Abstract
Introduction
Agrin is essential for the formation and maintenance of neuromuscular junctions (NMJs). NT-1654 is a C-terminal fragment of mouse neural agrin. In this study, we determined the effects of NT-1654 on the severity of experimental autoimmune myasthenia gravis (EAMG).
Methods
EAMG was induced in female Lewis rats by immunization with the Torpedo acetylcholine receptor (tAChR) and complete Freund’s adjuvant. NT-1654 was dissolved in PBS and injected daily s.c. into tAChR immunized rats during the first 10 days after immunization, and then every other day for the following 20 days.
Results
Experimental data showed that NT-1654 attenuated clinical severity, effectively promoted the clustering of AChRs at NMJs, and alleviated the impairment of NMJ transmission and the reduction of muscle-specific kinase (MuSK) in EAMG rats.
Conclusion
NT-1654 is a promising agent to improve NMJ transmission in EAMG. Future investigations are needed to test the potential of NT-1654 to treat human myasthenia gravis.
Keywords: Agrin, myasthenia gravis, acetylcholine receptor, neuromuscular junctions
Introduction
Agrin is essential for the formation and maintenance of neuromuscular junctions (NMJs). NT-1654 is a C-terminal fragment of mouse neural agrin. NT-1654 and agrin share the same mechanism of action by binding to the low-density lipoprotein receptor-related protein 4 (LRP4) to activate muscle-specific kinase (MuSK)/docking protein 7 (DOK7) signaling and subsequently inducing rapsyn dependent acetylcholine receptor (AChR) assembly and aggregation at the NMJ.1–6 Unlike endogenous neural agrin, NT-1654 is engineered to be highly soluble and resistant to cleavage by neurotrypsin, which is a protease that counteracts the activity of agrin in the synaptic cleft.7,8 NT-1654 has been shown to induce AChR clustering and to be effective in attenuating the sarcopenia-like phenotype in neurotrypsin-overexpressing (SARCO) mice.2
Myasthenia gravis (MG) is an immune-mediated neuromuscular disorder in which antibodies to the NMJ acetylcholine receptors or other antigens of postsynaptic skeletal muscle impair NMJ transmission and result in weakness of voluntary skeletal muscles.4,9–11 These pathogenic antibodies have several effects that cause clinical weakness; the most important of these is activation of the complement system and subsequent remodeling of the post-synaptic membrane of the NMJ.12 This remodeling results in decreased numbers of AChR, loss of the junctional folds that hold the receptors, reduced clustering of the receptors and, ultimately, long-term changes that may contribute to disability in affected patients. Untreated, the disease has high morbidity related to the frequent bulbar and respiratory weakness13. While current treatment with corticosteroids or immunomodulating agents can be effective at reducing this morbidity and alleviating symptoms, it also carries significant risks and adverse effects. There is an unmet need in this respect for agents that can help treat MG while reducing the doses of these immunomodulatory agents. Since standard immunotherapy involves reducing the pathogenic effects of disease causing antibodies14, strategies that enhance repair of these changes are attractive as potential adjunctive therapies to immunomodulation.
Experimental autoimmune MG (EAMG) reliably recapitulates many of the features seen in human disease. It is routinely induced in susceptible mouse or rat strains by immunization with Torpedo AChR (tAChR) and complete Freund’s adjuvant (CFA).15,16 After immunization, EAMG animals display reduced AChR clustering and impaired neurotransmission at their NMJs, which closely mimics the immunopathological and electrophysiological features of human disease.17,18
In this study, we evaluated the impact of NT-1654 on the severity of EAMG. In particular, we studied its ability to modify the clinical course of EAMG and its impact on the immunopathological and electrophysiological features of NMJs in EAMG.
Materials and Methods
Animals
Female Lewis rats, 8–10 weeks of age, were purchased from Charles River Laboratories International, Inc. (Wilmington, MA). A total of 48 rats (n=12 per group) were assigned to 4 groups: EAMG, EAMG treated with NT-1654 (2mg/kg), EAMG treated with NT-1654 (6mg/kg) and healthy naïve control. All rats were housed and handled in specific pathogen-free conditions according to approved protocol of the Institutional Animal Care and Use Committee at Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona.
