Abstract
This study examined behavioral, heart rate (HR), and event-related potential (ERP) correlates of attention and recognition memory for 4.5-, 6-, and 7.5-month-old infants (N = 45) during stimulus encoding. Attention was utilized as an independent variable using HR measures. The Nc ERP component associated with attention and the Late Slow Wave (LSW) associated with recognition memory were analyzed. 7.5-month-olds demonstrated a significant reduction in Nc amplitude with stimulus repetition. This reduction in Nc was not found for younger infants. Additionally, infants only demonstrated differential LSW amplitude based on stimulus type on attentive trials as defined by HR changes. These findings indicate that from 4.5 to 7.5 months, infants’ attentional engagement is influenced by an increasingly broader range of stimulus characteristics.
The ability to pay attention, encode to memory, and subsequently recognize a visual stimulus is a fundamental cognitive function which emerges early in human development. Each of the sub-processes involved in this cognitive function has been studied extensively in research on infant cognitive development (see reviews, Colombo, 2001; Reynolds, Courage, & Richards, 2013; Rose, Feldman, and Jankowski, 2004). The end product, recognition memory, is characterized by differential responsiveness to familiar stimuli in comparison to novel stimuli. Inferences made based on the direction of this differential responsiveness have been the source of longstanding debate within the developmental literature (e.g., Fisher-Thompson & Peterson, 2004). In the current study, we utilized behavioral, psychophysiological, and neural measures of attention and recognition memory to address questions regarding the functional significance of infant novelty and familiarity preferences in relation to processing repeated and non-repeated visual stimuli.
Preferential Looking Measures of Infant Visual Attention and Recognition Memory
The visual paired comparison (VPC) task is the most commonly used preferential looking procedure for examining recognition memory in infant participants. The VPC task involves the paired and simultaneous presentation of two visual stimuli. Participants are typically given prior exposure to one of the stimuli, whereas the other stimulus is novel during testing. Although preferential looking to either stimulus indicates discrimination of the “familiar” from the “novel” stimulus, one of the main sources of debate in the extant literature concerns the functional significance of familiarity and novelty preferences in relation to stimulus processing and recognition memory.
In one of the earlier hypotheses regarding the development of infant visual preferences, Hunt (1963) proposed a two stage developmental sequence characterized by familiarity preferences until around 6 months of age followed by a transition to novelty preferences at older ages. However, findings from Fantz’s (1964) research on habituation were inconsistent with a developmental shift from familiarity to novelty preferences. In Fantz’s procedure, infants were exposed to repeated VPC trials pairing a repeated stimulus with non-repeated (novel) stimuli. Infants showed a reduction in looking to the repeated stimulus and coinciding shift to greater looking to novel stimuli which was assumed to occur as the infant became increasingly familiar with the repeated stimulus. Fantz’s (1964) findings showing within-session decreases in looking to repeated relative to novel stimuli fit more with the possibility that infant visual preferences represent some aspect of information processing as opposed to developmental status.
Early examples of hypotheses relating visual preference behavior to information processing were based on the comparator model (Sokolov, 1963) and the discrepancy hypothesis (e.g., McCall & Kagan, 1967, 1970). Under both of these models, infant looking is proposed to reflect a process of comparing the visual stimulus with a previously encoded engram (or schema). A mismatch between the current stimulus and existing engrams results in longer looking and active stimulus encoding, and a match results in brief looking (i.e., recognition of a familiar stimulus). The discrepancy hypothesis provided a further prediction that look duration to novel stimuli should show an inverted-U shaped pattern based on amount of discrepancy from familiar stimuli. Findings from several studies support this hypothesis with infants demonstrating the longest looking to stimuli that differ from the familiar to a moderate extent (McCall & McGhee, 1977; see also: Kidd, Piantadosi, & Aslin, 2012; Piantadosi, Kidd, & Aslin, 2014).
Several cognitive models of infant visual preferences have been proposed based on the premise that looking during visual preference tasks reflects active perceptual or cognitive processing (e.g., Bahrick & Pickens, 1995; Hunter & Ames, 1988; Wagner & Sakovits, 1986). For example, Hunter and Ames (1988) proposed that with repeated presentations of a familiar stimulus during initial processing, an infant’s visual preference behavior progresses from a null preference prior to the onset of stimulus processing in early trials, to a familiarity preference as the infant is actively engaged in encoding features of the familiar stimulus, to a novelty preference indicative of recognition memory of a fully encoded familiar stimulus. This proposal is consistent with Rose and colleagues’ (1982) finding that 3.5-month-olds given 10 s of familiarization with a visual stimulus subsequently demonstrated familiarity preferences in a VPC task; however, infants of the same age given 30 s of familiarization demonstrated novelty preferences.
The majority of findings supporting the familiarity-novelty curve proposed by Hunter and Ames (1988) have come from studies in which group averages of preference scores were analyzed as opposed to examining the trajectory of preference scores throughout testing sessions for individual participants. Roder, Bushnell, and Sasseville (2000) used a procedure similar to Fantz’s (1964) preferential looking task to examine the progression of individual infant’s visual preferences during the course of visual processing. 4.5-month-old infants were shown VPC trials using the same familiar stimulus paired with a novel stimulus for every comparison. The familiar stimulus was presented on the same side for every trial for each participant. Infants shifted from familiarity preferences on early trials to novelty preferences on later trials. However, almost 40% of participants never reached criterion for a novelty run or showed strong side biases throughout testing. Fisher-Thompson and Peterson (2004) found that after controlling for side biases in a similar procedure, infants tended to demonstrate short novelty runs throughout testing as opposed to demonstrating a shift from familiarity preferences in early trials to novelty preferences in later trials. The authors (Fisher-Thompson & Peterson, 2004) concluded that infant looking in the VPC task fluctuates from trial to trial based on competition between the tendency to prefer novelty versus the tendency to look back to previous locations.
Neural Correlates of Infant Visual Attention and Recognition Memory
The ERP technique has been extensively used as a measure of neural activity associated with infant visual attention and recognition memory (for review, de Haan, 2007). ERPs are EEG voltage oscillations which are time-locked with an event of interest and averaged across trials by experimental condition (Picton et al., 2000). ERP components associated with different stages of perceptual and cognitive processing can be identified in the averaged ERP waveform. The Nc and LSW components have been commonly associated with infant attention and recognition memory, respectively.
The Nc ERP component provides an index of infant attentional engagement and is often found to be greater in amplitude to novel stimuli in comparison to familiar stimuli (e.g., Courchesne, Ganz, & Norcia, 1981; de Haan & Nelson, 1999; Karrer & Ackles, 1987; Nikkel & Karrer, 1994; Reynolds & Richards, 2005, 2009; Richards, 2003; Webb, Long, & Nelson, 2005). Nc is a negatively polarized component typically located at midline electrodes with a peak latency occurring between 350 and 750 ms post stimulus onset. The LSW is most commonly found at temporal electrodes from 1 to 2 s post stimulus onset. A significant reduction in the amplitude of the LSW has been routinely observed across repeated stimulus presentations. Thus, the LSW is believed to be associated with stimulus encoding and infant recognition memory (de Haan & Nelson, 1999; Guy, Reynolds, Mosteller, & Dixon, 2017; Guy, Reynolds, & Zhang, 2014; Reynolds, Guy, & Zhang, 2011; Nelson & Collins, 1991, 1992; Snyder, 2010; Snyder, Webb, & Nelson, 2002; Webb, Long, & Nelson, 2005; Wiebe et al., 2006).
