Abstract
Background
HER2-targeted therapies are highly effective at preventing breast cancer recurrence but are associated with cardiotoxicity in some patients, with minimal data regarding racial disparities in incidence of this toxicity. We conducted a retrospective study to analyze the association of black or white race with treatment-induced cardiotoxicity, and incomplete therapy, in patients with HER2-positive early breast cancer.
Methods
Women with HER2-positive, stage I–III breast cancer who initiated (neo) adjuvant HER2-targeted therapy (trastuzumab +/− pertuzumab) from January 2005–March 2015 at our institution were eligible. We analyzed differences in cardiotoxicity (left ventricular ejection fraction [LVEF] decline to <50% AND absolute drop in LVEF of ≥10% from baseline) incidence and incomplete therapy (less than 52 weeks of HER2-targeted therapy) between black and white women via univariate and multivariable analysis.
Results
We identified 59 black and 157 white patients, with median follow-up 5.2 years. Median age was 53 and was similar for black and white patients. The 1-year cardiotoxicity incidence was 12% overall (95% confidence interval [CI]: 7–16%); 24% in black women (95% CI: 12–34%) and 7% in white women (95% CI: 3–11%). Black patients had significantly higher probability of incomplete therapy compared to white patients (Odds Ratio=4.61 [95% CI: 1.70–13.07, p-value=0.002]). We observed a high correlation between cardiotoxicity event and incomplete therapy (96% concordance).
Conclusion
Black patients have a higher rate of cardiotoxicity, and resultant incomplete adjuvant HER2-targeted therapy than white patients. This patient population may benefit from enhanced cardiac surveillance, cardio-protective strategies and early referral to cardiology where appropriate.
Keywords: breast cancer, HER2- targeted therapy, trastuzumab, pertuzumab, cardiotoxicity, race
Introduction
Human epidermal growth factor receptor-2 (HER2) is overexpressed or amplified in approximately 15–20% of invasive breast cancers.[1] Prior to the introduction of HER2-targeted therapies such as the monoclonal antibody trastuzumab (Herceptin®, Genentech, San Francisco, USA and Roche, Basel, Switzerland), HER2-positive breast cancer was associated with poor prognosis and shorter overall survival (OS) than other breast cancer subtypes.[2, 3] Large multicenter, randomized controlled studies evaluating the addition of trastuzumab to standard chemotherapy regimens have demonstrated approximately 35–60% improvement in disease-free survival (DFS) and 23–33% improvement in OS when compared to chemotherapy alone.[4–7] Studies evaluating the optimal duration of HER2-targeted therapy in this setting have supported use of one year of (neo) adjuvant trastuzumab.[8, 9] In recent years, additional HER2-targeted therapies have been developed, including the HER2-dimerization inhibitor pertuzumab (Perjeta®, Genentech, San Francisco, USA and Roche, Basel, Switzerland).[10]
Though generally well tolerated, HER2-targeted therapies are associated with cardiotoxicity in some patients. The majority of these patients experience asymptomatic decrease in left ventricular ejection fraction (LVEF) that resolves with discontinuation of the therapy.[11] The seven-year follow-up analysis of the NSABP B-31 trial demonstrated cardiotoxicity rates of 4.0% in the trastuzumab arm and 1.3% in the non-trastuzumab arm.[12] A SEER-Medicare database publication reported the rate of cardiotoxicity to be much higher at 32.1% (trastuzumab without anthracycline) and 41.9% (trastuzumab with anthracycline) in a study of 45,537 patients who were not participating in clinical trials, considering the definitions of cardiotoxicity in various studies differed.[13] Exposure to anthracycline therapy may be cardiotoxic and can also be a risk factor for development of HER2-targeted therapy-mediated cardiotoxicity, even though the mechanisms involved are distinct.[14] Anthracyclines cause type 1 cardiotoxicity associated with myocyte destruction through reactive oxygen species; while HER2-targeted therapies result in loss of contractility more similar to ‘stunning’ or ‘hibernation’ through inhibition of signaling pathways.[15]
In addition to prior anthracycline therapy, a number of clinical factors that predispose to this undesirable sequela have been identified, including older age, history of cardiac dysfunction, hypertension, and obesity.[16] However, the data on racial differences as a potential risk factor for development of cardiotoxicity are extremely scarce. Out of the larger prospective trials, only the HERA trial stratified data according to race, but the study enrolled just 20 black patients (<1%).[7] A large observational study reported a higher rate of ≥ grade 3 cardiac safety events in black patients (10.9%) versus white patients (7.9%) receiving trastuzumab for advanced breast cancer.[17] Two smaller retrospective studies have also shown that black patients have a higher risk of developing cardiotoxicity related to use of HER2-targeted therapies.[18, 19] We hypothesized that black patients with HER2-positive early breast cancer would have a higher probability of cardiotoxicity and an incomplete course of HER2-targeted therapy (incomplete therapy) than white patients. We designed a retrospective study to analyze the association of race with treatment-induced cardiotoxicity, and incomplete therapy, in patients with HER2-positive early breast cancer.
