Abstract
Most bodily functions vary over the course of a 24 hour day. Circadian rhythms in body temperature, sleep-wake cycles, metabolism, and blood pressure (BP) are just a few examples. These circadian rhythms are controlled by the central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus and peripheral clocks located throughout the body. Light and food cues entrain these clocks to the time of day and this synchronicity contributes to the regulation of a variety of physiological processes with effects on overall health. The kidney, brain, nervous system, vasculature, and heart have been identified through the use of mouse models and clinical trials as peripheral clock regulators of BP. The dysregulation of this circadian pattern of BP, with or without hypertension, is associated with increased risk for cardiovascular disease. The mechanism of this dysregulation is unknown and is a growing area of research. In this review, we highlight research of human and mouse circadian models that has provided insight into the roles of these molecular clocks and their effects on physiological functions. Additional tissue-specific studies of the molecular clock mechanism are needed, as well as clinical studies including more diverse populations (different races, female patients, etc.), which will be critical to fully understand the mechanism of circadian regulation of BP. Understanding how these molecular clocks regulate the circadian rhythm of BP is critical in the treatment of circadian BP dysregulation and hypertension.
Keywords: blood pressure, hypertension, non-dipping, circadian rhythm, humans, mouse
Graphical abstract
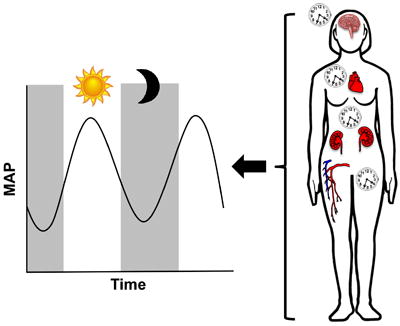
Introduction
The circadian clock evolved in order for organisms to better adapt to the 24 hr cycles of light/dark that occur on our planet. Circadian rhythms in physiological function have been identified in organisms ranging from cyanobacteria to humans. In higher eukaryotes and mammals, a central clock resides in the suprachiasmatic nucleus (SCN) of the brain and is directly entrained by light. Peripheral clocks are present in other areas of the brain and in most other cell types and tissues throughout the body. These clocks are synchronized in response to food and light cues and communicate via hormonal and neuronal signals. Desynchronization of the circadian system, such as what may occur in shift work or chronic jet lag, is associated with increased risk for a number of negative health outcomes. For an excellent review on this topic, please see Buijs et al. [1].
Mechanism of the Circadian Clock
The core molecular components of the circadian clock are a group of transcription factors that regulate gene expression [2]. These transcription factors function in a series of feedback loops that drive circadian gene expression for the core clock genes and an extensive number of target genes. In mammals, BMAL1 and CLOCK comprise the positive loop of the circadian mechanism in which they drive expression of the Period and Cryptochrome genes (encoding PER1-3, CRY1 and 2), and the Ror and Nr1d1/2 genes that encode the nuclear receptors ROR and REV-ERB (RORα-γ, REV-ERBα and β). In the negative feedback loops, PER and CRY antagonize BMAL1/CLOCK action. ROR and REV-ERB feedback on and mediate opposing action on BMAL1 gene expression. This transcriptional mechanism is regulated post-translationally through the action of key circadian kinases such as CK1δ/ε, and protein turnover is affected by FBXL family members leading to proteasome-mediated degradation. For an excellent and detailed review by recent Nobel Prize winner Dr. Michael Young on the molecular mechanisms of the clock, including new insights revealed by structural biology analysis of clock proteins, see [3].
Indeed, nearly 50% of all expressed genes throughout the entire body are subject to this mechanism of circadian regulation [4]. In a given tissue, 10-15% of genes are likely to be regulated by this molecular circadian clock, and the target genes of the clock vary in a tissue-specific manner. For example, the clock mechanism has been linked to regulation of ion transport genes in the kidney and metabolic pathway genes in the liver [5, 6]. We now know that the circadian clock components function in every cell type and tissue. This mechanism likely underlies the established daily variations in most physiological functions, including sleep/wake patterns, respiration, metabolism, body temperature, and blood pressure (BP). Circadian regulation of BP is an especially important topic. Although it is well-established that loss of the normal circadian rhythm of BP is associated with adverse cardiovascular outcomes, the mechanism of this effect is not understood. Moreover, evaluation of BP rhythms in humans is woefully underperformed. The purpose of this review is to consider the wealth of evidence supporting a role for the molecular clock in the regulation of BP. Here we review evidence from humans and rodent models that has lent considerable insight into the regulatory mechanisms and importance of circadian rhythms in BP control.
Blood Pressure Regulation
It has been known for several decades that BP exhibits a circadian rhythm in humans. BP dips at night during rest, undergoes a steep increase in the morning (known as the “morning surge”), and peaks typically in the late afternoon. This circadian rhythm of BP is present in the mouse and rat models that are commonly used to model human cardiovascular physiology.
BP is the product of cardiac output (CO) and total peripheral resistance (TPR): BP = CO x TPR. BP is the measure of the force that blood places on the walls of blood vessels. CO is determined by the difference between end diastolic volume and end systolic volume. Systolic BP (SBP) is the maximum arterial pressure when the heart beats, during the contraction of the left ventricle of the heart. Diastolic BP (DBP) is the minimum arterial pressure between heartbeats when the ventricles are relaxed and fill with blood. TPR is the sum of the resistance of all vessels in the circulation. BP is regulated by a number of different systems that contribute to CO and TPR, including the sympathetic nervous system, the central nervous system, the kidneys, the heart, the vasculature, and the immune system [7].
Circadian Blood Pressure Disorders in Humans
Both SBP and DBP have a circadian rhythm that repeats every 24 hrs in healthy humans. Healthy individuals experience a 10–20% decrease in BP at night. People who do not exhibit this “dip” of at least a 10% change in resting BP are termed “non-dippers.” Non-dipping hypertension is associated with activation of the renin-angiotensin-aldosterone system (RAAS) [8], increased risk of chronic kidney disease [9, 10] and adverse cardiovascular events [11-14]. The combination of salt-sensitivity and non-dipping hypertension is associated with significantly increased risk of cardiovascular mortality [15, 16]. African Americans have a higher incidence of salt-sensitive non-dipping hypertension, contributing to the established health disparities in this population [17, 18]. Non-dipping even in normotensive Black males is still associated with target organ damage [16]. Non-dipping hypertension leads to end organ damage and is associated with advanced diseases such as CKD and CVD [19].