Reynolds, Courage, and Richards (2010) conducted a multi-level analysis of visual attention and recognition memory in 4.5-, 6-, and 7.5-month-old infants. The authors designed a visual preference ERP (VP-ERP) procedure comprised of a familiarization phase followed by blocks of VPC trials alternated with blocks of ERP trials. This allowed for the analysis of relations between individual infant’s visual preference scores and neural responses to familiar and novel stimuli during ERP trials. Independent component analysis was used to identify and remove eye movement components in the EEG during VPC trials allowing for the analysis of ERPs during the VPC trials. Finally, heart rate was measured as psychophysiological index of infant attention throughout testing. Richards (Richards, 1997; Richards & Casey, 1992) defined the heart rate phases of attention which can be used to identify periods when the infant is attentive (referred to as sustained attention) as opposed to periods of time when the infant is inattentive (referred to as attention termination). Sustained attention is characterized by a maintained decrease in HR below prestimulus levels. Attention termination is characterized by a return of HR to prestimulus levels paired with continued looking at the stimulus. Nc was found to be greater in amplitude during sustained attention than attention termination. Furthermore, regardless of stimulus type, infants demonstrated greater amplitude Nc during looks to their visually preferred stimulus than during looks to their non-preferred stimulus. These results revealed convergent findings across multiple levels of analysis, and demonstrated the utility of the VP-ERP procedure for identifying brain-behavior relations during performance on recognition memory tasks.
Development of Attention Systems
Several theorists have proposed that the timing of developmental change in visual attention reflects the development of neural systems involved in attention (for reviews, see Colombo, 2001; Reynolds et al., 2013). Richards and colleagues (Reynolds et al., 2013; Richards 2008, 2010) proposed that a general arousal/attention system regulates state-related changes in arousal involved in attention. Areas of the brain involved in this system include the mesencephalic reticular formation, limbic system, and cardioinhibitory centers in the orbitofrontal cortex. Cortical areas involved in other aspects of attention (e.g., selective attention and executive control) will demonstrate enhanced activity when the infant is attentive and the general arousal/attention system is engaged. With increasing age in infancy, infants show gains in the amount of time spent in sustained attention and the magnitude of the HR response associated with sustained attention (Courage, Reynolds, & Richards, 2006; Richards, 2004).
Under the framework of Posner’s attention systems (Posner & Peterson, 1990), the posterior orienting system and the anterior attention system are proposed to be critical for the development of selective spatial attention and executive control components of attention. From 3 to 6 months of age, the posterior orienting system reaches functional maturity. This attention network includes the pulvinar nucleus of the thalamus, posterior parietal areas, and the frontal eye-fields; and is involved in the ability to voluntary disengage and shift visual attention (Johnson, Posner, & Rothbart, 1991; Posner & Rothbart, 2013). After 6 months of age, the anterior attention system associated with endogenous attentional control begins to develop. Areas of prefrontal cortex (e.g., orbitofrontal cortex, dorsolateral prefrontal cortex, and anterior cingulate cortex) are key components in this network involved in performance on tasks requiring early forms of executive function (e.g., Bell & Fox, 1994; Posner, 1995; Reynolds et al., 2015, 2016).
Statement of Purpose
In the current study, we used a VP-ERP procedure to examine brain-behavior relations while 4.5-, 6-, and 7.5-month-olds infants were actively engaged in the process of encoding a visual stimulus. This age range covers a major developmental transition in which the posterior orienting system reaches functional maturity, and rapid changes in the development of attention lead to gains in the volitional control of attention (e.g., Kwon, Setoodehnia, Baek, Luck, & Oakes, 2016; Posner & Rothbart, 2013; Ross-Sheehy, Schneegans, & Spencer, 2015). The procedure was a combination Fantz’s (1964) preferential looking task and Reynolds and colleagues’ (2010) VP-ERP procedure. Infants were shown repeated and non-repeated stimuli in a series of alternating blocks of VPC trials and ERP trials. Infants were shown one stimulus repeatedly until they demonstrated a stable novelty preference on VPC trials pairing the repeated stimulus with a non-repeated stimulus. Once the infant reached criterion for a novelty preference, the repeated stimulus was replaced with a new repeated stimulus. This allowed us to utilize neural correlates of attention and memory to provide insight into longstanding questions regarding whether behavioral progression from familiarity – novelty preferences represents underlying cognitive processes related to initial encoding of a novel stimulus (Hunter & Ames, 1988). Heart rate was also measured to determine ERP trials in which the infants were attentive and inattentive during testing.
If changes in the direction of infant visual preferences reflect stimulus processing (e.g., Hunter & Ames, 1988; Rose et al., 1982), then similar changes would be expected to occur in the amplitude of Nc and LSW. Consistency in the direction of changes in infant visual preferences and ERP component amplitude would be less likely to occur if the familiarity-novelty curve is simply an artifact of averaging look lengths across trials and infants. We predicted that as infants progressed from early repetition trials to trials preceding criterion for novelty preferences, they would shift from demonstrating greater amplitude Nc and LSW to the repeated stimulus to showing greater amplitude to the non-repeated stimuli. We also predicted that differences in ERP amplitude based on stimulus repetition and visual preference behavior would be significantly greater on attentive trials (as defined by HR changes) than inattentive trials.
Method
Participants
A total of 45 infants were tested in a cross-sectional design at 4.5 (M = 144 days, SD = 5.18, 8 F/8 M), 6 (M = 188 days, SD = 7.17, 5 F/7 M), or 7.5 (M = 226 days, SD = 6.2816, 7 F/10 M) months of age. An additional 19 infants were tested that did not provide useable data due to fussiness, inattentiveness, excessive artifact, or technical problems. All participants were born full-term (gestational age of 38 weeks or greater), weighed greater than 2500 g at birth, and had no history of pre- or perinatal medical complications. Only infants that maintained an alert, awake state throughout the procedure were retained in the study. Contact information for participants’ parents was obtained from commercial mailing lists. Parents were paid $30 for their infant’s participation in the study. The majority of participants were non-Hispanic and of Caucasian or African-American descent (Caucasian = 73%; African-American = 23%). Data collection was carried out from 2005 until 2008.
Apparatus and stimuli
A 29” color video monitor (NEC Multisync XM29) was used. The display was set to 1280 horizontal and 1024 vertical pixels. Throughout testing the infant was seated with their eyes located approximately 55 cm from the center of the monitor.
Camera and participant monitor
A video camera was located above the monitor for the purpose of judging infant visual fixation. Fixations were judged on-line using a video feed of the infant’s face. The video was recorded with the use of a Dell Workstation 610 computer equipped with a Broadway digital video card for digitizing video in an AVI format. Video resolution was limited to a single video frame (30 frames/sec, one frame = ~ 33 ms). A time code based upon frame number of the digitized video was used to synchronize physiological recordings, video information, and experimental events.
Visual Stimuli. Object bitmaps
The memory stimuli consisted of 139 photographed images of household objects presented against a static and relatively uniform background. The background scenery came from the Sesame Street television program (either a blue sky, a bedroom wall, or a bathroom wall). Static background scenery was used instead of a solid background to maintain infant interest levels and fixations when the memory stimuli were not on the screen. The background scenes were saved as photographs in bitmap format for experimental presentations. When presented on the monitor, each stimulus object image covered a 7” wide × 8” vertical area.
Sesame Street Characters
Videos of Sesame Street characters were used as attractor stimuli to attract initial fixation to the center of the display monitor before the onset of VPC trials and before the onset of blocks of ERP trials. The attractor stimuli were also used to regain the fixation of distracted infants throughout ERP trials. These stimuli covered a 2° by 3° rectangular area. The character was placed at the center of the monitor to attract infant fixation, once the infant shifted fixation to the character, the experimental presentations were resumed following a random delay of 300 – 800 ms.
