Abstract
Humans exhibit broad heterogeneity in affiliative social behavior. Twin and family studies show that individual differences in core dimensions of social behavior are heritable, yet there are knowledge gaps in understanding the underlying genetic and neurobiological mechanisms. Animal genetic reference panels (GRPs) provide a tractable strategy for examining the behavioral and genetic architecture of complex traits. Here, using males from 50 mouse strains from the BXD GRP, 4 domains of affiliative social behavior—social approach, social recognition, direct social interaction (DSI) (partner sniffing) and vocal communication—were examined in 2 widely used behavioral tasks—the 3‐chamber and DSI tasks. There was continuous and broad variation in social and nonsocial traits, with moderate to high heritability of social approach sniff preference (0.31), ultrasonic vocalization (USV) count (0.39), partner sniffing (0.51), locomotor activity (0.54‐0.66) and anxiety‐like behavior (0.36). Principal component analysis shows that variation in social and nonsocial traits are attributable to 5 independent factors. Genome‐wide mapping identified significant quantitative trait loci for USV count on chromosome (Chr) 18 and locomotor activity on Chr X, with suggestive loci and candidate quantitative trait genes identified for all traits with one notable exception—partner sniffing in the DSI task. The results show heritable variation in sociability, which is independent of variation in activity and anxiety‐like traits. In addition, a highly heritable and ethological domain of affiliative sociability—partner sniffing—appears highly polygenic. These findings establish a basis for identifying functional natural variants, leading to a new understanding typical and atypical sociability.
Keywords: Three‐chamber social interaction, activity, anxiety, direct social interaction, grooming, heritability, QTG, recombinant inbred, social approach, social recognition, ultrasonic vocalizations
1. INTRODUCTION
Affiliative social motivation and behavior (sociability) are foundational for the development of close social bonds and the endurance of social groups across species. Despite this importance, there is pronounced variation in sociability in typical humans1, 2, 3, 4 and in many vertebrate and invertebrate species.5, 6, 7, 8, 9, 10, 11, 12 The extremes of this continuum in humans are observed in many neurodevelopmental disorders (NDDs).13, 14, 15 Twin and family studies in humans,2, 16 and selection and population studies in animals,17, 18, 19, 20, 21, 22, 23 show that individual differences in core dimensions of social behavior are heritable. While major neurochemical systems involved in social‐emotional behavior (eg, oxytocin, vasopressin, dopamine, serotonin)2, 24, 25, 26 and genetic contributions to many NDDs,2, 13, 27, 28, 29 have been ascribed, the natural genetic variants that contribute to heterogeneity in affiliative social behavior are not well‐understood.
Research in human genetics has shown that many common disorders are the quantitative extremes of continuously varying traits in unaffected populations.30, 31, 32, 33 A continuum model of sociability is supported by evidence that genome‐wide polymorphisms (of unknown function) that impose genetic risk for autism spectrum (ASD) and other neuropsychiatric disorders, also influence a continuum of social behavior and developmental phenotypes in unaffected populations.34 In addition, continuous variation in social traits (including subclinical dimensions of impairment) are observed in the general population and in the unaffected family members of individuals with ASD and other NDDs.34, 35, 36, 37, 38, 39, 40, 41 These findings suggest that a combination of genes that contribute to heterogeneity in core dimensions of typical social behavior also influence disorder risk and symptom severity. Thus, determining the genetic basis of typical trait variation in humans and animals will lead to a greater understanding of the molecular and neural circuit underpinnings of typical and atypical sociability.
A tractable and unbiased approach to studying phenotypic variation in the laboratory has been the application of invertebrate and vertebrate genetic reference panels (GRPs) in which genetic variation is fixed and well‐cataloged across large families of genetically related recombinant inbred (RI) strains. Thousands of quantitative traits have been studied in GRPs, including fine mapping of genetic loci that contribute to continuous variation in complex physiological and neurobiological traits across species.42, 43, 44, 45, 46, 47, 48, 49 Mice are particularly advantageous for examining the genetic architecture of sociability because of their high levels and lifelong expression of affiliative behaviors.50, 51, 52, 53 Outbred and unrelated‐inbred mouse strains show differences in affiliative and aggressive social behavior9, 54, 55, 56 and mutant mice have been the focus of numerous candidate gene studies.57, 58
Nevertheless, the degree of behavioral heterogeneity, trait heritability and the genetic and behavioral structure of sociability in RI mouse strains are not known. Here, we used the BXD mouse GRP59, 60 and 2 widely used social behavior tasks—the 3‐chamber and direct social interaction (DSI) tasks—to address these questions. The tasks measure 4 core domains of sociality—social approach and avoidance (“social approach”), social recognition and novelty preference (“social novelty”), DSI using species‐typical behavior (partner sniffing, touching) and vocal communication. These behaviors are conserved across species.61, 62, 63, 64, 65 The tasks differ in several important ways, including the characteristics of the social partner (age, familiarity), extent of physical contact with the social partner, ethological similarity, session duration and chamber size, providing an opportunity to examine the “behavioral architecture” of sociability by determining the degree to which social (and nonsocial) traits co‐vary. Here, the BXD GRP is known to show continuous variation in a number of complex behaviors,46, 48, 49, 66, 67, 68, 69 including activity, anxiety and learning, providing an opportunity to examine whether there is heritable and correlated variation in nonsocial traits expressed during the social tasks. The studies show broad and heritable variation in social and nonsocial traits, which vary independently, providing an opportunity for whole‐genome mapping that shows overlapping, yet distinct, loci for vocal communication, social approach, activity and anxiety‐like behaviors. The foundational implications of the findings with regard to future studies of specific genes that influence typical and atypical social development are discussed.