Other circadian defects in 24 hr BP patterns include extreme-dipping (>20% nocturnal dip) and reverse-dipping (or riser) (>10% higher nighttime BP vs. daytime)[20]. One study demonstrated abnormalities in adrenergic, metabolic, and reflex function in reverse and extreme dippers [21]. Disruption of the BP circadian rhythm is associated with other morbidities as well. In a cross-sectional study of over 500 men and women, it was found that reverse-dipping was significantly associated with metabolic syndrome [22]. Interestingly, this association was found for men but not women. Nakano et al. found that reverse-dipping was predictive of end-stage renal failure in patients with diabetes mellitus [23].
Shift work that causes circadian disruption is associated with increased risk for a number of diseases in humans including cancer and cardiovascular disease [24-28] . In an effort to study the phenomenon of increased inflammation, increased hypertension, and increased CVD risk due to shift work, a recent randomized crossover study was carried out by Morris et al. [29]. Chronic shift workers were subjected to an acute circadian misalignment or a regular schedule as part of an in-clinic study. Ambulatory BP monitoring (ABPM) was utilized which allows an individual’s BP to be measured throughout the day without interfering with their day-to-day activities. The circadian misalignment caused a significant increase in SBP and DBP as well as C-reactive protein (CRP), an established marker of systemic inflammation. This same group conducted a longer term (8 days) study in healthy humans to test the effect of circadian misalignment on markers of CVD risk [30]. Human subjects on the circadian misalignment protocol exhibited increases in CRP as well as interleukin-6, resistin, and TNFα. Levels of interleukin-6, resistin, and TNFα have been shown to be involved in the progression of hypertension through inflammatory activation and endothelial dysfunction [31]. The increase of these inflammatory markers in the long-term study was accompanied by a decrease in wake time cardiac vagal modulation. Together these studies demonstrate a direct link between disruption of normal circadian rhythms and increases in markers of CVD risk.
Mechanisms of Circadian BP Regulation in Humans
It is well-established that the sympathetic nervous system contributes to the morning surge in BP [32, 33]. Early studies in humans aimed at studying 24 hours of BP patterns focused on postural effects and concluded that the central nervous system likely controlled the rhythmicity of BP and other cardiovascular parameters [34] which is corroborated by subsequent studies [35]. For example, in a small study with 9 human subjects with essential hypertension but normal dipping patterns, Sowers found that plasma norepinephrine was higher during wake time compared to sleep time [36]. Moreover, plasma NE also correlated with 24 hr MAP. Treatment of these patients with bromocriptine abolished the circadian variation in plasma NE and a dampening of the 24 hr BP rhythm, thus linking the sympathetic nervous system with 24 hr BP in humans. Liu et al. found that autonomic function was impaired in non-dippers and that non-dipping was a predictor of cardiovascular mortality [12].
Halberg and colleagues were pioneers in the field of chronobiological considerations for human health and linked circadian BP to cardiac function in both men and women [37]. A recent study in 51 non-dipper and 89 dipper humans, ABPM, and ambulatory impedance cardiography were used to assess both CO and TVR [38]. In these two groups, CO did not vary significantly between dippers and non-dippers whereas TVR decreased by two-fold greater in dippers vs. non-dippers. This study included 63 African-Americans and 77 white men and women. Non-dipping was more prevalent in African-Americans. For an excellent in-depth review in the area of the cardiac clock and its implications for human health, see Mistry et al. [39].
Clinical evidence also suggests a role for the vasculature in BP dipping. In a cross-sectional study of treated hypertensive patients and mostly untreated normotensive subjects, Hodgson et al. used forearm flow mediated dilation to examine the role of brachial artery vasodilator function in determining BP dipping [40]. Regardless of hypertension status, a decreased day/night BP difference was independently associated with reduced smooth muscle cell function.
There are several reports from the clinical literature linking Na transport in the kidney to aberrant circadian BP patterns, including the link between salt-sensitive hypertension and non-dipping [41-43]. For example, many patients suffering from aldosteronism exhibit the non-dipper pattern [44-46], and treatment with the angiotensin receptor blocker irbesartan corrected non-dipping in salt-sensitive hypertension patients [47]. Clinical studies revealed that the diuretic hydrochlorothiazide (Hctz) restored an appropriate decrease in nocturnal BP in non-dipping patients but had no effect in dippers [48]. It has also been shown that dietary Na restriction [49] and renal transplantation can restore the dipping pattern [50]. Consistent with this, unilateral nephrectomy led to non-dipping BP in a significant number of patients [51]. Inhibition of distal nephron Na transporters can restore the dipping pattern as well [52]. Moreover, in a study of over 300 African men and women representing more than 70 families, the presence of a nocturnal dip in BP was associated with higher daytime urinary sodium excretion compared to nighttime urinary sodium excretion [53]. These findings demonstrate a link between the timing of urinary sodium excretion and dipping BP in humans. Recent studies in humans and rodents suggest that the cardioprotective effects of SGLT2 inhibitors are possibly associated with normalization of the circadian rhythm of BP [54]. Taken together, these data from human studies support a role for the kidney and renal sodium handling in maintaining the normal circadian rhythm of BP.
Clinical and epidemiological studies in humans demonstrate the importance of the 24 hr BP rhythm and shed light on contributing mechanisms which likely include BP-regulatory systems such as the SNS, the CNS, kidney, the vasculature, and the heart. The rodent models discussed below provide molecular and genetic evidence linking the circadian clock genes to BP control and providing evidence for the tissue-specific roles played by the clock proteins.
Blood Pressure Phenotypes in Rodent Models
Convincing evidence from numerous animal studies clearly demonstrates an important role for the molecular clock in the regulation of blood pressure. Indeed, every clock gene mutant or knockout mouse that has been tested exhibited a blood pressure phenotype [55]. Below we review these mouse studies and consider the underlying mechanism of the BP phenotype. See Table 1 for a summary.
Table 1.
