Abstract
Approximately 60% of individuals with schizophrenia do not take their antipsychotic medications as prescribed, and nonadherence is associated with exacerbation of psychotic symptoms, increased hospital and emergency room use, and increased healthcare costs. Behavioral-tailoring strategies that incorporate medication taking into the daily routine and use environmental supports have shown promise as adherence-enhancing interventions. Informed by the Information-Motivation-Behavioral (IMB) Skills Model and using the iterative process of user-centered design, we collaborated with individuals with schizophrenia and psychiatrists to develop an interactive smartphone application and web-based clinician interface, MedActive, for improving adherence to oral antipsychotic treatment. MedActive facilitates the active involvement of individuals with schizophrenia in managing their antipsychotic medication regimen by providing automated reminders for medication administration and tailored motivational feedback to encourage adherence, and by displaying user-friendly results of daily ecological momentary assessments (EMAs) of medication adherence, positive psychotic symptoms, and medication side effects for individuals and their psychiatrists. In a 2-week open trial completed by 7 individuals with schizophrenia and their psychiatrists, MedActive was determined to be both feasible and acceptable, with patient participants responding to 80% of all scheduled EMAs and providing positive evaluations of their use of the application. Psychiatrist participants were interested in viewing the information provided on the MedActive clinician interface, but cited practical barriers to regularly accessing it and integrating into their daily practice.
Keywords: Schizophrenia, Antipsychotic, Adherence, Mobile, Technology, Smartphone
Introduction
Schizophrenia affects approximately 0.5% of adults worldwide (1) and is among the most severe and costly of psychiatric illnesses (2). When used as prescribed, antipsychotic medications are effective in reducing positive psychotic symptoms, with the aim of improving long-term functional outcomes and enhancing recovery. Despite the potential benefits, upwards of 60% of individuals with schizophrenia do not take their antipsychotic medications as prescribed (3–4). Commonly cited reasons for nonadherence to antipsychotic treatment include substance abuse, poor illness insight, cognitive deficits, lack of social support, poor therapeutic alliance, practical problems (i.e., financial, environmental, lack of routine), and problematic side effects (4). Partial or complete nonadherence to antipsychotic medications is associated with numerous adverse outcomes, including exacerbation of psychotic symptoms and increased risk of relapse, impaired functioning, increased suicide attempts, increased hospital and emergency room use, and high healthcare costs (5–7).
Studies of antipsychotic adherence-enhancing interventions developed for individuals with schizophrenia to date have demonstrated mixed findings, with some strategies indicating promise. The overwhelming majority of studies testing psychosocial interventions in which psychoeducation or cognitive behaviorally-oriented compliance therapy grounded in principles of motivational interviewing were provided alone were ineffective in improving adherence (8–11). However, behavioral-tailoring strategies that incorporate medication taking into the daily routine and use environmental supports (e.g., reminders, medication packaging), self-monitoring, or reinforcement (including monetary) have been shown to improve adherence to antipsychotic treatment (12–16). However, many of these interventions are logistically complex and difficult to deliver widely, requiring specialized therapist training or in-home visits, and may be financially unsustainable.
Mobile technology-based interventions have recently been used for disease management and prevention and have been found effective in engaging consumers in treatment, enhancing traditional clinical interventions, and improving both intermediate and clinical health outcomes (17–21). Several reviews of interventions utilizing mobile phone technology, primarily involving text messaging, have shown them to be effective in improving medication adherence for a number of non-psychiatric medical conditions (e.g., diabetes, asthma, HIV, obesity) (18–19, 21–23). Mobile phone technologies are increasingly being used for both the assessment and treatment of serious mental illness as well (24–32). Mobile phones, including smartphones, represent an ideal medium to improve medication adherence because of their availability and acceptability among individuals with schizophrenia (33), and their ability to enhance the ecological validity of mental health assessments and treatments as they take place in real time and in individuals’ natural settings (34–35). Increasingly, mobile phonetechnologies are being used to improve medication adherence among individuals with schizophrenia (24–29), though only one pilot study has employed a smartphone application (28–29) to date
The aims of this study were to employ a user-centered design approach (36–37) to develop a smartphone intervention, MedActive, designed to improve antipsychotic medication adherence in individuals with schizophrenia, and to conduct a short-term open trial to determine its preliminary acceptability and feasibility among individuals with schizophrenia and psychiatrists.
Methods
Theoretical Framework for the Intervention
The conceptualization of MedActive was informed by the Information-Motivation-Behavioral (IMB) Skills Model, an empirically supported framework for understanding and addressing nonadherence to highly active antiretroviral therapy for HIV (38–39), which shares many characteristics with psychiatric medication nonadherence (4). The IMB-Skills Model asserts that well-informed and well-motivated patients with adequate skills for enacting adherence-related behaviors will exhibit better adherence. In accordance with the IMB Skills Model, MedActive provides illness and medication information, increases motivation to adhere e.g., through personalized motivational feedback, and imparts the behavioral skills required for individuals with schizophrenia to adequately adhere to antipsychotic treatment, e.g., through daily medication reminders.