Material and Methods
Data collection
We conducted a retrospective chart review of electronic medical records, and paper records where appropriate, of individuals with stage I–III histologically confirmed HER2-positive invasive breast cancer, who received neoadjuvant or adjuvant trastuzumab with or without pertuzumab between January 2005 and March 2015 at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. Data were entered into an electronic research database and reviewed independently for completeness and accuracy prior to analysis. The project was reviewed and approved by the Johns Hopkins Institutional Review Board.
Data points comprised clinicopathologic features (i.e., patient age and race; tumor stage, grade, size, and hormone-receptor/HER2 status), select comorbidities, and antihypertensive medication use (Table 1). We also collected local and systemic treatments received including duration of HER2-targeted therapy in weeks, the development of disease recurrence, and survival. Cardiovascular risk factors collected from the medical record included age, gender, known hyperlipidemia or diabetes, antihypertensive medication use, smoking history.[20] HER2-positivity was defined as either 1) overexpression of the protein characterized as 3+ by immunohistochemistry (IHC) or 2) amplification of at least 2.0 by fluorescent in-situ hybridization (FISH).[21] Subjects were included if they were initiated on chemotherapy regimens with intent to administer at least one year of HER2-targeted therapy, HER2-targeted therapy was initiated, and if at least one year of follow-up from initiation of adjuvant therapy was available. The systemic chemotherapy regimens varied based on provider preference as well as patient tolerability, and included variants of FEC-H (F- 5 fluorouracil, E- epirubicin, C- cyclophosphamide, H- trastuzumab), AC-TH/AC-H (A-adriamycin, C- cyclophosphamide, T- paclitaxel, H- trastuzumab); and TCH/TCHP/TH/HP (T- Docetaxel, C- cyclophosphamide, H- trastuzumab, P- pertuzumab).
Table 1.
Patient Characteristics and Clinical Variables by Race
| Variables (n, %) | Entire Cohort (n=216) | White (n=157) | Black (n=59) | p-value* |
|---|---|---|---|---|
|
| ||||
| Patient age | 0.43 | |||
| Mean (sd) | 52 (12) | 53 (12) | 51 (11) | |
| Median(range) | 53 (26–82) | 53 (27–82) | 53 (26–76) | |
|
| ||||
| Stage | 0.31 | |||
|
| ||||
| 1,2 | 162 (75) | 122 (77.7) | 40 (67.8) | |
| 3 | 51 (23.6) | 33 (21.0) | 18 (30.5) | |
| missing | 3 | 2 | 1 | |
|
| ||||
| ER/PR | 0.08 | |||
|
| ||||
| Both Negative | 77 (35.6) | 50 (31.8) | 27 (45.8) | |
| Either Positive | 139 (64.4) | 107 (68.2) | 32 (54.2) | |
|
| ||||
| Duration of HER2 therapy (weeks) Median (range) | 52 (5,52) | 52 (12,52) | 52 (5,52) | < 0.001 |
|
| ||||
| Complete HER2 therapy | 0.002 | |||
|
| ||||
| No | 22 (10.2) | 9 (5.7) | 13 (22.0) | |
| Yes | 194 (89.8) | 148 (94.3) | 46 (78.0) | |
|
| ||||
| Radiation | 0.16 | |||
|
| ||||
| No | 119 (55.1) | 82 (52.2) | 37 (62.7) | |
| Yes | 96 (44.4) | 75 (47.8) | 21 (35.6) | |
| missing | 1 (0.5) | 0 | 1 | |
|
| ||||
| Anthracycline | 0.64 | |||
|
| ||||
| No | 95 (44.0) | 71 (45.2) | 24 (40.7) | |
| Yes | 121 (56.0) | 86 (54.8) | 35 (59.3) | |
|
| ||||
| Baseline LVEF | 0.85 | |||
|
| ||||
| <50 | 3 (1.4) | 3 (1.9) | 0 (0) | |
| 50–55 | 27 (12.5) | 20 (12.74) | 7 (11.9) | |
| >55 | 186 (86.1) | 134 (85.4) | 52 (88.1) | |
|
| ||||
| Risk factors CVD (n) | 0.02 | |||
|
| ||||
| 0 | 81 (37.5) | 67 (42.7) | 14 (23.7) | |
| >=1 | 135 (62.5) | 90 (57.3) | 45 (76.3) | |
|
| ||||
| Anti-hypertensive meds (n) | <0.001 | |||
|
| ||||
| 0 | 138 (63.9) | 117 (74.5) | 21 (35.6) | |
| >=1 | 78 (36.1) | 40 (25.5) | 38 (64.4) | |
|
| ||||
| Hypothyroidism | 0.09 | |||
|
| ||||
| No | 183 (84.7) | 129 (82.2) | 54 (91.5) | |