Summary of BP Phenotypes in Circadian Gene KO or Mutant Mice.
| Circadian Gene | BP Phenotype Relative to Control Mice* | Reference |
|---|---|---|
| Bmal1 KO | Lower with loss of dip | [56] |
| Clock KO | Lower | [60] |
| Clock D19 | Higher with decreased dip | [63] |
| Per2 mutant | DBP Lower | [65] |
| Cry1/Cry2 KO | Salt-sensitive hypertension | [67] |
| Per1 KO on 129/sv background | Lower | [68] |
| Per1 KO on C57BL/6 background | Non-dipping hypertension on high salt diet plus mineralocorticoid | [76] |
| Smooth muscle-specific Bmal1 KO | Lower with loss of dip | [79] |
| Renin-producing cell-specific Bmal1 KO | Lower | [80] |
These studies were conducted exclusively in male mice.
BMAL1
Curtis et al. generated global Bmal1 KO male C57Bl/6 mice, which exhibited significantly lower BP compared to control mice [56]. The circadian rhythm of BP was also affected. These mice displayed a “non-riser” phenotype in which BP was low and remained low throughout the 24 hr cycle, with a lack of increased BP during the active period. In subsequent studies from the Rudic group, vascular function was investigated as a putative mechanism contributing to this phenotype [57]. Anea et al. found that the BP phenotype of male global Bmal1 KO mice was associated with uncoupling of endothelial NO synthase and increased levels of superoxide [58]. These mice also develop dilated cardiomyopathy [59].
CLOCK
Zuber et al. characterized 24 hr BP in male WT and Clock KO mice on a C57BL/6 background [60]. Although the 24 hr rhythm of BP was not altered in the Clock KO, these mice exhibited significantly lower BP compared to WT mice with an ~10 mm Hg difference in MAP, SBP, and DBP, although the DBP difference did not reach statistical significance. Consistent with the lower BP, these mice exhibited differences in renal Na and K handling relative to control mice. Urine volume and urine osmolality comparisons also revealed the Clock KO mice had a mild diabetes insipidus. Subsequent work by this group linked the lower BP phenotype to increased urine Na excretion and decreased levels of 20-HETE, which regulates renal vascular tone [61], supporting a role for the kidney and renal Na handling in Clock-mediated BP control.
The Clock ∆19 mutation was induced by ENU mutagenesis and resulted in a loss of exon 19 and thus deletion of 51 amino acids in the C-terminus of the protein [62]. Clock mutant mice were crossed onto the Jcl/ICR background strain for analysis of cardiovascular rhythms. The Clock mutant male mice exhibited muted rhythms in BP and heart rate relative to control mice [63]. These mice also had lower plasma aldosterone levels relative to WT control mice. Importantly, the differences in BP and HR between Clock mutant and control mice were lost after adrenalectomy.
Taken together, these studies from either Clock KO or Clock mutant mice clearly demonstrate an important role for CLOCK in BP regulation. Interestingly, the Clock KO mice did not undergo an alteration of the BP rhythm whereas the Clock mutant mice had a decrease in amplitude and a shift in the acrophase of BP. Since the Clock mutant protein may act as a dominant negative, the differences between these two may be due to loss of the functional protein versus expression of a non-functional protein. The background strain differences between these studies also likely play a major role in the phenotype.
PER2
In Per2 mutant mice (Per2Brdm1), the Per2 protein lacks the domains that mediate protein-protein interactions [64]. Per2 mutant mice exhibit a mild cardiovascular phenotype of decreased DBP over 24 hr and a slight attenuation of the night/day difference, and elevated heart rate over 24 hr [65]. These BP studies were conducted only in male mice on a mixed 129/sv and C57Bl/6 background. Further evidence supporting a role for vascular function in circadian BP control comes from the study of these Per2 mutant mice. Male global Per2 mutant mice exhibit increased heart rate[65] and impaired endothelial relaxation as measured in aortic rings[66]. The aortic endothelial dysfunction observed in the Per2 mutant mice was associated with decreased NO and vasodilatory prostaglandin production and increased release of cyclooxygenase-1-derived vasoconstrictors [66].
CRY1/2
Doi et al. investigated BP in the double KO mouse lacking both Cry1 and Cry2. The male Cry1/2 KO mice exhibited salt-sensitive hypertension compared to WT control mice [67]. Using the mineralocorticoid receptor blocker eplerenone, these investigators showed that the salt-sensitive hypertension phenotype could be ameliorated. Indeed, the Cry1/2 KO mice had dramatically higher plasma aldosterone levels compared to control mice. Microarray analysis demonstrated that this effect was due to the overexpression of a specific enzyme in the zona glomerulosa cells of the adrenal gland, Hsd3b6. Although it was not clear from the data presented that the Cry1/2 KO mice exhibited a defect in circadian BP patterns, the results of this study clearly demonstrate a role for the adrenal gland clock components in overall BP regulation and particularly in the response to dietary salt.
PER1
Insight into the role of background genetics in the regulation of BP by the circadian clock has come from our lab. We first investigated the BP phenotype of global Per1 KO mice on a 129/sv background strain [68]. This strain of mice is hypertensive at baseline and has an increased sensitivity to dietary salt and renal injury compared to the salt-resistant C57Bl/6 strain of mice [69, 70]. On this background strain, KO of Per1 in male mice resulted in lower BP compared to WT control mice, suggesting that loss of Per1 was protective in the setting of hypertension and salt-sensitivity. In line with this, extensive cell culture experiments and whole animal studies in the 129/ sv mouse demonstrated that Per1 acted as a positive regulator of sodium reabsorption genes in the kidney [71-75]. In contrast, our study of male Per1 KO on the salt-resistant normotensive C57Bl/6 background revealed a distinct phenotype of non-dipping hypertension in response to a high salt diet and mineralocorticoid treatment [76].
It is tempting to speculate that this dramatic strain difference in Per1-dependent BP regulation is due to the presence of the extra renin gene in 129/sv mice and the salt-sensitive status of the mice. Consistent with this concept, heterozygous Per1 KO 129/sv mice exhibited lower plasma aldosterone and decreased renal Na reabsorption relative to WT control mice [77]. Indeed, male Per1 KO C57Bl/6 mice exhibit alterations in renal Na handling consistent with the non-dipping hypertension observed in these mice: Per1 KO mice exhibit a reduced night/day ratio of urine Na excretion relative to WT controls (Douma et al. 2017 in review). The differences in the RAAS between these strains of mice may be a contributing factor to the difference in Per1-dependent BP control, however, more detailed studies are needed to evaluate the relative contribution of RAAS to these distinct phenotypes.
