Key Points
Question
How accurate is fluorodeoxyglucose F18–labeled positron emission tomography with computed tomography in diagnosing malignancy across different populations with varying cancer prevalence?
Findings
In this multi-institutional cohort study, 18F-fluorodeoxyglucose positron emission tomography with computed tomography imaging demonstrated good sensitivity but poor specificity for the diagnosis of lung cancer.
Meaning
An abnormal positron emission tomography with computed tomography scan may be a reliable indicator that highly suspicious lung nodules may be cancerous, but a normal positron emission tomography with computed tomography scan is not a reliable indicator of the absence of disease.
Abstract
Importance
Clinicians rely heavily on fluorodeoxyglucose F18–labeled positron emission tomography (FDG-PET) imaging to evaluate lung nodules suspicious for cancer. We evaluated the performance of FDG-PET for the diagnosis of malignancy in differing populations with varying cancer prevalence.
Objective
To determine the performance of FDG-PET/computed tomography (CT) in diagnosing lung malignancy across different populations with varying cancer prevalence.
Design, Setting, and Participants
Multicenter retrospective cohort study at 6 academic medical centers and 1 Veterans Affairs facility that comprised a total of 1188 patients with known or suspected lung cancer from 7 different cohorts from 2005 to 2015.
Exposures
18F fluorodeoxyglucose PET/CT imaging.
Main Outcome and Measures
Final diagnosis of cancer or benign disease was determined by pathological tissue diagnosis or at least 18 months of stable radiographic follow-up.
Results
Most patients were male smokers older than 60 years. Overall cancer prevalence was 81% (range by cohort, 50%-95%). The median nodule size was 22 mm (interquartile range, 15-33 mm). Positron emission tomography/CT sensitivity and specificity were 90.1% (95% CI, 88.1%-91.9%) and 39.8% (95% CI, 33.4%-46.5%), respectively. False-positive PET scans occurred in 136 of 1188 patients. Positive predictive value and negative predictive value were 86.4% (95% CI, 84.2%-88.5%) and 48.7% (95% CI, 41.3%-56.1%), respectively. On logistic regression, larger nodule size and higher population cancer prevalence were both significantly associated with PET accuracy (odds ratio, 1.027; 95% CI, 1.015-1.040 and odds ratio, 1.030; 95% CI, 1.021-1.040, respectively). As the Mayo Clinic model–predicted probability of cancer increased, the sensitivity and positive predictive value of PET/CT imaging increased, whereas the specificity and negative predictive value dropped.
Conclusions and Relevance
High false-positive rates were observed across a range of cancer prevalence. Normal PET/CT scans were not found to be reliable indicators of the absence of disease in patients with a high probability of lung cancer. In this population, aggressive tissue acquisition should be prioritized using a comprehensive lung nodule program that emphasizes advanced tissue acquisition techniques such as CT-guided fine-needle aspiration, navigational bronchoscopy, and endobronchial ultrasonography.
This study determines the performance of fluorodeoxyglucose F18–labeled positron emission tomography with computed tomography in diagnosing lung malignancy across different populations with varying cancer prevalence.
Introduction
Following the results of the National Lung Screening Trial (NLST), lung cancer screening by low-dose computed tomography scans (LDCT) was adopted by the US Preventive Services Task Force and the Center for Medicare/Medicaid Services. Even more indeterminate pulmonary nodules (IPNs) will be discovered and require evaluation with increased penetrance of LDCT screening. The evidence-based algorithm for the evaluation of lung nodules by the National Cancer Center Network and the American College of Radiologists Lung CT Screening Reporting and Data System (Lung-RADS) practice guidelines for IPN diagnosis suggest that fluorodeoxyglucose F18–labeled positron emission tomography combined with computed tomography (FDG-PET/CT) should be used for evaluating lesions with at least 8-mm solid aspects.