Input from individuals with schizophrenia and their psychiatrists was carefully considered at each stage of developing MedActive through the iterative process of user-centered design (UCD) (36–37). UCD allows end users of a system to influence how a design takes shape to increase the ease with which a system can be learned and used. Five individuals with schizophrenia and seven psychiatrists participated in a usability testing protocol that was approved by the University of Maryland, Baltimore Institutional Review Board and informed consent was obtained from all participants. Throughout the development of MedActive, usability testing provided guidance on the specification of the format, appearance, and content of the intervention so that information was presented in a user-friendly and accessible manner to both groups of users. Individuals with schizophrenia interviewed all “strongly agreed” or “agreed” that they found the application to be easy to use and interesting, and could imagine using the application in their daily life. However, some commented that they had difficulty reading the text due to the font size and color, and had difficulty comprehending some terms, including “antipsychotic,” “cognitive problems,” and “rigidity.” All psychiatrists interviewed stated that they were “very interested” or “interested” in viewing summary reports of their patients’ adherence, symptoms, and side effects “daily” to “weekly,” and that this information would be “very useful” or “useful,” although some did express concerns about how patients’ cognitive impairments and literacy levels would interfere with their use of the application. Taking this feedback into consideration, several changes to the application were made, including alteration of the text size and color, and replacement of various terms to meet the literacy level of the patient population.
MedActive Smartphone Application User Interface: Overview
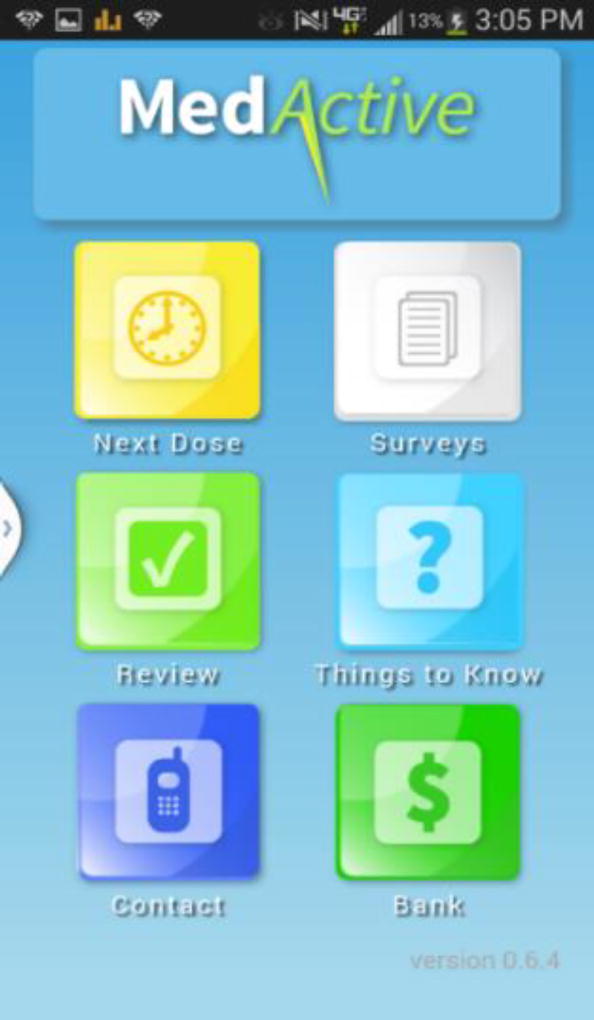
MedActive (Figure 1) consists of an Android-compatible smartphone application that reminds individuals to take their antipsychotic medications, stores and tracks information about their self-reported medication adherence, positive psychotic symptoms, and antipsychotic side effects, and allows them to research topics regarding schizophrenia and antipsychotic medications. Table 1 shows how the key features of MedActive map onto the components of the IMB Skills Model.
Figure 1.
MedActive smartphone application homepage.
Table 1.
Key Components of MedActive Smartphone Intervention
IMB-Skills Model: Adherence information
|
IMB-Skills Model: Adherence motivation
|
IMB-Skills Model: Adherence behavioral skills
|
MedActive Smartphone Application User Interface: Medication Reminders
MedActive reminds individuals to take their antipsychotic medication at scheduled times throughout the day (Supplemental (S.) Figure 2). Five minutes prior to a scheduled dose of medication, MedActive provides a personalized auditory and visual reminder to take that dose of medication. Five minutes later, individuals are asked whether they took that dose of medication. If individuals respond “yes,” rotating personalized motivational feedback messages of their choosing appear. If individuals respond “no,” they are then asked to select why they did not take that dose (options include: “It doesn’t help me,” “I don’t need it,” “It’s okay to skip it,” “I don’t have it with me,” and “The side effects make me feel bad”) and rotating personalized messages of their choice subsequently appear reminding individuals why taking their medication is helpful. If individuals respond that they are not taking their medication due to side effects, they are given the option to send an automated message to their psychiatrist via the clinician interface (see below). Individuals’ responses to these ecological momentary assessments (EMAs) of their adherence are displayed within a user-friendly calendar that appears during the EMA or can be accessed through the homepage at any time. If individuals do not respond to the initial EMA, they receive a single additional query thirty minutes later. As an incentive to respond to EMAs, participants receive points for each survey they respond to, which can be viewed as they accumulate via an icon on the homepage.