| Yes | 33 (15.3) | 28 (17.8) | 5 (8.5) | |
|
| ||||
| Smoking | 0.04 | |||
|
| ||||
| No | 154 (71.3) | 118 (75.2) | 36 (61.0) | |
| Yes | 62 (28.7) | 39 (24.8) | 23 (39.0) | |
|
| ||||
| Diabetes | < 0.001 | |||
|
| ||||
| No | 189 (87.5) | 149 (94.9) | 40 (67.8) | |
| Yes | 27 (12.5) | 8 (5.1) | 19 (32.2) | |
|
| ||||
| Hyperlipidemia | 0.04 | |||
|
| ||||
| No | 175 (81.0) | 133 (84.7) | 42 (71.2) | |
| Yes | 41 (19.0) | 24 (15.3) | 17 (28.8) | |
CVD, cardiovascular disease; ER, estrogen receptor; HER2, human epidermal growth factor receptor-2; LVEF, left ventricular ejection fraction; PR, progesterone receptor
P-values were based on Wilcoxon test for continuous variables and chi-square or fisher exact test for categorical variables without accounting missing data
Exclusion criteria included male patients, those not designated as of black or white race, and those with metastatic disease at diagnosis. In addition, patients who switched care to another health facility while on HER2-targeted therapy, discontinued therapy secondary to psychosocial reasons, or had two or fewer determinations of LVEF while on HER2-targeted therapy were also excluded.
Cardiac evaluation
LVEF was measured either by multiple gated acquisition scanning (MUGA) or by echocardiography. If a value was reported as a range of +/−5%, the mean value was used. If a baseline LVEF was not available, the LVEF following anthracycline-based therapy was used where applicable. Cardiotoxicity was defined as LVEF decline to <50% AND absolute drop in LVEF of ≥10% from baseline.[22]
Statistical Analysis
Demographics and clinicopathologic features were summarized by race using descriptive statistics. The clinical outcomes examined in this retrospective analysis included cumulative incidence of cardiotoxicity at one year, completion of one year of HER2-targeted therapy (incomplete therapy was defined as less than 52 weeks of HER2-targeted therapy), and descriptive statistics of recurrence-free survival (RFS) and OS. No other event was deemed to be a competing event of cardiotoxicity. Cumulative incidence of cardiotoxicity at one year was estimated as time-to-event outcome after the initial date of HER2-targeted therapy, where the event was the occurrence of cardiotoxicity, and patients without cardiotoxicity were censored at the last follow-up date or date of death. Relationship between incomplete therapy and cardiotoxicity was displayed via contingency table and concordance rate was defined as a proportion of identical results between incomplete therapy and cardiotoxicity in the study cohort. OS was calculated as the time from initiation of HER2-targeted therapy to death due to any cause, with patients who were alive censored at the time of last follow-up. RFS was calculated as the time from initiation of HER2-targeted therapy to the time of first documentation of breast cancer recurrence at any site (i.e., in-breast, axillary, chest wall or distant metastases) or deaths due to breast cancer, whichever occurred first.
Cumulative incidence of cardiotoxicity, probabilities of RFS and OS were estimated based on Kaplan-Meier method.[23, 24] Univariate and multivariable Cox regression analyses were performed to evaluate the prognostic impact of various clinicopathologic factors on cumulative incidence of cardiotoxicity. The factors adjusted in the multivariable analyses for race effect on cardiotoxicity were selected based on published reports and investigators’ clinical knowledge. In this study, no hypotheses of RFS and OS were tested due to the small number of events. All statistical tests were two-sided with significance level considered at P<0.05 and the analyses were carried out using the statistical software R (version 3.3.1).
