Abstract
Purpose
To assess the effect of macroscopic susceptibility gradients on the gas-phase referenced dissolved-phase 129Xe (DPXe) chemical shift (CS) and to establish the robustness of a water-based referencing system for in vivo DPXe spectra.
Methods
Frequency shifts induced by spatially-varying magnetic susceptibility are calculated by finite-element analysis for the human head and chest. Their effect on traditional gas-phase referenced DPXe CS is then assessed theoretically and experimentally. A water-based referencing system for the DPXe resonances that uses the local water protons as reference is proposed and demonstrated in vivo in rats.
Results
Across the human brain, macroscopic susceptibility gradients can induce an apparent variation in the DPXe CS of up to 2.5 ppm. An additional frequency shift as large as 6.5 ppm can exist between DPXe and gas-phase resonances. By using nearby water protons as reference for the DPXe CS, the effect of macroscopic susceptibility gradients is eliminated and consistent CS values are obtained in vivo, regardless of shimming conditions, region of interest analyzed, animal orientation, or lung inflation. Combining in vitro and in vivo spectroscopic measurements finally enables confident assignment of some of the DPXe peaks observed in vivo.
Conclusions
In order to use hyperpolarized xenon as a biological probe in tissues, the DPXe CS in specific organs/tissues must be reliably measured. When the gas-phase is used as reference, variable CS values are obtained for DPXe resonances. Reliable peak assignments in DPXe spectra can be obtained by using local water protons as reference.
Keywords: chemical shift, hyperpolarized xenon, xenon spectroscopy, susceptibility gradients, magnetic resonance spectroscopy
INTRODUCTION
In vivo NMR spectroscopy enables one to interrogate, non-invasively, tissue anatomy and function at the molecular level. Among all nuclei, xenon presents exciting opportunities for biomolecular NMR applications since the gas is inert, relatively soluble in tissues (1–3), and has a chemical shift (CS) that is extremely sensitive to the chemical environment (3–6). Thanks to the more than 10-fold improvement in xenon polarization efficiency (7–13), applications of dissolved-phase xenon (DPXe) imaging and spectroscopy have increased considerably over the past 5 years. Such applications include the monitoring of gas exchange (14) and alveolar septa thickness in the lungs (15–21), monitoring of brain perfusion and blood oxygenation (22–26), detection of brown adipose tissue (27), temperature imaging (28), and renal MRI and magnetic resonance spectroscopy (MRS) (29).
In order to be able to use DPXe as a probe for tissue anatomy and/or function, one has to be able to reliably identify spectral lines based on their CS. Historically, the CS of DPXe has been measured with respect to the xenon gas-phase resonance (17,22,30–33). However, referencing the DPXe peaks to the gas phase presents several pitfalls: first, depending on the specific organ/tissue investigated, the gas phase may not always be visible; second, even when visible, the gas-phase CS is sensitive to a number of factors that, as recently pointed out (34), typically vary during an MR experiment, including level of lung inflation and partial pressure of the different gases in the lungs (35–38); lastly, because the gas-phase and the dissolved-phase signals originate from tissue compartments or organs with different magnetic properties, local magnetic fields experienced by these two phases are expected to be very different such that, even if the relative gas concentration in the lungs were known and could be kept constant during an experiment, macroscopic magnetic susceptibility gradients would make the dissolved-phase frequency highly variable (39). To alleviate the problem, one might consider minimizing macroscopic field variations by shimming. In practice, however, shimming conditions can be optimized either for the dissolved phase or the gas phase, but not both (32). As a result, it is not surprising that variable DPXe CS values have been reported in the literature (Table 1), making the identification of the source of some of these peaks very challenging.
Table 1.
Summary of DPXe chemical shift values and their assignments found in the literature.
| Study | Investigation | Peak Position (ppm) (assignment) |
||||
|---|---|---|---|---|---|---|
| Swanson, et al (31) | Rat (body) | 192 (epicardial fat) | --- | --- | 199 (tissue) | 210 (blood) |
| Duhamel, et al (56) | Rat (head) | --- | --- | 194 (intravascular) | 199 (brain) | 194.0±1.0 (intravascular) |
| Duhamel, et al (57) | Rat (head) | --- | --- | 194.5 (lipid emulsion) | 199 (brain tissue) | 194.0±1.0 (intravascular) |
| Wakai, et al (58) | Rat (body) | 189 (epicardial fat) | --- | --- | 199 (tissue) | 212 (blood) |
| Nakamura, et al (33) | Rat (head) | 188.8–189.5 (non-brain) | 192 --- | 194.5–195.2 (brain) | 198 --- | 210 (blood) |
| Kilian, et al (59) | Human (head) | --- | 192–194 (white matter) | 195.5–197.5 (gray matter) | --- | --- |
| Kershaw, et al (32) | Rat (head) | 187–192 (jaw muscle and or fat tissue) | 191–194 (white matter) | 193–197 (gray matter) | 197–201 (jaw muscle and or fat tissue) | 216 (blood) |
| Zhou, et al (54) | Rat (head) | 189 (non-brain) | 191.6 --- | 194.7 (brain) | 197.8 --- | 209.5 (blood) |
| Mazzanti, et al (22) | Rat (head) | 189 (non-brain tissue) | 191.6 --- | 194.7 (gray matter) | 197.8 --- | 209.5 (blood) |
| Rao, et al (25) | Human (head) | 187–189 (soft muscular tissue) | 192–192.7 (white matter) | 195.4–196 (gray matter) | 199–200 (interstitial fluid/plasma, fat tissue, CSF) | 217 (blood) |
In this paper, we first analyze the effect of macroscopic susceptibility gradients on the gas-phase referenced DPXe CS using methods similar to those in previous studies (40–43). We then propose an alternative reference for the DPXe spectrum that, unlike the gas phase, leads to DPXe CS values that are immune to variations in field strength, lung oxygenation, and shimming conditions. The consistency of the DPXe CS values obtained by using this water-based reference is then tested in vivo in rats at 9.4 T and finally used, in combination with high resolution in vitro spectroscopic measurements, to identify the origin of some of the DPXe peaks observed in vivo.
METHODS
Human susceptibility model preparation
A human susceptibility model was constructed from a 3D CT data set of the human body acquired in a healthy human volunteer under approval of the institutional review board at the University of North Carolina at Chapel Hill and conducted in accordance with the Helsinki Declaration. CT images were acquired with a resolution of 0.91 mm × 0.91 mm × 1.5 mm by using 5 bed positions to cover a region from the crown of the head to the lower abdomen. The 3D image dataset was thresholded with a cutoff value of −400 HU to separate the soft tissue from airspaces using VivoQuant software (Invicro, Boston, MA, USA). Each region was then assembled into a standard tessellation language mesh using 3D Slicer open-source software (http://www.slicer.org) (44). In preparation for finite-element analysis simulations, the mesh models were reconstructed into watertight surfaces using screened Poisson surface reconstruction (45) and quadratic edge collapse decimation, as implemented in MeshLab (http://www.meshlab.net) (46).
Magnetic field simulations using finite-element analysis software
From the 3D human model, a 3D susceptibility map was generated by assigning a susceptibility value of −9.05 ppm to soft tissue and 0.36 ppm to air (47). Magnetic susceptibility gradients were computed via finite-element analysis calculations (COMSOL Multiphysics, Stockholm, Sweden). The computation was performed by axially aligning the 3D human model with an external static magnetic field of 3 T within a sphere of air 1.6 m in diameter. In order to avoid distortion in the field, the model was bounded by an infinite-element domain. The resulting boundary value problem was solved for the scalar magnetic potential, from which the reduced magnetic flux density was calculated throughout the body.