2. MATERIALS AND METHODS
2.1. Mice
The BXD GRP comprises >100 RI strains generated from progressive matings of C57BL/6 (B6) and DBA/2 (D2) offspring (B × D).59, 60, 70 The parental strains show high levels of sequence variation (~5M SNPs, 500K INDELS, 55K CNVs), which is cataloged at >7500 informative SNPs in the offspring strains.71 Experimental mice from the B6 and D2 parental strains, F1 cross (B6D2F1) and 47 BXD strains were obtained from The Jackson Laboratory (Bar Harbor, ME) at 6 to 10 weeks of age. Male mice were used in all studies. Experimental mice were tested in 7 cohorts over 3 years, with 9 to 12 mice/BXD strain and 70 to 75 mice/parental strain (~10/cohort) and were tested between 2 and 4.5 months of age, at an average age of 3 months. Mice were allowed to acclimate to the facility for 2 to 4 weeks prior to testing and were maintained on a 12 h light/dark cycle (lights on 6:00 am to 6:00 pm) with ad libitum access to food and water, except during testing. Behavioral tests were conducted during the light cycle (between 8:00 am and 5:00 pm). Mice were transported to the testing rooms at least 30 min prior to testing and tasks were performed under 80 lx lighting. All procedures were approved by the Institutional Animal Care and Use Committee at USC and conformed to National Institutes of Health guidelines. Mice were tested in the 3‐chamber social interaction task followed by the DSI task, with an average intertest interval of 3 weeks. As part of a separate study, mice were tested in a fear‐conditioning task following the social tasks (methods and results reported in Knoll et al46). The fear learning data are included here in the factor analysis in order to identify shared and independent sources of trait variance. A total of 637 mice entered the study. Mice were housed in pairs, except in the event of cage mate death or fighting, which resulted in single housing. Because single housing is stressful for mice and can increase subsequent affiliative and aggressive social interactions,72, 73, 74 we decided a priori to remove singly housed mice from the analyses. This affected 15 mice from 5 strains in the 3‐chamber social interaction task (B6, BXD28, 31, 32 and 43) and 30 mice from 10 strains in the DSI task (B6, BXD15, 27, 28, 31, 39, 43, 60, 91 and 100). Two strains (BXD27 and 28) showed high levels of intermale aggression and subsequent single housing (6 of 10 mice for each strain). To ensure that removing singly housed mice did not unduly affect the mapping results, we performed mapping with and without singly housed mice. Mapping results were highly concordant (data not shown). For the 3‐chamber task, adult B6 stimulus mice were obtained from The Jackson Laboratory at 8 weeks of age and housed 4/cage. One week prior to behavioral testing the stimulus mice were singly housed. For the DSI task, juvenile B6 stimulus mice were bred in house from breeders obtained from The Jackson Laboratory and were housed 2 to 3 per cage following weaning on postnatal day (P) 21. All mapping was performed with data collected using B6 partners. In a separate study, we examined sociability in the parental strains in the 3‐chamber and DSI tasks using 129S1/SvImJ (129) partners obtained from The Jackson Laboratory. We observed similar rank‐order differences in sociability between the parental strains when tested with B6 or 129 partners, providing evidence that lower sociability in B6 mice is not dependent upon being paired with a same‐strain partner (Figure S1, Supporting Information; see section 4).
2.2. Three‐chamber social interaction task
Social approach (a measure of affiliation) and social novelty preference (a measure of social recognition) were assessed using a modified 3‐chamber social interaction task.51, 75 In this task, mice are assessed for their propensity to approach and interact with unfamiliar adult male partners (stimulus mice), which are confined beneath inverted wire cups located in the side chambers. As described previously,75 all habituation, social approach and social novelty test sessions were performed sequentially in a custom‐designed Plexiglas 3‐chamber arena (63 × 42 × 23 cm, L × W × H). The rectangular apparatus was divided into 2 side chambers (24.5 × 42 × 23 cm, L × W × H) and a central chamber (11.5 × 42 × 23 cm, L × W × H) using 2 transparent Plexiglas interior walls. Each interior wall contained a central entryway (10 × 10 cm, H × W; 14.5 cm from the exterior walls). Entryways were closed off with transparent plastic doors, which could be raised simultaneously to allow experimental mice unbiased access to the side chambers. Inverted wire cups (Galaxy Cup, Spectrum Diversified Designs, Streetsboro, Ohio; 11 × 10 cm, H × W) were placed in the upper quadrant of each side chamber (5.0 cm from the top and side walls) and were weighed down to prevent mice from moving the cups or climbing on top of them. Stimulus mice were acclimated to the wire cups for 4 days prior to testing. During habituation, experimental mice freely explored the apparatus and empty wire cups for 20‐min. A longer habituation session (compared with the standard 10‐min session) was used to ensure all strains fully explored the chambers, as BXD strains show a broad range of exploratory and anxiety‐like behavior.49, 66 Prior to the interaction sessions, experimental mice were confined briefly to the center chamber while stimulus mice were introduced beneath the cups. During the social approach session, a stimulus mouse was placed beneath one of the cups (the initial location was counterbalanced across mice) and the experimental mouse explored the entire chamber for 10‐min. A social approach “chamber” preference score was calculated as the duration of time the experimental mouse spent in the chamber containing the stimulus mouse minus time spent in the chamber without a stimulus mouse (empty cup). The duration of time mice spent sniffing, or in close proximity to (within 1 cm), the wire cups was used as an additional measure of social approach “sniff” preference, calculated as the duration of time the experimental mouse spent sniffing the cup with the stimulus mouse minus time spent sniffing the empty cup. During the social novelty session, an additional stimulus mouse was placed on the opposite side and the experimental mouse explored the chamber for 10‐min. A social novelty “chamber” preference score was calculated as the duration of time mice spent in the chamber containing the novel, minus the familiar, stimulus mouse. Social novelty “sniff” preference was calculated as the duration of time the experimental mouse spent sniffing the cup with the novel, minus the familiar, stimulus mouse. Entries made into the side‐chambers during each session were scored as measures of locomotor activity, as previously reported.51, 76 This measure of activity was positively correlated with total distance traveled (R 2 = .80, P < .0001; data not shown) and generated indistinguishable mapping results (data not shown). Latency to exit the center chamber during the habituation session and the percentage of time mice spent in the center chamber during all sessions were scored as measures of locomotor activity and possible indirect measures of anxiety‐like behavior (see factor analysis). Latency to discover the social partners during the social approach and novelty sessions were scored and mice that failed to discover the social partner within the first 5‐min of the session were excluded; a total of 15 mice from 11 strains were excluded for this reason. After each complete test, the apparatus was cleaned with water and 70% ethanol and then dried. Sessions were videotaped and automatically scored for chamber entries, durations and cup sniffing and proximity using CleverSys TopScan™ software version 3.0 (Reston, Virginia).