Procedure
Infants were held on a parent’s lap during testing approximately 55 cm from the monitor during testing. A schematic diagram of the steps involved in the testing procedure is provided in Figure 1. Throughout the procedure, VPC trials were alternated with brief stimulus ERP presentations. Each trial block began with a dynamic attractor stimulus presented in the center of the presentation monitor (Step 1). Once the infant was centrally fixated on the attractor stimulus, the experimenter initiated the first VPC trial via button press on the experimental control PC keyboard. The VPC trials consisted of two stimuli presented simultaneously 10˚ to the left and right of midline (Step 2). One of the stimuli was randomly chosen to be the repeated stimulus. This remained the repeated stimulus until the novelty preference criterion was reached. A second stimulus was chosen (randomly) to be the non-repeated stimulus for the VPC trial. Side of presentation of the repeated stimulus varied at random across VPC trials. After 4 s of accumulated looking in the VPC trial, an attractor stimulus was presented again to regain central fixation (Step 3), followed by 4 brief-stimulus ERP trials (Step 4). These ERP trial sequence consisted of two 500 ms presentations of the repeated stimulus and two 500 ms presentations of the non-repeated stimulus. The 4 ERP trials were presented in random order. Each 500 ms ERP presentation was followed by a static presentation of the background slide for a duration that varied at random between 1300 – 1800 ms. Thus, the duration of the inter-stimulus interval (ISI) between each ERP trial varied at random between 1800 – 2300 ms. Following the block of 4 ERP trials, a new non-repeated stimulus was chosen for the next block of VPC/brief stimulus trials. Each block began with presentation of an attractor stimulus (Step 5), followed by another VPC trial (Step 6), another presentation of an attractor stimulus (Step 7), and 4 additional ERP trials with the repeated and new non-repeated stimulus (Step 8).
Figure 1.
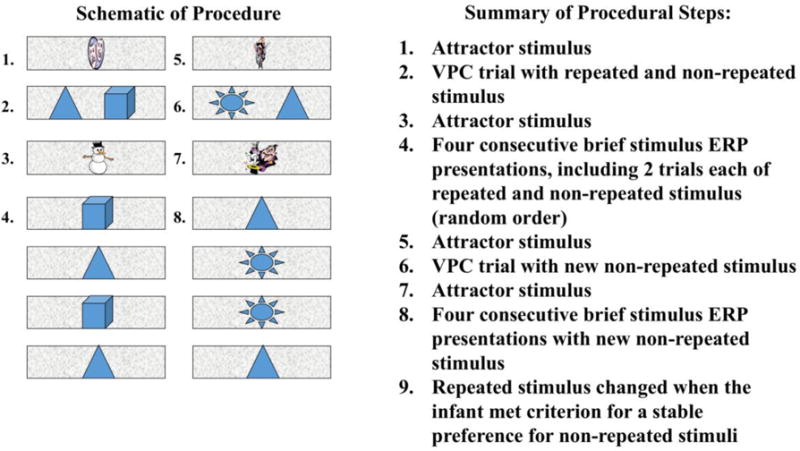
A schematic diagram of the procedural steps is shown on the left panel. A summary of the procedural steps is presented on the right panel.
Looks were coded online to determine visual preferences. Visual preference scores were only calculated on VPC trials in which the infant looked at both the left and right stimuli. The repeated stimulus remained the same object image until the infant demonstrated novelty preferences (i.e., > .55 of total looking to non-repeated stimulus) on 4 consecutive VPC trials, at which point a new block began with the repeated stimulus being replaced with a new stimulus (Step 9). This sequence of alternating VPC and ERP trials was repeated until the end of testing. Infants were tested until they were no longer on task. The 4 s duration chosen for the VPC trials, and the criterion used to determine a stable novelty preference were based on protocols used successfully in previous studies (e.g., Reynolds et al., 2010; Rose, Feldman, & Jankowski, 2002).
Fixation judgments
Infant fixations were judged online by an observer in an adjacent experiment control room to determine the timing of stimulus presentations. During VPC trials, the observer viewed the video feed of the participant and pressed a keyboard button for the duration of each look to the left or right stimulus. Custom software was used to calculate the length of each look and sum the accumulated looking. Off-line judgments were used for the purposes of data processing and analysis. Trials which the observer judged that the infant was not fixated on the monitor during stimulus presentation were not included in the analysis.
Fixation direction
For VPC trials, two observers judged fixation direction off-line for approximately 33% of the participants. Observers were blind to the experimental conditions for each trial. The average agreement between observers that a look occurred (right, left, away) was 91%. The average difference between the duration of the looks toward the stimuli was 0.7 s. The correlation between the two observers for the duration of the looks was 0.859. Novelty preferences were calculated by dividing the total time looking toward the non-repeated stimulus by the total time of accumulated looking during a VPC trial.
Measurement and quantification of HR
The electrocardiogram (ECG) was recorded using Ag-AgCl electrodes placed on each infant’s chest with disposable electrode collars. The Electrical Geodesics Incorporated (EGI) system was used to amplify and digitize the ECG. The ECG was sampled at 250 Hz. A custom computer algorithm was used to identify the QRS complex and to define the inter-beat interval (IBI) for each successive R – R interval. An algorithm developed by Quigley, Jang, and Boysen (1990) in combination with visual inspection was used to identify artifacts in the ECG. For a more detailed description of the approach to HR processing, the interested reader is referred to Courage and colleagues (2006).
HR-defined attention phases
Each experimental trial was classified by HR changes into “attentive” and “inattentive”. The “attentive” periods were defined by the onset of a deceleration in HR (lengthening of the IBI) continuing until the HR returned to pre-deceleration level. HR decelerations were defined as five successive beats with IBIs longer than the median of the five beats preceding stimulus presentation. A return of HR to its prestimulus level was defined as five successive beats with IBIs shorter than the median IBI of the five prestimulus beats, following a deceleration. Any period of time from when the infant looked at the stimulus before a HR deceleration began was defined as “inattentive”. Periods of time in between the return of HR to pre-deceleration levels and the onset of a subsequent HR deceleration were also defined as “inattentive”.
Measurement and quantification of EEG
EEG was measured using a high-density 128 channel EEG EGI (Electrical Geodesics Incorporated, Eugene, Oregon) recording system. The Netstation software package produced by EGI was used for A/D sampling, data storage, zero and gain calibration for each channel, and impedance measurement. The electrode net was placed on the infant’s head, and impedances were assessed until below 100 kΩ. The sampling rate of the EEG was 250 Hz. The EEG was referenced to Cz during recording, and algebraically re-referenced to the average reference after recording. The EEG amplification was set to 20K. A band-pass filter from 0.1 to 100 Hz was applied during EEG recording. A further low pass filter set at 45 Hz was applied off-line prior to ERP segmentation. The EEG recordings were manually inspected and individual channels within trials were eliminated from the analyses if artifacts, poor recordings, or blinks occurred. Blinks were defined on the basis of a difference between the two electrodes on the sensor net on the outside canthii of the eye and the two electrodes above the eye and were defined as electrooculogram (EOG) changes >150 μV in the vertical direction. Trials in which greater than 10% of the electrode channels were marked bad were excluded from analysis. Further details of the equipment and procedures may be found in Reynolds and colleagues (2005, 2010).