These strain differences are not likely the result of differences in how the clock genes are expressed in 129/sv versus C57BL/6 mice. Indeed, comparing clock gene expression in WT 129/sv mice [78] to that observed in C57BL/6 mice [4] reveals that the patterns of mRNA expression are quite similar between the two strains and amongst different tissues. A more likely explanation involves genetic variation between mouse strains. A survey of the Jax lab genome database reveals extensive single nucleotide polymorphisms (SNPs) between C57Bl/6 and 129/sv mice (http://www.informatics.jax.org/strains_SNPs.shtml). These SNPs are found across the entire genome. Supporting the notion that overall genetic variation contributes to differences in BP regulation, the Jax lab phenome database shows stark differences in baseline BP among a variety of mouse strains (https://phenome.jax.org/measures/45302 last accessed 10/05/2017). Over three dozen different mouse strains show a range of phenotypes in SBP not unlike a bell-shaped curve in BP values that one would observe across a wide swath of the human population. Indeed, a clear strength of studying BP traits across different mouse strains is the relevance to human health given and the large degree of genetic variation found across distinct human populations.
CRE/LOX KO MODELS
The limitation of performing BP studies in global KO mice is that investigation of underlying mechanisms is typically focused on the investigators’ tissue of interest. A given study may find evidence for the contribution of that single tissue to the BP phenotype, but those results do not rule out the contribution of other BP-regulatory systems. Recent developments in generating cell-type and tissue-specific KO mouse models use CRE/LOX methods have begun to shed light on the magnitude of the contribution each particular BP-regulatory tissue has on circadian BP control.
In a convincing study, Xie et al. investigated the role of smooth muscle Bmal1 in BP regulation [79]. KO of Bmal1 specifically in smooth muscle resulted in decreased BP and reduced amplitude of the circadian rhythm of BP. The magnitude of change was greater for DBP than SBP in smooth muscle Bmal1 KO mice. Importantly, these effects occurred in the absence of any change in circadian heart rate or locomotor activity, suggesting that the effect was specific to BP and the peripheral clock rather than the SCN. Comparison of these results to that of the global Bmal1 KO lends insight into the magnitude of contribution by smooth muscle to BP regulation. The difference in the MAP between global Bmal1 KO and control mice was ~10 mm Hg whereas the difference between smooth muscle Bmal1 KO and control mice was ~5 mm Hg. These studies were performed nearly a decade apart by different labs at various institutions, so this difference constitutes, at best, a rough approximation. Still, it is tempting to speculate that the vascular component of Bmal1’s contribution to overall BP regulation may represent nearly 50% of the dramatic phenotype observed in global Bmal1 KO mice.
Firsov and colleagues were the first to generate a kidney-specific Bmal1 KO. Tokonami et al. crossed floxed Bmal1 mice with Ren1d Cre mice to KO Bmal1 in renin-producing cells including cells of the JGA, TAL, and CD [80]. Relative to Cre negative floxed control mice, the cell-type specific Bmal1 KO mice exhibited lower plasma aldosterone levels and decreased BP. Lower BP was observed both in SBP and DBP. As discussed above for the work of Xie et al., comparison of the tissue-specific KO with the global KO can shed light on the relative contribution of a given tissue to the overall phenotype. Global Bmal1 KO have decreased BP (~10 mm Hg) and loss of the active-phase increase in BP. KO of Bmal1 specifically in renin-producing cells of the kidney led to a ~5 mm Hg reduction in BP, but the circadian rhythm was preserved. Keeping the smooth muscle Bmal1 KO phenotype in mind (decrease in BP and loss of rhythm), it may be the case that Bmal1 in the kidney contributes to BP control without affecting the rhythm whereas Bmal1 in smooth muscle also affects BP rhythm.
Together these studies in rat and mouse models all provide evidence linking the molecular circadian clock to the regulation of BP and circadian rhythms in BP. Limitations of these studies include the use of male mice only and in many cases, the lack of interventional studies to investigate the mechanisms by which clock proteins contribute to BP control.
Chronotherapy and Clinical Implications
Hypertension is the primary risk factor for cardiovascular disease (CVD), the leading cause of death of Americans. More than one-third of adults in the U.S., approximately 80 million people, are hypertensive and the American Heart Association predicts that this will increase to more than 40% of the population by 2030[81]. Despite the availability of several classes of anti-hypertensive agents, more than half of high-risk patients do not have adequate BP control [82]. Undoubtedly, this contributes to the correlation between hypertension and decreased life expectancy. Although it is widely known that non-dipping hypertension leads to increased cardiovascular mortality, the use of ambulatory blood pressure monitoring is underutilized. Missed opportunities associated with undiagnosed non-dipping hypertension likely contribute to the lack of appropriate BP control and the epidemic of hypertension and CVD.
Further evidence for the importance of the circadian clock in the regulation of BP and circadian rhythms in BP comes from human molecular studies. In a compelling study spanning mouse, rat, and human genetics, Woon et al. showed a strong association between BMAL1 and susceptibility to both type 2 diabetes and hypertension [83]. In the human component of the study, two BMAL1 single nucleotide polymorphisms (SNPs) were associated with diabetes and hypertension using over 10000 individuals from over 400 families. Marques et al. performed unbiased transcriptomic studies using kidney tissue from normotensive compared to hypertensive humans. PER1 was significantly up-regulated in the kidneys of hypertensive humans versus normotensives [84]. This finding is consistent with our report that loss of Per1 resulted in decreased BP in 129/sv mice [68].
Recently, Dashti et al. examined clock gene SNPs in two distinct cohorts of Caucasians (GOLDN [85]) and Puerto Ricans (BPRHS [86]) [87]. The analysis was done using office BP measurements, so the association study provided evidence linking clock gene SNPs to SBP only at a single time point. A number of SNPs in the core clock genes PER1, CRY1, CLOCK, and PER3 were significant in the GOLDN cohort (n=819, p≤0.01). Ancillary clock genes DBP, DEC2, PPARG, and RORA were significant in the GOLDN cohort as well (n=819, p≤0.01). This interesting study provides the first evidence linking the clock to BP control at a genetic level. Further studies using ABPM in larger populations that are more ethnically diverse are certainly needed to investigate this link.