2.3. Direct social interaction (DSI) task
The DSI task was used to assess fine details of adult DSI and ultrasonic vocalization (USV) communication with a juvenile male. Juvenile B6 males (postnatal day (P) 26‐30) were used in order to minimize aggressive and sexual behavior. A small number of stimulus mice at P31 to 39 were used early in the study (paired with <5% of total adult mice tested), with no measured differences compared with the younger juveniles used for ~95% of the DSI runs. Testing was conducted in a rectangular Plexiglas chamber (30 × 19 × 19 cm, L × W × H) equipped with an ultrasonic microphone (Condenser Microphone CM16/CMPA, Avisoft Bioacoustics, Brandenburg, Germany) to record USVs. During the test session, the experimental mouse was acclimated to the chamber for 10‐min prior to the addition of the juvenile. Mice then freely interacted for 6‐min. Test sessions were videotaped (top and front views) and manually scored using MOOSES™ software version 3.0 (Jon Tapp, Vanderbilt Kennedy Center, Nashville, Tennessee) for the duration of self‐grooming and affiliative, aggressive and sexual behavior initiated by the experimental mouse. As a result of differences in coat colors and behavior across strains, observers were only partially blinded to strain identity. Affiliative behavior was defined as nonaggressive sniffing directed toward any portion of the juvenile’s body (“partner sniffing”). Two experimenters scored separate sets of videos. A subset of videos from 31 strains (39 mice), which showed broad heterogeneity in partner sniffing, were scored by both experimenters, with high inter‐rater reliability (R 2 = .97, partner sniffing; R 2 = .91, self‐grooming; Figure S2). Juveniles rarely initiated social interaction and there was no evidence that juvenile behavior varied across strains or influenced adult‐initiated social behavior (Figure S2). Distance traveled in the chamber during the habituation session was scored as a measure of locomotor activity using CleverSys TopScan™ software. USVs were recorded using an ultrasound recorder (UltraSoundGate 116Hb) and recording software (Recorder USGH version 4.2, both Avisoft Bioacoustics, Brandenburg, Germany) and ultrasounds between 25 and 125 kHz were automatically counted using Avisoft SASLab Pro software version 5.2. All USVs were attributed to the adult (experimental) male based on several lines of evidence that juvenile males do not vocalize during this task: (1) we did not observe overlapping vocalization streams, consistent with a single vocalizer, (2) vocalizations were highly synchronous with adult sniffing of the juvenile, (3) we observed broad heterogeneity in the number of USVs generated by different BXD strains, but highly consistent levels within a strain, despite all strains being paired with a B6 juvenile and (4) MUPET custom USV analysis software provided evidence of distinct syllable types, which were driven by the identity of the experimental strain, rather than the genetic identity of the juvenile,77 consistent with evidence that mouse syllable types are under strong genetic control.78, 79, 80
2.4. Quantitative trait locus (QTL) mapping
Genome‐wide simple interval mapping (SIM) was performed using the genome mapping tools within GeneNetwork (GN; http://www.genenetwork.org) to identify suggestive and significant QTLs. We used composite interval mapping (CIM) to identify additional suggestive QTLs masked by linkage and pair‐scanning to identify epistatic interactions. CIM was performed by controlling for the peak with the highest LRS score. The B6 and D2 parental strains show substantial sequence variation.81 All mapping was performed with 2017 genotypes in GN (mm10 GRCm38 assembly; 7320 informative markers), without parental strain data and without strain weighting. When appropriate, outlier values for specific traits were winsorized. In no case did winsorizing alter the location of a QTL peak. We performed permutation tests (5000) in GN to determine the likelihood ratio statistic (LRS) thresholds for suggestive and significant QTLs, corresponding to genome‐wide probabilities of .63 and .05, respectively.82 The proportion of phenotypic variance accounted for by each significant locus was estimated using the square of the Pearson correlation coefficient (r) between the trait and the peak marker. We used a 1.0‐LOD drop support interval for QTLs with LOD scores greater than 3.0 and a more conservative 0.5‐LOD drop support interval for suggestive peaks with LOD scores below 3.0 based on evidence that a smaller drop support interval prevented the inclusion of a large number of SNPs with P‐values >.9 on either side of the peak. Within these intervals, we identified positional candidate quantitative trait genes (QTGs) of potential interest for future investigation, based on 3 criteria: (1) evidence from QTLMiner83 that the gene is (A) expressed in representative brain regions based on GN mRNA databases for the hippocampus (Hippocampus Consortium M430v2 [Jun06] PDNN,84), amygdala (INIA Amygdala Cohort Affy MoGene 1.0 ST [Mar11] RMA,85), or hypothalamus (INIA Hypothalamus Affy MoGene 1.0 ST [Nov10],85) and (B) cis‐regulated within 1 or more of these regions, and that the gene contains (C) nsSNPs or (D) INDELs between the parental strains, (2) genes whose expression in one of these representative regions is correlated with the parent trait (i.e. the trait that generated the QTL) or correlated with a related “activity” or “social” behavior trait based on the factor analysis and (3) literature evidence of gene involvement in human neurological disorders (NCBI databases: OMIM®, ClinVar and MedGen) or phenotypes related to sociability, communication, activity or anxiety.