Quantification of ERP
The ERP averages for the brief stimulus presentations were segmented from 50 ms before stimulus onset through 2 s after onset. Each ERP segment was baseline corrected using the average of the 50 ms pre-stimulus baseline period. The Nc component is typically located at midline frontal and central electrodes. We analyzed the mean data from clusters of electrodes that corresponded to these regions. Nc mean amplitude was analyzed from the intervals from 350 ms to 750 ms following stimulus onset from midline frontal (4, 10, 11, 16, 19, and 20) and central (7, 32, 55, 81, and 107) electrode locations. For the LSW analysis, mean amplitude of the ERP from 1000 to 1750 ms post stimulus onset was analyzed from clusters of electrodes at left temporal (51, 58, 59, 64, 65, and 66) and right temporal (85, 91, 92, 96, 97, and 98) locations. The positions of these electrode clusters are indicated in the shaded boxes on the Geodesic Sensor Net (GSN) montage shown in the top left panel of Figure 3. Only infants that contributed a minimum of 8 artifact-free ERP trials per condition were included in the analysis. On average, infants contributed 47.76 trials (SE = 3.0; range = 78) for the repeated stimulus condition, 46.62 trials (SE = 2.95; range = 66) for the non-repeated stimulus condition, 42.43 trials (SE = 3.08; range = 92) during attention, 52.80 trials (SE = 5.33; range = 120) during inattention, 35.24 (SE = 2.04; range = 54) for the early repetition condition, and 27.90 trials (SE = 2.28; range = 54) for the late repetition condition.
Design for Statistical Analysis
The design for the study included between-subjects factors of testing age (3: 4.5, 6, 7.5 months) and attention phase (2: attention, inattention), and within-subjects factors of stimulus type (2: repeated, non-repeated) and repetition (2: early repetition, late repetition). Electrode location was utilized as an additional within-subjects factor. The electrode locations varied for this factor for the Nc analysis (2: midline frontal, midline central) and the LSW analysis (2: left temporal, right temporal). For the repetition factor, late repetition files were defined as trials which were over halfway through a block of trials (but not including criterion trials). Only trial blocks comprised of at least a total of 32 ERP trials were included in the repetition analysis. ANOVAs for the analyses were done using “Proc GLM” in SAS. Scheffe-type methods were used to control for inflation of test wise error rate, and all significant tests are reported at p < .05. Effect sizes are reported using eta squared (η2) on significant experimental effects.
Results
Descriptive Summary of Looking Behavior on VPC Trials
We primarily utilized the participants’ preferential looking on VPC trials and HR data to define the stimulus repetition and attention factors used in the ERP analyses. The following summary of the characteristics of participants’ preferential looking behavior on VPC trials is included for descriptive purposes only. For a review of the extensive body of research examining relations between infant looking behavior and HR measures of attention, the interested reader is referred to Reynolds and Richards (2008). On average, infants completed 19.83 (SE = .75) VPC trials. The majority of infants reached criterion for a stable novelty preference at least once during testing (N = 35). However, 10 infants failed to reach criterion for a stable novelty preference.
The average number of times infants reached criterion within a testing session was 1.2 (SD = .96). No differences were found across age groups for number of times a participant reached criterion during a testing session, F(2, 43) .83, p = .42. Across age groups, the average number of VPC trials infants completed before reaching criterion for a stable novelty preference was 10.22 (SE = .59). By age group, the average number of VPC trials to reach criterion was 10.03 (SE = .98) for 4.5-month-olds, 11.23 (SE = 1.13) for 6-month-olds, and 9.55 (SE = .96) for 7.5-month-olds. Figure 2 shows a backwards plot of the average preference scores for the non-repeated stimulus across blocks leading up to meeting the criterion of 4 consecutive VPC trials with greater than .55 proportion of looking toward the non-repeated stimulus (i.e., a novelty preference). As can be seen in this plot, infants generally shifted from familiarity preferences for the repeated stimulus on early trials to null preferences prior to reaching criterion line.
Figure 2.
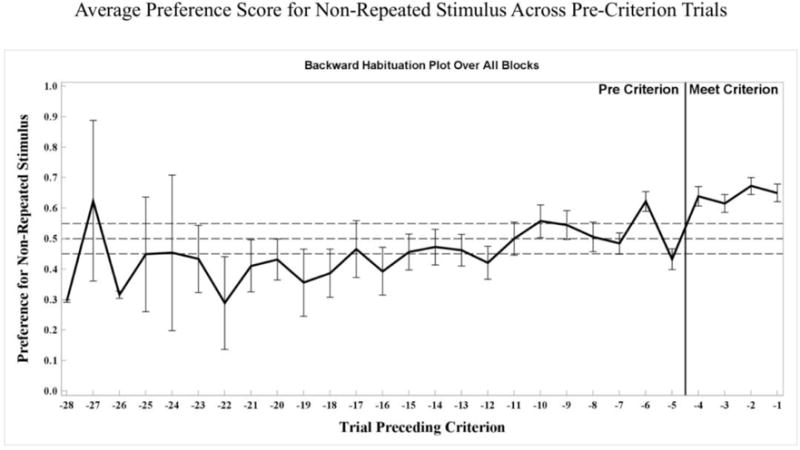
Average preference score (SE) for the non-repeated stimulus from VPC trials preceding criterion trials. Preference score for the non-repeated stimulus is shown on the Y-Axis, and trials preceding criterion are shown on the X-Axis.
ERP Data Analysis
The ERP grand averages are shown by electrode location and stimulus type in Figure 3. The Nc component can be seen as a negatively-polarized deflection in the waveform occurring between 350 to 750 ms post stimulus onset at midline frontal and central leads. The LSW can be seen from 1000 to 1750 ms post stimulus onset at temporal electrodes. A summary of significant experimental effects is provided in Table 1 and described in the sections that follow.
Figure 3.
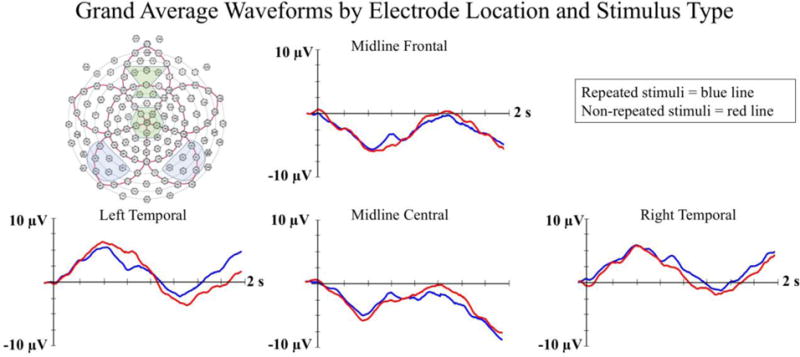
The grand average ERP waveforms by stimulus type and electrode location. Change in amplitude of the ERP relative to the prestimulus baseline is represented on the Y-axis (in microvolts), and time following stimulus onset is represented on the X-axis. The electrodes included in each electrode cluster used in the analyses are indicated in shaded boxes in the GSN Sensor Net montage shown in the upper left.
Table 1.
Summary of significant experimental effects and post hoc analyses by ERP component.
| Nc Component Analysis | |
|---|---|
| Significant Effects | Post Hoc Analyses |
| Attention × Stimulus Repetition × Electrode Location interaction | Greater amplitude Nc during attention on early repetition trials |
| Age × Stimulus Repetition interaction | Greater amplitude Nc on late repetition trials for 6-month-olds |
| Age × Stimulus Type × Stimulus Repetition interaction | Reduced amplitude Nc to repeated stimuli from early to late trials for 7.5-month-olds at central electrodes |
| LSW Component Analysis | |
| Significant Effects | Post Hoc Analyses |
| Attention × Stimulus Type interaction | Differences in LSW amplitude based on stimulus type only found on attentive trials |
The Nc Component
Nc was analyzed as the mean amplitude of the ERP waveform occurring between 350 and 750 ms post-stimulus onset at midline frontal and central electrodes. There was a significant three-way interaction between attention, stimulus repetition, and electrode location, F(2, 79) 5.63; p < .01, η2 = .12. Follow up analyses revealed a significant interaction of attention and stimulus repetition at midline central electrodes, F(1, 32) 4.32; p < .05, η2 = .12 (see Figure 4). On early repetition trials, infants showed significantly greater amplitude Nc during attention (M = −8.93, SD = 21.84) than during inattention (M = −4.37, SD = 21.63). On late repetition trials, there were no differences between attentive (M = −7.19, SD = 22.22) and inattentive (M = −8.10, SD = 23.50) trials. There were no significant differences based on attention and/or stimulus repetition at midline frontal electrodes (all ps > .35).
