life history costs and consequences of rapid reproductive maturation in female rhesus macaques. Behavioral Ecology and Sociobiology. 1993;32:103–109. [Google Scholar]
- Berman CM. Maternal condition and offspring sex ratio in a group of free-ranging rhesus monkeys: An 11-year study. American Naturalist. 1988;131:307–328. [Google Scholar]
- Berman CM, Schwartz S. A nonintrusive method for determining relative body fat in free-ranging monkeys. American Journal of Primatology. 1988;14(1):53–64. doi: 10.1002/ajp.1350140105. [DOI] [PubMed] [Google Scholar]
- Boesch C. Evidence for dominant wild female chimpanzees investing more in sons. Animal Behaviour. 1997;54:811–815. doi: 10.1006/anbe.1996.0510. [DOI] [PubMed] [Google Scholar]
- Borries C, Koenig A, Winkler P. Variation of life history traits and mating patterns in female langur monkeys (Semnopithecus entellus) Behavioral Ecology and Sociobiology. 2001;50(5):391–402. doi: 10.1007/s002650100391. [DOI] [Google Scholar]
- Brockman DK, van Schaik CP. Seasonality and reproductive function. In: Brockman DK, VanSchaik CP, editors. Seasonality in primates: Studies of living and extinct human and non-human primates. Vol. 44. 2005. pp. 269–306. [Google Scholar]
- Bronikowski A, Altmann J. Foraging in a variable environment: Weather patterns and the behavioral ecology of baboons. Behavioral Ecology and Sociobiology. 1996;39:11–25. [Google Scholar]
- Bronson FH. Mammalian reproduction: An ecological perspective. Biology of Reproduction. 1985;32(1):1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproductive biology. Chicago: University of Chicago Press; 1989. [Google Scholar]
- Brown GR. Sex-biased investment in nonhuman primates: Can Trivers & Willard’s theory be tested. Animal Behaviour. 2001;61:683–694. doi: 10.1006/abne.2000.1659. [DOI] [Google Scholar]
- Bulger J, Hamilton WJ., III Rank and density correlates of inclusive fitness measures in a natural chacma baboon (Papio ursinus) troop. International Journal of Primatology. 1987;8:635–650. [Google Scholar]
- Byrne RW, Whiten A, Henzi SP, McCulloch FM. Nutritional constraints on mountain baboons (Papio ursinus): Implications for baboon socioecology. Behavioral Ecology and Sociobiology. 1993;33:233–246. [Google Scholar]
- Charpentier MJE, Fontaine MC, Cherel E, Renoult JP, Jenkins T, Benoit L, Barthes N, Alberts SC, Tung J. Genetic structure in a dynamic baboon hybrid zone corroborates behavioural observations in a hybrid population. Molecular Ecology. 2012;21:715–731. doi: 10.1111/j.1365-294X.2011.05302.x. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson SE, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. International Journal of Primatology. 2004;25(2):401–428. doi: 10.1023/b:ijop.0000019159.75573.13. [DOI] [Google Scholar]
- Clements MN, Clutton-Brock TH, Albon SD, Pemberton JM, Kruuk LEB. Gestation length variation in a wild ungulate. Functional Ecology. 2011;25(3):691–703. doi: 10.1111/j.1365-2435.2010.01812.x. [DOI] [Google Scholar]
- Cleveland WS, Devlin SJ. Locally weighted regression: An approach to regression analysis by local fitting. Journal of the American Statistical Association. 1988;83(403):596–610. doi: 10.2307/2289282. [DOI] [Google Scholar]
- Deschner T, Boesch C. Can the patterns of sexual swelling cycles in female Tai chimpanzees be explained by the cost of sexual attraction hypothesis? International Journal of Primatology. 2007;28(2):389–406. doi: 10.1007/s10764-007-9120-1. [DOI] [Google Scholar]
- Dixson AF. Observations on the evolution and behavioral significance of sexual skin in female primates. Advances in the Study of Behavior. 1983;13:63–106. [Google Scholar]
- Drent RH, Daan S. The prudent parent: Energetic adjustments in avian breeding. Ardea. 1980;68(1-4):225–252. [Google Scholar]
- Ellison PT. On fertile ground: A natural history of human reproduction. Harvard University Press; Cambridge, MS: 2001. pp. 169–180. [Google Scholar]