2.5. Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM). Differences in means were tested using one‐way analysis of variance (ANOVA). Heritability (h 2) was estimated by comparing within‐ and between‐strain variances using ANOVA R 2 values.42, 46, 66 Spearman correlations were calculated to show relationship between traits and significance was defined as Bonferroni‐corrected P values <.003 in order to correct for 15 comparisons (Table 1). Analyses were performed using GraphPad Prism version 6 (GraphPad Software, Inc, La Jolla, CA).
Table 1.
Spearman rank order correlations
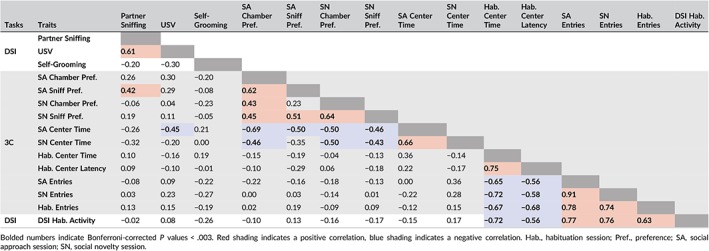
|
2.6. Principal component analysis (PCA)
The 2 tasks generated 17 measures of social and nonsocial behavior. Two measures in the DSI task, mounting and aggression, had a low frequency of occurrence and were not included in the PCA analysis (Figure S2). To determine how phenotypic variance in the remaining 15 measures are related to each other we performed exploratory factor analysis to determine the principal components using data collected from the 50 strains. Descriptive statistics (including tests of normality) of variables were performed. To avoid undue influence from any one variable, skewed variables were transformed and variables were standardized and centered. Factors were determined by those with an eigen value >1.0. The fraction of overall variance explained by each factor was calculated (Table 2). Analyses were performed using SPSS version 23 (IBM, Armonk, NY); α = .05.
Table 2.
PCA to identify factors explaining trait variance across tasks
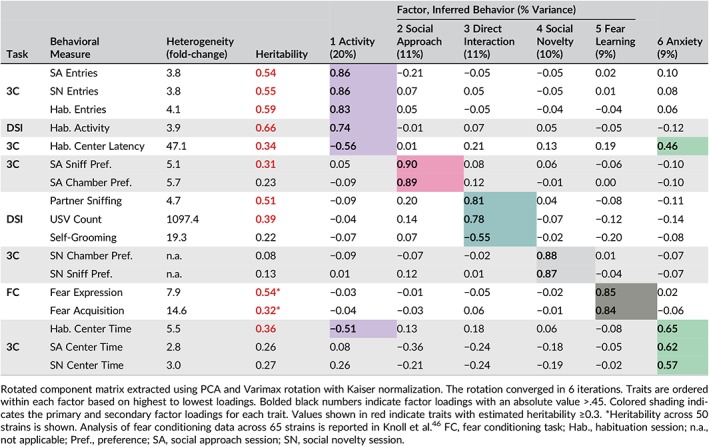
|
2.7. Power calculations
We performed power calculations with R/qtlDesign software version 1.442, 86 to estimate our ability to detect individual loci accounting for different proportions of trait variance as a function of strain number. Environmental variance was estimated from the within‐strain trait variance, genetic variance was set to 100 units, and the detectable variance generated by a single QTL was estimated based on 80% power, with 10 replicates per strain (Figure S7).
3. RESULTS
Across 50 mouse strains, we found broad heterogeneity in all traits measured in the 3‐chamber and DSI tasks (Figures 1 and 2, S2 and S3). Table 1 provides the Spearman rank order correlations and Table 2 summarizes the heterogeneity, heritability and PCA results for all traits (discussed below).
Figure 1.
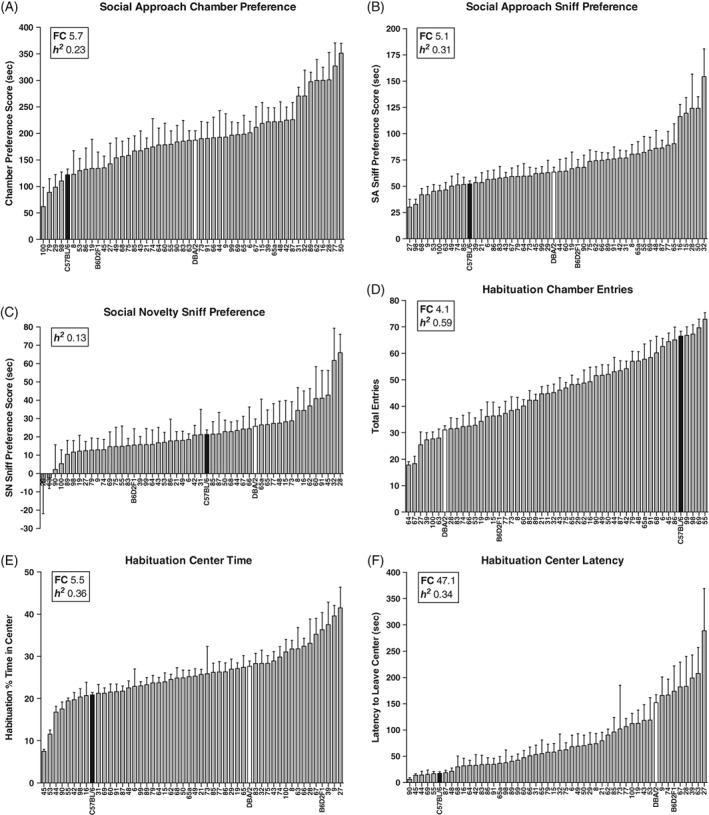
Behavioral heterogeneity in the 3‐chamber social interaction task. The BXD panel shows continuous variation in (A), social approach chamber preference, (B), social approach sniff preference and (C) social novelty sniff preference. During habituation to the 3‐chamber, the BXD panel shows continuous variation in (D), chamber entries, (E), the percentage of time spent in the center chamber and (F), the latency to leave the center chamber. Data are means ± SEM. Parental strains are shown in black (C57BL/6) and white (DBA/2). The F1 cross (B6D2F1) and 47 BXD strains are shown in gray. The inset in each graph shows the estimated heritability (h 2) and fold‐change (FC) for each measure
Figure 2.