Initially, FDG-PET/CT was found to have high sensitivity (97%) and fairly high specificity (78%) for diagnosing IPNs suspicious for lung cancer, thereby reducing unnecessary invasive testing. A 2014 meta-analysis examining FDG-PET/CT accuracy to diagnose cancer in regions of endemic infectious lung diseases found the specificity of FDG-PET/CT to be significantly less in endemic regions (61%, translating to a 39% false-positive rate) compared with the specificity observed in nonendemic regions (77%). Infectious lung diseases, such as histoplasmosis, coccidioidomycosis, or tuberculosis, can generate benign lung granulomas. These cancer-mimicking lung granulomas frequently cause false-positive imaging by CT or FDG-PET/CT. False-positive imaging increases unnecessary surgical biopsies and the significant morbidity associated with invasive operations to the lung.
Nevertheless, clinicians still rely heavily on FDG-PET/CT imaging to evaluate lung nodules suspicious for cancer. We sought to evaluate the performance of FDG-PET/CT for the diagnosis of malignancy in 7 different populations with varying cancer prevalence and methods of nodule discovery from across the United States.
Methods
Study Population
We performed a multicenter retrospective cohort study of patients with known or suspected lung cancer from 7 different sources. This population was a subset analysis of a re-estimation of a predictive model for lung cancer, including all patients with PET imaging. Table 1 provides an overview of the 7 cohorts included.
Table 1. Cohort Descriptions.
| Cohort Grouping | Cohort | Cohort Description | Location | No. |
|---|---|---|---|---|
| Pulmonary nodule cohorts | 1 | High-risk nodule clinic | Tennessee | 111 |
| 2 | High-risk nodule clinic | Arizona | 104 | |
| Thoracic surgery clinic cohorts | 3 | Thoracic surgical clinic | Tennessee | 383 |
| 4 | Lung-RADS 4 population | Massachusetts | 50 | |
| Surgical resection cohorts | 5 | Veteran surgical cohort | Tennessee | 196 |
| 6 | Surgical cohort | Virginia | 238 | |
| 7 | Surgical cohort | Tennessee | 106 |
Abbreviation: RADS, radiology and data systems.
Cohort 1 comprised patients evaluated by a pulmonary nodule clinic at the Vanderbilt University Medical Center in Nashville, Tennessee. Cohort 2 included patients evaluated by a pulmonary nodule clinic at the Mayo Clinic in Phoenix, Arizona. Cohort 3 included patients evaluated by a thoracic surgery clinic at Vanderbilt University Medical Center in Nashville, Tennessee, and has been described previously. Cohort 4 included patients from the Lahey Hospital and Medical Center in Burlington, Massachusetts, who underwent lung cancer screening and had a Lung-RADS 4 nodule or mass. Cohort 5 included patients who underwent a lung resection for known or suspected lung cancer at the Tennessee Valley Healthcare System Veterans Affairs hospital in Nashville from 2005 to 2015. Cohort 6 included patients who underwent a lung resection for known or suspected lung cancer at the University of Virginia in Charlottesville as part of the Lung Cancer Biospecimen Resource Network collaboration. Cohort 7 included patients who underwent a lung resection for known or suspected lung cancer at Vanderbilt University Medical Center in Nashville, Tennessee.
Cohorts 1 and 2 were high-risk pulmonary nodule cohorts enrolled from pulmonary nodule clinics whose IPNs were evaluated by pulmonologists. Cohorts 3 and 4 represent patients typically referred to a thoracic surgery clinic. Cohort 3 was from a thoracic specialist surgical clinic population. Cohort 4 was a Lung-RADS 4 population from a lung cancer screening program who were evaluated either in a multidisciplinary conference or in clinic by a surgeon. Indeterminate pulmonary nodule diagnosis for these 4 cohorts was either by radiographic determination of benign disease, 18 months of radiographic surveillance, or pathological tissue diagnosis. Cohorts 5, 6, and 7 were pure surgical cohorts of patients who underwent lung resections for known or suspected lung cancer. All diagnoses were pathologically determined in these 3 surgical cohorts. Table 2 shows the grouping of the 7 cohorts that reflect the similar populations and cancer prevalences (cohorts 1 and 2, cohorts 3 and 4, and cohorts 5, 6, and 7). The institutional review board of each facility approved this study, with waivers of individual patient consent.