Results
We identified 216 eligible patients (of whom 13 had received pertuzumab in addition to trastuzumab), including 59 (27.3%) black women and 157 (72.7%) white women. The median patient age was 53 (range, 26–82 years). At baseline, black women had a higher frequency of cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and smoking history, and were more likely to be prescribed a higher number of anti-hypertensive medications when compared to white women (Table 1). Black women presented with a higher stage of cancer at the time of diagnosis, were more likely to have hormone receptor-negative breast cancer, and were as likely to receive an anthracycline-based treatment regimen as white women.
Median time to cardiotoxicity event was 179 days (range 70–448) and only two patients experienced cardiotoxicity later than one-year after trastuzumab (at 377 and at 448 days, respectively). The 1-year probability of a cardiotoxicity event was 0.12 (95% confidence interval [CI] 0.07–0.16) overall, 0.24 (95% CI 0.12–0.34) in black women, and 0.07 (95% CI 0.03–0.11) in white women (Figure 1). In univariate analysis, black women had a higher risk of cardiotoxicity compared to white women (HR 3.22 [95% CI 1.51–6.84], P= 0.002, Supplementary Table 1). Multivariable Cox regression analysis also revealed that race was a significant predictor of cardiotoxicity (HR 2.73 [95% CI 1.24–6.01], P=0.012, Table 2) when controlling for patient age, stage, presence of cardiovascular risk factors and prior anthracycline use.
Figure 1.
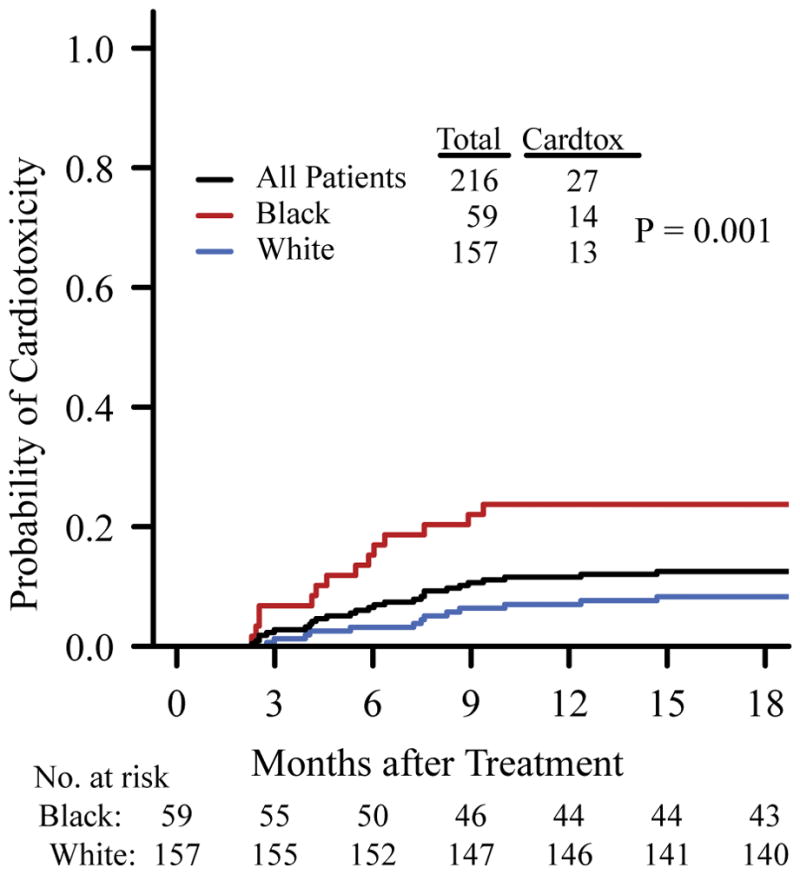
Cumulative Incidence of Cardiotoxicity by Race. Curves were truncated at 18 months after treatment. P-value was based on log rank test
Table 2.