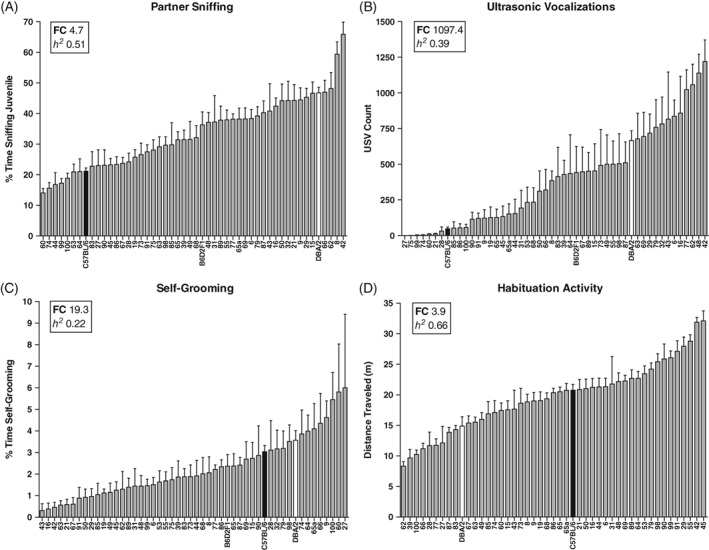
Behavioral heterogeneity in the DSI task. During the social interaction session, the BXD panel shows continuous variation in (A), partner sniffing, (B), ultrasonic vocalization count and (C), self‐grooming. During habituation to the testing chamber, the BXD panel shows continuous variation in (D), locomotor activity. Data are means ± SEM. Parental strains are shown in black (C57BL/6) and white (DBA/2). The F1 cross (B6D2F1) and 47 BXD strains are shown in gray. The inset in each graph shows the estimated heritability (h 2) and fold‐change (FC) for each measure
3.1. Three‐chamber social interaction
As summarized in Figure 1, there is continuous variation in social approach and social novelty preference, as well as in measures of activity and anxiety‐like behavior, expressed during the 3‐chamber task. Social approach “chamber” and “sniff” preference varied ~5 to 6‐fold across the panel (chamber preference: 62.1 ± 36.0, BXD100 to 351.3 ± 18.5, BXD50; F 49,549 = 3.37, P < .001, Figure 1A; sniff preference: 30.3 ± 7.4, BXD27 to 154.3 ± 26.5, BXD32; F 49,549 = 5.12, P < .001, Figure 1B) and these measures had low‐to‐moderate heritability (h 2 = .23‐.31, Table 2). Social novelty “chamber” and “sniff” preference also varied across the panel (Figures 1C and S3), but because of relatively high within‐strain variability, these traits had low heritability (h 2 = .08‐.13; Table 2). PCA (discussed below) showed activity and anxiety‐like traits expressed during the 3‐chamber task, which also exhibited variation across strains. Chamber entries were measured during the habituation, social approach and social novelty sessions and varied ~4‐fold across strains in each session (habituation: 17.8 ± 1.3, BXD64 to 72.9 ± 2.5, BXD55; F 49,549 = 16.1, P < .001, Figure 1D; social approach and novelty, Figure S3). Chamber entries were positively correlated across sessions (Table 1, discussed below) and highly heritable (h 2 = .54‐.59, Table 2). During the habituation session, there was continuous variation in the percentage of time that the strains spent in the center chamber (“habituation center time”: 7.5 ± 0.5, BXD45 to 41.5 ± 4.9, BXD27; F 49,549 = 6.19, P < .001, Figure 1E), as well as the latency for strains to exit the center (“habituation center latency”: 6.1 ± 3.4, BXD90 to 289.0 ± 80.1, BXD27; F 49,549 = 5.84, P < .001, Figure 1F). These putative measures of activity and anxiety‐like behavior were modestly heritable (h 2 = .34‐.36, Table 2). The percentage of time strains spent in the center during the social approach and novelty sessions also showed continuous variation across strains and these measures had low heritability (Figure S3).