Table 2. Demographics and Clinical Characteristics of Cohorts.
| Characteristic | Total Population (n = 1188) |
Pulmonary Nodule Cohorts (n = 215) |
Thoracic Surgery Clinic Cohorts (n = 433) |
Surgical Resection Cohorts (n = 540) |
|---|---|---|---|---|
| Age, median (IQR), y | 66 (59-73) | 70 (60-75) | 65 (57-71) | 66 (60-72) |
| Male, No. (%) | 714 (60) | 109 (51) | 232 (54) | 373 (69) |
| Smokers, No. (%) | 1011 (85) | 165 (77) | 361 (83) | 485 (90) |
| Pack-years, median (IQR) | 40 (20-60) | 30 (0.6-50) | 40 (20-60) | 45 (25-68) |
| Lesion size, median (IQR), mm | 22 (15-33) | 18 (13-25) | 22 (15-36) | 23 (16-34) |
| Cancer prevalence, No. (%) | 962 (81) | 112 (52) | 355 (82) | 495 (92) |
| Mayo Clinic Model-predicted probability of cancer (IQR) | 67.5 (39.6-90.6) | 54.9 (30.4-79.2) | 72.4 (41.3-94.2) | 69.4 (42.8-91.5) |
Abbreviation: IQR, interquartile range.
Our study consisted of participants in these 7 cohorts who had the presence of nodules after chest PET imaging. Exclusion criteria for surgical patients included multiple nodules suspicious for cancer, evidence of benign diseases (eg, benign calcification, infiltrates, bronchiolitis obliterans organizing pneumonia, or empyema), preoperative neoadjuvant chemotherapy and/or radiation, and known metastatic disease. For patients who underwent a second lung resection for a new primary or recurrence during the study period, only the first resection was included. Exclusion criteria for nonsurgical patients also included less than 2 years of radiographic surveillance or clinical follow-up from the date of their initial nodule evaluation.
Study Procedures and Definitions
A Research Electronic Data Capture form was created to facilitate data entry across multiple sites. Reference standard information (outcome, cancer, or benign disease) was determined by either pathological tissue diagnosis, radiographic evidence of benign disease (eg, calcifications), or 18 months of radiographic surveillance among patients not undergoing a diagnostic procedural biopsy. Positron emission tomography avidity was determined by radiologist impression of the nodule or standardized uptake value of 2.5 or greater when impression was not reported.
The estimated probability that a suspicious lesion was cancer was calculated for each patient using the published formula of the Mayo Clinic model ex/(1 + ex), where x = −6.8272 + (0.0391 × Age) + (0.7917 × Smoking History) + (1.3388 × Previous Nonthoracic Cancer) + (0.1274 × Lesion Size) + (1.0407 × Spiculated Lesion Edge) + (0.7838 × Upper Lobe Location).
Statistical Analysis
Analyses were performed in R, version 3.2.3 (R Programming) and Stata, version 12.1 (StataCorp). We calculated the overall accuracy (area under the curve), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of PET imaging for each cohort and the entire data set. Using a multivariable logistic regression model, we examined the association between population cancer prevalence, lesion size, and FDG-PET accuracy. A logistic regression model was developed to determine the degree to which population cancer prevalence and lesion size predicted FDG-PET accuracy. The primary purpose of this analysis was to determine whether population cancer prevalence was a primary driver of FDG-PET accuracy. The association between Mayo Clinic model–predicted cancer probability and PET performance was plotted. All analyses used a 2-sided P value of .05 as indication of statistical significance.
Results
Our multicenter population included a total of 1188 patients (Table 1). The overall lung cancer prevalence was 81% (962 of 1188 patients). Most patients were male smokers older than 60 years (Table 2). Cohort 4 had a smoking prevalence of 100% because it was drawn from a lung cancer screening population constituted entirely of smokers. Median nodule size ranged from 16 mm (interquartile range [IQR], 13-22 mm) in cohort 4 (Lung-RADS 4 screening population) to 24 mm (IQR, 16-35 mm) in cohort 5 (veterans surgical population). As expected, the median nodule size was larger in the surgical cohorts than in the pulmonary nodule clinic cohorts. The median Mayo Clinic model–predicted probability of cancer was 67.5% (IQR, 39.6%-90.6%).