Multivariable Analysis for Effect of Race on Risk of Cardiotoxicity
| Risk of Cardiotoxicity | ||
|---|---|---|
|
| ||
| Variables | HR (95% CI) | p-value |
|
| ||
| Patient race | ||
|
| ||
| White | 1 | |
| Black | 2.73 (1.24–6.01) | 0.012 |
|
| ||
| Patient age (continuous) | 0.96 (0.92–1.00) | 0.047 |
|
| ||
| Risk factors CVD | ||
|
| ||
| 0 | 1 | |
| >=1 | 1.41 (0.53–3.79) | 0.49 |
|
| ||
| Anthracycline | ||
|
| ||
| No | 1 | |
| Yes | 2.35 (0.93–5.91) | 0.07 |
|
| ||
| Stage | ||
|
| ||
| 1,2 | 1 | |
| 3 | 1.90 (0.83–4.34) | 0.13 |
CAD, cardiovascular disease; CI, confidence interval; HR, hazard ratio
The recommended course of HER2-directed therapy (one year) was not completed in 22 patients (10.2% entire cohort; 5.7% of white patients, 22% of black patients [Table 1]), due to cardiotoxicity in 20/22 cases. Six of these patients (2 white, 4 black) were placed on heart failure medications (e.g., ACEI, beta blockers) and were able to resume HER2-targeted therapy for a duration after an improvement in LVEF. The median duration of HER2-targeted therapy for these patients was 27.5 weeks (range: 5–49) with no statistical difference (p=0.31) between white and black patients (28 weeks [range 12–49] vs. 22 weeks [range 5–43], respectively). Cardiotoxicity events and incomplete therapy were thus highly correlated with 96% concordance rate (Table 3). For those patients who did not experience cardiotoxicity, 99% (187/189) completed all prescribed HER2-directed therapy. For those patients experiencing cardiotoxicity, only 26% (7/27) completed prescribed HER2-directed therapy. Reasons for the low rate of completion included lack of recovery of LVEF, physician referral practices to cardiology over time, and patient preference. Black patients had significantly higher probability of incomplete therapy compared to white patients (Odds Ratio=4.61 [95% CI: 1.70–13.07, p-value=0.002]).
Table 3.
Cardiotoxicity and Completion of HER2-targeted Therapy
| N (%) | Complete Therapy (n=194) | Incomplete therapy (n=22) |
|---|---|---|
| No Cardiotoxicity (n=189) | 187 (99) | 2 (1) |
|
| ||
| White (n=144) | 143 (99) | 1 (1) |
| Black (n=45) | 44 (98) | 1 (2) |
|
| ||
| Cardiotoxicity (n=27) | 7 (26) | 20 (74) |
|
| ||
| White (n=13) | 5 (38) | 8 (62) |
| Black (n=14) | 2 (14) | 12 (86) |
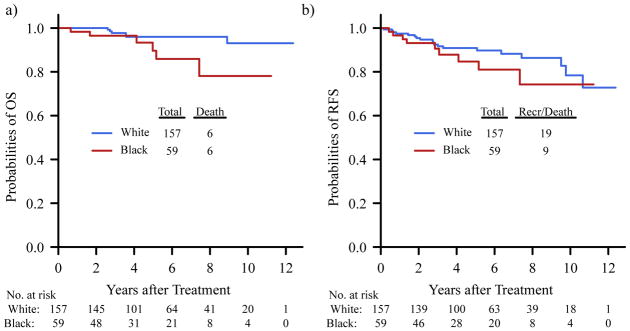
With median follow-up of 5.2 years (range 238 days–12.4 years) based on reverse Kaplan-Meier method, there were 12 deaths during the study period and 28 relapse or death events. The 5-year RFS and OS were relatively lower in black compared to white patients; 0.85 (95% CI: 0.74–0.96) vs. 0.91 (95% CI: 0.86–0.96) for RFS, 0.90 (95% CI: 0.80–1.00) vs. 0.96 (0.93–1.00) for OS, respectively (Figure 2).
Figure 2.
Kaplan-Meier Curves of Overall Survival (OS) and Recurrence-Free Survival (RFS) by Race
Discussion
(Neo) adjuvant HER2-targeted therapy has become the standard of care for women with HER2-positive breast cancer. The majority of patients tolerate the therapy well; however, a small proportion of patients experience potentially serious side effects such as cardiotoxicity which can lead to treatment delays and early discontinuation of therapy. We hypothesized that an incomplete course of HER2-targeted therapy secondary to drug toxicity might be more prevalent in black patients. Our study reports a cardiotoxicity rate at 1 year of 12.5% in the overall study population, which is consistent with the rates observed in the large prospective adjuvant trials (ranging from 5.7% to 18.0%).[4–7] However, as hypothesized, black women in our study were more likely to develop cardiotoxicity in the setting of HER2-targeted therapy compared to white women (24% versus 7% at 1 year). A high correlation was also observed between the development of cardiotoxicity and incomplete therapy. These results complement the findings of the observational registHER study (n=1,001) which reported a higher rate of ≥ grade 3 cardiac safety events in black patients (10.9%) versus white patients (7.9%) receiving trastuzumab for advanced breast cancer.[17] Our results, however, are novel as we investigated both the association of race with treatment-induced cardiotoxicity, and incomplete therapy, in an early breast cancer population.