In vitro high-resolution 129Xe NMR spectroscopy
High-resolution NMR measurements of 129Xe dissolved in cerebrospinal fluid (CSF; Lee Biosolutions Inc., Maryland Heights, MO, USA), excised rat white adipose tissue (WAT) and excised rat and mouse muscle were performed on a 500 MHz Varian Inova spectrometer (Varian NMR Systems, Palo Alto, California, USA). Samples were prepared by partially filling a high-pressure NMR tube (Daedalus Innovations, LLC, Aston, Pennsylvania, USA) with either CSF, freshly excised WAT, or freshly excised muscle. The sample was then connected to a vacuum pump and frozen in liquid nitrogen. Air was removed with the aid of a rotary pump and the sample was thawed. The freeze-thaw cycle was repeated 3 times to remove any possible oxygen from the sample. After degassing, enriched 129Xe gas (>86% isotopic enrichment) was introduced at a given pressure in the sample, which was then allowed to equilibrate for 24 hours under xenon atmosphere to a pressure of 1.04 atm (CSF), 1.03 atm (WAT), or 2.86 atm (muscle). Equilibrium pressures were kept low to avoid Xe-Xe dipolar interactions that could shift the DPXe frequencies. A slightly higher pressure was used for the muscle samples, given the lower solubility of xenon in muscle compared to WAT and the inability to shim the sample to a linewidth similar to that of CSF.
Samples were then placed in the NMR spectrometer and allowed to equilibrate for at least 1 hour at a temperature of 31.3°C (CSF), 31.0°C (WAT), or 30.8°C (muscle), regulated and maintained by a temperature controller. This temperature value was calibrated with an accuracy of 0.1 °C at the beginning of each spectroscopy experiment by using the CS separation between the methyl and hydroxyl protons in a 100% methanol temperature standard. Sample temperature was maintained at 31°C to enable direct comparison with the in vivo MRS and CSI experiments described below.
All samples were manually shimmed using up to the 7th order z shim and 4th order x and y shims. The 129Xe spectrum was acquired with a center frequency (CF) of 138.2675304 MHz (138.2694672 MHz for muscle), repetition time (TR) of 34.5 s (30.5 s for WAT, 30.1 s for muscle), spectral width (SW) of 60015 Hz, 524288 points (8192 for muscle), 500 averages (7200 for CSF and 5300 for muscle) and a 90° flip angle. Proton spectra were acquired before and after the acquisition of each 129Xe spectrum (to ensure consistency) with a CF of 499.7835792 MHz, TR of 9 s, SW of 5998 Hz, 47984 points, 16 averages, and 23° flip angle. The CS of the DPXe signal was then measured with respect to the water CS as described below.
Method for dissolved-phase 129Xe referencing
Referencing of the DPXe frequencies to the 1H water frequency was performed similarly to the method employed by Zhang, et al for in vivo absolute 129Xe MR thermometry (48). First, a 1H spectrum was acquired to obtain a measurement of the water resonance frequency. This frequency was then scaled by 3.6152950216, i.e. the ratio between the 129Xe gyromagnetic ratio (γXe=11.7767392 MHz/T) and 1H gyromagnetic ratio (γH=42.5763866 MHz/T) (49), to obtain a water-based 0 ppm 129Xe CF, from which the experimental DPXe CS was calculated. Figure 1 shows a cartoon of the referencing procedure. An example of how the CS of the main peak observed in the rat head was computed is also provided in Table 2. This water-based referencing method led to CS values typically ~4 ppm higher than those obtained by using the xenon gas phase as reference, at least in vivo from the rat head.
FIG. 1.
(a) Susceptibility-induced frequency shift in the human body. (b) Cartoon showing the proposed reference system for DPXe resonances. The 0 ppm reference in the xenon spectrum is obtained by scaling the water resonance frequency by the gyromagnetic ratios of the two nuclei. The DPXe chemical shift can then be measured with respect to this water-based reference. This referencing system eliminates the effect of macroscopic susceptibility gradients. An example of how chemical shift values are calculated is provided in Table 2.
Table 2.
Summary of the experimental frequencies measured in all 4 rat brains: The water 1H resonance frequency, the xenon gas-phase frequency (since this peak was quite broad, a range of CS values is provided), the primary DPXe peak (previously attributed to gray matter) frequency and the water-based DPXe reference. The water-based DPXe reference was calculated by dividing the water resonance frequency by 3.6152950216. The last two columns report the DPXe chemical shift calculated with respect to the water-based DPXe reference and the gas-phase reference.
| Subject | Water (MHz) |
129Xe Gas (MHz) |
DPXe (MHz) |
Water-based DPXe Reference (MHz) |
Gas-ref DPXe CS (ppm) |
Water-ref DPXe CS (ppm) |
|---|---|---|---|---|---|---|
| Rat #1 | 400.3263440 | 110.7315642–110.7317303 | 110.7532861 | 110.7313073 | 194.6–196.1 | 198.5 |
| Rat #2 | 400.3261221 | 110.7315811 | 110.7532519 | 110.7312459 | 195.7 | 198.7 |
| Rat #3 | 400.3264150 | 110.7316491 | 110.7533335 | 110.7313269 | 195.8 | 198.7 |
| Rat #4 | 400.3262054 | 110.7315610–110.7316060 | 110.7532815 | 110.7312689 | 195.9–196.3 | 198.8 |
Dissolved-phase 129Xe MRS and CSI in rats
All in vivo animal studies were conducted under animal protocols approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. For in vivo studies, a total of 4 male Fisher rats, age 8–10 weeks old, were used. Prior to the experiments, rats were anesthetized with a 60 mg/kg intraperitoneal injection of pentobarbital and intubated with a 20-gauge catheter. Rats were mechanically ventilated with approximately 25-vol% O2 and 75-vol% N2 using a home-built ventilator (50) at a rate of 60 breaths/min with a tidal volume of about 2 mL. Before the acquisition of xenon spectra, N2 gas was replaced by an equivalent HP 129Xe gas volume.
Xenon gas was polarized up to 14% with a commercial polarizer (Model 9800, Polarean, Inc., Durham, North Carolina, USA) using a gas mixture of 1% enriched xenon (>86% isotopic enrichment), 10% N2, and 89% He (Global Specialty Gases, Bethlehem, Pennsylvania, USA). The polarizer oven temperature was maintained at 403 K with a pre-saturation region held at 438 K. Gas flow was regulated by a mass flow controller to a rate of 1.5 standard liters per minute and cryogenically collected for 20 minutes. Frozen xenon was then sublimated into a 350 mL Tedlar bag (Jensen Inert Products, Coral Springs, Florida, USA) and connected to the ventilator.
In vivo MRS and MRI experiments were performed on a 9.4 T Bruker BioSpec 94/30 spectrometer (Bruker, Billerica, Massachusetts, USA) controlled by a console running Paravision software. Rats were placed with the anatomical region of interest in the sensitive region of a 1-cm diameter 129Xe surface coil. The rat and the 129Xe surface coil were then placed inside a 72-mm diameter 1H volume coil, used to collect reference anatomical images and 1H spectra. All 129Xe acquisitions were triggered to the breathing cycle to optimize the DPXe signal to noise ratio. Rat rectal temperature was monitored via a fiber optic rectal temperature probe and maintained at 31° C by a forced heated-air system.