Antigen preparation, immunization, and clinical evaluation of EAMG
The tAChR was purified from the electric organs of Torpedo californica (Pacific Bio-Marine Laboratories, Inc., Venice, CA) by affinity chromatography on an α-cobratoxin-agarose resin (Sigma-Aldrich Co., LLC, St. Louis, MO) as previously described.19–21 A total of 36 rats were subcutaneously injected in the hind foot pads and at the base of the tail on day 0 with 50 μg of purified tAChR emulsified in CFA for a total volume of 200 μl.22 For clinical examination, body weight and assigned clinical scores were evaluated blindly at least by two investigators from day1 to day 49 after immunization. Disease severity of muscle weakness was assessed as has been previously described and scored as follows: 0, healthy normal rats with no signs of EAMG; 1, mildly decreased activity and weak grip with fatigability; 2, weakness, hunched posture at rest, decreased body weight, and tremor; 3, severe generalized weakness, marked decrease in body weight, moribund; 4, death.15,23,24 All rats were sacrificed at day 49 after initial tAChR immunization.
NT-1654 administration
NT-1654 was provided gratis by Neurotune (Neurotune AG, Schlieren-Zurich, Switzerland) and was dissolved in PBS at a concentration of 2 mg/ml. From day 1 to day 30 after tAChR immunization, EAMG rats received subcutaneous injections of either vehicle PBS only, vehicle containing 2 mg/kg NT-1654, or vehicle containing 6 mg/kg NT-1654. Injections were administrated once a day from day 1 to day 10 and then once every other day from day 11 to day 30. Naïve control rats were subjected to subcutaneous injections of vehicle PBS using the same injection schedule.
Electromyography (EMG)
Repetitive nerve stimulation was performed to record compound muscle action potentials (CMAPs) at day 49 after immunization. Rats were anesthetized with a cocktail of ketamine and xylazine (60 mg/kg and 10 mg/kg body weight, respectively) and were maintained at 37°C on a heating pad throughout the process. The stimulating electrode containing an anode and a cathode was placed near sciatic nerve in the thigh of the left leg. The recording needle electrode was inserted into the middle of the tibialis anterior muscle of the left leg, and the reference needle electrode was inserted near the calcaneal tendon. The reference and recording electrodes were connected to a Sierra Wave amplifier (Cadwell, Kennewick, WA). Repetitive nerve stimulation was performed on the sciatic nerve with 10 trains of 10 stimuli at several frequencies (1, 10, 20, and 40 Hz) according to other previous studies.10,25 Thereafter, CMAPs were recorded and peak-to-peak data were measured with Sierra Wave 11.0 (Cadwell, Kennewick, WA).
Immunostaining
The animals were sacrificed at day 49 after immunization. The tibialis anterior muscles were obtained and were fixed overnight in 4% paraformaldehyde (PFA) before paraffin embedding. For whole-mount muscle tissue staining and analysis, the tissue blocks were cut into 6-μm cross-sections and every twentieth section was stained. For immunofluorescence, muscle sections (three sections per tissue block) were incubated in blocking buffer consisting of 5% goat serum with 1% bovine serum albumin (BSA) in 0.1% triton X-100 in PBS (PBST) for 2 hours. Subsequently, the muscle sections were incubated overnight at 4°C with α-bungarotoxin conjugated with Alexa Fluor® 594 (1:2,000; lot No. B13423, Thermo Fisher Scientific, Inc., Anthem, AZ) and antibodies against neurofilament heavy chain (NF-H) (1:100; lot No. sc-20112, Santa Cruz Biotechnology, Paso Robles, CA), synaptic vesicle glycoprotein 2A (SV2A) (1:100; lot No. sc-376234, Santa Cruz Biotechnology, Paso Robles, CA). After the tissue sections were washed three times in PBS, they were incubated with Alexa Fluor 488-conjugated secondary antibody (1: 1,000; Thermo Fisher Scientific, Inc., Anthem, AZ) for 2 hours at room temperature. Finally, the slices were incubated with fluoroshield mounting medium with DAPI (Abcam, Inc, Cambridge, MA) for 5 minutes before images (four random fields per section) were taken with a fluorescence microscopy (model BX61, Olympus, Center Valley, PA). For immunohistochemical analysis, tissue sections (10 sections per tissue block) were stained with hematoxylin-eosin, and microscopic images (four random fields per section) were obtained (model BX61, Olympus, Center Valley, PA). All image data were analyzed and quantified by using ImageJ open-source software (U.S. National Institutes of Health, Washington, DC).