The racial disparity we observed in rates of cardiotoxicity could relate to the fact that black women in this cohort had more cardiac comorbidities (diabetes, hypertension, hyperlipidemia and smoking history). Clinical risk factors for the development of cardiotoxicity from HER2-targeted therapy have been identified previously and indeed include older age, prior exposure to or concurrent anthracycline use, high body mass index (BMI), lower baseline LVEF and history of hypertension.[16, 25] Interestingly, coronary artery disease, diabetes and valvular dysfunction were not found to be associated with the development of cardiotoxicity in the large prospective trials of adjuvant trastuzumab. Diabetes has, however, been found to be a risk factor in elderly patients receiving trastuzumab in the adjuvant or metastatic setting.[26] Genetic variations may also contribute to variable rates of cardiotoxicity from HER2-targeted therapy. A recent study of 140 women with breast cancer receiving trastuzumab revealed that the HER2 Pro 1170 Ala polymorphism was associated with an increased risk of cardiotoxicity by 2.6 fold.[27] Other polymorphisms have also been identified such as Ile655Val.[28] These studies did not suggest that these polymorphisms were more prevalent in black patients. Additional research is necessary to ascertain the link between genetic polymorphisms and the development of cardiotoxicity associated with HER2-targeted therapy.
Whether black patients receiving chemotherapy and HER2-targeted therapy should undergo more intensive screening for cardiotoxicity is unclear. It is routine in clinical practice to monitor patients receiving these agents with serial LVEF evaluation but this is not necessarily supported by strong evidence.[29] The American Society of Clinical Oncology and American College of Cardiology are in the process of developing collaborative guidelines for patients receiving HER2- targeted therapy. Ideally these recommendations can be supported by evidence from well-designed clinical trials or large retrospective analyses, and consider closer surveillance for “at risk” populations.
As the field of cardio-oncology is expanding,[30] research is ongoing to develop both preventive and therapeutic strategies for patients who are at risk for, or develop cardiotoxicity. Trastuzumab-induced cardiotoxicity was found to be related to activation of the renin-angiotensin system (RAS) in a murine model, and inhibition of RAS with angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs) and the renin inhibitor aliskerin were found to potentially prevent its development.[31] The PRADA trial (n=120) showed improvement in rates of cardiotoxicity with single agent candesartan (ARB) but not with metoprolol (beta blocker) in patients receiving adjuvant anthracycline-containing therapy with or without trastuzumab for early breast cancer.[32] Patients selected for this study were at low risk for the development of cardiotoxicity, and the follow up period was short. The MANTICORE trial evaluated whether perindopril (ACEI) or bisoprolol (beta blocker) could prevent trastuzumab-related cardiotoxicity.[33] The primary outcome of trastuzumab-mediated left ventricular remodeling was not prevented by either agent. However, trastuzumab-associated declines in LVEF were slightly reduced by the agents, when compared to placebo. Further evaluation of promising cardio-protective agents in larger prospective trials is thus warranted before routine use. The ongoing SAFE-HEART trial is also investigating the safety of initiating or continuing HER2-targeted therapy in patients with asymptomatic mildly reduced LVEF (40–49%, NCT01904903).