Anatomical axial 1H images were collected by using a gradient echo multi-slice sequence with a field of view of 4 × 4 cm. Before the collection of 1H and 129Xe spectra, manual first and second order local shimming was performed to achieve narrow water linewidths (between 0.1 – 0.5 ppm, depending on the size and localization of the shimming voxel). 1H spectra, without water suppression, were acquired using a point-resolved spectroscopy (PRESS) sequence from the same voxel before and after the acquisition of a xenon spectrum, performed by maintaining the same shimming conditions used for the acquisition of the 1H spectra. 1H and 129Xe chemical shift imaging (CSI) was also performed in the rat head. 129Xe CSI data were collected using the xenon surface coil with a field of view (FOV) of 4 × 4 cm, without slice selection, 8192 complex points, TR of 6 s, SW of 500 ppm, CF of 110.7532358 MHz, and CSI matrix size of 8 × 8. 1H CSI data were collected using the 1H volume coil with a FOV of 4 cm by 4 cm, 1 slice, 1 cm slice thickness, 2048 complex points, TR of 2 s, SW of 10 ppm, CF of 400.326207 MHz and CSI matrix size of 8 × 8. The CSI results of both nuclei were reconstructed to a matrix size of 16 × 16 using Paravision software.
RESULTS
Susceptibility-induced frequency shifts
A map of the reduced magnetic flux density and the induced frequency shift produced by magnetic susceptibility gradients between tissue and air in the human body is shown in Figure 1. This map outlines the challenge associated with trying to use the gas phase as a reference for the DPXe frequency. For example, at 3T, the DPXe signal originating from brain tissue experiences a local field up to 24 µT lower than that experienced by the gas phase in the lungs or nasal cavities. This field difference produces an apparent difference in CS between the gas phase in the lung and the DPXe in the brain of up to 6.5 ppm.
Figure 2a shows axial, sagittal, and coronal views of the frequency shift produced by magnetic susceptibility gradients in the human head, as well as the corresponding CT slices showing the details of the skull, nasal cavity, brain, and throat. These maps show that, across the human head, if shimming is not applied, macroscopic susceptibility gradients can produce apparent frequency shifts of up to 3 ppm, depending on the region analyzed. Figure 2b shows the frequency distribution for individual voxels previously selected to investigate the origin of DPXe resonances in the human head (25). The field and frequency distribution is particularly inhomogeneous for individual spectra acquired near the nasal cavity or for those encompassing the brain from top to bottom. Across the brain, macroscopic susceptibility gradients can produce an apparent frequency shift between 1.5 – 2 ppm, of the same order of magnitude as variations observed for the DPXe peaks in the human head by Rao et al (25).
FIG. 2.
(a) Representative maps (left column) of the frequency shift induced by magnetic susceptibility gradients at 3 T in the human head along with (right column) corresponding original CT slices used in the model. In CT slices, white represents bones, gray represents soft tissue, and black represents air. (b) Histograms of frequency shift (left) present within the highlighted voxels shown in the 3D human head rendering (right). Note that anatomical features of the model located in front of the planes and voxels of interest are visible in the model rendering.
In vivo MRS in rats
Figure 3 shows representative xenon spectra acquired from the head of one of the rats at 34°C and 37°C. In addition to the gas phase peak and the RBC peak, these spectra show 3 additional peaks, with only one of them shifting by an average of −0.2 ppm/°C. The location of these peaks with respect to the gas phase and the water-based xenon reference is shown in Table 3, along with a summary of previous peak assignments. Table 4 reports the CS value of the major peak attributed to xenon in the brain for all rats analyzed. Experimental resonance frequencies from which the CS values were calculated are reported in Supporting Table S1. As is clear from Table 4, our reference system provides consistent CS values, whereas the gas-phase reference provides CS values that varied by more than 1.5 ppm across the 4 animals. In this case, smaller variations, on the order of 0.3 ppm, were observed also across spectra acquired in the same animal under different shimming conditions.
FIG. 3.
Representative in vivo xenon spectra obtained from the head of one of the rats at 34°C and 37°C. CS values are reported with respect to the water-based xenon reference calculated from the water resonance frequency measured in the region of interest. Only the peak at 193 ppm shows a clear upfield shift with increased temperature. Also shown are the anatomical regions from which the reference 1H spectra were acquired after a manual shimming procedure (blue (5 × 5 × 5 mm) and red (7 × 15 × 14 mm) boxes corresponding to the 34°C and 37 °C spectra, respectively), and the location of the xenon surface coil (yellow line). A larger reference region was used in the 34°C spectrum to better resolve the lipid peak.
Table 3.
Summary of the chemical shift of the major peaks observed in this study and their assignments. Chemical shift values are reported with respect to the gas phase and to our water-based reference, obtained by scaling the water resonance frequency. Included is a summary of previous peak assignments.
| Chemical Shift (ppm) Gas-referenced |
Chemical Shift (ppm) Water-referenced |
Previous Peak Assignments |
Peak Assignments from this study |
|---|---|---|---|
| 189.9 @ 31°C and 188.7@ 37°C | 192.9 @ 31°C and 191.8@ 37°C | jaw muscle, fat | fat |
| 192.3 | 194.9 | brain tissue, white matter | CSF |
| 195.7–196.2 | 198.7 | brain tissue, gray matter | brain tissue, gray matter and maybe other tissues |
| 195.6a | 198.8 | --- | muscular tissue, plasma |
| 199.3a | 202.5 | fat tissue | muscular tissue |
| 210–212 | 213–214 | red blood cells | red blood cells |
Since no gas-phase 129Xe signal was present in the in vivo muscle spectrum, these gas-referenced values were estimated by taking the CS difference between the water-referenced DPXe CS values for these peaks and the primary brain tissue peak, and adding the gas-referenced DPXe CS value for gray matter as reported in Rao et al (25).
Table 4.
Chemical shift values of the major peak observed from the heads of all rats. Chemical shift values are reported with respect to the xenon gas phase and our water-based reference, calculated from the resonance frequency of water protons. The gas phase in Rats 1 and 4 was split into multiple peaks giving a range of possible chemical shift values.