Chronotherapy offers one putative treatment for defects associated with disrupted circadian rhythms in physiological function. As early as the 1970s, it was recognized by Bartter, Halberg, and colleagues that the timing of medication delivery could be optimized for the treatment of hypertension[88-90]. Recent work in this area has been spearheaded by a former trainee of Dr. Halberg, Dr. Ramon Hermida [91, 92]. Hermida and colleagues have published dozens of papers in the area of ABPM, chronotherapy, and a variety of pathological human conditions including CKD and diabetes. The work of this group demonstrates that non-dipping hypertensives appear to receive cardiovascular benefit from nighttime dosing of anti-hypertensive medication. The specific class of antihypertensive medications administered at night in these studies is not always clear. Future studies that carefully control the class of medication given in the morning versus the night are needed. Nevertheless, this work highlights the necessity for performing ABPM in at-risk patients [93]. Importantly, in line with the results of Hermida and colleagues, groups in Japan [94], Italy [95], China [96], and Nigeria [97], have demonstrated a beneficial effect of nighttime administration of antihypertensive medications on 24 hr BP, nocturnal BP, or both. It should be noted that some studies have not noted a benefit of nighttime dosing compared to morning administration [98, 99]. Differences among these reports include study size, class of anti-hypertensive drug used, and ethnic differences. Larger trials across more diverse populations are needed to further determine who will benefit from chronotherapy in hypertension. Currently, several clinical trials are in progress [100, 101](also ClinicalTrials.gov identifiers: NCT02922023).
Free Radical Biology and Circadian BP Regulation
Little information is available regarding the connection between free radical biology and regulation of BP by the molecular circadian clock. There is ample evidence however demonstrating that redox signaling is closely linked with the circadian clock mechanism. Indeed, Rodrigo and Herbert have written a compelling review article in this issue focusing on vascular function and BP in relation to circadian variations in redox signaling [102]. One area that is likely to provide clues to how redox signaling and the clock may interact to regulated BP rhythms is related to melatonin. Melatonin is a circadian hormone secreted from the pineal gland in the dark and its antioxidant properties are well-established [103]. Scheer and colleagues have demonstrated beneficial effects of melatonin on BP in hypertensive humans [104]. Moreover, this group has also demonstrated that induced circadian misalignment in humans increases levels of C-reactive protein (CRP)[29], a marker of inflammation that positively correlates with cardiovascular risk and redox state. These connections among melatonin, BP, and redox state provide tantalizing evidence that free radical biology contributes to circadian regulation of BP.
In conclusion, clinical and epidemiological studies clearly show the importance of the BP circadian rhythm. Loss of this rhythm is associated with an increased risk of stroke and other adverse cardiovascular outcomes. Correction of this rhythm is associated in most cases with improved outcomes. Extensive work from rodent models demonstrates that the molecular clock directly affects BP regulation. These pre-clinical studies provide insight into the contribution of a number of BP-regulating systems to circadian BP control. Together, this broad array of studies from humans and rodents supports the concept that the molecular clock and its targets located within the kidney, vasculature, heart, brain, and nervous system all contribute to BP regulation (Figure 1). Additional studies are needed to study the function of the tissue-specific clocks in different pathological conditions related to hypertension. Female animal models have been underutilized in this field, and this is an important future direction for the field. Better understanding of clock function and the signaling amongst the various peripheral clocks will increase our understanding and provide new therapeutic targets in the treatment of hypertension.
Figure 1. Molecular circadian clocks throughout the body contribute to the circadian rhythm of blood pressure.
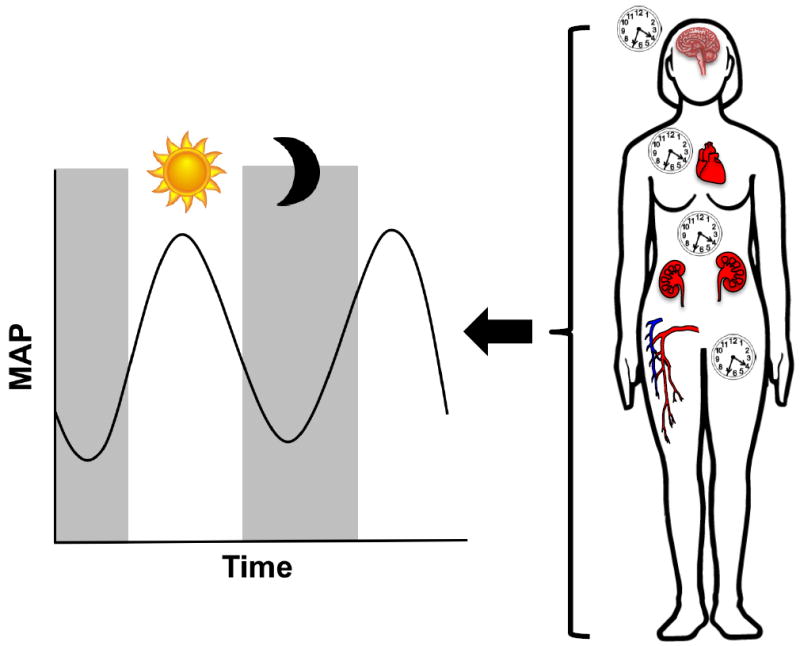
Studies with rodents and humans suggest that clock components within the kidneys, brain, nervous system, heart, and vasculature all contribute to the regulation of the BP circadian cycle. The molecular circadian clock within these tissues regulates the physiological functions that contribute to overall BP regulation. BP has a 24-hour cycle, with a peak in BP during the day and a 10-20% dip in BP at night. Dysregulation of the BP circadian rhythm can have profound effects, including a greater risk of stroke and cardiovascular disease.
Highlights.