- Ellison PT. Energetics and reproductive effort. American Journal of Human Biology. 2003;15(3):342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Endocrinology, energetics, and human life history: A synthetic model. Hormones and Behavior. 2017;91:97–106. doi: 10.1016/j.yhbeh.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Panterbrick C, Lipson SF, Orourke MT. The ecological context of human ovarian function. Human Reproduction. 1993;8(12):2248–2258. doi: 10.1093/oxfordjournals.humrep.a138015. [DOI] [PubMed] [Google Scholar]
- Fedigan LM, Carnegie SD, Jack KM. Predictors of reproductive success in female white-faced capuchins (Cebus capucinus) American Journal of Physical Anthropology. 2008;137(1):82–90. doi: 10.1002/ajpa.20848. [DOI] [PubMed] [Google Scholar]
- Foley R. Another unique species: Patterns in human evolutionary ecology. New York: Wiley; 1987. [Google Scholar]
- Foley RA. The influence of seasonality on hominid evolution. In: Ulijaszek SJ, Strickland SS, editors. Seasonality and human ecology: 35th symposium volume of the society for the study of human biology. Cambridge, UK: Cambridge University Press; 1993. pp. 17–37. [Google Scholar]
- Frisch RE. Body fat, puberty and fertility. Biological Reviews of the Cambridge Philosophical Society. 1984;59(2):161–188. doi: 10.1111/j.1469-185X.1984.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Frisch RE, McArthur JW. Menstrual cycles: Fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185(4155):949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- Garcia C, Lee PC, Rosetta L. Dominance and reproductive rates in captive female olive baboons, Papio anubis. American Journal of Physical Anthropology. 2006;131(1):64–72. doi: 10.1002/ajpa.20405. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Altmann J, Khan MZ, Couret J, Yu JC, Endres CS, Wango EO. Coming of age: Steroid hormones of wild immature baboons (Papio cynocephalus) American Journal of Primatology. 2005;67(1):83–100. doi: 10.1002/ajp.20171. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Beehner J, Khan MZ, Lynch JW, Fox E, Altmann J. Amboseli Baboon Research Project laboratory procedures for fecal hormones. 2017 Retrieved July 15, 2017, from https://amboselibaboons.nd.edu/assets/75656/altmann_lab_protocols_jan08.pdf.
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, Alberts SC, Altmann J. Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus) Hormones and Behavior. 2008;54(3):410–416. doi: 10.1016/j.yhbeh.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: Sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Hormones and Behavior. 2007;51(1):114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Gillman J. Pregnancy in the baboon. South African Journal of Medical Science. 1951;16:115–124. [PubMed] [Google Scholar]
- Gillman J. Effects on the perineal swelling and on the menstrual cycle of single injections of combinations of estradiol benzoate and progesterone given to baboons in the first part of the cycle. Endocrinology. 1942;30(1):54–60. [Google Scholar]
- Gladstone M, White S, Kafulafula G, Neilson JP, van den Broek N. Post-neonatal mortality, morbidity, and developmental outcome after ultrasound dated preterm birth in rural Malawi: A community based cohort study. PLOS Medicine. 2011;8(11):e1001121. doi: 10.1371/journal.pmed.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasier A. Chapter 34: Contraception. In: Jameson JL, De Groot LJ, de Krester D, Giudice LC, Grossman A, Melmed S, Potts JT Jr, Weir GC, editors. Endocrinology: Adult and pediatric. Seventh. Philadelphia: Saunders Elsevier; 2015. pp. 2297–2309. [Google Scholar]
- Graham L, Schwarzenberger F, Mostl E, Galama W, Savage A. A versatile enzyme immunoassay for the determination of progestogens in feces and serum. Zoo Biology. 2001;20(3):227–236. doi: 10.1002/zoo.1022. [DOI] [Google Scholar]
- Hamilton WJI, Buskirk RE, Buskirk WH. Omnivory and utilization of food resources by chacma baboons, Papio ursinus. American Naturalist. 1978;112:911–924. [Google Scholar]