An important question, which this study was not designed to answer, is whether the higher rates of treatment-induced cardiotoxicity and incomplete therapy observed in black patients is associated with inferior breast cancer outcomes. It has been known for a number of decades that black patients with breast cancer in the United States have a higher population-based breast cancer mortality than white patients.[34, 35] This is not fully understood but may relate to differences in socio-economic access to care, genetic and lifestyle factors as well as a propensity to develop an aggressive breast cancer subtype (triple-negative breast cancer). When adjusted for age only due to the small number of events in an exploratory analysis, our data indeed indicated that black patients had significantly worse OS compared to white patients (results not shown). It must be noted that black patients were more likely to have higher stage disease and hormone receptor-negative breast cancer. However, no significant associations were observed between the occurrence of cardiotoxicity and RFS/OS in our patient cohort, which may relate to the small sample size. Future studies with larger patient cohorts could help determine better the association between development of cardiotoxicity and breast cancer outcomes in black patients.[34]
Although this study, to our knowledge, is the largest to date investigating racial differences in cardiotoxicity and incomplete therapy in the setting of HER2-targeted therapy, it has some limitations. This was a retrospective study with small sample size, though larger than some recent reports.[18, 19] There were insufficient data on certain baseline patient characteristics such as BMI at the time of diagnosis, the number of years since diagnosis of hypertension and diabetes, and whether these medical conditions were well or poorly controlled. Data on symptomatic cardiac dysfunction was not collected. The number of events (death and relapse) was small and longer follow-up would be preferable to strengthen the associations reported. Another limitation is that transthoracic echocardiogram was utilized for most patients, which is less precise and more operator dependent than MUGA, although it is more readily available, less expensive and is not associated with radiation exposure.[36]
In summary, this study has demonstrated that the rate of cardiotoxicity is higher in black women and is highly concordant with receipt of an incomplete course of HER2-targeted therapy. Enhanced surveillance for cardiotoxicity and indeed preventive measures may be warranted in this patient population. We propose that black patients receiving HER2-targeted treatment be closely monitored for early signs and symptoms of cardiotoxicity. Early referral to cardiology and interventions with ACEI/ARB agents, beta blockers, and diuretics may provide improved cardiac function that will allow for re-initiation of HER2-targeted therapy, although this needs to be further studied in clinical trials. The use of concurrent chemotherapy regimens that avoid anthracyclines has also become more common in recent years and their use will likely minimize cardiotoxicity and maximize optimal therapy for patients with HER2-positive breast cancer.[4, 37] Moving forward, clinical trials should aim to include more minority patients and indeed have specific cohorts which enroll minorities, in order to expand our knowledge of safety and efficacy of breast cancer regimens in these cohorts. Finally, the evaluation of data from randomized controlled trials and larger patient cohorts is also required to confirm our study findings and evaluate predisposing factors for the development of cardiotoxicity including genetic predisposition, and may provide stronger rationale for a more intense surveillance approach in at risk populations.
Supplementary Material
Univariate Analysis for Risk of Cardiotoxicity
Acknowledgments
Funding sources: SKCCC Core Grant (P30-CA006973), AVON Center of Excellence (01-2008-012).
We thank Dr. Christopher Umbricht for database assistance.
Footnotes
Previous presentation: None
Conflict of Interest: SR served as a consultant for Genentech. VS has received research grants from Merck, Celgene Corporation, Abbvie, Pfizer, Novartis, Medimmune, and Puma Biotechnology. RC has received research grants from Novartis, Puma Biotechnology, Genentech, Merrimack, Clovis, Merck. LE has served as a consultant for Celgene, Vaccinex, Amgen, Astrazeneca, Syndax, Peregrine, Bayer, eTHeRNA, Molecuvax, and Gritstone, and has received research funding from Genentech/Roche, EMD Serono, Merck, Astrazeneca, Aduro Biotech, and Corvus.