Median pack-year history of smoking ranged from 29 (IQR, 1-48) in cohort 1 (pulmonary nodule clinic population) to 50 (IQR, 40-80) in cohort 5 (veterans surgical population). Cancer prevalence was lowest (n = 112 of 115; 52%) in cohorts 1 and 2 (pulmonary nodule clinic populations), moderately high (n = 355 of 433; 82%) in cohorts 3 and 4 (typical thoracic surgery clinic populations), and high (n = 495 of 540; 92%) in cohorts 5 and 7 (pure surgical populations).
The overall sensitivity and specificity of FDG-PET to diagnose lung cancer was 90.1% (95% CI, 88.1%-91.9%) and 39.8% (33.4%-46.5%), respectively. The sensitivity of PET ranged from 77% in cohort 2 (high-risk nodule population from Arizona) to 96% in cohort 5 (veteran surgical cohort from Tennessee) (Table 3). Sensitivity was significantly lower (mean, 79.1%; 95% CI, 72.2%-86.0% compared with 92.3%; 95% CI 90.2%-94.4% in the cohorts with a median lesion size > 2 cm; P < .001) in the 3 cohorts with a median lesion size of less than 2 cm. Specificity was lowest at 22% in the 2 pure surgical cohorts from Tennessee and Virginia. Specificity was highest at 75% in cohort 4, the Lung-RADS 4 population from Massachusetts, albeit with wide range (95% CI, 19%-99%) owing to the smaller cohort size. Cohort 4 was the only truly nonendemic cohort with regard to fungal lung disease. Specificity was also generally higher in cohorts that relied on a combination of pathology and radiographic surveillance.
Table 3. Performance of PET Imaging in Different Cohorts.
| Variables | Variable (95% CI) | |||
|---|---|---|---|---|
| Total Population (n = 1188) |
Pulmonary Nodule Cohorts (n = 215) |
Thoracic Surgery Clinic Cohorts (n = 433) |
Surgical Resection Cohorts (n = 540) |
|
| Sensitivity | 90.1 (88.1-91.9) | 81.3 (72.8-88.0) | 89.3 (85.6-92.3) | 92.7 (90.1-94.9) |
| Specificity | 39.8 (33.4-46.5) | 45.6 (35.8-55.7) | 41.0 (30.0-52.8) | 24.4 (12.9-39.5) |
| PPV | 86.4 (84.2-88.5) | 61.9 (53.5-69.8) | 87.3 (83.5-90.6) | 93.1 (90.5-95.2) |
| NPV | 48.7 (41.3-56.1) | 69.1 (56.7-79.8) | 45.7 (33.7-58.1) | 23.4 (12.3-38.0) |
| Lesion size, median (IQR), mm | 22 (15-33) | 18 (13-25) | 22 (15-36) | 23 (16-34) |
| Cancer prevalence | 81.0 (78.6-83.2) | 52.1 (45.2-58.9) | 82.0 (78.0-85.5) | 91.7 (89.0-93.9) |
| Mayo Clinic model–predicted probability of cancer (IQR) | 67.5 (39.6-90.6) | 54.9(30.4-79.2) | 72.4 (41.3-94.2) | 69.4 (42.8-91.5) |
Abbreviations: IQR, interquartile range; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value.
The overall PPV was 86.4% and by population ranged from 55% to 97%. The overall NPV was 48.7%, ranging from 17% to 70%. To quantify the association between PET accuracy and both nodule size and cancer prevalence, a logistic model was fit. Based on this model, both larger nodule size and higher population cancer prevalence were significantly associated with PET accuracy (odds ratio, 1.027; 95% CI 1.015-1.040; P < .001 and 1.030; 95% CI, 1.021-1.040; P < .001, respectively).