3.2. Direct social interaction
As summarized in Figure 2, direct interaction (“partner sniffing”) varied 4.7‐fold across strains (Figure 2A, 14.1 ± 1.4, BXD60 to 65.9 ± 4.0, BXD42; F 49,512 = 10.90, P < .001) and was highly heritable (h 2 = .51, Table 2). Similarly, USV count varied ~1100‐fold across the 49 strains that vocalized (Figure 2B, 1.1 ± 0.4, BXD75 to 1219.3 ± 151.8, BXD42; F 49,514 = 6.61, P < .001) and was moderately heritable (h 2 = .39, Table 2). For one strain (BXD27), 6 of 10 mice were single housed because of cage mate aggression and therefore were excluded from the analysis. The remaining 4 mice did not vocalize, but we note that the single housed mice emitted an average of 217.5 ± 134.5 USVs (range 0‐841), demonstrating that this strain is capable of vocalizing. Partner sniffing and USV counts were positively correlated (Figure S2, Table 1, discussed below). Self‐grooming occurred relatively infrequently during the interaction session, yet showed continuous variation across strains (0.3 ± 0.3, BXD43 to 6.0 ± 3.4, BXD27; F 49,512 = 2.89, P < .001; Figure 2C) and had low heritability (h 2 = .22, Table 2). Interaction sessions were conducted with juvenile males to facilitate affiliative interactions; we observed few instances of aggressive or sexual behavior across strains (Figure S2). Finally, locomotor activity during the habituation session varied ~4‐fold across strains (8.3 ± 0.7, BXD62 to 32.1 ± 1.6, BXD45; F 46,368 = 15.34, P < .001; Figure 2D) and was highly heritable (h 2 = .66, Table 2).
3.3. Correlations
Spearman rank‐order correlations across behavioral measures from the 3‐chamber and DSI tasks are reported in Table 1. Within the DSI task, partner sniffing and USV count are positively correlated (r s = .61). This is consistent with our observation that vocalizations are often synchronous with adult‐initiated sniffing (data not shown), and that vocalizations are important components of affiliative, same‐sex social encounters.52, 87, 88, 89 Across tasks, partner sniffing is positively correlated with 3‐chamber social approach (sniff preference; r s = .42). Thus, despite salient differences in task requirements and attributes of the social partner, variation in affiliative sociability across strains is generally maintained across tasks (see section 4). Within the 3‐chamber task, chamber and sniff preference scores are positively correlated (r s = .62‐.64), providing evidence that both chamber choice and active investigation of the wire cups containing the stimulus mice are informative measures of social motivation, as previously reported.90 There was a positive relationship between social approach and social novelty preferences scores for both chamber and sniff preference (eg, sniff r s = .51). Social approach and novelty measures are inversely correlated with time spent in the center chamber during the social interaction sessions (social approach, r s = −.69; social novelty r s = −.50), but not during the habituation session. This relationship suggests that center time is an informative measure of social motivation (antisocial behavior), but only when measured in the presence of social partners. Chamber entries during the habituation, social approach and social novelty sessions were positively correlated (r s = .74‐.91) and total chamber entries were very similar across sessions (see Figures 1D and S3). In addition, these activity measures were positively correlated with distance traveled in the DSI task (r s = .63‐.77). Together, these data provide evidence of stable differences in activity across strains and tasks, irrespective of differences in the size and features of the testing chamber or the sequential order of the tasks. Importantly, activity measures did not co‐vary with social approach, social novelty, partner sniffing or USV count, consistent with prior evidence that sociability and activity measures are independent behaviors.9, 91 Finally, habituation center time and habituation center latency are positively correlated with each other (r s = .75) and inversely correlated with activity (r s = −.56 to −.72). These results, together with PCA analyses (below) provide evidence that exploration during habituation to the 3‐chamber provides measures of activity and anxiety‐like behavior.
3.4. Principal component analysis (PCA)
The heterogeneity across behavioral measures in each task is summarized in Table 2 as fold‐changes between the lowest and highest trait means across the BXD strains. The estimated heritability of each measure is also shown. Nine traits had heritabilities ≥.30 (bolded in red), which is amenable for exploratory QTL mapping in a panel of this size (see below and section 4).42, 92 We used PCA to determine whether shared or independent factors explain the observed variation in social and nonsocial phenotypes expressed during the social tasks. To further examine the factor structure of these traits, we analyzed conditioned fear acquisition and expression data that were measured in the same mice (see46). Six factors describe nearly 70% of the overall phenotypic variance (Table 2) and include putative measures of activity (Factor 1), social approach preference (Factor 2), DSI motivation (Factor 3), social novelty preference (Factor 4), fear learning (Factor 5) and anxiety‐like behavior (Factor 6). Despite Spearman statistical testing showing positive correlations among social approach, social novelty and DSI measures of social motivation (Table 1), these traits loaded on 3 separate factors (Factors 2‐4). Expected measures of activity from both tasks (chamber entries, distance traveled) loaded positively on Factor 1 and had ~4‐fold heterogeneity across the panel and were highly heritable in both tasks. Habituation center latency had a primary, negative loading on Factor 1, as well as a secondary, negative loading on Factor 6 (anxiety‐like behavior), suggesting that this trait is impacted by differences in both activity and anxiety‐like behaviors. Center time during each session of the 3‐chamber task loaded positively on Factor 6, which we hypothesize is a measure of anxiety‐like behavior. Here, habituation center time also had a secondary, negative loading on Factor 1, suggesting that time spent in the center in the absence of a social partner is impacted by differences in both activity and anxiety‐like behaviors. Although center time during the social approach and social novelty sessions was inversely correlated with sociability (Table 1), these traits loaded on Factor 6 rather than on the social factors, suggesting that they may reflect anxiety that generalizes beyond the social context (see section 4). We found that measures of conditioned fear learning loaded together on Factor 5 and were unrelated to sociability, activity, or anxiety‐like behavior.