| Subject | Chemical Shift (ppm) Gas-referenced |
Chemical Shift (ppm) Water-Referenced |
|---|---|---|
| Rat #1 | 194.6–196.1 | 198.5 |
| Rat #2 | 195.7 | 198.7 |
| Rat #3 | 195.8 | 198.7 |
| Rat #4 | 195.9–196.3 | 198.8 |
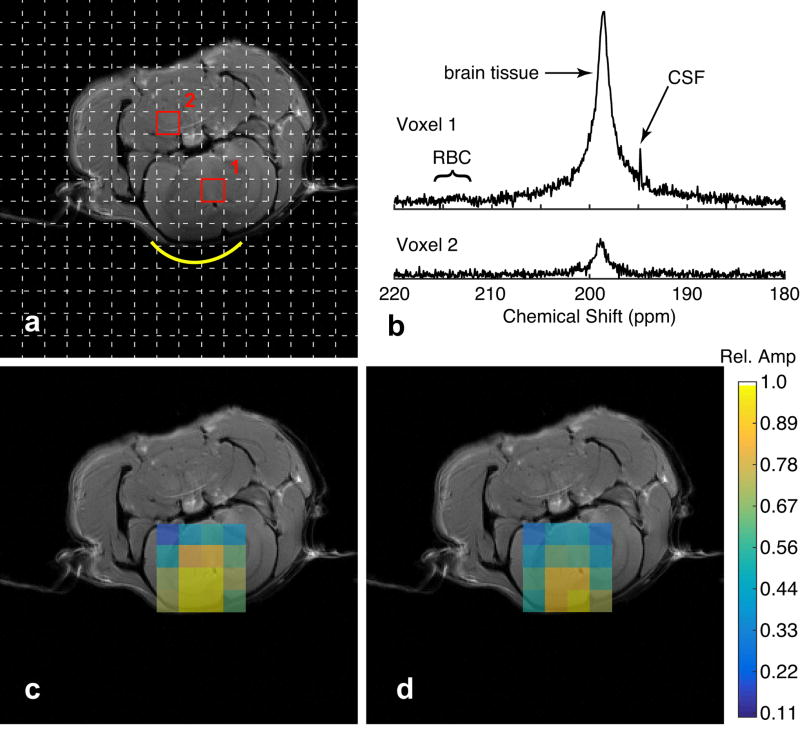
Figure 4 shows representative spectra obtained from a voxel located inside the brain and a voxel located outside the brain (Fig. 4a). In the voxel located inside the brain, only two major peaks are observed: one at 198.7 ppm and one at 194.9 ppm (Fig. 4b), previously attributed to DPXe in gray and white matter, respectively (25). A very weak signal is observed in the voxel outside the brain, most likely due to leakage from strong signals originating from brain tissue (51). Fig. 4c and 4d show CSI maps of the relative amplitude of the brain/gray matter and CSF DPXe peaks, respectively. In both cases, the signal originates from the expected regions of the brain.
FIG. 4.
Xenon CSI maps in the rat brain. (a) CSI matrix depicting two representative voxels, one inside (1) and one outside (2) the brain, along with (b) the 129Xe spectra referenced to the water protons in each voxel. (c, d) CSI maps of the primary brain (c) and CSF (d) peaks. The color scale depicts the peak amplitude relative to the maximum peak in each map.
Figure 5 shows representative xenon spectra acquired from different locations on the rat, along with xenon spectra acquired in vitro in CSF and in excised WAT and muscle. The anatomical regions and 129Xe surface coil positions for each in vivo spectrum is given in Supporting Figures S1 and S2. In the rat leg, four peaks are clearly visible: two prominent peaks, located at 193 ppm and 202.5 ppm, and two small, broad peaks, located at 198.8 ppm and at 214 ppm, the latter known to originate from DPXe in RBC. Comparison of the in vivo spectra with the in vitro spectra acquired from excised muscle and adipose tissues (Fig. 6) clearly indicates that the peak at 193 ppm (originally reported at 188–189 ppm from the gas phase (25)) originated from xenon dissolved in fat, not muscle. Also, the shift of this peak observed in the rat head spectra acquired at the two different temperatures is consistent with the previously measured temperature-induced shift of the CS of xenon dissolved in lipids (48).
FIG. 5.
In vivo and in vitro DPXe spectra. (a–d) show representative DPXe spectra acquired in vivo from the rat head, leg muscle, kidney, and liver, respectively. All chemical shifts were calculated using the water-based 129Xe reference calculated from the 1H PRESS signal acquired from the voxels shown in Supporting Figures S1 and S2. (e–g) show DPXe spectra acquired in vitro in samples of CSF, excised rat white adipose tissue, and excised rat muscle, respectively. In vitro spectra corroborate the peak identification of CSF at 194.9 ppm and lipids at 193 ppm in vivo and 193.8 ppm in vitro at 31°C. Liver and kidney spectra show broad peaks centered around 200–202 ppm that may have contributions from muscle outside of the homogeneous field region as well as unidentified compartments within each organ.
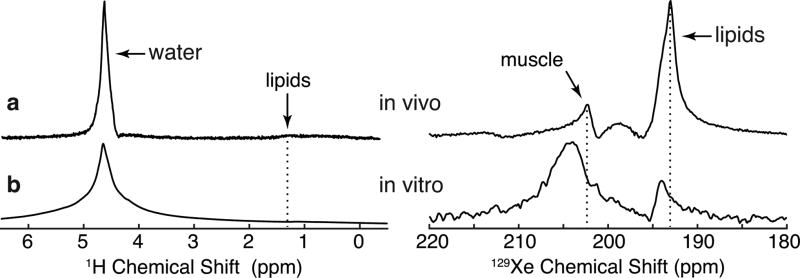
FIG. 6.
Comparison of in vivo and in vitro proton and DPXe muscle spectra. (a) In vivo 1H (left) and 129Xe (right) muscle spectra. (b) In vitro 1H (left) and 129Xe (right) muscle spectra acquired with a sample of excised muscle placed under 2.86 atm of xenon gas. Note that all spectra were acquired by optimizing shim conditions for the water protons. The use of a 3 atm xenon pressure for the in vitro studies produced a downfield shift of the DPXe lipid peak, as expected based on the previously reported pressure dependence of this peak (48). Likewise, the muscle DPXe peak in vitro is shifted downfield from the in vivo peak by about 1.5 ppm, most likely because of a pressure dependence of the DPXe muscle peak (see Supporting Fig. S4) and post-mortem changes in muscular structure and/or chemistry. A very broad peak centered at around 198.8 ppm can also be seen in the DPXe spectrum.
Since the peak at 193 ppm can be confidently assigned to xenon dissolved in lipids, the peak at 202.5 ppm and the broad peak at 198.8 ppm, the only other peaks seen in the spectra from the rat leg and not seen in the spectra from excised adipose tissue, can only be assigned to xenon dissolved in muscle. In vitro, the peak at 202.5 ppm appears to be much broader and shifted slightly downfield, while the broad peak at 198.8 ppm was only visible in the spectrum acquired from excised mouse muscle. These differences between the in vivo and in vitro muscle spectra may be due to post mortem changes in tissue structure and composition.
Similarly, by comparing the in vivo brain spectra to the spectra acquired in vitro from CSF samples, we can identify the peak at 194.9 ppm (upfield ~192 ppm from the gas phase and 3.9 ppm from the major peak) observed in the rat head spectra as coming from CSF.
DISCUSSION
To take full advantage of the sensitivity of the DPXe CS to the chemical environment in vivo in tissues, a robust referencing system needs to be established. As showed by our simulations, the gas-phase peak represents a suboptimal reference; when the DPXe peaks are referenced to the gas-phase peak, magnetic susceptibility gradients can produce apparent variation in the CS values that make comparison between in vivo xenon spectra very difficult (see Table 1). In addition, considering that in vivo, in tissues, the CS of DPXe resonances spans a range of ~15 ppm, these variations prevent us from recognizing peaks originating from the same tissue/organ, or from exploiting the sensitivity of some of the DPXe chemical shifts to monitor important physiological parameters such as blood oxygenation (52,53) or tissue temperature (48).