MedActive Smartphone Application: EMAs of Positive Psychotic Symptoms and Antipsychotic Side Effects
In addition to the adherence EMAs, individuals are prompted to respond to once daily EMAs regarding their positive psychotic symptoms (S. Figure 3) and antipsychotic medication side effects (S. Figure 4) at random times within a timeframe that they specify they are available.
The EMA of positive psychotic symptoms queries individuals about 7 positive psychotic symptoms, including hallucinations, disorganized thought, paranoia, having a special mission or purpose, being able to mind read, thought insertion, and communication with the TV or music (35). Similarly, the EMA of medication side effects asks individuals about 9 common side effects of antipsychotic treatment, including weight gain, difficulty concentrating, difficulty falling or staying asleep, daytime sleepiness, restlessness, lack of energy, not feeling like oneself, sexual difficulties, and muscle stiffness. Each question in both of the EMAs has a simple multiple-choice “yes/no” response, and an affirmative response leads to subsequent questions pertaining to the frequency of occurrence or the severity of the symptoms or side effects. If individuals report that they are always bothered by one or more of the symptoms or side effects, they are given the option of letting their psychiatrist know via an automated message displayed on the clinician interface (see below). If individuals do not initially respond to a request for an EMA, they are prompted to do so thirty minutes later. Individuals receive a “point” as an incentive for completing each EMA.
In addition to these prompted EMAs, individuals can also elect to report symptoms and side effects at any time during the day by accessing these surveys via an icon on the MedActive homepage. Also via the homepage, individuals can view pictorial representations of their reporting of symptoms and side effects over time (S. Figures 2 and 3), as well as a resource guide that provides concise descriptions of positive psychotic symptoms (S. Figure 5) and antipsychotic medication side effects (S. Figure 6), including brief tips on self-management of side effects, as well as tips for talking with their doctor (S. Figure 7).
MedActive Clinician Interface
MedActive also consists of a clinician interface which is a secure website that enables psychiatrists to review their patients’ daily antipsychotic medication adherence (S. Figure 8), positive psychotic symptoms (S. Figure 9), and antipsychotic side effects at any time (S. Figure 10). Alerts appear if individuals report not taking a dose of medication due to side effects or if they report always being bothered by a symptom or side effect in the previous day. To facilitate communication about medication concerns, psychiatrists could also view when their patients send them automated requests indicating their desire to speak with them at the next appointment about a specific medication, symptom, or side effect concern.
Open Trial of MedActive
Study Overview
We conducted an open trial in which individuals with schizophrenia and psychiatrists used the MedActive application and clinician interface for 2 weeks between March and June 2014. Outpatients with a diagnosis of a schizophrenia spectrum disorder received an Android smartphone and used the MedActive application for approximately 2 weeks, during which time their psychiatrists could monitor their antipsychotic medication adherence, positive psychotic symptoms, and side effects on the clinician interface. The study protocol was approved by the University of Maryland, Baltimore Institutional Review Board and informed consent was obtained from all participants.
Setting and Sample
Individuals with schizophrenia and their psychiatrists were recruited from 2 outpatient mental health clinics within the University of Maryland Division of Community Psychiatry that serve adults in low-income areas of Baltimore. Seven psychiatrists were recruited, and referred patients who met inclusion criteria for the study. The patients were diagnosed with a schizophrenia spectrum disorder as reported by their psychiatrists, between the ages of 18 and 64 years, prescribed and self-administered one or more oral antipsychotic medications, English speaking, and able to read English at a 7th grade reading level or above (per the Wide Range Achievement Test). Because the primary aim of this pilot study was to evaluate the initial feasibility and acceptability of MedActive rather than its effects on medication adherence, we did not preferentially enroll individuals who had difficulties adhering to antipsychotic treatment. Patient-participants were excluded if they had a visual, hearing, voice, or motor impairment that would prevent completion of the study procedures or use of a mobile phone. Patient-participants were paid $50 upon completion of the trial and return of the smartphone.