3.5. QTL mapping of social behaviors
DSI partner sniffing is the most heritable measure of sociability (0.51) and yet the genome‐wide mapping did not identify any suggestive or significant QTLs (Figure 3A). The data suggest that this trait is highly polygenic and will likely require mapping with additional strains to generate sufficient power to detect loci with relatively small effect size (see section 4). For USV count, we identified 1 significant locus located on Chr 18 (68.188 Mb, LRS = 18.79), which explains 31% of trait variation across strains (Figure 3B). We also identified suggestive loci for USV count (Figure 3B) and social approach sniff preference (Figure 3C), with the same suggestive peak on Chr 19 identified for both. Peak locations and gene attributes are summarized in Figure 3D and discussed below. To understand possible linkage between loci, we conducted CIM using the markers with the highest LRS scores for each trait, showing additional suggestive loci (Figures 3D and S4). To identify epistatic interactions between pairs of loci, we conducted pair‐scan mapping in GN. For USV count, we identified a significant epistatic interaction between loci on Chr 1 (66.159‐66.197 Mb) and Chr 18 (68.176‐68.189 Mb) (LRS full = 36.450, LRS additive = 32.163; LRS interact = 4.287, P < .05; Figure S6).
Figure 3.
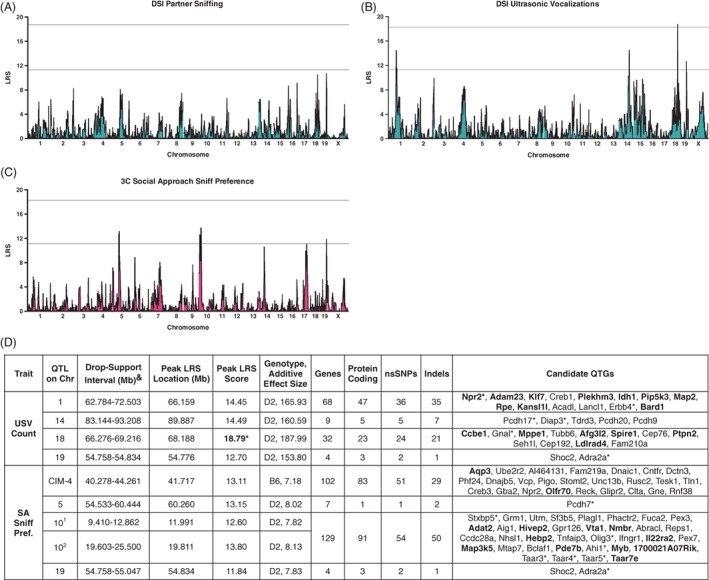
Genome‐wide mapping to identify quantitative trait loci (QTLs) for social behaviors measured in the direct (DSI) and 3‐chamber (3C) social interaction tasks. (A), DSI partner sniffing is not associated with any suggestive or significant loci. (B), DSI ultrasonic vocalization (USV) count is associated with a significant QTL on Chr 18 (upper line) and 3 suggestive loci on Chr 1, 14 and 19 (lower line). (C), 3C social approach sniff preference is associated with 3 suggestive loci on Chr 5, 10 and 19. Mapping color corresponds to the color of the primary factor containing each trait that was identified using PCA in Table 2. (D), Summary of QTL locations, peak LRS scores and locations, additive effect sizes and genotypes, gene numbers and number of genes with sequence variants, and candidate quantitative trait genes (QTGs). Candidate QTGs are listed in order of location on each on each Chr and were selected based on evidence of (1) QTLMiner ratings based on gene expression in brain, cis‐regulation and presence of nonsynonymous SNPs or Indels, (2) significant correlation between trait means and transcript expression in the hippocampus, hypothalamus or amygdala (P < .01, expression datasets in GN, bolded) or (3) involvement in human neurological disorders or key phenotypes related to social behavior or communication (starred). CIM controlling for the social approach peak on Chr 10 revealed an additional suggestive locus on Chr 4 (CIM‐4; data shown in Figure S4). Significant LRS scores are bolded and starred. Superscripts indicate that the loci on Chr 10 are linked (Spearman ρ .795) and are considered a single QTL. Chr, chromosome; CIM, composite interval mapping; Indels, insertions/deletions; LRS, likelihood ratio statistic; nsSNPs, nonsynonymous single nucleotide polymorphisms; SA, social approach session; QTGs, quantitative trait genes. &Drop‐support intervals were 0.5‐LOD for loci with LOD scores below 3.0 and 1.0‐LOD for loci with LOD scores above 3.0. CIM loci were required to have LOD scores of 2.50 or greater to be listed. Note: The 4 genes in QTL 19 are adjacent to the locus on either side; there are no genes within the locus
3.6. QTL mapping of nonsocial behaviors
For chamber entries made during the 3‐chamber task (Figure 4A‐C) as well as habituation center latency and center time (Figures 4E,F), we identified a consistent QTL on distal Chr X (133.525 Mb). This locus was significant for habituation entries (LRS = 20.50) and social novelty entries (LRS = 20.53) (Figure 4A,C) and accounted for 35% of trait variation across strains. Suggestive peaks on distal Chr X were present for the other measures (Figure 4B,E,F). Given that both habituation center latency and center time had primary (center latency) or secondary (center time) loadings on Factor 1 (Table 2), we hypothesize that this locus on distal Chr X contributes to variation in activity. Although this locus was not present in the map for DSI activity (Figure 4D), previous studies have found a suggestive QTL at this location on Chr X for ethanol‐induced differences in locomotor activity.49, 93 Across tasks, DSI activity and 3‐chamber entries had a consistent suggestive locus on Chr 12 (peaks between 91.520 and 98.800 Mb), representing a potential additional activity locus (Figure 4A‐D), which is consistent with previous reports.49 CIM using the markers with the highest LRS scores for each trait showed additional suggestive loci (Figure 4G and S5). For example, CIM controlling for the significant locus on Chr X using marker rs29271731 showed a suggestive QTL on Chr 17 (peak LRS at 78.925 Mb) for social novelty entries, but did not show additional peaks for habituation entries, despite very similar locus maps for these traits. As discussed above, habituation center latency and time are complex traits that appear to be comprised of both activity and anxiety‐like behaviors. Consistent with this hypothesis, for habituation center latency we identified suggestive peaks on Chr 11 (98.136 Mb) and Chr 13 (95.514 Mb), in addition to the peak on Chr X (Figure 4E). Interestingly, the locus on Chr 13 is within a few Mb of a peak that we identified for conditioned fear (QTL 13a: peak located at 84.648 Mb, 1.5 LOD confidence interval 78.26‐96.47 Mb, see46). Although conditioned fear measures and center latency loaded on separate factors (Table 2), these data suggest that QTL 13a contains genes that influence both fear acquisition and anxiety‐like behavior (see section 4). Finally, in addition to the QTLs on Chr 12 and X, we identified a suggestive locus on distal Chr 1 for habituation center time (Figure 4F), which overlaps with a locus identified for locomotor activity in an anxiety assay (see GN trait 12401, Cook et al, unpublished data) and is near a “QTL hotspot” for brain‐based behavioral phenotypes.94 Pair‐scan mapping did not identify any significant epistatic interactions for the nonsocial traits.