Magnetic susceptibility gradients can also negatively affect the apparent CS of DPXe peaks, even within the same organ. A variation of about 2 ppm, similar to the one recently reported in the human head by Rao et al (25), was indeed computed just across the human brain. Although in vivo shimming is typically applied to reduce some of these frequency variations, since shimming can only be optimized either for the gas phase or the dissolved phase, but not both, some of these frequency variations are expected to remain
In this paper, we showed that the effect of macroscopic susceptibility gradients can be mitigated by referencing the DPXe resonances to that of nearby water protons. By using water protons in close physical proximity as a reference for the DPXe peaks, consistent CS values for the DPXe resonances were obtained. In addition, we could directly compare spectra acquired from different tissues/organs or spectra acquired in vivo with those acquired in vitro. These comparisons have proven to be indispensable to correctly identify the origin of common and uncommon xenon peaks in spectra acquired in vivo.
Specifically, in addition to the broad peak at 213 – 214 ppm, which is well-known to originate from xenon dissolved in red blood cells (RBC), we were able to identify three additional peaks: one around 193 ppm, one broad peak around 198.8 ppm, and one around 202.5 ppm. The first peak, which corresponds to the peak seen between 187–189 ppm in Mazzanti et al (22), Zhou et al (54), Nakamura et al (33) and Rao et al (25) in spectra acquired from rat and human heads, was previously attributed to non-brain tissue and jaw muscle. In this study, comparison of in vivo spectra with in vitro spectra CS values obtained from xenon dissolved in adipose tissue clearly shows that this peak originates from regions outside the brain and, specifically, from xenon dissolved in adipose tissue. The CS of this peak nicely matches the CS previously measured for xenon dissolved in adipose tissue in our lab (55), as well as the major peak seen from brown adipose tissue in humans during stimulation of thermogenesis (27) (see Supporting Table S1). Not surprisingly, this peak is very broad or can appear to be split in multiple components, when shimming conditions are optimized for the water signal (25). See Supporting Figure S3 for further discussion. Additionally, the −0.2 ppm/°C temperature-induced upfield shift seen in one of the rats is consistent with the temperature-induced shift of xenon dissolved in adipose tissue used previously for in vivo temperature imaging in mice (48). The small frequency variations seen in this peak across the in vivo spectra are more likely indicative of a temperature gradient present in the anesthetized rat. For example, the same peak appears to have a small upfield shift in the spectrum collected from the rat leg, which was placed right next to the heating hose inside the magnet. In vitro experiments, where temperature could be more precisely controlled and shimming conditions optimized for the methylene peak, place the lipid DPXe peak more accurately at 193.8 ppm at 31°C (see Supporting Table S2).
The identification of the peak at 193 ppm as a peak originating from xenon dissolved in adipose tissue leads to the identification of the two peaks at 202.5 ppm and 198.8 ppm as originating from muscle tissue. While the peak at 202.5 ppm, elsewhere reported at 199–200 ppm downfield from the gas-phase resonance, was previously identified as originating from xenon dissolved in interstitial fluid, plasma, fat tissue, and jaw muscle (25,32), the peak at 198.8 ppm has never been reported. Since these are the only two peaks seen in the rat leg spectrum, along with the peaks attributed to xenon dissolved in fat and RBC, we can confidently assign these two peaks to xenon dissolved in muscle tissue, although a contribution from xenon dissolved in plasma for the 198.8 ppm peak cannot be excluded.
The peak at 194.9 ppm corresponds to the peak observed between 191–194 ppm and assigned to white matter plus blood plasma in Kershaw et al (32), and between 192–192.7 ppm and assigned to white matter in Rao et al (25). Comparison of in vitro spectra acquired in CSF samples and in vivo rat head spectra strongly suggests that this peak originates or has contribution from xenon dissolved in CSF. This peak, which in vivo can derive from passive diffusion of xenon across the blood-CSF barrier, was indeed visible only in voxels located within the brain that contained the lateral ventricles in our rat head CSI studies and in Rao et al’s (25) human head CSI studies. Naturally, the assignment of the peak at 194.9 ppm to xenon dissolved in CSF raises the question of where the peak corresponding to xenon dissolved in white matter is. Unfortunately, high-resolution spectroscopy studies of excised brain tissue, which were tried in our lab, could not help in this case since spectra from excised brain tissue presented a single, very broad resonance frequency line. This resonance frequency line, like the resonance frequency of xenon dissolved in excised muscle, was much broader than the frequency lines observed in the corresponding 1H spectra, indicating possible fast exchange of xenon dissolved in different compartments whose structure and/or chemistry was probably compromised post mortem during excision (Supporting Figures S4 and S5).
Although the water-referencing method can successfully address most of the problems associated with gas-phase referencing, it does require an extra step: the acquisition of a 1H spectrum, and thus the use of either a dual-tuned 1H/129Xe RF coil or, at 3 T, the use of a 1H body coil. In addition, a necessary condition for the correct application of this method is the use of the same shim currents, which in clinical scanners are often modified when transitioning from one nucleus to another.
CONCLUSIONS
Macroscopic susceptibility gradients produce large shifts in the apparent CS of DPXe resonances and prevent correct identification and assignment of peaks that can be found in xenon spectra acquired from different animals or organs. By referencing the DPXe CS to that of nearby water protons, we can remove the effect of macroscopic susceptibility gradients and obtain consistent CS values for xenon signals originating from the same organ or tissue compartments. Such a referencing system allows us to directly compare CS values obtained in vivo to those obtained in vitro from excised tissue or biological samples, and to correctly identify the origin of several peaks observed in vivo.
Supplementary Material
Table S1: Comparison of chemical shift values of DPXe in rat white adipose tissue at 31°C as reported in Zhang, et al and as recalculated using the method presented in this work. The difference in ppm between the two methods is due to the difference in precision used for the gyromagnetic ratios: In Zhang, et al, the values used were γXe=11.777 MHz/T and γH=42.576 MHz/T for the 129Xe and 1H gyromagnetic ratios whereas here we used values of γXe=11.7767392 MHz/T and γH=42.5763866 MHz/T, respectively.
Table S2: Comparison of lipid peaks from in vivo and in vitro experiments. All lipid peaks were fit with a Voigt profile in MATLAB using an iterative least-squares algorithm. RMS error indicates the RMS difference, in percent, between the fitted model and data in the fitted spectral region. Some In vivo regions were composed of a main peak with a sizable shoulder on the upfield side of the peak, likely from contributions outside of the shimmed ROI. Most likely, small temperature gradients present across the anesthetized rat body also contributed to the small variation in the chemical shift of the lipid peak.
Figure S1. Regions of interest analyzed for the acquisition of rat (a) kidney, (b) liver, and (c,d) leg DPXe spectra. 1H spectra were acquired from the voxels outlined in magenta, after a manual localized shimming procedure, using a 1H PRESS sequence, right before the acquisition of 129Xe spectra. 129Xe spectra were acquired with a 90°-acquisition sequence with the use of a surface coil, whose position is indicated by the yellow line. The anatomical image for the liver (b) was acquired post-mortem since movement of the diaphragm significantly distorted the liver image.
Figure S2. 1H anatomical coronal image of the rat kidney with DPXe gradient-echo image overlay. The magenta box outlines the shimmed region. Notice that most of the DPXe signal originates from a region within the kidney.
Figure S3. Comparison of PRESS 1H spectra and single-pulse acquisition DPXe spectra in a rat liver, with shimming conditions optimized either for water (top) or fat (bottom) signal. For the 1H PRESS spectra (right), narrow bandwidth refocusing pulses were used to suppress the fat (water) signal in the top (bottom) spectrum. Shimming conditions were found to strongly affect the shape of the DPXe spectrum. We believe that the splitting of the peak at 193 ppm found when shimming on the fat proton signal is most likely due to the presence of different lipid compartments in which xenon dissolves. Since this splitting is not observed in the 1H PRESS spectrum obtained from the shimmed voxel, one could speculate that, while one peak originates from xenon spins dissolved in the lipid compartments within the shimmed voxel, the other peak originates from xenon dissolved in the surrounding fat tissues.