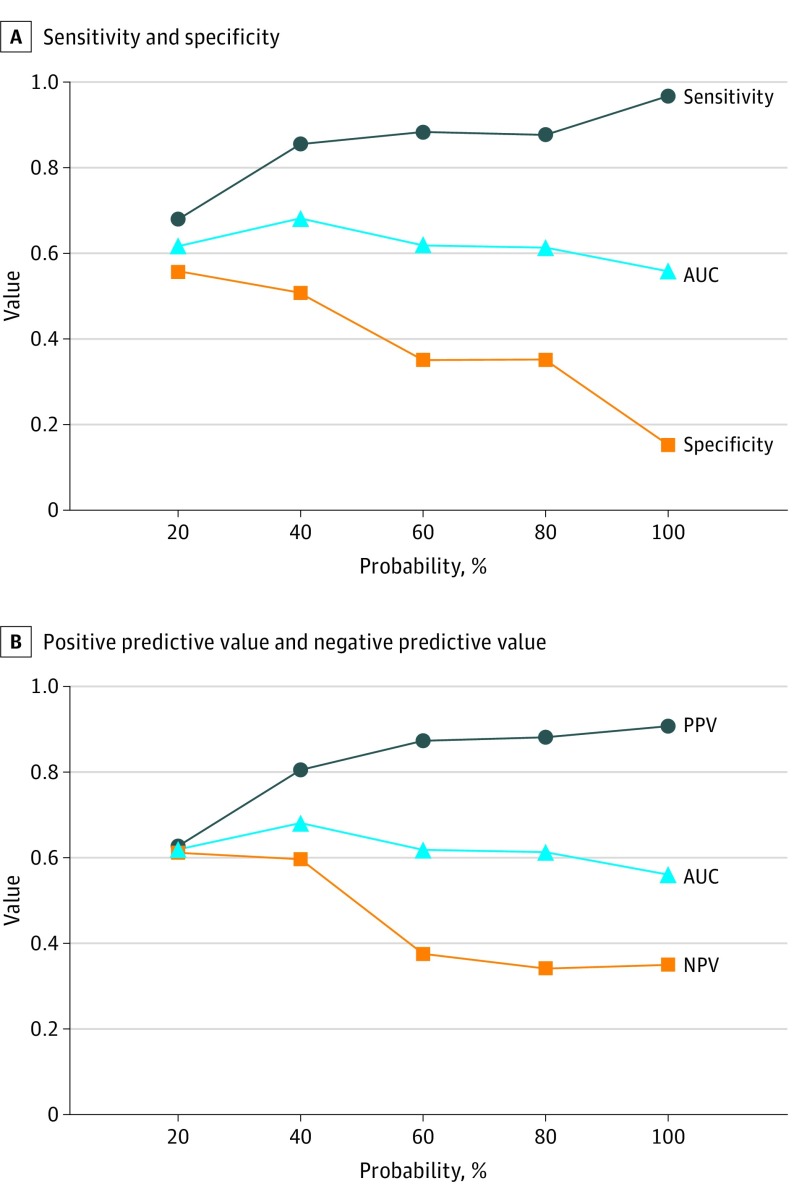
As the Mayo Clinic model–estimated IPN probability of cancer increased, the sensitivity and PPV of PET/CT imaging increased, whereas the specificity and NPV dropped (Figure). The area under the curve for the ability of PET imaging to predict cancer diagnosis did not vary appreciably by probability of cancer quintile (results not shown). When the Mayo Clinic model estimated individual predicted probabilities of cancer to be less than 40%, PET imaging had greater than 50% specificity, sensitivity, NPV, and PPV.
Figure. Probability of Cancer and Positron Emission Tomography (PET) Performance.
The Mayo Clinic model probability of cancer is calculated from the following variables: patient age, smoking history (yes/no), history of previous nonthoracic cancer, lesion size, spiculation, and lesion location. AUC indicates area under the curve for the ability of PET to predict cancer diagnosis on logistic regression.
Discussion
Our findings suggest that FDG-PET/CT has more limited value than previously recognized to diagnose IPNs suspicious for lung cancer across a variety of clinical contexts, geographic regions, and nodule sizes. We report an overall sensitivity and specificity of FDG-PET to diagnose lung cancer of 90.1% (95% CI, 88.1%-91.9%) and 39.8% (33.4%-46.5%), respectively. These compare less favorably to the early reports of a sensitivity of 97% and specificity of 78% for diagnosing lung cancer.