- Hausfater G. Dominance and reproduction in baboons (Papio cynocephalus) Vol. 7. Basel: Karger; 1975. [PubMed] [Google Scholar]
- Hausfater G, Altmann J, Altmann S. Long term consistency of dominance relations among female baboons (Papio cynocephalus) Science. 1982;217:752–755. doi: 10.1126/science.217.4561.752. [DOI] [PubMed] [Google Scholar]
- Henson MC, Castracane VD. Leptin in pregnancy: An update. Biology of Reproduction. 2006;74(2):218–229. doi: 10.1095/biolreprod.105.045120. [DOI] [PubMed] [Google Scholar]
- Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: Evaluating gestation specific risks of fetal and infant mortality. British Journal of Obstetrics and Gynaecology. 1998;105:169–173. doi: 10.1111/j.1471-0528.1998.tb10047.x. [DOI] [PubMed] [Google Scholar]
- Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. American Journal of Human Biology. 2009;21(4):512–519. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, N. R. C. Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- Janson C, Verdolin J. Seasonality of primate births in relation to climate. In: Brockman DK, VanSchaik CP, editors. Seasonality in primates: Studies of living and extinct human and non-human primates. Vol. 44. 2005. pp. 307–350. [Google Scholar]
- Janson CH. Food competition in brown capuchin monkeys (Cebus apella): Quantitative effects of group size and tree productivity. Behaviour. 1988;105:53–76. doi: 10.1163/156853988x00449. [DOI] [Google Scholar]
- Johnson SE. Life history and the competitive environment: Trajectories of growth, maturation, and reproductive output among chacma baboons. American Journal of Physical Anthropology. 2003;120(1):83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- Jolly CJ. Species, subspecies, and baboon systematics. In: Kimbel WH, Martin LB, editors. Species, Species Concepts, and Primate Evolution. Plenum Press; New York: 1993. pp. 67–107. [Google Scholar]
- Jonsson KI. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos. 1997;78(1):57–66. doi: 10.2307/3545800. [DOI] [Google Scholar]
- Jukic AM, Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. Length of human pregnancy and contributors to its natural variation. Human Reproduction. 2013;28(10):2848–2855. doi: 10.1093/humrep/det297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: Evaluating the storage of fecal samples fo steroid analysis. General and comparative endocrinology. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Kiltie RA. Intraspecific variation in the mammalian gestation period. Journal of Mammalogy. 1982;63(4):646–652. doi: 10.2307/1380270. [DOI] [Google Scholar]
- Klein RG. The human career: Human biological and cultural origins. Second. Chicago: University of Chicago Press; 1999. [Google Scholar]
- Kline J, Stein Z, Susser M. Conception to birth: Epidemiology of prenatal development. New York: Oxford University Press; 1989. [Google Scholar]
- Koyama N, Takahata Y, Huffman MA, Norikoshi K, Suzuki H. Reproductive parameters of female japanese macaques: 30 years data from the Arashiyama troops, Japan. Primates. 1992;33(1):33–47. doi: 10.1007/bf02382761. [DOI] [Google Scholar]
- Lager C, Ellison PT. Effect of moderate weight loss on ovarian function assessed by salivary progesterone measurements. American Journal of Human Biology. 1990;2(3):303–312. doi: 10.1002/ajhb.1310020312. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Coward WA, Lawrence F, Cole TJ, Whitehead RG. Fat gain during pregnancy in rural African women: The effect of season and dietary status. American Journal of Clinical Nutrition. 1987;45(6):1442–1450. doi: 10.1093/ajcn/45.6.1442. [DOI] [PubMed] [Google Scholar]
- Lee PC. The meanings of weaning: Growth, lactation, and life history. Evolutionary Anthropology. 1997;5:87–96. [Google Scholar]
- Lee PC, Brennan EJ, Else JG, Altmann J. The ecology and behavior of vervet monkeys in a tourist lodge habitat. In: Else JG, Lee PC, editors. Primate ecology and conservation. Cambridge, UK: Cambridge University Press; 1986. pp. 229–236. [Google Scholar]