Western blot
The tibialis anterior muscles were obtained at day 49 after immunization and sacrifice, and were lysed in RIPA buffer (Cell Signaling Technology, Inc., Danvers, MA) supplemented with a protease inhibitor cocktail (Sigma-Aldrich Co., LLC, St. Louis, MO) for 1 hour at 4°C. Then protein concentration was measured and tissue lysates were subjected to Western blotting. Whole tissue lysates containing 40 μg of protein were resolved by 10% SDS-PAGE and transferred to polyvinyl difluoride (PVDF) membranes using a transfer apparatus (Bio-Rad Laboratories, Inc., Philadelphia, PA) according to the manufacturer’s protocol. The PVDF membranes were incubated with 5% non-fat milk in Tris-buffered saline and Tween 20 buffer containing primary antibodies against MuSK (1:100; lot No. sc-6009, Santa Cruz Biotechnology, Paso Robles, CA) or α-tubulin (1: 1,000; lot No. sc-23948, Santa Cruz Biotechnology, Paso Robles, CA) at 4°C overnight. After the PVDF membranes were washed twice with TBST, they were incubated with horseradish peroxidase–(HRP)-conjugated rat anti-mouse IgG (1: 2,000; Thermo Fisher Scientific, Inc., Anthem, AZ) or rabbit anti-goat IgG (1: 1,000; Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Immunoreactive bands were detected using enhanced chemiluminescence (Thermo Fisher Scientific, Inc., Rockford, IL) and captured with an Odyssey Fc Imaging System (LI-COR, Inc., Lincoln, NE). Western blot data were analyzed with ImageJ software.
Statistics
Data were normally distributed and analyzed using a two-tailed unpaired Student’s t-test for two groups or analysis of variance (ANOVA) for multiple comparisons, followed by Tukey post hoc test using GraphPad Prism 7.01 (GraphPad Software. Inc). Data were expressed as mean ± s.e.m. unless otherwise indicated. A P value less than 0.05 was considered significant and expressed in figures as p<0.05 or p<0.01.
Results
NT-1654 administration ameliorates EAMG
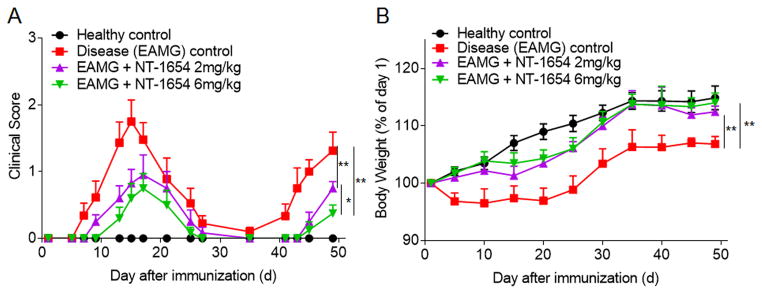
After tAChR immunization, Lewis rats receiving vehicle PBS served as disease (EAMG) controls and developed typical two-phase EAMG, with an early phase of muscular weakness followed by a late phase of progressive muscular weakness during the 7-week observation period (Fig. 1A). EAMG rats treated with NT-1654 were found to have less severe clinical scores in both the early and late phases of disease. At day 49 after immunization, 2 mg/kg NT-1654 and 6mg/kg NT-1654 treated EAMG rats had a reduction in clinical scores of 42.86% and 71.43%, respectively, when compared to disease control rats, suggesting that the therapeutic effect of NT-1654 is dose dependent (Fig. 1A). 2 mg/kg NT-1654 and 6 mg/kg NT-1654 treated EAMG rats also had more weight gain by 4.12% and 5.70%, respectively, when compared to disease control rats at day 49 (Fig. 1B). In addition, no difference was seen in percent body weight gain among the healthy and treated rats. Overall, these results suggest that early treatment with NT-1654 can attenuate the clinical severity of EAMG.
Figure 1. Effects of NT-1654 on EAMG manifestation.
(A) Clinical scores were recorded for rats randomly assigned to the healthy control group (Naïve controls [no vehicle or NT-1654 treatment]), the EAMG + vehicle group (EAMG rats treated with PBS), the EAMG + NT-1654 (2 mg/kg) group (EAMG rats treated with 2 mg/kg NT-1654), and the EAMG + NT-1654 (6 mg/kg) group (EAMG rats treated with 6 mg/kg NT-1654). (B) Body weight measurements for each group of rats as a percentage of body weight on day 1. Data are expressed as mean ± s.e.m.; n = 12 rats per group; *p<0.05; **p<0.01.