We found PET diagnostic performance to be particularly poor in smaller lesions from populations with lower cancer prevalence, where a normal test result does not always rule out cancer. The overall NPV of PET/CT was 48.7 (95% CI, 41.3-56.1), although it was higher at 69.1 (95% CI, 56.7-79.8) in the 2 pulmonary nodule cohorts. Both PET specificity and NPV drop as the Mayo Clinic model–predicted cancer prevalence increases: essentially, a normal PET/CT did not rule out cancer in a meaningful way in this population. Of note, only at low predicted probabilities of cancer (less than 40%) did PET imaging have greater than 50% specificity, sensitivity, NPV, and PPV. Positive predictive value and NPV vary naturally with cancer prevalence.
18F fluorodeoxyglucose is a combination of deoxyglucose, a glucose analogue, and the positron-emitting flourine 18 radionuclide. This radiographic biomarker of metabolic activity disperses quickly when injected and accumulates in tissues that use glycolysis such as the kidneys and brain. Concentrations of the fluorine radionuclide are measured by PET. Normal lung tissue is not metabolically active and does not accumulate the fluorine emitter. However, active infections, growing cancers, and some benign lung processes, such as sarcoidosis, metabolize glucose and accumulate FDG in the lung. Conversely, slow-growing cancers of the lung, such as adenocarcinoma in situ and carcinoid, do not actively use glucose and often generate false-negative scan results.
On the heels of the National Lung Screening Trial, the United States is experiencing a veritable epidemic of lung nodules, driven largely by imaging, many of which turn out to be false-positives and lead to unnecessary invasive biopsies and/or surgeries. An estimated 1.6 million incidentally discovered IPNs are found annually, with a 1% to 12% chance of malignancy. These nodules represent a significant diagnostic burden. The National Lung Screening Trial demonstrated a reduction in lung cancer and overall mortality but also a 96% false-positive imaging rate, 24% benign operative rate, and 1.2% mortality in patients undergoing diagnostic surgical procedures. Extrapolating from the results of the National Lung Screening Trial to health care use from a national lung cancer screening program, an additional 1.5 million follow-up CT scans, approximately 250 000 FDG-PET/CT scans, and 120 000 diagnostic operations will be performed annually. Among the 120 000 diagnostic operations, 29 000 operations in the first 3 years would result in benign disease, subjecting patients to harm with little therapeutic benefit.
18F fluorodeoxyglucose PET/CT scans are currently recommended for noninvasive clinical diagnosis of lung cancer. However, infectious fungal diseases, such as histoplasmosis, blastomycosis and coccidioidomycosis, are well documented to cause false-positive scans. In addition, mycobacterium, including mycobacterium tuberculosis and Mycobacterium avium-intracellulare, may result in false-positive imaging.
Limitations
Although the procedure costs between $2000 and $3000, FDG-PET/CT is still considered standard of care for the noninvasive diagnosis of IPNs. However, our work has questioned the use of FDG-PET/CT for the diagnosis of suspicious IPNs where fungal lung diseases are endemic. Limitations of this study include our reliance on data from areas, with endemic fungal infections as well as the lack of prospective data collection with multiple cohorts specifically designed to represent a range of cancer prevalence, exposure to fungal lung disease, and other variables. Positron emission tomography specificity was highest in the Massachusetts cohort, a population with minimal fungal lung diseases and whose measured endemic rate is far lower than that of the other states’ cohorts (1.1%). In addition, there is some potential for verification bias in this retrospective study because in some cohorts, PET-avid lesions were more likely to have a definitive diagnosis. This was particularly true for cohort 4, where at least half of the Lung-RADS 4 source population did not have sufficient follow-up duration to prove benign disease on imaging alone. Accounting for this verification bias would likely shift the specificity for cohort 4 closer to 85%.
Current National Cancer Center Network and American College of Radiologists algorithms for the evaluation of lung nodules recommend the use of FDG-PET/CT imaging before biopsy or surgical excision for pathological tissue diagnosis. In a cost-effectiveness study comparing diagnostic FDG-PET/CT scan with aggressive tissue acquisition, we have previously reported that clinicians should pursue diagnosis by pathological tissue procedures instead of a diagnostic FDG-PET/CT scan when the specificity of FDG-PET/CT is less than 72%. Frequent use of preoperative tissue acquisition strategies has been shown to result in lower rates of nontherapeutic lung resections.