Procedures
At the beginning of the study, participants received an Android smartphone with the MedActive application uploaded, and completed a brief training session on how to operate them. The phone was customized with personalized medication reminders, timeframes for completion of symptom and side effect EMAs, and motivational feedback messages. The smartphone and accessories were provided free of charge with unlimited data and text messaging capabilities and limited call time for the 2-week study period. Participants were contacted on days 2 and 7 of the 2-week study period to troubleshoot any difficulties with the phone or app. If a participant did not respond to any of the adherence, symptom, or side effects EMAs over any 2-day period during the trial, they were contacted by research staff. If participants did not respond to any EMAs after 7 days and could not otherwise be contacted, they were considered lost to follow-up and the phone was deactivated. Participants were expected to return the smartphone and its accessories at the follow-up appointment.
Psychiatrist-participants were given access to the clinician interface to monitor their patients over the 2-week period in which up to 2 of their patients were using the MedActive application. They were not given a minimum frequency with which they should access the interface, and responding to requests from patients to discuss symptoms or side effects was considered optional, as would be the case in regular clinical practice.
Measures
At baseline, patient-participants completed surveys regarding their demographic information and prior use of mobile phones. During the 2-week study period, all patient-participant use of the application (i.e., button presses, responses EMAs) and psychiatrist-participant use of the clinician interface were continuously uploaded to a secure study server. At follow-up, psychiatrist- and patient-participants completed surveys querying them about the acceptability and likeability of the application and clinician interface, respectively.
The primary outcomes of the open trial were the feasibility and acceptability of the application and clinician interface. Medactive was considered “feasible” if 100% of patient- participants completed the trial, 100% returned the smartphone in working condition, and patient-participants responded to at least 70% of all EMAs. Acceptability was based on patient- and psychiatrist-participant ratings of the application and clinician interface.
Results
Participants
Of the 7 psychiatrists enrolled in the study, 6 referred patients to participate. Five of the 6 psychiatrists referred 1 patient, and 1 psychiatrist referred 2 patients. Of the 9 patients recruited for the study, 1 patient declined to participate prior to consent after hearing the description of the study and 1 did not return to complete the pre-trial surveys and mobile phone training. The remaining 7 individuals were enrolled, and all were African American males with a mean age of 47.6 (SD=10.4). Participants had been diagnosed with schizophrenia at a mean age of 24.3 (SD=12.3) and had completed an average of 10 (± 2) years of schooling. Overall, 57% (n=4) of participants were currently unemployed and all were single or never married. Six participants (86%) took their medication once per day, while the other participant (14%) took his medication twice per day. Five participants (71%) stated that they owned a mobile phone, and of these, 2 (29%) reported owning a smartphone.
Feasibility
All 7 patient-participants completed the approximate 2 week trial (range=14–18 days) and returned their phones in working order. Only 1 participant experienced minor technical difficulties in operating the phone and received re-training. Overall, participants responded to 80% of all EMAs. They responded to 85% of adherence EMAs, 79% of the EMAs of positive psychotic symptoms, and 75% of the EMAs of medication side effects. Participants reported taking their antipsychotic medication in 100% of the adherence EMAs to which they responded. The majority of participants reported experiencing symptoms and side effects in response to those EMAs (Table 2). Participants reported experiencing one or more symptoms in 62% of the EMAs to which they responded, of which “voices or visions,” “confused thinking,” and “thought insertion” were most commonly reported. Participants reported experiencing one or more side effects in 82% of the EMAs to which they responded, with “low energy” being the most commonly cited. Over half of participants also reported experiencing “concentration difficulties,” “concerns about weight,” “not feeling like oneself”, “muscle stiffness/trembling”, and “excess sedation” at least once during the trial.
Table 2.
Responses to Daily Assessments of Positive Symptoms and Antipsychotic Side Effects.
| Symptom/Side Effect Reported |
Number of participants who reported symptom/side effect at least once (%) |
Mean number of times symptom/side effect reported |
|---|---|---|
| Symptoms | ||
| Voices or Visions | 4 (57) | 8.75 ± 5.12 |
| Confused Thinking | 4 (57) | 8.50 ± 3.11 |
| Thought Insertion | 4 (57) | 5.25 ± 5.32 |
| Thought Broadcasting | 3 (43) | 5.67 ± 6.43 |
| Special Powers/Missions | 3 (43) | 5.00 ± 6.93 |
| Paranoia | 1 (14) | 13.0 |
| TV or Music Communicating | 1 (14) | 13.0 |
| Side Effects | ||
| Low Energy | 5 (71) | 6.80 ± 4.92 |
| Concentration Difficulties | 4 (57) | 8.75 ± 4.72 |
| Concerns about Weight | 4 (57) | 8.50 ± 5.80 |
| Not Feeling Like Oneself | 4 (57) | 5.75 ± 2.87 |
| Muscle Stiffness/Trembling | 4 (57) | 4.50 ± 4.51 |
| Excess Sedation | 4 (57) | 8.00 ± 3.83 |
| Insomnia | 3 (43) | 7.33 ± 5.69 |
| Sexual Problems | 3 (43) | 5.33 ± 5.86 |
| Restlessness | 3 (43) | 6.00 ± 4.36 |
With regard to psychiatrists’ use of the clinician interface, 4/6 psychiatrists (67%) used the website at least one time during the 2 week trial, logging in an average of 3 (±3) times. Three of the 4 clinicians (75%) who logged in viewed information on their patients’ medication adherence (an average of 5 (±4) times per patient), positive symptoms (9 (±10) times per patient), and antipsychotic side effects (9 (±10) times patient). This corresponded to 4/7 patients (57%) having their psychiatrists view information on their adherence and antipsychotic side effects, and 3/7 patients (43%) having their clinicians view information on their positive symptoms during the 2 weeks.