Maintaining normal circadian rhythms in BP is critical for cardiovascular survival
Circadian clock proteins are master regulators of gene expression in most tissues
Clock proteins function in every tissue that contributes to BP regulation
Clock-mediated regulation of target genes underlies circadian rhythms in BP
Acknowledgments
This work was supported by NIH DK109570, AHA Grant-in-aid, and NIH AG052861 to MLG and T32DK104721 to LGD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buijs FN, Leon-Mercado L, Guzman-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, Buijs RM. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology (Bethesda) 2016;31:170–181. doi: 10.1152/physiol.00037.2015. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nature reviews Genetics. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crane BR, Young MW. Interactive features of proteins composing eukaryotic circadian clocks. Annu Rev Biochem. 2014;83:191–219. doi: 10.1146/annurev-biochem-060713-035644. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solocinski K, Gumz ML. The Circadian Clock in the Regulation of Renal Rhythms. Journal of biological rhythms. 2015;30:470–486. doi: 10.1177/0748730415610879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atger F, Mauvoisin D, Weger B, Gobet C, Gachon F. Regulation of Mammalian Physiology by Interconnected Circadian and Feeding Rhythms. Frontiers in endocrinology. 2017;8:42. doi: 10.3389/fendo.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nature medicine. 2011;17:1402–1409. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 8.Pati P, Fulton DJ, Bagi Z, Chen F, Wang Y, Kitchens J, Cassis LA, Stepp DW, Rudic RD. Low-Salt Diet and Circadian Dysfunction Synergize to Induce Angiotensin II-Dependent Hypertension in Mice. Hypertension. 2016;67:661–668. doi: 10.1161/HYPERTENSIONAHA.115.06194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiology international. 2010;27:1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 10.Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clinical and experimental hypertension. 2004;26:177–189. doi: 10.1081/ceh-120028556. [DOI] [PubMed] [Google Scholar]
- 11.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. Journal of hypertension. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, Watanabe M, Toriyama T, Kawahara H, Matsuo S. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:563–569. doi: 10.1093/ndt/18.3.563. [DOI] [PubMed] [Google Scholar]
- 13.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645–653. doi: 10.1038/jhh.2009.9. [DOI] [PubMed] [Google Scholar]
- 14.Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens. 2008;21:92–97. doi: 10.1038/ajh.2007.7. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertension research : official journal of the Japanese Society of Hypertension. 2017;40:413–422. doi: 10.1038/hr.2016.166. [DOI] [PubMed] [Google Scholar]
- 16.Mezue K, Isiguzo G, Madu C, Nwuruku G, Rangaswami J, Baugh D, Madu E. Nocturnal Non-dipping Blood Pressure Profile in Black Normotensives Is Associated with Cardiac Target Organ Damage. Ethn Dis. 2016;26:279–284. doi: 10.18865/ed.26.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA. International Society on Hypertension in, B. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 18.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28:640–648. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27:680–687. doi: 10.1093/ajh/hpt175. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara T, Tomitani N, Sato K, Okura A, Suzuki N, Kario K. The relationship between a blunted morning surge and a reversed nocturnal blood pressure dipping or “riser” pattern. Journal of clinical hypertension. 2017 doi: 10.1111/jch.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008;52:925–931. doi: 10.1161/HYPERTENSIONAHA.108.116368. [DOI] [PubMed] [Google Scholar]
- 22.Yan B, Yan H, Sun L, Yan X, Peng L, Wang Y, Wang G. Novel Association Between the Reverse-Dipper Pattern of Ambulatory Blood Pressure Monitoring and Metabolic Syndrome in Men But Not in Women. Medicine (Baltimore) 2015;94:e2115. doi: 10.1097/MD.0000000000002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano S, Ogihara M, Tamura C, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm independently predicts endstage renal failure in non-insulin-dependent diabetes mellitus subjects. J Diabetes Complications. 1999;13:224–231. doi: 10.1016/s1056-8727(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 24.Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neuroscience and biobehavioral reviews. 2014;40:80–101. doi: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Baron KG, Reid KJ. Circadian misalignment and health. International review of psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reutrakul S, Knutson KL. Consequences of Circadian Disruption on Cardiometabolic Health. Sleep Med Clin. 2015;10:455–468. doi: 10.1016/j.jsmc.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pimenta AM, Kac G, Souza RR, Ferreira LM, Silqueira SM. Night-shift work and cardiovascular risk among employees of a public university. Revista da Associacao Medica Brasileira. 2012;58:168–177. [PubMed] [Google Scholar]
- 28.Ohlander J, Keskin MC, Stork J, Radon K. 0170 Shift work and hypertension: prevalence and analysis of disease pathways in German car manufacturing workers. Occupational and environmental medicine. 2014;71(Suppl 1):A81. doi: 10.1002/ajim.22437. [DOI] [PubMed] [Google Scholar]
- 29.Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. Journal of biological rhythms. 2017;32:154–164. doi: 10.1177/0748730417697537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113:E1402–1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang C, Lei J, Zhou SX, Zhang YL, Yuan GY, Wang JF. Association of higher resistin levels with inflammatory activation and endothelial dysfunction in patients with essential hypertension. Chin Med J (Engl) 2013;126:646–649. [PubMed] [Google Scholar]
- 32.Sayk F, Becker C, Teckentrup C, Fehm HL, Struck J, Wellhoener JP, Dodt C. To dip or not to dip: on the physiology of blood pressure decrease during nocturnal sleep in healthy humans. Hypertension. 2007;49:1070–1076. doi: 10.1161/HYPERTENSIONAHA.106.084343. [DOI] [PubMed] [Google Scholar]
- 33.Lambert EA, Chatzivlastou K, Schlaich M, Lambert G, Head GA. Morning surge in blood pressure is associated with reactivity of the sympathetic nervous system. Am J Hypertens. 2014;27:783–792. doi: 10.1093/ajh/hpt273. [DOI] [PubMed] [Google Scholar]