NT-1654 improves electrophysiologic measures of neuromuscular transmission
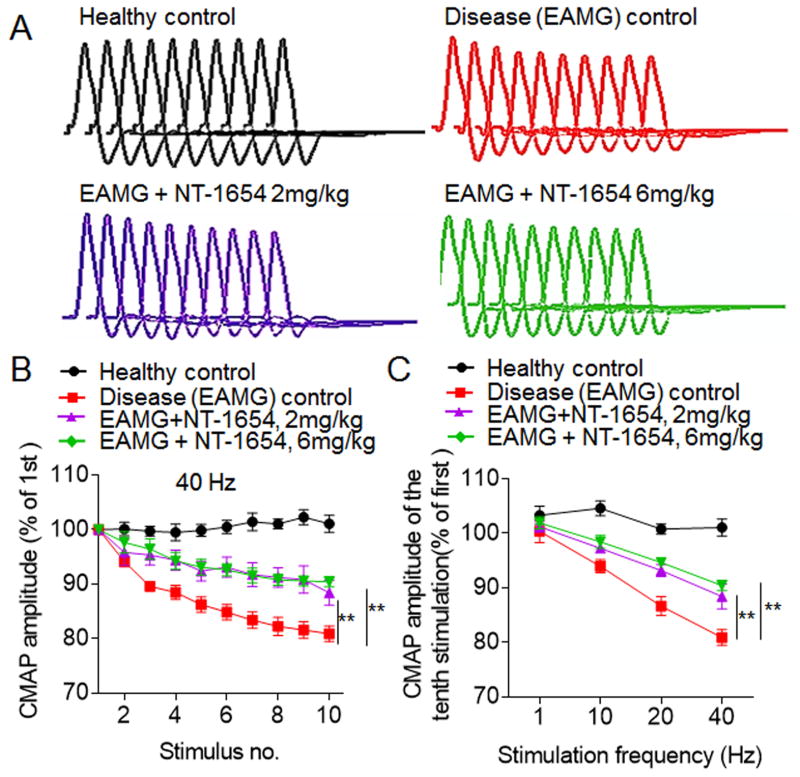
In healthy control rats, there was little or no decrement after 10 consecutive nerve stimuli at1 Hz, 10 Hz, 20 Hz and 40 Hz. In contrast to healthy controls, disease control rats stimulated at 40 Hz had a mean decrement response of 11.1% by the fourth stimulus and 19.9% by the tenth stimulus (Fig. 2A and B). In contrast, EAMG rats treated with 2 mg/kg and 6 mg/kg of NT-1654 and stimulated at 40 Hz had a mean decremental response of 5.1% (p= 0.073) and 5.3% (p= 0.089) by the fourth stimulus and of 12.8 % and 10.4% by the tenth stimulus, respectively compared to healthy control’s (Fig. 2B). This effect appeared to be greater in the 6 mg/kg group than in the 2 mg/kg group (Fig. 2C), in keeping with the dose-dependent response observed in our other measures of disease severity. Overall, these data suggest that NT-1654 can attenuate neuromuscular transmission dysfunction in treated EAMG rats compared to disease control rats.
Figure 2. NT-1654 treatment attenuates CMAP decrement in EAMG rats.
CMAPs in the tibialis anterior were recorded in response to a train of 10 submaximal stimuli at different frequencies in control and EAMG rats at day 49 after immunization. The first stimulus response in control rats was designated as 100%. (A) Representative CMAP traces in response to 10 successive stimulations at 40 Hz for healthy control, EAMG + vehicle, EAMG + NT-1654 (2 mg/kg), and EAMG + NT-1654 (6 mg/kg) rats. (B) Effects of NT-1654 on CMAP decrement in EAMG rats. NT-1654 attenuated the decrement of CMAP amplitudes at 40 Hz in EAMG rats. (C) NT-1654 CMAP attenuated the decrement of CMAP amplitudes of the tenth stimulation at different stimulation frequencies. Data are expressed as mean ± s.e.m.; n = 12 rats per group; **p<0.01.
Effects of NT-1654 treatment on neuromuscular junction pathology
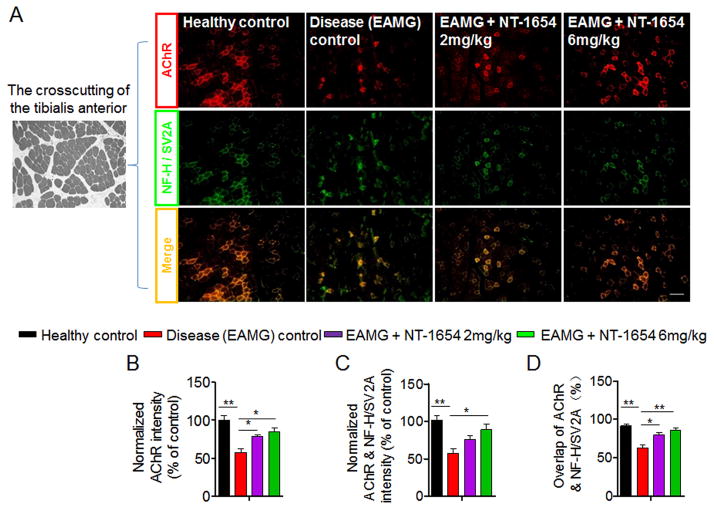
Quantification of the immunofluorescence intensity of AChR showed a reduction of 42.1% in disease control rats relative to healthy controls (Fig. 3A and B). In addition to the loss of AChR clusters, the intensity of AChR and nerve terminals (α-bungarotoxin and NF-H / SV2A double positive) were reduced by 43.8% in disease control rats compared to healthy controls (Fig. 3A and C). When we compared EAMG rats treated with 2 mg/kg and 6 mg/kg of NT-1654 to disease controls, there was increased immunofluorescence intensity of AChR of 21.3% and 27.1%, respectively (Fig. 3A and B). Moreover, the overlap percentage of AChR to nerve terminals was also significantly increased by 17.4% and 23.9% in EAMG rats receiving 2 mg/kg and 6 mg/kg of NT-1654, respectively (Fig. 3D). In keeping with the ameliorated clinical disease severity and neuromuscular transmission dysfunction seen after NT-1654 treatment, these data showed that NT-1654 also attenuates the loss of AChR in NMJ of EAMG rats.