Conclusions
Given the overall poor specificity and NPV across our 7 populations of patients with IPNs, these findings reiterate the need for a comprehensive lung nodule program that emphasizes advanced tissue acquisition techniques, such as image-guided fine-needle aspiration, navigational bronchoscopy, and endobronchial ultrasonography, to obtain tissue, avoid thoracoscopic procedures driven by FDG-PET/CT inaccuracies, and ultimately reduce nontherapeutic resection rates. Positron emission tomographic imaging is still useful for cancer staging after pathological diagnosis, but a normal PET should not be relied on to rule out cancer in patients with a high likelihood of cancer.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Network NCC. Non-Small Cell Lung Cancer v4.2016. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Fort Washington, PA: National Comprehensive Cancer Network; 2016. [Google Scholar]
- 3.Radiology ACo Lung CT Screening Reporting and Data System. Reston, VA: Lung-RADS; 2014. [Google Scholar]
- 4.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285(7):914-924. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri GA, Gonzalez AV, Jantz MA, et al. . Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e211S-e250S. [DOI] [PubMed] [Google Scholar]
- 6.Deppen SA, Blume JD, Kensinger CD, et al. . Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. JAMA. 2014;312(12):1227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason R, Broaddus V, Martin T. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed Philadelphia, PA: Saunders; 2010. [Google Scholar]
- 8.Grogan EL, Deppen SA, Ballman KV, et al. . Accuracy of fluorodeoxyglucose-positron emission tomography within the clinical practice of the American College of Surgeons Oncology Group Z4031 trial to diagnose clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2014;97(4):1142-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deppen SA, Blume JD, Aldrich MC, et al. . Predicting lung cancer prior to surgical resection in patients with lung nodules. J Thorac Oncol. 2014;9(10):1477-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe VJ, Fletcher JW, Gobar L, et al. . Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol. 1998;16(3):1075-1084. [DOI] [PubMed] [Google Scholar]
- 11.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. [PubMed] [Google Scholar]
- 12.Edwards PQ, Klaer JH. Worldwide geographic distribution of histoplasmosis and histoplasmin sensitivity. Am J Trop Med Hyg. 1956;5(2):235-257. [PubMed] [Google Scholar]
- 13.Gould MK, Tang T, Liu IL, et al. . Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Sáez N, González-Álvarez I, Vilar J, et al. . Prevalence and variables associated with solitary pulmonary nodules in a routine clinic-based population: a cross-sectional study. Eur Radiol. 2014;24(9):2174-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacMahon H, Austin JH, Gamsu G, et al. ; Fleischner Society . Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. [DOI] [PubMed] [Google Scholar]
- 16.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):e142S-e165S. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385. [DOI] [PubMed] [Google Scholar]
- 18.Gould MK. Cost effectiveness of positron emission tomography for characterizing pulmonary nodules. PET Clin. 2006;1(4):339-346. [DOI] [PubMed] [Google Scholar]
- 19.Coleman MT, Chen RY, Lee M, et al. . PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci Transl Med. 2014;6(265):265ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deppen S, Putnam JB Jr, Andrade G, et al. . Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011;92(2):428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deppen SA, Davis WT, Green EA, et al. . Cost-effectiveness of initial diagnostic strategies for pulmonary nodules presenting to thoracic surgeons. Ann Thorac Surg. 2014;98(4):1214-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards LB, Acquaviva FA, Livesay VT. Further observations on histoplasmin sensitivity in the United States. Am J Epidemiol. 1973;98(5):315-325. [DOI] [PubMed] [Google Scholar]
- 23.Barta JA, Henschke CI, Flores RM, Yip R, Yankelevitz DF, Powell CA. Lung cancer diagnosis by fine needle aspiration is associated with reduction in resection of nonmalignant lung nodules. Ann Thorac Surg. 2017;103(6):1795-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]