Acceptability
As shown in Table 3, the majority of patient-participants found MedActive easy to use, reporting they had no difficulties understanding and responding to the questions presented within the application and operating the smartphone. The majority also agreed that their experience using MedActive was pleasant yet challenging, but not stressful, and did not interfere negatively with daily activities. Four participants (57%) “strongly agreed” or “agreed” that they needed technical support. All patient-participants “strongly agreed” or “agreed” that they liked that their psychiatrists could view their information in real time, that MedActive was interesting and easy to use, and that the application helped them to remember to take their medication. Six participants (86%) “strongly agreed” or “agreed” that MedActive helped them talk to their psychiatrist about their medication.
Table 3.
Patient Acceptability of the MedActive Smartphone Application.
| Statement | Number of Participants Responding “Quite a Bit” or “Very Much” (%) |
| “I had difficulties understanding the questions in the MedActive application.” | 0 (0) |
| “I had difficulties typing my responses to the questions in the MedActive application.” | 0 (0) |
| “I had difficulties operating the smartphone.” | 0 (0) |
| “The smartphone was comfortable for me to carry.” | 4 (57) |
| “The reminders in MedActive interfered negatively with my activities.” | 0 (0) |
| “Overall, this experience of using MedActive was pleasant.” | 5 (71) |
| “Overall, this experience of using MedActive was challenging.” | 5 (71) |
| “Overall, this experience of using MedActive was stressful.” | 0 (0) |
| “I would be interested in participating in similar studies in the future.” | 6 (86) |
| “I would recommend to others to participate in a similar study.” | 7 (100) |
| Statement | Number of Participants Responding “Strongly Agree” or “Agree” (%) |
| “I liked that I was able to view on the phone a calendar that showed me how many days I took my medication.” | 7 (100) |
| “I liked that I was able to view on the phone a calendar that showed me how many days I reported experiencing side effects of my medication.” | 7 (100) |
| “I liked that I was able to view on the phone a calendar that showed me how many days I reported experiencing symptoms of schizophrenia.” | 7 (100) |
| “I liked that my psychiatrist was able to view how many days I took my medications.” | 7 (100) |
| “I liked that my psychiatrist was able to view how many days I reported experiencing side effects of my medications.” | 7 (100) |
| “I liked that my psychiatrist was able to view how many days I reported experiencing symptoms of schizophrenia.” | 7 (100) |
| “I liked that I was able to let my psychiatrist know when I had a question or a problem with my medications.” | 7 (100) |
| “I thought MedActive was easy to use.” | 7 (100) |
| “I found MedActive to be interesting.” | 7 (100) |
| “MedActive helped me talk to my psychiatrist about my medication.” | 6 (86) |
| “I needed technical support while using MedActive.” | 4 (57) |
| “Overall, MedActive helped me remember to take my medications.” | 6 (86) |
Five of the 6 psychiatrist-participants (83%) stated that they were “interested” in continuing to be able to view summary reports of their patients’ adherence, symptom, and side effect information on a secure website. Psychiatrists indicated that they would use the interface frequently outside of the study, with 1 (17%) stating he/she would access the interface every day, 1 (17%) 2–3 times weekly, 3 (50%) once weekly, and 1 (17%) less than once weekly. Three psychiatrists (50%) stated that they were “successful” in implementing the interface into their practice, that it was “helpful” to log onto the interface to view patients’ adherence, symptom, and side effect information, and that it was “not burdensome” to log on to the interface. Among the open-ended feedback that the psychiatrists provided, two stated that integrating the interface information into a patient’s medical records would be helpful, and two suggested providing alerts when new information became available in the interface.