Figure 3. Effects of NT-1654 on AChR in the muscle of EAMG rats.
Muscle tissue sections of the tibialis anterior were obtained at day 49 after immunization and stained with α-bungarotoxin (red) to label AChR and antibodies against NF-H/SV2A (green) to label nerve branches and terminals. (A) Representative images (10x magnification) show AChR and nerve endings of healthy control, EAMG + vehicle, EAMG + NT-1654 (2 mg/kg), and EAMG + NT-1654 (6 mg/kg) rats in tibialis anterior tissue. Scale bar: 100 μm. (B–D) Summarized results show that NT-1654 improved AChR covering the nerve terminals in EAMG rats. Normalized pixel density of AChR relative to healthy controls (B), normalized pixel density of AChR and nerve terminals relative to healthy controls (C), and the percentage of AChR covering nerve terminals relative to total AChR (D). Data are expressed as mean ± s.e.m.; n = 6 rats per group; *p<0.05; **p<0.01.
NT-1654 treatment ameliorates muscular atrophy in EAMG
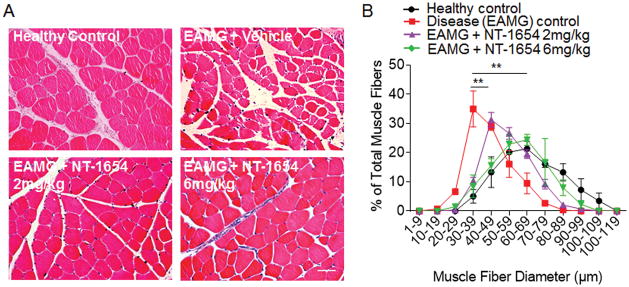
Tissue cross-sections of the tibialis anterior muscle were obtained at day 49 after EAMG induction. Muscle fiber atrophy was visualized in EAMG muscle cross-sections stained with hematoxylin-eosin (Fig. 4A). Quantitative morphometric evaluation of the fiber size distribution demonstrated the obvious shift toward smaller fibers in disease (EAMG) control rats than in healthy control muscles (Fig. 4B). Treatment with NT-1654 significantly reversed the size distribution of muscle fibers (Fig. 4B). This result suggests that NT-1654 treatment can at least partially prevent muscular atrophy in EAMG.
Figure 4. NT-1654 treatment attenuates the reduction of muscle fiber diameter in EAMG rats.
(A) Representative hematoxylin-eosin–stained muscle cross-sections (10x magnification) of the tibialis anterior muscle show muscle fibers of healthy control, EAMG + vehicle, EAMG + NT-1654 (2 mg/kg), and EAMG + NT-1654 (6 mg/kg) rats at day 49 after immunization. Scale bar: 50 μm. (B) Summarized results of muscle fiber size distribution show attenuated reduction of muscle fiber diameter in EAMG rats after NT-1654 treatment. Values represent relative numbers of fibers in a given diameter class. Data are expressed as mean ± s.e.m.; n= 6 rats per group; **p<0.01.