- 34.Sindrup JH, Kastrup J, Christensen H, Jorgensen B. Nocturnal variations in peripheral blood flow, systemic blood pressure, and heart rate in humans. Am J Physiol. 1991;261:H982–988. doi: 10.1152/ajpheart.1991.261.4.H982. [DOI] [PubMed] [Google Scholar]
- 35.Chen CW, Kuo TB, Chen CY, Yang CC. Reduced capacity of autonomic and baroreflex control associated with sleep pattern in spontaneously hypertensive rats with a nondipping profile. Journal of hypertension. 2017;35:558–570. doi: 10.1097/HJH.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 36.Sowers JR. Dopaminergic control of circadian norepinephrine levels in patients with essential hypertension. J Clin Endocrinol Metab. 1981;53:1133–1137. doi: 10.1210/jcem-53-6-1133. [DOI] [PubMed] [Google Scholar]
- 37.Zachariah PK, Cornelissen G, Halberg F. Ambulatory cardiovascular monitoring of healthy adults in Rochester, Minnesota: chronobiologic assessment. Prog Clin Biol Res. 1990;341A:243–254. [PubMed] [Google Scholar]
- 38.Sherwood A, Hill LK, Blumenthal JA, Hinderliter AL. Circadian hemodynamics in men and women with high blood pressure: dipper vs. nondipper and racial differences. Journal of hypertension. 2017 doi: 10.1097/HJH.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mistry P, Duong A, Kirshenbaum L, Martino TA. Cardiac Clocks and Preclinical Translation. Heart Fail Clin. 2017;13:657–672. doi: 10.1016/j.hfc.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Hodgson JM, Woodman RJ, Croft KD, Ward NC, Bondonno CP, Puddey IB, Lukoshkova EV, Head GA. Relationships of vascular function with measures of ambulatory blood pressure variation. Atherosclerosis. 2014;233:48–54. doi: 10.1016/j.atherosclerosis.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Uzu T, Kazembe FS, Ishikawa K, Nakamura S, Inenaga T, Kimura G. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension. 1996;28:139–142. doi: 10.1161/01.hyp.28.1.139. [DOI] [PubMed] [Google Scholar]
- 42.Kimura G, Dohi Y, Fukuda M. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertension research : official journal of the Japanese Society of Hypertension. 2010;33:515–520. doi: 10.1038/hr.2010.47. [DOI] [PubMed] [Google Scholar]
- 43.Sinha AD, Agarwal R. The complex relationship between CKD and ambulatory blood pressure patterns. Advances in chronic kidney disease. 2015;22:102–107. doi: 10.1053/j.ackd.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzu T, Nishimura M, Fujii T, Takeji M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Changes in the circadian rhythm of blood pressure in primary aldosteronism in response to dietary sodium restriction and adrenalectomy. Journal of hypertension. 1998;16:1745–1748. doi: 10.1097/00004872-199816120-00006. [DOI] [PubMed] [Google Scholar]
- 45.Takakuwa H, Shimizu K, Izumiya Y, Kato T, Nakaya I, Yokoyama H, Kobayashi K, Ise T. Dietary sodium restriction restores nocturnal reduction of blood pressure in patients with primary aldosteronism. Hypertension research : official journal of the Japanese Society of Hypertension. 2002;25:737–742. doi: 10.1291/hypres.25.737. [DOI] [PubMed] [Google Scholar]
- 46.Williams D, Croal B, Furnace J, Ross S, Witte K, Webster M, Critchen W, Webster J. The prevalence of a raised aldosterone-renin ratio (ARR) among new referrals to a hypertension clinic. Blood pressure. 2006;15:164–168. doi: 10.1080/08037050600772615. [DOI] [PubMed] [Google Scholar]
- 47.Polonia J, Diogo D, Caupers P, Damasceno A. Influence of two doses of irbesartan on non-dipper circadian blood pressure rhythm in salt-sensitive black hypertensives under high salt diet. Journal of cardiovascular pharmacology. 2003;42:98–104. doi: 10.1097/00005344-200307000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 49.Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Determinants of circadian blood pressure rhythm in essential hypertension. Am J Hypertens. 1999;12:35–39. doi: 10.1016/s0895-7061(98)00182-4. [DOI] [PubMed] [Google Scholar]
- 50.Gatzka CD, Schobel HP, Klingbeil AU, Neumayer HH, Schmieder RE. Normalization of circadian blood pressure profiles after renal transplantation. Transplantation. 1995;59:1270–1274. [PubMed] [Google Scholar]
- 51.Goto N, Uchida K, Morozumi K, Ueki T, Matsuoka S, Katayama A, Haba T, Tominaga Y, Fukuda M, Nakao A, Kimura G. Circadian blood pressure rhythm is disturbed by nephrectomy. Hypertension research : official journal of the Japanese Society of Hypertension. 2005;28:301–306. doi: 10.1291/hypres.28.301. [DOI] [PubMed] [Google Scholar]
- 52.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Current hypertension reports. 2011;13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 54.Rahman A, Hitomi H, Nishiyama A. Cardioprotective effects of SGLT2 inhibitors are possibly associated with normalization of the circadian rhythm of blood pressure. Hypertension research : official journal of the Japanese Society of Hypertension. 2017;40:535–540. doi: 10.1038/hr.2016.193. [DOI] [PubMed] [Google Scholar]
- 55.Richards J, Diaz AN, Gumz ML. Clock genes in hypertension: novel insights from rodent models. Blood Press Monit. 2014;19:249–254. doi: 10.1097/MBP.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, Yao L, Ali MI, Merloiu AM, Stepp DW, Black SM, Fulton DJ, Rudic RD. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res. 2012;111:1157–1165. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefta M, Campbell KS, Feng HZ, Jin JP, Esser KA. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart Circ Physiol. 2012;303:H475–485. doi: 10.1152/ajpheart.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23:1019–1026. doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sei H, Oishi K, Chikahisa S, Kitaoka K, Takeda E, Ishida N. Diurnal amplitudes of arterial pressure and heart rate are dampened in Clock mutant mice and adrenalectomized mice. Endocrinology. 2008;149:3576–3580. doi: 10.1210/en.2007-1714. [DOI] [PubMed] [Google Scholar]
- 64.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 65.Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani JP. Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 2010;298:R627–634. doi: 10.1152/ajpregu.00404.2009. [DOI] [PubMed] [Google Scholar]
- 66.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 67.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nature medicine. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 68.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59:1151–1156. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:1999–2004. doi: 10.1093/ndt/gfg299. [DOI] [PubMed] [Google Scholar]