Figure S4. Pressure-induced frequency shift measured in vitro for xenon dissolved in human CSF, rat WAT, and rat muscle. The pressure-induced shift of the DPXe CS seems to strongly depend on the tissue in which xenon dissolves. For example, the average shift seen in CSF was only about 0.05 ppm/atm, whereas in muscle it was about 0.27 ppm/atm.
Figure S5. Comparison of (a) DPXe spectrum obtained in vivo from rat muscle, to DPXe spectra obtained from excised muscle tissue from different rats euthanized with CO2 (b–d) and from a mouse euthanized with pentobarbital (e). The in vivo spectrum clearly shows 4 peaks: one from xenon dissolved in RBC, one from xenon dissolved in lipids (interestingly, in vivo, this peak is shifted slightly upfield, possibly because the temperature of the rat leg, situated closed to the heating hose, was slightly higher than the rectal rat temperature), and two from xenon dissolved in muscle. These two peaks are extensively broadened in the in vitro spectra and have a slightly higher CS, possibly due to a post-mortem change in tissue structure and/or chemistry. Also, given that the broad peak at 198.8 ppm is about 15 ppm upfield from the RBC, based on the previous literature report, we cannot exclude some contribution from xenon dissolved in plasma.
Acknowledgments
The authors would like to thank professor Cynthia Jameson of the University of Illinois at Chicago for helpful discussions on the methodology used for early spectroscopic measurements of DPXe chemical shifts. This work was supported by NIH grant fund R01 DK108231.
References
- 1.Sase S, Takahashi H, Shigefuku R, Ikeda H, Kobayashi M, Matsumoto N, Suzuki M. Measurement of blood flow and xenon solubility coefficient in the human liver by xenon-enhanced computed tomography. Med. Phys. [Internet] 2012;39:7553. doi: 10.1118/1.4767759. [DOI] [PubMed] [Google Scholar]
- 2.Isbister WH, Schofield PF, Torrance HB. Measurement of the Solubility of Xenon-133 in Blood and Human Brain. Phys. Med. Biol. [Internet] 1965;10:243–250. doi: 10.1088/0031-9155/10/2/308. [DOI] [PubMed] [Google Scholar]
- 3.Cherubini A, Bifone A. Hyperpolarised xenon in biology. Prog. Nucl. Magn. Reson. Spectrosc. 2003;42:1–30. doi: 10.1016/S0079-6565(02)00052-3. [DOI] [Google Scholar]
- 4.Miller KW, Reot NV, Uiterkampt AJMS, Stengle DP, Stengle TR, Williamson KL. Xenon NMR : Chemical shifts of a general anesthetic in common solvents, proteins, and membranes. Proc. Natl. Acad. Sci. 1981;78:4946–4949. doi: 10.1073/pnas.78.8.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodson BM. Nuclear magnetic resonance of laser-polarized noble gases in molecules, materials, and organisms. J. Magn. Reson. 2002;155:157–216. doi: 10.1006/jmre.2001.2341. [DOI] [PubMed] [Google Scholar]
- 6.Pietraiß T, Gaede HC. Optically Polarized 129Xe in NMR Spectroscopy. Adv. Mater. [Internet] 1995;7:826–838. doi: 10.1002/adma.19950071003. [DOI] [Google Scholar]
- 7.Happer W. Optical Pumping. Rev. Mod. Phys. 1972;44:169–249. doi: 10.1103/RevModPhys.44.169. [DOI] [Google Scholar]
- 8.Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev. Mod. Phys. 1997;69:629–642. doi: 10.1103/RevModPhys.69.629. [DOI] [Google Scholar]
- 9.Driehuys B, Cates GD, Miron E, Sauer K, Walter DK, Happer W. High-volume production of laser-polarized 129Xe. Appl. Phys. Lett. [Internet] 1996;69:1668. doi: 10.1063/1.117022. [DOI] [Google Scholar]
- 10.Fink A, Brunner E. Optimization of continuous flow pump cells used for the production of hyperpolarized 129Xe: A theoretical study. Appl. Phys. B [Internet] 2007;89:65–71. doi: 10.1007/s00340-007-2754-z. [DOI] [Google Scholar]
- 11.Hersman FW, Ruset IC, Ketel S, et al. Large Production System for Hyperpolarized 129Xe for Human Lung Imaging Studies. Acad. Radiol. 2008;15:683–692. doi: 10.1016/j.acra.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norquay G, Parnell SR, Xu X, Parra-Robles J, Wild JM. Optimized production of hyperpolarized 129Xe at 2 bars for in vivo lung magnetic resonance imaging. J. Appl. Phys. 2013;113 doi: 10.1063/1.4776763. [DOI] [Google Scholar]
- 13.Freeman MS, Emami K, Driehuys B. Characterizing and modeling the efficiency limits in large-scale production of hyperpolarized 129Xe. Phys. Rev. A [Internet] 2014;90:23406. doi: 10.1103/PhysRevA.90.023406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang YV, Quirk JD, Ruset IC, Atkinson JJ, Hersman FW, Woods JC. Quantification of human lung structure and physiology using hyperpolarized 129Xe. Magn. Reson. Med. 2014;71:339–344. doi: 10.1002/mrm.24992. [DOI] [PubMed] [Google Scholar]
- 15.Qing K, Mehrad B, Mugler JP, et al. Proceedings of the International Society of Magnetic Resonance in Medicine. Singapore: 2016. Assessing Functional Changes in Lungs with Idiopathic Pulmonary Fibrosis using Hyperpolarized Xenon-129 MRI; p. 1147. [Google Scholar]
- 16.Kaushik SS. Translational Imaging of Pulmonary Gas-Exchange Using Hyperpolarized 129Xe Magnetic Resonance Imaging. Duke University; 2014. [Google Scholar]
- 17.Robertson SH, Virgincar RS, Bier EA, He M, Schrank GM, Smigla RM, Rackley C, McAdams HP, Driehuys B. Uncovering a third dissolved-phase 129 Xe resonance in the human lung: Quantifying spectroscopic features in healthy subjects and patients with idiopathic pulmonary fibrosis. Magn. Reson. Med. [Internet] 2016:0. doi: 10.1002/mrm.26533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart NJ, Leung G, Norquay G, et al. Experimental validation of the hyperpolarized 129 Xe chemical shift saturation recovery technique in healthy volunteers and subjects with interstitial lung disease. Magn. Reson. Med. [Internet] 2015;74:196–207. doi: 10.1002/mrm.25400. [DOI] [PubMed] [Google Scholar]
- 19.Ruppert K, Altes TA, Mata JF, Ruset IC, Hersman FW, Mugler JP. Detecting pulmonary capillary blood pulsations using hyperpolarized xenon-129 chemical shift saturation recovery (CSSR) MR spectroscopy. Magn. Reson. Med. [Internet] 2016;75:1771–1780. doi: 10.1002/mrm.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Araki T, Okajima Y, Albert M, Hatabu H. Pulmonary hyperpolarized noble gas MRI: Recent advances and perspectives in clinical application. Eur. J. Radiol. [Internet] 2014;83:1282–1291. doi: 10.1016/j.ejrad.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Mugler JP, Altes TA, Ruset IC, Dregely IM, Mata JF, Miller GW, Ketel S, Ketel J, Hersman FW, Ruppert K. Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21707–21712. doi: 10.1073/pnas.1011912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzanti ML, Walvick RP, Zhou X, Sun Y, Shah N, Mansour J, Gereige J, Albert MS. Distribution of hyperpolarized xenon in the brain following sensory stimulation: Preliminary MRI findings. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Sun Y, Mazzanti M, Henninger N, Mansour J, Fisher M, Albert M. MRI of stroke using hyperpolarized 129Xe. NMR Biomed. [Internet] 2011;24:170–175. doi: 10.1002/nbm.1568. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Hane FT, Lawrence-Dewar JM, Hassan A, Granberg K, Pellizzari RM, Plata JA, Albert MS. Proceedings of the International Society of Magnetic Resonance in Medicine. Honolulu: 2017. Using Hyperpolarized 129Xe MRI to Detect Impaired Cerebral Perfusion in Human Brain with Alzheimer’s Disease; p. 487. [Google Scholar]