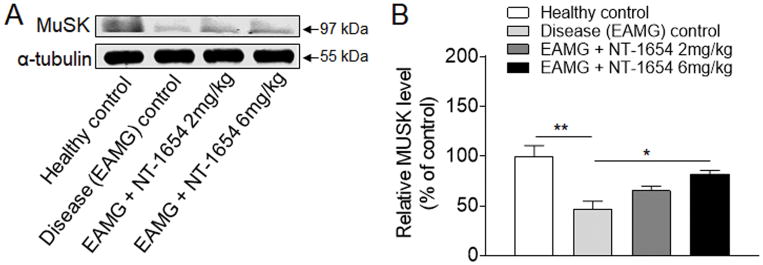
NT-1654 treatment enhances resilience of the MuSK protein in EAMG
The reduction of MuSK expression was evident in disease (EAMG) control rats compared to healthy controls (Fig. 5), indicating the loss of MuSK in conjunction with abnormal NMJ structures in EAMG. In EAMG rats treated with NT-1654, protein level of MuSK was significantly higher than in disease control rats when analyzed by Western blotting analysis (Fig. 5A and B). The protein level of MuSK was not significantly different between groups of EAMG rats receiving 2 mg/kg versus 6 mg/kg NT-1654. Different from the finding in MuSK protein levels, we found that NT-1654 treatment did not significantly alter the mRNA level of MuSK in EAMG rats, suggesting that mechanisms other than mRNA upregulation might be involved in the augment of MuSK protein levels after NT-1654 treatment (data not shown). Together, these findings suggest that NT-1654 attenuate the reduction of MuSK protein in injured NMJ of EAMG rats.
Figure 5. Improved MuSK expression in EAMG rats after NT-1654 treatment.
(A) Western blot images show improved expression of MuSK in EAMG rats after NT-1654 treatment. For an internal control, α-tubulin was used. (B) Quantitative analysis performed by Image J shows the relative expression of MuSK normalized to healthy controls. Data are expressed as mean ± s.e.m.; n = 5 rats per group; *p<0.05; **p<0.01.
Discussion
The mainstay of treatment for MG has been a combination of acetylcholinesterase inhibitors that improve availability of acetylcholine at the NMJ and immunomodulating agents that blunt the immune attack on AChRs at the NMJ. Although effective, these treatments are associated with substantial long and short term adverse effects.26–28 Additionally, none of these treatments directly improve or reverse the structural changes that occur at the post-synaptic membrane secondary to the immune attack on the AChRs. In this study, we demonstrate that the recombinant agrin fragment NT-1654 improves AChR clustering and NMJ transmission in EMAG rats, supporting further therapeutic development of this compound.
Our pathologic data supported the effect of NT-1654 on neuromuscular junction repair. The number of AChRs identified by immunofluorescence was increased by treatment with NT-1654 and, importantly, these were localized at the NMJ in the muscle. Improvement in fiber size distribution after NT-1654 treatment was also observed. Muscle atrophy has been reported to be related to functional denervation of muscle fibers but also likely to weakness-induced disuse.29 Thus, this is likely to be a secondary effect of decreased weakness and increased AChR numbers at the NMJ rather than a direct effect on muscle fibers.
The effect of agrin on AChR stabilization is thought to be concentration dependent.30–32 In keeping with that, we found that rats treated with the higher dose (6 mg/kg) of NT-1654 had greater AChR clustering and clinical response than rats treated with the lower dose (2 mg/kg). However, given the small cohort sizes, no definite conclusions can be drawn, and further investigation is necessary. Furthermore, it remains to be determined whether higher doses of NT-1654 may be more effective or whether concomitant treatment with immunomodulatory agents, which are commonly used in treatment of MG, may alter the dosing or have a synergistic effect.
Our data support further investigation of NT-1654 in EAMG and human MG. This study could not address some questions that would be relevant to human MG. In the current study, NT-1654 was administered immediately after EAMG induction and the clinical benefits were observed over the course of the disease, suggesting that the effect was long lasting. However, in humans, MG is treated only after symptoms have developed and antibody generation is well underway. It is not clear whether treatment with NT-1654 would be beneficial if initiated during the chronic phase of EAMG after the immune attack on the NMJ has had time to cause structural end-plate changes. This should be a focus of further studies in the development of NT-1654.
Another observation was that, despite administration of the last dose of NT-1654 on day 30, the clinical assessments, muscle pathology and electrophysiological testing performed on day 49 showed beneficial effect, which suggests a relatively long-lasting effect on the NMJ. Because agrin and its targets including MuSK and rapsyn play an essential role in inducing AChR clustering and maintaining the structure and function of NMJ,6,33 one possibility is that treatment with NT-1654 helps to prevent the loss of proteins including MuSK of NMJs, allowing NMJ function to endure the immune attack and makes the NMJ less susceptible to the chronic phase of EAMG after the compound is no longer present. This could indicate that the effectiveness of the compound is due to its action during the acute phase of EAMG, and that it may not be useful in human MG. Thus, the long-lasting clinical improvement after completion of administration of NT-1654 suggests that NT-1654 should be evaluated by administration during later time points in the disease process and by use in other animal models of myasthenia gravis.