Figure 4.
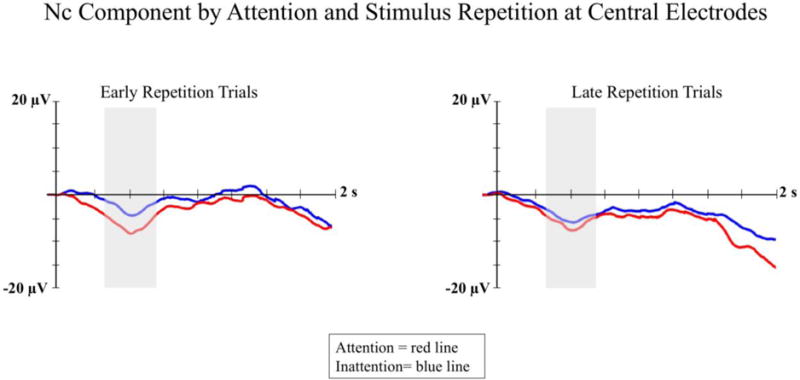
Nc ERP amplitude is presented by attention and stimulus repetition at the midline central electrode cluster. The left panel shows ERP waveforms for early repetition trials, and the right panel shows ERP waveforms for late repetition trials. Trials in which infants were engaged in sustained attention (measured with heart rate) are represented with red lines and trials in which infants were inattentive are represented with blue lines. The shaded areas on the waveform plots indicate the time-window for the Nc analysis (i.e., 350 – 750 ms).
Age interacted with several factors. First, there was an age by stimulus repetition interaction, F(2, 41) 3.75; p < .05, η2 = .14. Follow-up analyses done by age group revealed that 6-month-olds demonstrated a significant increase in Nc amplitude from early trials (M = −3.22, SD = 21.29) to late trials (M = −6.31, SD = 20.82), regardless of stimulus type. No differences were found based exclusively on stimulus repetition for the 4.5-month-olds and 7.5-month-olds. However, there was a marginally significant interaction of age, stimulus type, and stimulus repetition, F(2, 39) 3.09; p = .057, η2 = .12. In contrast to the 6-month-olds who demonstrated increased amplitude Nc based on stimulus repetition, 7.5-month-olds showed significant differences in Nc amplitude based on stimulus type with greater amplitude Nc following non-repeated stimulus presentations (M = −6.85; SD = 21.21) in comparison to repeated stimulus presentations (M = −4.58, SD = 21.19). No significant differences were found based on stimulus type for 4.5-month-olds or 6-month-olds.
There was also a significant interaction between age, stimulus type, and electrode location on Nc amplitude, F(2, 42) 3.35; p < .05, η2 = .08. Follow up ANOVAs were run separately at each electrode location to test one of our primary hypotheses that infants would demonstrate a shift from showing greater Nc amplitude to the repeated stimulus on early repetition trials to greater Nc amplitude to non-repeated stimuli on late repetition trials. The ANOVA run at midline central electrodes revealed a significant interaction of age, stimulus type, and stimulus repetition, F(2, 30) 6.28; p < .05, η2 = .29. As shown in Figure 5, 7.5-month-olds showed a significant reduction in Nc amplitude to the repeated stimulus from early repetition (M = −13.46, SD = 20.86) to late repetition trials (M = −1.72, SD = 19.25), but did not demonstrate a reduction in Nc amplitude to the non-repeated stimuli from early trials (M = −11.08, SD = 21.11) to late trials (M = −11.17, SD = 21.33). The 4.5- and 6-month-old groups did not demonstrate significant interactions of stimulus type by stimulus repetition. There were no significant differences found in comparisons between repeated and non-repeated stimuli.
Figure 5.
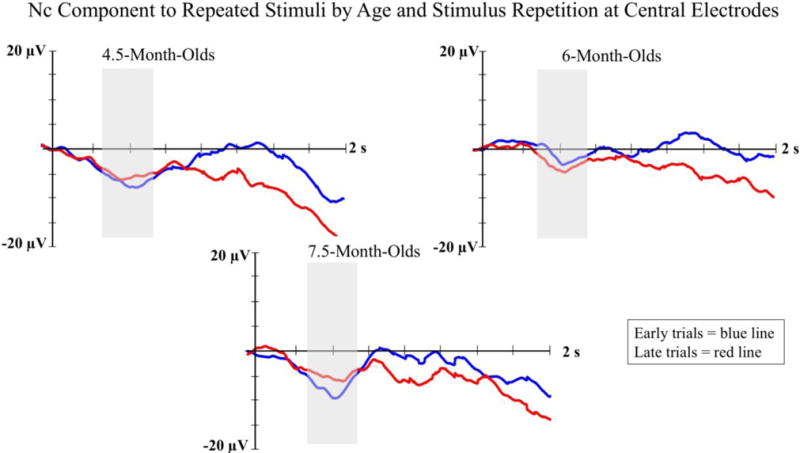
Nc ERP amplitude to repeated stimuli is presented by age and stimulus repetition at the midline central electrode cluster. ERP waveforms for each age group are presented in separate panels. Early repetition trials are represented with blue lines and late repetition trials are represented with red lines. The shaded areas on the waveform plots indicate the time-window for the Nc analysis (i.e., 350 – 750 ms).
The Late Slow Wave
The LSW was analyzed as the mean amplitude of the ERP waveform occurring between 1000 and 1750 ms post-stimulus onset at left and right temporal electrodes. There was a significant interaction between attention and stimulus type on LSW amplitude, F(1, 29) 7.15; p < .01; η2 = .10 (see Figure 6). On attentive trials, infants showed significant differences in LSW amplitude between the repeated stimulus (M = .86, SD = 21.97) and the non-repeated stimuli (M = −2.03, SD = 21.95). On inattentive trials, no differences were found between LSW amplitude to the repeated stimulus (M = −.50, SD = 22.61) and the non-repeated stimuli (M = −.30, SD = 21.44).
Figure 6.
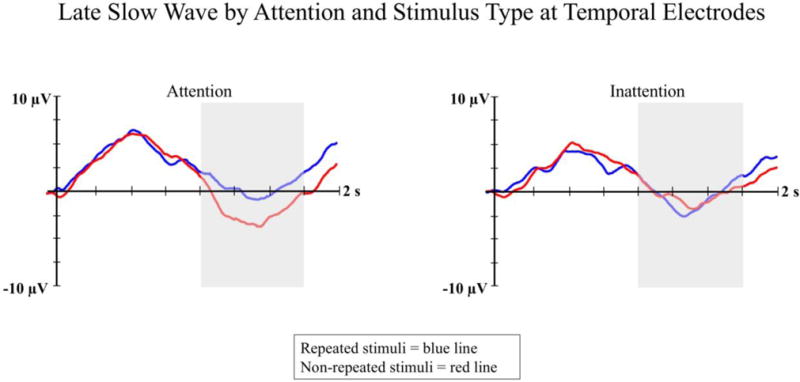
LSW ERP amplitude is presented by attention and stimulus type at temporal electrodes. The averaged ERP waveform from attentive trials is shown in the left panel, and the averaged ERP waveform from inattentive trials is shown in the right panel. Blue lines represent responses to the repeated stimuli and the red lines represent responses to non-repeated stimuli. The shaded areas on the waveform plots indicate the time-window for the LSW analysis (i.e., 1000 – 1750 ms).