- 70.Ma LJ, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 2003;64:350–355. doi: 10.1046/j.1523-1755.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 71.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochimica et biophysica acta. 2010;1799:622–629. doi: 10.1016/j.bbagrm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of alphaENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1delta/epsilon. Am J Physiol Renal Physiol. 2012;303:F918–927. doi: 10.1152/ajprenal.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem. 2014;289:11791–11806. doi: 10.1074/jbc.M113.531095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol. 2015;309:F933–942. doi: 10.1152/ajprenal.00197.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, Wingo CS, Gumz ML. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta physiologica. 2017;220:72–82. doi: 10.1111/apha.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richards J, Cheng KY, All S, Skopis G, Jeffers L, Lynch IJ, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Renal Physiol. 2013;305:F1697–1704. doi: 10.1152/ajprenal.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 79.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–336. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML, Firsov D. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol. 2014;25:1430–1439. doi: 10.1681/ASN.2013060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics, C.; Stroke Statistics, S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong ND, Dede J, Chow VH, Wong KS, Franklin SS. Global cardiovascular risk associated with hypertension and extent of treatment and control according to risk group. Am J Hypertens. 2012;25:561–567. doi: 10.1038/ajh.2012.2. [DOI] [PubMed] [Google Scholar]
- 83.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58:1093–1098. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 85.Corella D, Arnett DK, Tsai MY, Kabagambe EK, Peacock JM, Hixson JE, Straka RJ, Province M, Lai CQ, Parnell LD, Borecki I, Ordovas JM. The -256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53:1144–1152. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- 86.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dashti HS, Aslibekyan S, Scheer FA, Smith CE, Lamon-Fava S, Jacques P, Lai CQ, Tucker KL, Arnett DK, Ordovas JM. Clock Genes Explain a Large Proportion of Phenotypic Variance in Systolic Blood Pressure and This Control Is Not Modified by Environmental Temperature. Am J Hypertens. 2016;29:132–140. doi: 10.1093/ajh/hpv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bartter FC, Delea CS, Baker W, Halberg F, Lee JK. Chronobiology in the diagnosis and treatment of mesor-hypertension. Chronobiologia. 1976;3:199–213. [PubMed] [Google Scholar]
- 89.Hermida RC, Halberg F, Haus E, Lakatua D, Kawasaki T, Uezono K, Omae T. Human mesor-hypertensive chronorisk. Chronobiologia. 1990;17:227–251. [PubMed] [Google Scholar]
- 90.Hermida RC, Fernandez JR, Ayala DE, Iglesias M, Halberg F. Time-dependent effects of ASA administration on blood pressure in healthy subjects. Chronobiologia. 1994;21:201–213. [PubMed] [Google Scholar]
- 91.Smolensky MH, Hermida RC, Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep medicine reviews. 2017;33:4–16. doi: 10.1016/j.smrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Hermida RC, Ayala DE, Fernandez JR, Mojon A, Crespo JJ, Rios MT, Smolensky MH. Bedtime Blood Pressure Chronotherapy Significantly Improves Hypertension Management. Heart Fail Clin. 2017;13:759–773. doi: 10.1016/j.hfc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 93.Hermida RC, Ayala DE, Rios MT, Fernandez JR, Mojon A, Smolensky MH. Around-the-clock ambulatory blood pressure monitoring is required to properly diagnose resistant hypertension and assess associated vascular risk. Current hypertension reports. 2014;16:445. doi: 10.1007/s11906-014-0445-9. [DOI] [PubMed] [Google Scholar]
- 94.Takeda A, Toda T, Fujii T, Matsui N. Bedtime administration of long-acting antihypertensive drugs restores normal nocturnal blood pressure fall in nondippers with essential hypertension. Clinical and experimental nephrology. 2009;13:467–472. doi: 10.1007/s10157-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 95.Minutolo R, Gabbai FB, Borrelli S, Scigliano R, Trucillo P, Baldanza D, Laurino S, Mascia S, Conte G, De Nicola L. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;50:908–917. doi: 10.1053/j.ajkd.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 96.Zeng J, Jia M, Ran H, Tang H, Zhang Y, Zhang J, Wang X, Wang H, Yang C, Zeng C. Fixed-combination of amlodipine and diuretic chronotherapy in the treatment of essential hypertension: improved blood pressure control with bedtime dosing-a multicenter, open-label randomized study. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34:767–772. doi: 10.1038/hr.2011.36. [DOI] [PubMed] [Google Scholar]
- 97.Okeahialam B, Ohihoin E, Ajuluchukwu J. Chronotherapy in Nigerian hypertensives. Ther Adv Cardiovasc Dis. 2011;5:113–118. doi: 10.1177/1753944711402119. [DOI] [PubMed] [Google Scholar]
- 98.Asmar R, Gosse P, Quere S, Achouba A. Efficacy of morning and evening dosing of amlodipine/valsartan combination in hypertensive patients uncontrolled by 5 mg of amlodipine. Blood Press Monit. 2011;16:80–86. doi: 10.1097/MBP.0b013e328344c6db. [DOI] [PubMed] [Google Scholar]
- 99.Rahman M, Greene T, Phillips RA, Agodoa LY, Bakris GL, Charleston J, Contreras G, Gabbai F, Hiremath L, Jamerson K, Kendrick C, Kusek JW, Lash JP, Lea J, Miller ER, 3rd, Rostand S, Toto R, Wang X, Wright JT, Jr, Appel LJ. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension. 2013;61:82–88. doi: 10.1161/HYPERTENSIONAHA.112.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rorie DA, Rogers A, Mackenzie IS, Ford I, Webb DJ, Willams B, Brown M, Poulter N, Findlay E, Saywood W, MacDonald TM. Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: the Treatment In Morning versus Evening (TIME) study. BMJ Open. 2016;6:e010313. doi: 10.1136/bmjopen-2015-010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carter BL, Chrischilles EA, Rosenthal G, Gryzlak BM, Eisenstein EL, Vander Weg MW. Efficacy and safety of nighttime dosing of antihypertensives: review of the literature and design of a pragmatic clinical trial. Journal of clinical hypertension. 2014;16:115–121. doi: 10.1111/jch.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodrigo GC, Herbert KE. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic Biol Med. 2017 doi: 10.1016/j.freeradbiomed.2017.10.381. [DOI] [PubMed] [Google Scholar]
- 103.Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P. The role of melatonin in acute myocardial infarction. Front Biosci (Landmark Ed) 2012;17:2433–2441. doi: 10.2741/4063. [DOI] [PubMed] [Google Scholar]
- 104.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]