- Lipson SF, Ellison PT. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Human Reproduction. 1996;11(10):2090–2096. doi: 10.1093/oxfordjournals.humrep.a019055. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. Concentrations of four fecal steroids in wild baboons: Short-term storage conditions and consequences for data interpretation. General and comparative endocrinology. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Markham AC, Gesquiere LR, Alberts SC, Altmann J. Optimal group size in a highly social mammal. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(48):14882–14887. doi: 10.1073/pnas.1517794112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RD. Scaling of the mammalian brain: The maternal energy hypothesis. News in Physiological Sciences. 1996;11:149–156. [Google Scholar]
- Martin RD. The evolution of human reproduction: A primatological perspective. In: Stinson S, editor. Yearbook of physical anthropology. Vol. 50. 2007. pp. 59–84. [DOI] [PubMed] [Google Scholar]
- McFarland Symington M. Sex ratio and maternal rank in wild spider monkeys: When daughters disperse. Behavioral Ecology and Sociobiology. 1987;20(6):421–425. doi: 10.1007/bf00302985. [DOI] [Google Scholar]
- McNeilly AS. Lactational control of reproduction. Reproduction Fertility and Development. 2001;13(7-8):583–590. doi: 10.1071/rd01056. [DOI] [PubMed] [Google Scholar]
- Mueck AO, Sitruk-Ware R. Nomegestrol acetate, a novel progestogen for oral contraception. Steroids. 2011;76(6):531–539. doi: 10.1016/j.steroids.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Mysterud A, Roed KH, Holand O, Yoccoz NG, Nieminen M. Age-related gestation length adjustment in a large iteroparous mammal at northern latitude. Journal of Animal Ecology. 2009;78(5):1002–1006. doi: 10.1111/j.1365-2656.2009.01553.x. [DOI] [PubMed] [Google Scholar]
- Norton GW, Rhine RJ, Wynn GW, Wynn RD. Baboon diet: A five-year study of stability and variability in the plant feeding and habitat of the yellow baboons (Papio cynocephalus) of Mikumi National Park, Tanzania. Folia Primatologica. 1987;48:78–120. doi: 10.1159/000156287. [DOI] [PubMed] [Google Scholar]
- Onyango PO, Gesquiere LR, Wango EO, Alberts SC, Altmann J. Persistence of maternal effects in baboons: Mother’s dominance rank at son’s conception predicts stress hormone levels in sub-adult males. Hormones and Behavior. 2008;54:319–324. doi: 10.1016/j.yhbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer C, Collins DA, Sindimwo A, Goodall J. Reproductive constraints on aggressive competition in female baboons. Nature. 1995;373(6509):60–63. doi: 10.1038/373060a0. [DOI] [PubMed] [Google Scholar]
- Pollock JI. Behavioral ecology and body condition changes in new forest ponies. Sussex, England: RSPCA Scientific Publications; 1980. [Google Scholar]
- Potts R. Evolution and climate variability. Science. 1996;273:922–923. [Google Scholar]
- Potts R. Environmental hypotheses of hominin evolution. Yearbook of Physical Anthropology. 1998a;41:93–136. doi: 10.1002/(sici)1096-8644(1998)107:27+<93::aid-ajpa5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Potts R. Variability selection in hominid evolution. Evolutionary Anthropology. 1998b;7:81–96. [Google Scholar]
- Racey PA. Environmental factors affecting the length of gestation in mammals. In: Gilmore D, Cook B, editors. Environmental factors in mammal reproduction. London: MacMillan; 1981. pp. 208–213. [Google Scholar]
- Rawlins RG, Kessler MJ. Demography of the free-ranging Cayo Santiago macaques (1976-1983) In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques. Albany: State University of New York Press; 1986. pp. 47–72. [Google Scholar]
- Riney T. A field technique for assessing physical condition of some ungulates. The Journal of Wildlife Management. 1960;24(l):92–94. doi: 10.2307/3797362. [DOI] [Google Scholar]
- Riopelle AJ, Hale PA. Nutritional and environmental factors affecting gestation length in rhesus monkeys. American Journal of Clinical Nutrition. 1975;28(10):1170–1176. doi: 10.1093/ajcn/28.10.1170. [DOI] [PubMed] [Google Scholar]
- Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD. Age-related patterns of reproductive success among female mountain gorillas [Article] American Journal of Physical Anthropology. 2006;131(4):511–521. doi: 10.1002/ajpa.20474. [DOI] [PubMed] [Google Scholar]
- Robinson JJ. Changes in body composition during pregnancy and lactation. Proceedings of the Nutrition Society. 1986;45(1):71–80. doi: 10.1079/pns19860037. [DOI] [PubMed] [Google Scholar]
- Rosetta L, Lee PC, Garcia C. Energetics during reproduction: A doubly labeled water study of lactating baboons. American Journal of Physical Anthropology. 2011;144(4):661–668. doi: 10.1002/ajpa.21475. [DOI] [PubMed] [Google Scholar]
- Sadlier RMFS. Ecology of reproduction in wild and domestic mammals. London: Methuen & Co., Ltd.; 1969. [Google Scholar]
- Setchell JM, Lee PC, Wickings EJ, Dixson AF. Reproductive parameters and maternal investment in mandrills (Mandrillus sphinx) International Journal of Primatology. 2002;23(1):51–68. doi: 10.1023/a:1013245707228. [DOI] [Google Scholar]
- Setchell JM, Wickings EJ. Social and seasonal influences on the reproductive cycle in female mandrills (Mandrillus sphinx) American Journal of Physical Anthropology. 2004;125(1):73–84. doi: 10.1002/ajpa.10375. [DOI] [PubMed] [Google Scholar]
- Shaikh AA, Celaya CL, Gomexz I, Shaikh SA. Temporal relationship of hormonal peaks to ovulation and sex skin deturgescence in the baboon. Primates. 1982;23:444–452. [Google Scholar]
- Silk J, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) International Journal of Primatology. 1993;14(1):95–104. doi: 10.1007/bf02196505. [DOI] [Google Scholar]
- Silk JB. Eating for 2: Behavioral and environmental correlates of gestation length among free-ranging baboons (Papio cynocephalus) International Journal of Primatology. 1986;7(6):583–602. doi: 10.1007/bf02736663. [DOI] [Google Scholar]
- Sitruk-Ware R. New progestagens for contraceptive use. Human Reproduction Update. 2006;12(2):169–178. doi: 10.1093/humupd/dmi046. [DOI] [PubMed] [Google Scholar]
- Smuts B, Nicolson N. Reproduction in wild female olive baboons. American Journal of Primatology. 1989;19(4):229–246. doi: 10.1002/ajp.1350190405. [DOI] [PubMed] [Google Scholar]
- Stearns SC. Trade-offs in life-history evolution. Functional Ecology. 1989;3(3):259–268. doi: 10.2307/2389364. [DOI] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- Stevenson RD, Woods WA., Jr Condition indices for conservation: New uses for evolving tools. Integrative and Comparative Biology. 2006;46(6):1169–1190. doi: 10.1093/icb/icl052. [DOI] [PubMed] [Google Scholar]
- Stewart KJ, Harcourt AH, Watts DP. Determinants of fertility in wild gorillas and other primates. In: Diggory P, Potts M, Teper S, editors. Natural human fertility: Social and biological determinants. London: Palgrave Macmillan UK; 1988. [Google Scholar]
- Strum SC, Western JD. Variations in fecundity with age and environment in olive baboons (Papio anubis) American Journal of Primatology. 1982;3(1-4):61–76. doi: 10.1002/ajp.1350030106. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Ohsawa H. Population dynamics of japanese monkeys with special reference to the effect of artificial feeding. Folia Primatologica. 1982;39(3-4):238–263. doi: 10.1159/000156080. [DOI] [PubMed] [Google Scholar]
- Thompson ME. Reproductive ecology of female chimpanzees. American Journal of Primatology. 2013a;75(3):222–237. doi: 10.1002/ajp.22084. [DOI] [PubMed] [Google Scholar]
- Thompson ME. Comparative reproductive energetics of human and nonhuman primates. Annual Review of Anthropology. 2013b;42:287–304. doi: 10.1146/annurev-anthro-092412-155530. [DOI] [Google Scholar]
- Thompson ME, Muller MN, Wrangham RW. The energetics of lactation and the return to fecundity in wild chimpanzees. Behavioral Ecology. 2012;23(6):1234–1241. doi: 10.1093/beheco/ars107. [DOI] [Google Scholar]