Discussion
In this report, we describe a novel Smartphone-based intervention, MedActive, designed to increase adherence to antipsychotic treatment in individuals with schizophrenia. Consistent with the Information-Motivation-Behavioral (IMB) Skills Model (38–39), MedActive is expected to improve medication adherence by conveying medication-taking knowledge and heuristics, by employing personal and social motivators for adherence, and by imparting behavioral skills for sustaining medication self-administration e.g., through automated reminders. While MedActive shares some features common to other mobile technology interventions for this population, including inquiries about medication adherence (24–29) and EMAs of positive psychotic symptoms (28–29), MedActive also possesses several unique attributes. For example, whereas most of these interventions have largely relied on text messaging (24–27) and included medication adherence among one of several intervention domains (25–29), MedActive is a smartphone application that has medication adherence as its exclusive focus. MedActive incorporates personalized reminders to take medications at the specific times they are prescribed, and includes assessment of reasons for nonadherence coupled with provision of motivational feedback personally tailored to an individual’s self-identified reasons for taking medication. In addition to EMAs of adherence and positive psychotic symptoms, MedActive uniquely captures in-the-moment information on common antipsychotic side effects and provides user-friendly tips for self-management. In addition, MedActive provides longitudinal summaries of EMAs of adherence, psychiatric symptoms, and side effects in user-friendly displays for both patients and clinicians, in an effort to facilitate shared decision-making to overcome barriers to adherence.
The present study demonstrated that an antipsychotic adherence-enhancing smartphone application is feasible among individuals with schizophrenia, with 100% of participants completing the study and returning the smartphone in working condition, and participants responding to 80% of all EMAs of adherence, symptoms and side effects. Further, these individuals found the MedActive application to be acceptable, with everyone endorsing that MedActive was easy to use and helpful in remembering to take their medication, and the majority agreeing that MedActive helped them talk to their psychiatrist about their medication. It should be noted that only two participants owned their own smartphones prior to the study. Because several individuals found using MedActive to be “challenging” and felt in need of technical support, it is important that initial training and ongoing technical assistance be made available for some individuals with serious mental illness who may not be familiar with or have reservations about using newer technologies such as smartphones. Overall, our findings are consistent with past studies examining mobile phone interventions in this population, contributing to the growing body of literature demonstrating that technologies, including personal digital assistants (31), text messaging (24–27), and smartphone applications (28–29) are acceptable and feasible resources by which to address medication adherence difficulties in individuals with serious mental illness.
In this study, 85% of patient-participants self-endorsed high rates of adherence at baseline and this continued throughout the study, with participants self-reporting taking their antipsychotic medication in 100% of adherence EMAs to which they responded. This level of adherence is significantly higher than the poor adherence commonly cited in this population (3–4). This is not necessarily surprising as we did not enroll individuals based upon their medication adherence status and subjective assessments of non-adherence are known to be unreliable (40–41). Future studies evaluating the effectiveness of MedActive and similar adherence-enhancing interventions should enroll individuals with known adherence difficulties (4) and should employ more objective measures of medication adherence (e.g., unannounced pill counts (15–16), pharmacy refill records (13, 15), electronic pill caps (16, 40)) along with self-reports (41).
Despite such high rates of self-reported adherence, a significant proportion of individuals nevertheless reported experiencing positive psychotic symptoms during the 2-week study period, endorsing them in over 60% of EMAs, of which “voices or visions”, “confused thinking”, and “thought insertion” were the most commonly cited. An even greater proportion of participants experienced one or more antipsychotic side effects, having endorsed them in over 80% of EMAs, of which “concentration difficulties,” “concerns about weight” and “low energy” were mentioned most frequently. While a participating psychiatrist noted that at least one of her patients was responding to the EMAs as though they were being asked if they had ‘ever’ experienced the symptom or side effect rather than reporting over the past day, this was likely not the case for all participants. This suggests that many individuals with schizophrenia are frequently experiencing multiple psychotic symptoms and antipsychotic side effects that could be interfering with their daily lives, which could be brought to the attention of clinicians through the use of a tool like MedActive.
With regard to psychiatrists’ reactions to MedActive, the findings were more varied, with the interface being used by approximately 70% of psychiatrists who logged in an average of 3 times during the 2-week study period. While the majority of psychiatrists noted being interested in continuing to view summary reports of their patients’ adherence, symptom, and side effect information, only half considered themselves to be “successful” in implementing the interface into their regular practice. This is not necessarily surprising given the time pressures that psychiatrists face treating often complex patients in 15–20 minute visits, and not typically having access to clinical information available to them in real-time. More work is needed to understand how to better enable service systems and providers to integrate real-time patient information into existing workflows in order to facilitate measurement-based care (42). Suggestions provided by psychiatrists in our study included integrating MedActive into the existing electronic medical record system and providing alerts when new patient information becomes available.
Limitations of this study include its small sample size and limited demographic mix, short duration, and lack of a comparison group. While appropriate for feasibility testing, these shortcomings precluded any investigation of the impact of MedActive on its intended targets, including improved antipsychotic adherence and enhanced shared decision-making, as well as more distal outcomes including improved psychotic symptoms and reduced psychiatric hospitalizations. Despite these limitations, the results of this study provide the foundation for future testing of innovative smartphone interventions that incorporate principles of behavioral tailoring as a promising strategy for improving adherence to antipsychotic treatment in individuals with serious mental illness.