Discussion
The current study examined the effects of attention and stimulus repetition on object recognition for 4.5-, 6-, and 7.5-month-old infants. We predicted that as infants progressed from early repetition trials to late repetition trials, they would shift from demonstrating greater amplitude Nc and LSWs to the repeated stimulus to showing greater amplitude to the non-repeated stimuli. This prediction was partially supported by an interaction of age, stimulus type, and stimulus repetition at central electrodes with 7.5-month-olds demonstrating reduced amplitude Nc to the repeated stimulus on late repetition trials. We also predicted that differences in ERP amplitude based on stimulus repetition and visual preference behavior would be greater on attentive trials (defined by HR) than inattentive trials. This prediction was supported by an interaction of stimulus type and attention on LSW amplitude. Infants only demonstrated significant differences in LSW amplitude based on stimulus type during attention. Our analyses revealed several additional effects which we discuss in detail in the sections that follow.
Preferential Looking Data
On average, infants completed approximately 10 VPC trials before reaching criterion for a stable novelty preference, and infants reached criterion an average of 1.2 times during testing. Given the procedure used in this study, the finding that most infants reached criterion once during testing is to be expected. Unlike the majority of research in the area, there was no familiarization phase in this study. Familiarization phases for studies with infants in this age range are typically 20 – 30 s long (e.g., Reynolds et al., 2005, 2010, 2011; Richards, 2003; Vogel, Monesson, & Scott, 2012). In the current study, infants were shown very brief presentations of the repeated and non-repeated stimuli. On an average block of trials, infants would have seen the repeated stimulus for approximately 30 s of accumulated looking spread out across the VPC and brief stimulus ERP trials.
Similar results have been found in previous studies that have utilized modifications of the Fantz (1964) procedure. For example, the VPC component of the current procedure was largely based on a procedure used by Rose, Feldman, and Jankowski (2002). They presented 5-, 10-, and 12-month-olds with a series of VPC trials pairing a repeated stimulus with a non-repeated stimulus, and continued testing until the infant met criterion for a novelty preference. Not all of the infants in their study met criterion during testing. Five-month-olds viewed 19 VPC trials on average prior to reaching criterion, 7-month-olds viewed 15 VPC trials on average prior to reaching criterion, and 12-month-olds viewed 10 VPC trials on average prior to reaching criterion. Although infants in the current study only viewed 10 VPC trials on average before reaching criterion, they were also shown 4 ERP trials in between each VPC trial, which would sum to 10 s of additional accumulated looking to the repeated stimulus within a block. Although the VPC and HR data were primarily used to define the repetition and attention independent variables in the analysis of the ERP data, it is interesting to note that across blocks infants did appear to shift from familiarity preferences to null preferences prior to reaching criterion for a novelty preference. Although this is consistent with the idea that infants demonstrate familiarity preferences during the initial stages of stimulus processing, it is not entirely consistent with the Hunter and Ames’ (1988) null preference – familiarity preference – novelty preference curve.
The Nc Component
Several interesting findings were revealed in our analysis of the Nc component. First, there was an interaction of attention and stimulus repetition on Nc amplitude (see Figure 3). Infants showed greater amplitude Nc during attention in comparison to inattention, but only on early repetition trials. No differences in Nc amplitude based on attention were found on late repetition trials. Courchesne (1983) proposed that Nc amplitude is associated with activation of the reticular activating system. Similarly, Richards and colleagues (Reynolds et al., 2010, 2013; Richards, 2003) have proposed that both increases in Nc amplitude and reductions in infant HR are separate components reflecting activation of a general arousal system involved in attention. When this system is activated, arousal levels are maintained at an optimal level for attention, perceptual processing, and learning. Thus, the increased impact of attention on early trials may be associated with greater levels of attention and arousal for infants engaged in early stages of visual processing. This is consistent with Fisher-Thompson and Peterson’s (2004) proposal that infant performance on the VPC task is influenced by a number of factors including general arousal level.
Our analysis of Nc provides insight into differences across age groups in attention and stimulus processing on this task. No differences in Nc amplitude were found for 4.5-month-olds based on stimulus repetition or stimulus type. However, 6-month-olds demonstrated greater amplitude Nc on late repetition trials in comparison to early repetition trials. This finding was somewhat surprising and may indicate that the 6-month-olds became increasingly engaged in visual processing as the procedure progressed. Finally, there was an interaction of age, stimulus type, stimulus repetition. The 7.5-month-olds showed a significant reduction in Nc amplitude to the repeated stimulus across early to late trials that was not found for the younger age groups (see Figure 5). These findings indicate gains in processing efficiency from 4.5 to 7.5 months. While the youngest group did not demonstrate any significant effects on Nc amplitude, the 6-month-olds demonstrated an increase in Nc amplitude from early to late trials to both stimulus types that may reflect repetition enhancement. In contrast, the 7.5-month-olds specifically demonstrated a decrease in Nc amplitude to the repeated stimulus across early to late trials indicative of repetition suppression. Past research (Gagnepain et al., 2008; Henson, Shallice, & Dollan, 2000) indicates that repetition enhancement may occur during active formation of a memory representation for a partially processed stimulus whereas repetition suppression occurs in response to more fully processed stimuli (Nordt, Hoehl, & Weigelt, 2016).
The finding that the effects of stimulus type were not apparent in the Nc analysis until after 6 months of age is consistent with previous research on infant look duration. Courage and colleagues (2006) examined look duration to a range of different stimulus types for infants from 3 to 12 months of age. Across 3 – 6 months, infant look duration dropped significantly, regardless of stimulus type. However, from 6 months on, infant looking to basic stimuli stayed low; whereas, their looking to complex stimuli increased. The authors interpreted the drop in look duration from 3 to 6 months as reflecting further development of eye movement control and the posterior orienting system. The finding that look duration was dependent on stimulus type after 6 months was interpreted as reflecting increased volitional control of visual attention coinciding with initial development of the anterior attention system.
The current findings show consistency across visual preference scores and differential amplitude of the Nc component for the 7.5-month-old group. The decrease in looking to the repeated stimulus that occurred as 7.5-month-olds reached criterion was preceded by a corresponding decrease in neural responsiveness associated with visual attention to the repeated stimulus. This trend of decreasing amplitude to the repeated stimulus across early to late trials is consistent overall with the prediction that infants would show a shift from familiarity to novelty preference with stimulus repetition. However, instead of manifesting the shift as an increase in Nc amplitude to the non-repeated stimulus, the shift was manifested as a decrease in Nc amplitude to the repeated stimulus similar to a decrease in look duration to the repeated stimulus. This indicates that visual preference scores do reflect underlying cognitive processes associated with visual processing, and provides support at a general level for information processing models of infant look duration (e.g., Hunter & Ames, 1988; Rose et al., 1982).
Given that no differences were found across age groups behaviorally in number of VPC trials needed to reach criterion for stable novelty preferences; the interaction of age, stimulus type, and stimulus repetition in the analysis of Nc is of great interest. We propose that the ERP data provide greater insight into the effects of stimulus repetition and stimulus type on attention and memory across these age groups. Although all three age groups showed evidence of recognition memory both in their visual preference behavior and LSW amplitude, there were significant differences across groups in attentional engagement which could not be parceled out by exclusively analyzing look duration data.