- 25.Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM. High resolution spectroscopy and chemical shift imaging of hyperpolarized 129Xe dissolved in the human brain in vivo at 1.5 tesla. Magn. Reson. Med. [Internet] 2016;75:2227–2234. doi: 10.1002/mrm.26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MS, Couch MJ, Albert MS. In: Chapter 22. Magnetic Resonance Imaging of the Brain using Hyperpolarized 129Xe. Reimer P, Parizel PM, Meaney JFM, Stichnoth FA, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. pp. 407–425. [DOI] [Google Scholar]
- 27.Branca RT, Zhang L, Burant A, Katz L, McCallister A. Proceedings of the International Society of Magnetic Resonance in Medicine. Singapore: 2016. Detection of human brown adipose tissue by MRI with hyperpolarized Xe-129 gas and validation by FDG-PET/MRI; p. 1054. [Google Scholar]
- 28.Burant A, Zhang L, McCallister A, Branca RT. In Vivo Temperature Imaging Using Lipid-dissolved Hyperpolarized Xenon-129; Proceedings of 56th Experimental Nuclear Magnetic Resonance Conference; 2015. p. 87. [Google Scholar]
- 29.Miller GW, Cates GD, Keder D, Altes TA, Mata JF, Qing K, Ruset I, Hersman FW, Mugler JP. Proceedings of the International Society of Magnetic Resonance in Medicine. Honolulu: 2017. Dynamic Spectroscopy of Dissolved-Phase Xenon-129 in the Human Kidney; p. 1182. [Google Scholar]
- 30.Norquay G, Stewart NJ, Wild JM. Proceedings of the International Society of Magnetic Resonance in Medicine. Honolulu: 2017. Evaluation of 129Xe-RBC signal dynamics and chemical shift in the cardiopulmonary circuit using hyperpolarized 129Xe NMR; p. 3327. [Google Scholar]
- 31.Swanson SD, Rosen MS, Coulter KP, Welsh RC, Chupp TE. Distribution and dynamics of laser-polarized 129Xe magnetization in vivo. Magn. Reson. Med. 1999;42:1137–1145. doi: 10.1002/(SICI)1522-2594(199912)42:6<1137::AID-MRM19>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Kershaw J, Nakamura K, Kondoh Y, Wakai A, Suzuki N, Kanno I. Confirming the existence of five peaks in 129Xe rat head spectra. Magn. Reson. Med. [Internet] 2007;57:791–797. doi: 10.1002/mrm.21186. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Kondoh Y, Wakai A, Kershaw J, Wright D, Kanno I. 129Xe spectra from the heads of rats with and without ligation of the external carotid and pterygopalatine arteries. Magn. Reson. Med. [Internet] 2005;53:528–534. doi: 10.1002/mrm.20399. [DOI] [PubMed] [Google Scholar]
- 34.Virgincar RS, Robertson SH, Nouls J, Degan S, Schrank GM, He M, Driehuys B. Establishing an accurate gas phase reference frequency to quantify 129Xe chemical shifts in vivo. Magn. Reson. Med. [Internet] 2017;77:1438–1445. doi: 10.1002/mrm.26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jameson CJ, Jameson AK, Cohen SM. Temperature and density dependence of 129Xe chemical shift in rare gas mixtures. J. Chem. Phys. [Internet] 1975;62:4224–4226. doi: 10.1063/1.430304. [DOI] [Google Scholar]
- 36.Jameson CJ, Jameson AK, Cohen SM. Temperature and density dependence of 129Xe chemical shift in xenon gas. J. Chem. Phys. [Internet] 1973;59:4540–4546. doi: 10.1063/1.1680652. [DOI] [Google Scholar]
- 37.Jameson CJ, Jameson AK. Density dependence of 129Xe N.M.R. chemical shifts in O2 and NO. Mol. Phys. [Internet] 1971;20:957–959. doi: 10.1080/00268977100100961. [DOI] [Google Scholar]
- 38.Jameson CJ, Jameson AK, Cohen SM. 129Xe contact shift in oxygen gas. Mol. Phys. [Internet] 1975;29:1919–1927. doi: 10.1080/00268977500101671. [DOI] [Google Scholar]
- 39.Marques JP, Bowtell R. Application of a fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2005;25:65–78. doi: 10.1002/cmr.b.20034. [DOI] [Google Scholar]
- 40.Bhagwandien R, Moerland MA, Bakker CJG, Beersma R, Lagendijk JJW. Numerical analysis of the magnetic field for arbitrary magnetic susceptibility distributions in 3D. Magn. Reson. Imaging [Internet] 1994;12:101–107. doi: 10.1016/0730-725X(94)92357-4. [DOI] [PubMed] [Google Scholar]
- 41.Truong T-K, Clymer BD, Chakeres DW, Schmalbrock P. Three-dimensional numerical simulations of susceptibility-induced magnetic field inhomogeneities in the human head. Magn. Reson. Imaging [Internet] 2002;20:759–770. doi: 10.1016/S0730-725X(02)00601-X. [DOI] [PubMed] [Google Scholar]
- 42.Collins CM, Yang B, Yang QX, Smith MB. Numerical calculations of the static magnetic field in three-dimensional multi-tissue models of the human head. Magn. Reson. Imaging. 2002;20:413–424. doi: 10.1016/S0730-725X(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 43.Dewal RP, Yang QX. Volume of interest-based fourier transform method for calculation of static magnetic field maps from susceptibility distributions. Magn. Reson. Med. [Internet] 2016;75:2473–2480. doi: 10.1002/mrm.25747. [DOI] [PubMed] [Google Scholar]
- 44.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging [Internet] 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazhdan M, Hoppe H. Screened poisson surface reconstruction. ACM Trans. Graph. [Internet] 2013;32:1–13. doi: 10.1145/2487228.2487237. [DOI] [Google Scholar]
- 46.Cignoni P, Callieri M, Corsini M, Dellepiane M, Ganovelli F, Ranzuglia G. MeshLab: an Open-Source Mesh Processing Tool; Sixth Eurographics Ital. Chapter Conf; 2008. pp. 129–136. [DOI] [Google Scholar]
- 47.Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. [Internet] 1996;23:815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Burant A, McCallister A, Zhao V, Koshlap KM, Degan S, Antonacci M, Branca RT. Accurate MR thermometry by hyperpolarized 129Xe. Magn. Reson. Med. [Internet] 2016;0:1–10. doi: 10.1002/mrm.26506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan I, Knappe-Grüuneberg S, Voigt J, et al. Direct measurement of the {γ} He {γ} Xe ratio at ultralow magnetic field; J. Phys. Conf. Ser; 2016. [DOI] [Google Scholar]