No adverse effects of NT-1654 were seen during the study. The nature of NT-1654’s structure and mechanism raise certain potential safety concerns. The compound could induce antibody formation or an immune response that could either inhibit its activity or cause hypersensitivity reactions. Our data suggests that anti-agrin antibodies are not produced after treatment with NT-1654. Another study of NT-1654 immunogenicity in rats also did not demonstrate a significant immune response after 8 weeks of treatment (personal communication, S. Hettwer, PhD). An additional concern is the theoretical possibility of ectopic AChR formation which could be a potential dose-limiting feature of the compound. Although we did not address this in our study, this has been investigated by others, and no dose toxicity was seen in animals up to 30 mg/kg (personal communication S. Hettwer, PhD). Moreover, in addition to subcutaneous administration, future studies will also evaluate effects of this compound via other routes including intraperitoneal, intravenous or intramuscular injection.
In conclusion, we believe that our study provides preliminary evidence that NT-1654 decreases the clinical severity of EAMG, reduces muscle fiber atrophy and improves neurophysiological measures of neuromuscular transmission, most likely by facilitating clustering of AChRs and preventing the loss of MuSK. Given this promising pilot data, there is potential for the use of NT-1654 as an adjunctive treatment for human MG in the future. Further investigations in EAMG are planned to confirm this data and answer some of the questions raised by this preliminary work.
Acknowledgments
This study was supported in part by National Basic Research Program of China (Grant 2013CB966900), National Key-Project of Clinical Neurology, National Science Foundation of China (Grants 81230028, 81301044, 81471535), Tianjin Education Commission Foundation (Grant 14JCYBJC42000). US National Institutes of Health Grant R01NS092713; and American Heart Association Grant (16SDG27250236), and National Multiple Sclerosis Society Research Grant (RG-1507-05318).
We thank Neurotune AG, Schlieren-Zurich, Switzerland, for providing NT-1654. We thank Armin Mader and Steffan Hettwer, Neurotune AG for generously providing information on NT-1654. We thank David Richman, MD and Mark Aguis, MD for their critical review and of the manuscript and /or project.
Abbreviations
- CMAPs
compound muscle action potentials
- DOK7
docking protein 7
- EAMG
experimental autoimmune myasthenia gravis
- LRP4
low-density lipoprotein receptor-related protein 4
- MuSK
muscle-specific kinase
- NF-H
neurofilament heavy chain
- NMJs
neuromuscular junctions
- PBST
phosphate-buffered Saline with 0.1% tritonX-100
- PFA
paraformaldehyde
- PVDF
polyvinyl difluoride
- SARCO
sarcopenia-like phenotype in neurotrypsin-overexpressing
- SV2A
synaptic vesicle glycoprotein 2A
- tAChR
Torpedo acetylcholine receptor
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
References
- 1.Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85(4):513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 2.Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, Ruegg MA, Vrijbloed JW. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PloS one. 2014;9(2):e88739. doi: 10.1371/journal.pone.0088739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tezuka T, Inoue A, Hoshi T, Weatherbee SD, Burgess RW, Ueta R, Yamanashi Y. The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(46):16556–16561. doi: 10.1073/pnas.1408409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuhrer C, Gautam M, Sugiyama JE, Hall ZW. Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(15):6405–6416. doi: 10.1523/JNEUROSCI.19-15-06405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18(4):623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 7.Bolliger MF, Zurlinden A, Luscher D, Butikofer L, Shakhova O, Francolini M, Kozlov SV, Cinelli P, Stephan A, Kistler AD, Rulicke T, Pelczar P, Ledermann B, Fumagalli G, Gloor SM, Kunz B, Sonderegger P. Specific proteolytic cleavage of agrin regulates maturation of the neuromuscular junction. Journal of cell science. 2010;123(Pt 22):3944–3955. doi: 10.1242/jcs.072090. [DOI] [PubMed] [Google Scholar]
- 8.Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wolfel J, Luscher D, Zurlinden A, Stephan A, Ahmed S, Baici A, Ledermann B, Kunz B, Sonderegger P. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(13):3468–3478. doi: 10.1096/fj.07-8800com. [DOI] [PubMed] [Google Scholar]