Kagan (2008) has argued for the importance of utilizing multiple measures when studying perceptual and cognitive processes in infancy (see also: Nelson, Bloom, Cameron, Amaral, Dahl, & Pine, 2002; Quinn, 2008; Reynolds & Guy, 2012). He noted that relations between novelty and infant look duration often follow a curvilinear trend based on amount of discrepancy between the novel and familiar stimuli as opposed to a basic linear trend based on degree of novelty (Kagan, 2002; McCall & McGhee, 1977), thus calling into question basic interpretations of look duration reflecting stimulus encoding. In line with Kagan’s (2002) conclusions, the current findings highlight the importance of utilizing multiple measures in research on infant attention and memory processes. The increased sensitivity of 7.5-month-olds to stimulus repetition and stimulus type in comparison to 4.5- and 6-month-olds was only revealed through the combined analysis of the looking data and the Nc component.
The Late Slow Wave
Our prediction that differences in LSW amplitude based on stimulus repetition and visual preference behavior would be greater on attentive trials than inattentive trials was partially supported by the data. There was an interaction of attention and stimulus type on LSW amplitude at left and right temporal electrodes. Infants showed significant differences in LSW amplitude to the repeated stimulus in comparison to non-repeated stimuli on attentive trials. No differences were found in LSW amplitude based on stimulus type on inattentive trials. Using basic visual patterns, Reynolds and Richards (2005) found a similar interaction of attention and stimulus type with infants only demonstrating greater amplitude LSW to novel compared to familiar patterns during attention.
Past studies have consistently found differential LSW amplitude based on amount of prior exposure or level of familiarity (de Haan & Nelson, 1999; Guy et al., 2013, 2017; Nelson & Collins, 1991, 1992; Snyder, 2010; Snyder, Webb, & Nelson, 2002; Snyder et al., 2010; Webb, Long, & Nelson, 2005; Wiebe et al., 2006). Snyder (2010) found that infants who show significant decreases in LSW amplitude during habituation are more likely to demonstrate novelty preferences in subsequent VPC testing than infants who do not show a significant reduction in the LSW during habituation. Using the HR phases and preferential looking, Richards (1997) found that infants require less familiarization time to demonstrate novelty preferences in subsequent testing if familiarization occurs when the infant is engaged in sustained attention. Taken together with these previous findings, the current findings indicate that attention fosters infant visual processing, and attention is integral to performance on recognition memory tasks across this age range. Although infants demonstrated significant differences in LSW amplitude between repeated and non-repeated stimuli during attention, they did not demonstrate a significant reduction in LSW amplitude from early to late trials. This lack of an effect of stimulus repetition is somewhat unexpected due to previous findings (e.g., Guy, Reynolds, & Zhang, 2014; Snyder, 2010; Snyder, Webb, & Nelson, 2002), but may be due to the complex procedure used in the current study. The repeated presentations of attractor stimuli and VPC trials alternating with brief stimulus ERP trials may have led to a lack of a reduction in the amplitude of the LSW from early to late trials.
The results of both the Nc and LSW analyses demonstrate the strength of a multi-level approach for examining perceptual processing in infancy. We have proposed that convergent responses in preferential looking, heart rate, and ERPs reflect the influence of a general arousal/attention system on infant visual processing and recognition memory (Reynolds et al., 2010, 2016; Richards, 2008, 2010). This general arousal/attention system is comprised of the noradrenergic and cholinergic neurotransmitter systems (Robbins & Everitt, 1995; Sarter, Givens, & Bruno, 2001), and neuroanatomical connections between the reticular activation system and the cortex (Heilman, Watson, Valenstein, & Goldberg, 1987; Mesalum, 1983). Activation of this system during infant attention fosters an optimal state of arousal for perceptual processing, learning, and recognition memory.
The current findings provide evidence of the importance of infant attention for visual processing and object recognition. The significant interactions found between organismic variables (age and attention) and environmental variables (stimulus type and stimulus repetition) exemplify the complex and multidetermined nature of early cognitive development. Neural correlates of attention and recognition memory were not simply influenced by a single factor, such as age or stimulus type. Given the growing body of literature demonstrating the importance of infant attention for early learning and cognitive development (e.g., Cuevas & Bell, 2014; Frick & Richards, 2001; Kovack-Lesh, Oakes, & McMurray, 2012; Markant & Amso, 2016; Rose, Feldman, & Jankowski, 2012), further research is needed to elucidate the dynamic internal (e.g., arousal, neural responsiveness) and external (e.g., stimulus events, social experience) processes that influence attention, perceptual processing, and learning in early development.
Acknowledgments
Research reported in this article and the writing of this article was supported by the National Institute of Child Health and Human Development Grants R03 HD05600 and R21HD065042 to GDR, and the National Institute of Child Health and Human Development Grant R37 HD18942 to JER. Partial support for this research was provided by a consortium grant from the McDonnell Foundation (220020096, R. Aslin PI).
Contributor Information
Greg D. Reynolds, University of Tennessee, Knoxville
John E. Richards, University of South Carolina
References
- Bahrick LE, Pickens JN. Infant memory for object motion across a period of three months: Implications for a four-phase attention function. Journal of Experimental Child Psychology. 1995;59:343–371. doi: 10.1006/jecp.1995.1017. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life: Relations between EEG frequency and coherence and cognitive and affective behaviors. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 314–345. [Google Scholar]
- Casey BJ, Richards JE. Sustained visual attention measured with an adapted version of the visual preference paradigm. Child Development. 1988;59:1514–1521. [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Courage ML, Reynolds GD, Richards JE. Infants’ attention to patterned stimuli: Developmental change from 3 to 12 months of age. Child Development. 2006;77:680–695. doi: 10.1111/j.1467-8624.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- Cuevas K, Bell MA. Infant attention and early childhood executive function. Child Development. 2014;85:397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and Event-Related Potentials. New York: Psychology Press; 2007. pp. 101–144. [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Fantz JF. Visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Fisher-Thompson D, Peterson JA. Infant side biases and familiarity–novelty preferences during a serial paired-comparison task. Infancy. 2004;5:309–340. [Google Scholar]
- Freeseman LJ, Colombo J, Coldren JT. Individual differences in infant visual attention: Four-month-olds’ discrimination and generalization of global and local stimulus properties. Child Development. 1993;64:1191–1203. [PubMed] [Google Scholar]
- Gagnepain P, Chetelat G, Landeau B, Dayan J, Eustache F, Lebreton K. Spoken word memory traces within the human auditory cortex revealed by repetition priming and functional magnetic resonance imaging. Journal of Neuroscience. 2008;28:5281–5289. doi: 10.1523/JNEUROSCI.0565-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy MW, Reynolds GD, Mosteller SM, Dixon KC. The effects of stimulus symmetry on hierarchical processing in infancy. Developmental Psychobiology. 2017;59:279–290. doi: 10.1002/dev.21486. [DOI] [PubMed] [Google Scholar]
- Guy MW, Reynolds GD, Zhang D. Visual attention to global and local stimulus properties in six-month-old infants: Individual differences and event-related potentials. Child Development. 2013;84:1392–1406. doi: 10.1111/cdev.12053. [DOI] [PubMed] [Google Scholar]
- Guy MW, Zieber N, Richards JE. The cortical development of specialized face processing in infancy. Child Development. 2016 doi: 10.1111/cdev.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Goldberg ME. Attention: Behavior and neural mechanisms. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of Physiology, Section 1: The nervous system. PPS. V. Bethesda, MD: American Physiological Society; 1987. pp. 461–481. [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Hunt JM. Piaget’s observations as a source of hypotheses concerning motivation. Merrill-Palmer Quarterly of Behavior and Development. 1963;9:263–275. [Google Scholar]