- Tung J, Charpentier MJE, Garfield DA, Altmann J, Alberts SC. Genetic evidence reveals temporal change in hybridization patterns in a wild baboon population. Molecular Ecology. 2008;17:1998–2011. doi: 10.1111/j.1365-294X.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- Valeggia C, Ellison PT. Lactational amenorrhoea in well nourished Toba women of Formosa, Argentina. Journal of Biosocial Science. 2004;36(5):573–595. doi: 10.1017/s0021932003006382. [DOI] [PubMed] [Google Scholar]
- Valeggia C, Ellison PT. Interactions between metabolic and reproductive functions in the resumption of postpartum fecundity. American Journal of Human Biology. 2009;21(4):559–566. doi: 10.1002/ajhb.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik CP, van Noordwijk MA, Deboer RJ, Dentonkelaar I. The effect of group size on time budgets and social behavior in wild long-tailed macaques (Macaca fascicularis) Behavioral Ecology and Sociobiology. 1983;13(3):173–181. [Google Scholar]
- Vitzthum VJ, Spielvogel H, Thornburg J. Interpopulational differences in progesterone levels during conception and implantation in humans. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(6):1443–1448. doi: 10.1073/pnas.0302640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neuroscience and Biobehavioral Reviews. 1992;16(2):235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Wango TL, Musiega D, Mundia CN, Altmann J, Alberts SC, Tung J. Climate and land cover analysis suggest no strong ecological barriers to gene flow in a natural baboon hybrid zone. International Journal of Primatology. 2017 doi: 10.1007/s10764-017-9989-2. In press. [DOI] [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. General and Comparative Endocrinology. 2000;120(3):260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Monfort SL, Southers J, Wildt DE. Excretion rates and metabolites of estradiol and progesterone in baboon (Papio cynocephalus cynocephalus) feces. Journal of Reproduction and Fertility. 1994;101(1):213–220. doi: 10.1530/jrf.0.1010213. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Norton GW, Kleindorfer S, Rhine RJ. Population trend alters the effects of maternal dominance rank on lifetime reproductive success in yellow baboons (Papio cynocephalus) Behavioral Ecology and Sociobiology. 2004;56(4):338–345. doi: 10.1007/s00265-004-0797-2. [DOI] [Google Scholar]
- Wasser SK, Norton GW, Rhine RJ, Klein N, Kleindorfer S. Ageing and social rank effects on the reproductive system of free-ranging yellow baboons (Papio cynocephalus) at Mikumi National Park, Tanzania. Human Reproduction Update. 1998;4(4):430–438. doi: 10.1093/humupd/4.4.430. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Risler L, Steiner RA. Excreted steroids in primate feces over the menstrual cycle and pregnancy. Biology of Reproduction. 1988;39(4):862–872. doi: 10.1095/biolreprod39.4.862. [DOI] [PubMed] [Google Scholar]
- Whiten A, Byrne RW, Barton RA, Waterman PG, Henzi SP. Dietary and foraging strategies of baboons. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1991;334(1270):187–197. doi: 10.1098/rstb.1991.0108. [DOI] [PubMed] [Google Scholar]
- Widdowson EM. Changes in the body and its organs during lactation: Nutritional implications. In: Elliott K, Fitzsimons DW, editors. Breast-feeding and the mother. The Hague: Mouton & Co.; 1976. pp. 103–118. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Doyle LL, Stone SC, Harrison RM. Correlation of perineal swelling with serum ovarian hormone levels, vaginal cytology, and ovarian follicular development during the baboon reproductive cycle. Primates. 1977;18:261–270. [Google Scholar]
- Winder IC. The biogeography of the Papio baboons: a GIS-based analysis of range characteristics and variability. Folia Primatologica. 2015;85(5):292–318. doi: 10.1159/000362545. [DOI] [PubMed] [Google Scholar]
- Wood JW. Dynamics of human reproduction: Biology, biometry, demography. New York: Aldine de Gruyter; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