Contribution of Authors: Conceptualization, VS, AL, RC, DA, JF, ACW and LE; Methodology, VS, RC, AL, BB, SR, HLT, and GR; Investigation, AL, BB, RS, RC, and SJ; Validation, BB, RC and SJ; Formal Analysis, HLT and GR; Writing – Original Draft, AL, BB, RC and VS; Writing – Review & Editing, VS, SR, DA, JF, HLT, GR, ACW, LE and RC; Funding Acquisition, VS; Resources, RC, VS, DA, JF, ACW, LE; Supervision, VS, SR, GR and RC.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2010;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE, Jr, Martino S, Mamounas EP, Kaufman PA, Wolmark N. Four-Year Follow-Up of Trastuzumab Plus Adjuvant Chemotherapy for Operable Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Joint Analysis of Data From NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 8.Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan CC, Connolly RM. Pertuzumab and its accelerated approval: evolving treatment paradigms and new challenges in the management of HER2-positive breast cancer. Oncology (Williston Park) 2014;28(3):186–194. 196. [PubMed] [Google Scholar]
- 11.Fiuza M. Cardiotoxicity associated with trastuzumab treatment of HER2+ breast cancer. Adv Ther. 2009;26(Suppl 1):S9–17. doi: 10.1007/s12325-009-0048-z. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE, Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31):3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 14.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23(13):2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 16.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugo HS, Brufsky AM, Ulcickas Yood M, Tripathy D, Kaufman PA, Mayer M, Yoo B, Abidoye OO, Yardley DA. Racial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;141(3):461–470. doi: 10.1007/s10549-013-2697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron KB, Brown JR, Heiss BL, Marshall J, Tait N, Tkaczuk KH, Gottlieb SS. Trastuzumab-induced cardiomyopathy: incidence and associated risk factors in an inner-city population. J Card Fail. 2014;20(8):555–559. doi: 10.1016/j.cardfail.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Snider J, Ahmed S, Paba C, et al. Cardiotoxicity of Trastuzumab Treatment in African American Women and Older Women in the Non Trial Setting. Cancer Res. 2009;69(24 Supplement):5087. doi: 10.1158/0008-5472.SABCS-09-5087. [DOI] [Google Scholar]
- 20.Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63(25 Pt A):2886. doi: 10.1016/j.jacc.2014.02.606. [DOI] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 22.Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25(25):3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 23.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10:51. doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, Sarti S, Cecconetto L, Pietri E, Ferrario C, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013;99(9):634–639. doi: 10.1136/heartjnl-2012-303151. [DOI] [PubMed] [Google Scholar]
- 26.Serrano C, Cortes J, De Mattos-Arruda L, Bellet M, Gomez P, Saura C, Perez J, Vidal M, Munoz-Couselo E, Carreras MJ, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol. 2012;23(4):897–902. doi: 10.1093/annonc/mdr348. [DOI] [PubMed] [Google Scholar]
- 27.Stanton SE, Ward MM, Christos P, Sanford R, Lam C, Cobham MV, Donovan D, Scheff RJ, Cigler T, Moore A, et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer. 2015;15:267. doi: 10.1186/s12885-015-1298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemieux J, Diorio C, Cote MA, Provencher L, Barabe F, Jacob S, St-Pierre C, Demers E, Tremblay-Lemay R, Nadeau-Larochelle C, et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013;33(6):2569–2576. [PubMed] [Google Scholar]
- 29.Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, Norton L, Hudis CA. Cardiac Surveillance Guidelines for Trastuzumab-Containing Therapy in Early-Stage Breast Cancer: Getting to the Heart of the Matter. J Clin Oncol. 2016;34(10):1030–1033. doi: 10.1200/JCO.2015.64.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantini L, Massimo Gulizia M, Di Lenarda A, Maurea N, Giuseppe Abrignani M, Bisceglia I, Bovelli D, De Gennaro L, Del Sindaco D, Macera F, et al. ANMCO/AIOM/AICO Consensus Document on clinical and management pathways of cardio-oncology: executive summary. Eur Heart J Suppl. 2017;19(Suppl D):D370–D379. doi: 10.1093/eurheartj/sux019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akolkar G, Bhullar N, Bews H, Shaikh B, Premecz S, Bordun KA, Cheung DY, Goyal V, Sharma AK, Garber P, et al. The role of renin angiotensin system antagonists in the prevention of doxorubicin and trastuzumab induced cardiotoxicity. Cardiovasc Ultrasound. 2015;13:18. doi: 10.1186/s12947-015-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland A, Storas TH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol. 2017;35(8):870–877. doi: 10.1200/JCO.2016.68.7830. [DOI] [PubMed] [Google Scholar]
- 34.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA surgery. 2017;152(5):485–493. doi: 10.1001/jamasurg.2017.0005. [DOI] [PubMed] [Google Scholar]
- 35.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol. 2015;33(20):2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker J, Bhullar N, Fallah-Rad N, Lytwyn M, Golian M, Fang T, Summers AR, Singal PK, Barac I, Kirkpatrick ID, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol. 2010;28(21):3429–3436. doi: 10.1200/JCO.2009.26.7294. [DOI] [PubMed] [Google Scholar]
- 37.Yu AF, Mukku RB, Verma S, Liu JE, Oeffinger KC, Steingart RM, Hudis CA, Dang CT. Cardiac safety of non-anthracycline trastuzumab-based therapy for HER2-positive breast cancer. Breast Cancer Res Treat. 2017 doi: 10.1007/s10549-017-4362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate Analysis for Risk of Cardiotoxicity