Supplementary Material
Clinical Implications.
Many individuals with schizophrenia have difficulties adhering to antipsychotic treatment, and behavioral tailoring strategies that incorporate medication taking into the daily routine and use environmental supports are showing promise in improving adherence. Relatively low-cost technologies like mobile smartphones whose availability is growing possess many attributes that make them particularly suitable for behavioral tailoring interventions. The results of this study show that a novel intervention, MedActive, in which a smartphone application provides automated medication reminders and tailored motivational feedback to encourage adherence, and collects and displays daily ecological momentary assessments of medication adherence, positive psychotic symptoms, and medication side effects for both patients and their clinicians, is a feasible and acceptable approach to addressing adherence problems in individuals with schizophrenia. Additional research is needed to determine whether such smartphone-based interventions are effective in improving adherence to antipsychotic treatment and associated therapeutic outcomes.
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health (R34 MH094555 to J.K.).
We would like to acknowledge David C. Mohr, Ph.D. and his staff at the Center for Behavioral Intervention Technologies (CBITs) within the Northwestern University Feinberg School of Medicine for their contributions to the development of MedActive.
Footnotes
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990–2013: a systematic literature review. BMC Psychiatry. 2015;15:193. doi: 10.1186/s12888-015-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 3.Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437–452. doi: 10.1016/j.psc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series: Adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1–46. [PubMed] [Google Scholar]
- 5.Offord S, Lin J, Mirski D, Wong B. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30(3):286–97. doi: 10.1007/s12325-013-0016-5. [DOI] [PubMed] [Google Scholar]
- 6.Novick D, Haro JM, Suarez D, Perez V, Dittmann RF, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2–3):109–113. doi: 10.1016/j.psychres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Gilmer TP, Dolder CR, Lacro JP, Folsom DP, Lindamer L, Garcia P, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 8.Barkhof E, Meijer CJ, de Sonneville LMJ, Linszen DH, de Haan L. Interventions to improve adherence to antipsychotic medication in patients with schizophrenia-A review of the past decade. Eur Psychiatry. 2012;27(1):9–18. doi: 10.1016/j.eurpsy.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, Lehman A, Tenhula WN, Calmes C, Pasillas RM, Peer J, Kreyenbuhl J. The 2009 Schizophrenia PORT Psychosocial Treatment Recommendations and Summary Statements. Schizophr Bull. 2010;36(1):48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lincoln TM, Wilhelm K, Nestoriuc Y. Effectiveness of psychoeducation for relapse, symptoms, knowledge, adherence and functioning in psychotic disorders: A meta-analysis. Schizophr Res. 2007;96(1–3):232–245. doi: 10.1016/j.schres.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Barkhof E, Meijer CJ, de Sonneville LMJ, Linszen DH, de Haan L. The effect of motivational interviewing on medication adherence and hospitalization rates in nonadherent patients with multi-episode schizophrenia. Schizophr Bull. 2013;39(6):1242–1251. doi: 10.1093/schbul/sbt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priebe S, Yeeles K, Bremner S, Lauber C, Eldridge S, Ashby D, David AS, O'Connell N, Forrest A, Burns T. Effectiveness of financial incentives to improve adherence to maintenance treatment with antipsychotics: cluster randomized controlled trial. BMJ. 2013;347:f5847. doi: 10.1136/bmj.f5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenstein M, Kavanagh J, Lee T, Reilly P, Dalack GW, Grabowski J, Smelson D, Ronis DL, Ganoczy D, Woltmann E, Metreger T, Wolschon P, Jensen A, Poddig B, Blow FC. Using a pharmacy-based intervention to improve antipsychotic adherence among patients with serious mental illness. Schizophr Bull. 2011;37(4):727–36. doi: 10.1093/schbul/sbp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montes JM, Maurino J, Diez T, Saiz-Ruiz J. Telephone-based nursing strategy to improve adherence to antipsychotic treatment in schizophrenia: a controlled trial. Int J Psychiatry Clin Pract. 2010;14(4):274–281. doi: 10.3109/13651501.2010.505343. [DOI] [PubMed] [Google Scholar]