- 9.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. The Journal of clinical investigation. 2006;116(11):2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen C, Lu Y, Zhang B, Figueiredo D, Bean J, Jung J, Wu H, Barik A, Yin DM, Xiong WC, Mei L. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. The Journal of clinical investigation. 2013;123(12):5190–5202. doi: 10.1172/JCI66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357(9274):2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 12.Cavalcante P, Bernasconi P, Mantegazza R. Autoimmune mechanisms in myasthenia gravis. Current opinion in neurology. 2012;25(5):621–629. doi: 10.1097/WCO.0b013e328357a829. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Annals of the New York Academy of Sciences. 1981;377:670–677. doi: 10.1111/j.1749-6632.1981.tb33765.x. [DOI] [PubMed] [Google Scholar]
- 14.Gold R, Schneider-Gold C. Current and future standards in treatment of myasthenia gravis. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2008;5(4):535–541. doi: 10.1016/j.nurt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losen M, Martinez-Martinez P, Molenaar PC, Lazaridis K, Tzartos S, Brenner T, Duan RS, Luo J, Lindstrom J, Kusner L. Standardization of the experimental autoimmune myasthenia gravis (EAMG) model by immunization of rats with Torpedo californica acetylcholine receptors--Recommendations for methods and experimental designs. Experimental neurology. 2015;270:18–28. doi: 10.1016/j.expneurol.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu B, Goluszko E, Christadoss P. Experimental autoimmune myasthenia gravis in the mouse. Current protocols in immunology. 2001;Chapter 15(Unit 15):18. doi: 10.1002/0471142735.im1508s21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clinical immunology. 2000;94(2):75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Goluszko E, Huda R, Tuzun E, Christadoss P. Experimental autoimmune myasthenia gravis in the mouse. Current protocols in immunology. 2011;Chapter 15(Unit 15):23. doi: 10.1002/0471142735.im1523s95. [DOI] [PubMed] [Google Scholar]
- 19.Lindstrom J, Einarson B, Tzartos S. Production and assay of antibodies to acetylcholine receptors. Methods in enzymology. 1981;74(Pt C):432–460. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Hao J, Dayao CS, Shi FD, Campagnolo DI. T-bet deficiency decreases susceptibility to experimental myasthenia gravis. Experimental neurology. 2009;220(2):366–373. doi: 10.1016/j.expneurol.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi FD. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. European journal of immunology. 2010;40(6):1577–1589. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi FD, Bai XF, Li HL, Huang YM, Van der Meide PH, Link H. Nasal tolerance in experimental autoimmune myasthenia gravis (EAMG): induction of protective tolerance in primed animals. Clinical and experimental immunology. 1998;111(3):506–512. doi: 10.1046/j.1365-2249.1998.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Lindstrom J. Antigen-specific immunotherapeutic vaccine for experimental autoimmune myasthenia gravis. Journal of immunology. 2014;193(10):5044–5055. doi: 10.4049/jimmunol.1401392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lennon VA, Lindstrom JM, Seybold ME. Experimental autoimmune myasthenia gravis: cellular and humoral immune responses. Annals of the New York Academy of Sciences. 1976;274:283–299. doi: 10.1111/j.1749-6632.1976.tb47693.x. [DOI] [PubMed] [Google Scholar]
- 25.Plomp JJ, Morsch M, Phillips WD, Verschuuren JJ. Electrophysiological analysis of neuromuscular synaptic function in myasthenia gravis patients and animal models. Experimental neurology. 2015;270:41–54. doi: 10.1016/j.expneurol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Sanders DB, Evoli A. Immunosuppressive therapies in myasthenia gravis. Autoimmunity. 2010;43(5–6):428–435. doi: 10.3109/08916930903518107. [DOI] [PubMed] [Google Scholar]
- 27.Lallana EC, Fadul CE. Toxicities of immunosuppressive treatment of autoimmune neurologic diseases. Current neuropharmacology. 2011;9(3):468–477. doi: 10.2174/157015911796557939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaishi A, Yukitake M, Kuroda Y. Long-term treatment of steroid-dependent myasthenia gravis patients with low-dose tacrolimus. Internal medicine. 2008;47(8):731–736. doi: 10.2169/internalmedicine.47.0513. [DOI] [PubMed] [Google Scholar]
- 29.Oosterhuis H, Bethlem J. Neurogenic muscle involvement in myasthenia gravis. A clinical and histopathological study. Journal of neurology, neurosurgery, and psychiatry. 1973;36(2):244–254. doi: 10.1136/jnnp.36.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85(4):525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 31.Bezakova G, Lomo T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers. The Journal of cell biology. 2001;153(7):1453–1463. doi: 10.1083/jcb.153.7.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bezakova G, Rabben I, Sefland I, Fumagalli G, Lomo T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9924–9929. doi: 10.1073/pnas.171539698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel MA, Valdez G, Tapia JC, Lichtman JW, Sanes JR. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PloS one. 2012;7(10):e46663. doi: 10.1371/journal.pone.0046663. [DOI] [PMC free article] [PubMed] [Google Scholar]