- 50.Nouls J, Fanarjian M, Hedlund L, Driehuys B. A constant-volume ventilator and gas recapture system for hyperpolarized gas MRI of mouse and rat lungs. Concepts Magn. Reson. Part B Magn. Reson. Eng. [Internet] 2011;39B:78–88. doi: 10.1002/cmr.b.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bashir A, Yablonskiy DA. Natural linewidth chemical shift imaging (NL-CSI) Magn. Reson. Med. [Internet] 2006;56:7–18. doi: 10.1002/mrm.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norquay G, Leung G, Stewart NJ, Tozer GM, Wolber J, Wild JM. Relaxation and exchange dynamics of hyperpolarized 129Xe in human blood. Magn. Reson. Med. 2015;74:303–311. doi: 10.1002/mrm.25417. [DOI] [PubMed] [Google Scholar]
- 53.Norquay G, Leung G, Stewart NJ, Wolber J, Wild JM. 129Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129Xe NMR. Magn. Reson. Med. [Internet] 2017;77:1399–1408. doi: 10.1002/mrm.26225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Mazzanti ML, Chen JJ, Tzeng Y-S, Mansour JK, Gereige JD, Venkatesh AK, Sun Y, Mulkern RV, Albert MS. Reinvestigating hyperpolarized 129Xe longitudinal relaxation time in the rat brain with noise considerations. NMR Biomed. [Internet] 2008;21:217–225. doi: 10.1002/nbm.1184. [DOI] [PubMed] [Google Scholar]
- 55.Branca RT, He T, Zhang L, Floyd CS, Freeman M, White C, Burant A. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proc. Natl. Acad. Sci. U.S. A. 2014;111:18001–18006. doi: 10.1073/pnas.1403697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duhamel G, Choquet P, Leviel J-L, et al. In vivo 129Xe NMR in rat brain during intra-arterial injection of hyperpolarized 129Xe dissolved in a lipid emulsion. Comptes Rendus l’Académie des Sci. - Ser. III - Sci. la Vie [Internet] 2000;323:529–536. doi: 10.1016/S0764-4469(00)00147-5. [DOI] [PubMed] [Google Scholar]
- 57.Duhamel G, Choquet P, Grillon E, Lamalle L, Leviel J-L, Ziegler A, Constantinesco A. Xenon-129 MR imaging and spectroscopy of rat brain using arterial delivery of hyperpolarized xenon in a lipid emulsion. Magn. Reson. Med. [Internet] 2001;46:208–212. doi: 10.1002/mrm.1180. [DOI] [PubMed] [Google Scholar]
- 58.Wakai A, Kershaw J, Nakamura K, Iida H, Tamura H, Kondoh Y, Kanno I. Magnetic Resonance Spectra of Hyperpolarized 129Xe in Human Blood and Living Rat Chest. Magn. Reson. Med. Sci. 2003;2:189–194. doi: 10.2463/mrms.2.189. [DOI] [PubMed] [Google Scholar]
- 59.Kilian W, Seifert F, Rinneberg H. Dynamic NMR spectroscopy of hyperpolarized 129Xe in human brain analyzed by an uptake model. Magn. Reson. Med. 2004;51:843–847. doi: 10.1002/mrm.10726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of chemical shift values of DPXe in rat white adipose tissue at 31°C as reported in Zhang, et al and as recalculated using the method presented in this work. The difference in ppm between the two methods is due to the difference in precision used for the gyromagnetic ratios: In Zhang, et al, the values used were γXe=11.777 MHz/T and γH=42.576 MHz/T for the 129Xe and 1H gyromagnetic ratios whereas here we used values of γXe=11.7767392 MHz/T and γH=42.5763866 MHz/T, respectively.
Table S2: Comparison of lipid peaks from in vivo and in vitro experiments. All lipid peaks were fit with a Voigt profile in MATLAB using an iterative least-squares algorithm. RMS error indicates the RMS difference, in percent, between the fitted model and data in the fitted spectral region. Some In vivo regions were composed of a main peak with a sizable shoulder on the upfield side of the peak, likely from contributions outside of the shimmed ROI. Most likely, small temperature gradients present across the anesthetized rat body also contributed to the small variation in the chemical shift of the lipid peak.
Figure S1. Regions of interest analyzed for the acquisition of rat (a) kidney, (b) liver, and (c,d) leg DPXe spectra. 1H spectra were acquired from the voxels outlined in magenta, after a manual localized shimming procedure, using a 1H PRESS sequence, right before the acquisition of 129Xe spectra. 129Xe spectra were acquired with a 90°-acquisition sequence with the use of a surface coil, whose position is indicated by the yellow line. The anatomical image for the liver (b) was acquired post-mortem since movement of the diaphragm significantly distorted the liver image.
Figure S2. 1H anatomical coronal image of the rat kidney with DPXe gradient-echo image overlay. The magenta box outlines the shimmed region. Notice that most of the DPXe signal originates from a region within the kidney.
Figure S3. Comparison of PRESS 1H spectra and single-pulse acquisition DPXe spectra in a rat liver, with shimming conditions optimized either for water (top) or fat (bottom) signal. For the 1H PRESS spectra (right), narrow bandwidth refocusing pulses were used to suppress the fat (water) signal in the top (bottom) spectrum. Shimming conditions were found to strongly affect the shape of the DPXe spectrum. We believe that the splitting of the peak at 193 ppm found when shimming on the fat proton signal is most likely due to the presence of different lipid compartments in which xenon dissolves. Since this splitting is not observed in the 1H PRESS spectrum obtained from the shimmed voxel, one could speculate that, while one peak originates from xenon spins dissolved in the lipid compartments within the shimmed voxel, the other peak originates from xenon dissolved in the surrounding fat tissues.
Figure S4. Pressure-induced frequency shift measured in vitro for xenon dissolved in human CSF, rat WAT, and rat muscle. The pressure-induced shift of the DPXe CS seems to strongly depend on the tissue in which xenon dissolves. For example, the average shift seen in CSF was only about 0.05 ppm/atm, whereas in muscle it was about 0.27 ppm/atm.
Figure S5. Comparison of (a) DPXe spectrum obtained in vivo from rat muscle, to DPXe spectra obtained from excised muscle tissue from different rats euthanized with CO2 (b–d) and from a mouse euthanized with pentobarbital (e). The in vivo spectrum clearly shows 4 peaks: one from xenon dissolved in RBC, one from xenon dissolved in lipids (interestingly, in vivo, this peak is shifted slightly upfield, possibly because the temperature of the rat leg, situated closed to the heating hose, was slightly higher than the rectal rat temperature), and two from xenon dissolved in muscle. These two peaks are extensively broadened in the in vitro spectra and have a slightly higher CS, possibly due to a post-mortem change in tissue structure and/or chemistry. Also, given that the broad peak at 198.8 ppm is about 15 ppm upfield from the RBC, based on the previous literature report, we cannot exclude some contribution from xenon dissolved in plasma.