- Hunter M, Ames E. A multifactor model of infant preferences for novel and familiar stimuli. In: Rovee-Collier C, Lipsitt LP, editors. Advances in infancy research. Vol. 5. Norwood, NJ: Ablex; 1988. pp. 69–95. [Google Scholar]
- Johnson MH, Posner M, Rothbart MK. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. Journal of Cognitive Neuroscience. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Kagan J. Surprise, uncertainty, and mental structures. Harvard University Press; 2002. [Google Scholar]
- Kagan J. In defense of qualitative changes in development. Child Development. 2008;79:1606–1624. doi: 10.1111/j.1467-8624.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- Karrer R, Ackles PK. Visual event-related potentials of infants during a modified oddball procedure. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Current trends in event-related potential research. Amsterdam: Elsevier Science Publishers; 1987. pp. 603–608. [PubMed] [Google Scholar]
- Kidd C, Piantadosi ST, Aslin RN. The Goldilocks effect: Human infants allocate attention to visual sequences that are neither too simple nor too complex. PloS one. 2012;7:e36399. doi: 10.1371/journal.pone.0036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovack-Lesh KA, Oakes LM, McMurray B. Contributions of attentional style and previous experience to 4-month-old infants’ categorization. Infancy. 2012;17:324–328. doi: 10.1111/j.1532-7078.2011.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MK, Setoodehnia M, Baek J, Luck SJ, Oakes LM. The development of visual search in infancy: Attention to faces versus salience. Developmental psychology. 2016;52:537. doi: 10.1037/dev0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Amso D. The Development of Selective Attention Orienting is an Agent of Change in Learning and Memory Efficacy. Infancy. 2016;21:154–176. doi: 10.1111/infa.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, Kagan J. Stimulus-schema discrepancy and attention in the infant. Journal of Experimental Child Psychology. 1967;5:381–390. doi: 10.1016/0022-0965(67)90066-5. [DOI] [PubMed] [Google Scholar]
- McCall RB, Kagan J. Individual differences in the infant’s distribution of attention to stimulus discrepancy. Developmental Psychology. 1970;2:90. [Google Scholar]
- McCall RB, McGhee PE. The Structuring of Experience. Springer; US: 1977. The discrepancy hypothesis of attention and affect in infants; pp. 179–210. [Google Scholar]
- Mesulam MM. The functional anatomy and hemispheric specialization for directed attention. Trends in Neuroscience. 1983;6:384–387. [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain–behavior relations in the context of typical and atypical development. Development and psychopathology. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19:105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nikkel L, Karrer R. Differential effects of experience on the ERP and behavior of 6-month-old infants: Trends during repeated stimulus presentation. Developmental Neuropsychology. 1994;10:1–11. [Google Scholar]
- Nordt M, Hoehl S, Weigelt S. The use of repetition suppression paradigms in developmental cognitive neuroscience. Cortex. 2016;80:61–75. doi: 10.1016/j.cortex.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Piantadosi ST, Kidd C, Aslin R. Rich analysis and rational models: Inferring individual behavior from infant looking data. Developmental Science. 2014;17:321–337. doi: 10.1111/desc.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Posner MI. Attention in cognitive neuroscience: An overview. In: Gazzaniga M, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 1995. pp. 615–624. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Development of attention networks. In: Kar B, editor. Cognition and brain development: Converging evidence from various methodologies. Washington, DC: American Psychological Association; 2013. pp. 61–83. [Google Scholar]
- Quinn PC. In defense of core competencies, quantitative change, and continuity. Child Development. 2008;79:1633–1638. doi: 10.1111/j.1467-8624.2008.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD. Infant visual attention and object recognition. Behavioural Brain Research. 2015;285:34–43. doi: 10.1016/j.bbr.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. Infant attention and visual preferences: Converging evidence from behavior, event-related potentials, and cortical source localization. Developmental Psychology. 2010;46:886–904. doi: 10.1037/a0019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. The development of attention. In: Reisberg D, editor. Oxford Handbook of Cognitive Psychology. Oxford University Press; New York, NY: 2013. pp. 1000–1013. [Google Scholar]
- Reynolds GD, Guy MW. Brain–behavior relations in infancy: Integrative approaches to examining infant looking behavior and event-related potentials. Developmental Neuropsychology. 2012;37:210–225. doi: 10.1080/87565641.2011.629703. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Guy MW, Zhang D. Neural correlates of individual differences in infant visual attention and recognition memory. Infancy. 2011;16:368–391. doi: 10.1111/j.1532-7078.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An ERP and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Infant heart rate: A developmental psychophysiological perspective. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology: Theory, Systems, and Applications. Cambridge University Press; 2008. pp. 173–212. [Google Scholar]
- Reynolds GD, Richards JE. Cortical source localization of infant cognition. Developmental Neuropsychology. 2009;34:312–329. doi: 10.1080/87565640902801890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Romano AC. The development of attention systems and working memory in infancy. Frontiers in Systems Neuroscience. 2016;10:1–12. doi: 10.3389/fnsys.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Zhang D, Guy MW. Infant attention to dynamic audiovisual stimuli: Look duration from 3 to 9 months of age. Infancy. 2013;18:554–577. [Google Scholar]
- Richards JE. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997;33:22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Attention in young infants: A developmental psychophysiological perspective. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA, US: MIT Press; 2008. pp. 479–497. [Google Scholar]
- Richards JE. Attention in the brain and early infancy. In: Johnson SP, editor. Neoconstructivism: The new science of cognitive development. New York, NY: Oxford University Press; 2010. pp. 3–31. [Google Scholar]
- Richards JE, Casey BJ. Development of sustained visual attention in the human infant. In: Campbell BA, Hayne H, editors. Attention and Information Processing in Infants and Adults: Perspectives from Human and Animal Research. Hillsdale, NJ: Erlbaum Publishing; 1992. pp. 30–60. [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga MS, editor. Cognitive Neurosciences. Cambridge, MA: MIT; 1995. pp. 703–720. [Google Scholar]
- Roder BJ, Bushnell EW, Sasseville AM. Infants’ preferences for familiarity and novelty during the course of visual processing. Infancy. 2000;1:491–507. doi: 10.1207/S15327078IN0104_9. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24:74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Implications of infant cognition for executive functions at age 11. Psychological Science. 2012;23:1345–1355. doi: 10.1177/0956797612444902. [DOI] [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar PM, Bridger WH. Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology. 1982;18:704–713. [Google Scholar]
- Ross-Sheehy S, Schneegans S, Spencer JP. The infant orienting with attention task: Assessing the neural basis of spatial attention in infancy. Infancy. 2015;20:467–506. doi: 10.1111/infa.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain research reviews. 2001;35(2):146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Snyder K. Neural correlates of encoding predict infants’ memory in the paired-comparison procedure. Infancy. 2010;15:487–516. doi: 10.1111/j.1532-7078.2009.00015.x. [DOI] [PubMed] [Google Scholar]
- Snyder K, Garza J, Zolot L, Kresse A. Electrophysiological signals of familiarity and recency in the infant brain. Infancy. 2010;15:487–516. doi: 10.1111/j.1532-7078.2009.00021.x. [DOI] [PubMed] [Google Scholar]
- Snyder K, Webb SJ, Nelson CA. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behavior and Development. 2002;25:466–494. [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Oxford: Pergamon Press; 1963. [Google Scholar]
- Wagner SH, Sakovits LJ. A process analysis of infant visual and cross-modal recognition memory: Implications for an amodal code. Advances in Infancy Research. 1986;4:195–217. [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Cheatham CL, Lukowski AF, Haight JC, Muehleck AJ, Bauer PJ. Infants’ ERP responses to novel and familiar stimuli change over time: Implications for novelty detection and memory. Infancy. 2006;9:21–44. [Google Scholar]