- 15.Velligan DI, Diamond PM, Mintz J, Maples N, Li X, Zeber J, Ereshefsky L, Lam YW, Castillo D, Miller AL. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull. 2008;34(3):483–93. doi: 10.1093/schbul/sbm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velligan D, Mintz J, Maples N, et al. A randomized trial comparing in person and electronic interventions for improving adherence to oral medications in schizophrenia. Schizophr Bull. 2013;39(5):999–1007. doi: 10.1093/schbul/sbs116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15(Pt 1):1–39. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J, Hollin I, Kachnowski S. A review of the use of mobile phone text messaging in clinical and healthy behavior interventions. J Telemed Telecare. 2011;17(1):41–48. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- 19.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD007459.pub2. CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health. 2015;36:393–415. doi: 10.1146/annurev-publhealth-031914-122855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park LG, Howie-Esquivel J, Dracup K. A quantitative systematic review of the efficacy of mobile phone interventions to improve medication adherence. J Adv Nurs. 2014;70(9):1932–53. doi: 10.1111/jan.12400. (2014) [DOI] [PubMed] [Google Scholar]
- 24.Montes JM, Medina E, Gomez-Beneyto M, Maurino J. A short message service (SMS)-based strategy for enhancing adherence to antipsychotic medication in schizophrenia. Psychiatry Res. 2012;200:89–95. doi: 10.1016/j.psychres.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Granholm E, Ben-Zeev D, Link PC, Bradshaw KR, Holden JL. Mobile Assessment and Treatment for Schizophrenia (MATS): a pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophr Bull. 2012;38(3):414–25. doi: 10.1093/schbul/sbr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beebe LH, Smith K, Crye C, et al. Telenursing intervention increases psychiatric medication adherence in schizophrenia outpatients. J Am Psychiatr Nurses Assoc. 2008;14(3):217–24. doi: 10.1177/1078390308318750. [DOI] [PubMed] [Google Scholar]
- 27.Beebe L, Smith KD, Phillips C. A comparison of telephone and texting interventions for persons with schizophrenia spectrum disorders. Issues Ment Health Nurs. 2014;35(5):323–9. doi: 10.3109/01612840.2013.863412. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Zeev D, Kaiser SM, Brenner CJ, Begale M, Duffecy J, Mohr DC. Development and usability testing of FOCUS: a smartphone system for self-management of schizophrenia. Psychiatr Rehabil J. 2013;36(4):289–96. doi: 10.1037/prj0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244–53. doi: 10.1093/schbul/sbu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Zeev D, Kaiser SM, Krzos I. Remote "hovering" with individuals with psychotic disorders and substance use: feasibility, engagement, and therapeutic alliance with a text-messaging mobile interventionist. J Dual Diagn. 2014;10(4):197–203. doi: 10.1080/15504263.2014.962336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenze SJ, Armey MF, Miller IW. Feasibility and Acceptability of a Mobile Intervention to Improve Treatment Adherence in Bipolar Disorder: A Pilot Study. Behav Modif. 2014;38(4):497–515. doi: 10.1177/0145445513518421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depp CA, Ceglowski J, Wang VC, Yaghouti F, Mausbach BT, Thompson WK, Granholm EL. Augmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trial. J Affect Disord. 2015;174:23–30. doi: 10.1016/j.jad.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Zeev D, Davis KE, Kaiser S, Krzsos I, Drake RE. Mobile technologies among people with serious mental illness: opportunities for future services. Adm Policy Ment Health. 2013;40(4):340–3. doi: 10.1007/s10488-012-0424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmier-Claus JE, Myin-Germeys I, Barkus E, Bentley L, Udachina A, Delespaul PA, Lewis SW, Dunn G. Experience sampling research in individuals with mental illness: reflections and guidance. Acta Psychiatr Scand. 2011;123(1):12–20. doi: 10.1111/j.1600-0447.2010.01596.x. [DOI] [PubMed] [Google Scholar]
- 35.Granholm E, Loh C, Swendsen J. Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophr Bull. 2008;34(3):507–514. doi: 10.1093/schbul/sbm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abras C, Maloney-Krichmar D, Preece J. User-centered design. In: Bainbridge W, editor. Berkshire Encyclopedia of Human-Computer Interaction. Great Barrington, MA: Berkshire Publishing Group; 2004. pp. 463–468. [Google Scholar]
- 37.Dabbs Ade V, Myers BA, Mc Curry KR, Dunbar-Jacob J, Hawkins RP, Begey A, Dew MA. User-centered design and interactive health technologies for patients. Comput Inform Nurs. 2009;27(3):175–83. doi: 10.1097/NCN.0b013e31819f7c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–73. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 39.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5(4):193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- 40.Velligan DI, Wang M, Diamond P, Glahn DC, Castillo D, Bendle S, Lam YW, Ereshefsky L, Miller AL. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–92. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- 41.Sajatovic M, Velligan DI, Weiden PJ, Valenstein MA, Ogedegbe G. Measurement of psychiatric treatment adherence. J Psychosom Res. 2010;69(6):591–9. doi: 10.1016/j.jpsychores.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young AS, Niv N, Chinman M, Dixon L, Eisen SV, Fischer EP, Smith J, Valenstein M, Marder SR, Owen RR. Routine Outcomes Monitoring to Support Improving Care for Schizophrenia: Report from the VA Mental Health QUERI. Community Ment Health J. 2011;47(2):123–35. doi: 10.1007/s10597-010-9328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.