Figure 4.
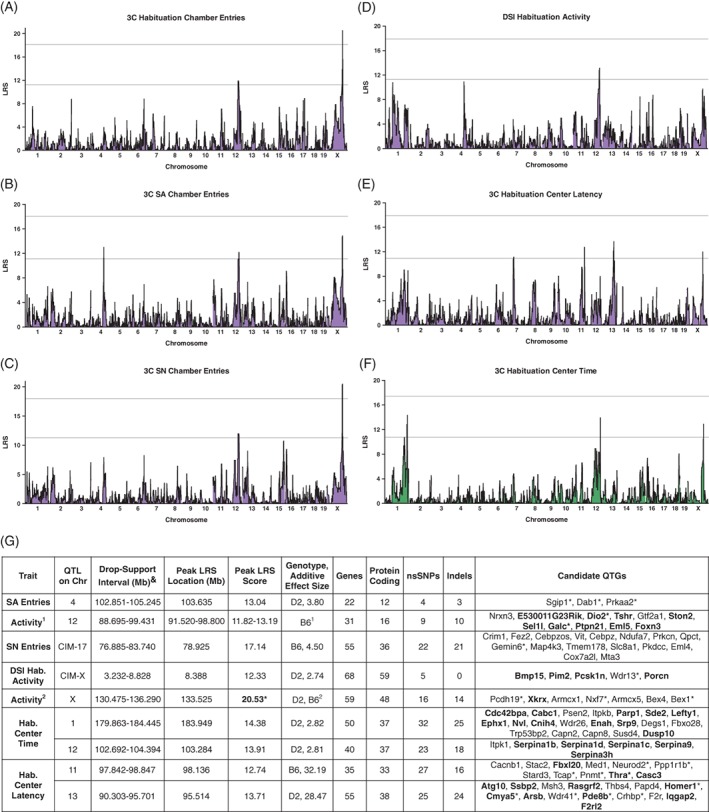
Genome‐wide mapping to identify quantitative trait loci (QTLs) for nonsocial behaviors measured in the direct (DSI) and 3‐chamber (3C) social interaction tasks. (A‐C) Chamber entries during each session of the 3C task are associated with consistent QTLs on Chr 12 and X. The QTL on Chr X is significant (upper line) for entries made during (A), habituation and (C), social novelty (SN) sessions and suggestive (lower line) for (B), social approach (SA) entries. SA entries are also associated with a suggestive QTL on Chr 4. (D), Activity during habituation to the DSI chamber is associated with a suggestive locus on Chr 12 at the same location as in (A‐C). During habituation to the 3‐chamber, (E), latency to leave the center is associated with suggestive loci on Chr 11, 13 and X and (F), time spent in the center is associated with suggestive loci on Chr 1, distal 12 and X. Mapping color corresponds to the color of the primary factor containing each trait that was identified using PCA in Table 2. (G), Summary of QTL locations, peak LRS scores and locations, additive effect sizes and genotypes, gene numbers and number of genes with sequence variants, and candidate quantitative trait genes (QTGs). Candidate QTGs are listed in order of location on each on each Chr and were selected based on evidence of (1) QTLMiner ratings based on gene expression in brain, cis‐regulation and presence of nonsynonymous SNPs or Indels, (2) significant correlation between trait means and transcript expression in the hippocampus, hypothalamus or amygdala (P < .01, expression datasets in GN, bolded) or (3) involvement in human neurological disorders or key phenotypes related to activity or anxiety‐like behavior (starred). CIM controlling for the peak on Chr X for SN entries revealed an additional suggestive locus on Chr 17 (CIM‐17); controlling for the peak on Chr 12 for DSI activity revealed an additional suggestive locus on Chr X (CIM‐X, data shown in Figure S5). 3C, 3‐chamber social interaction task; Chr, chromosome; CIM, composite interval mapping; DSI, direct social interaction task; Hab., habituation session; Indels, insertions/deletions; LRS, likelihood ratio statistic; nsSNPs, nonsynonymous single nucleotide polymorphisms; SA, social approach session; SN, social novelty session; QTGs, quantitative trait genes. (1) Putative activity locus on Chr 12 is present for 3C entries during habituation (additive effect size, 6.11), SA (3.76) and SN (4.48) sessions and DSI activity (2.71). Listed are the combined drop support intervals, peak LRS scores and locations and candidate QTGs. (2) Putative activity locus on Chr X is present for 3C entries during habituation (additive effect size, 7.85; genotype, D2), SA (4.09, D2) and SN (5.74, D2) entries and 3C center time (2.77, B6) and center latency (27.54, B6). Listed are the combined drop support intervals, peak LRS scores and locations and candidate QTGs. Significant LRS scores are bolded and starred. &Drop‐support intervals were 0.5‐LOD for loci with LOD scores below 3.0 and 1.0‐LOD for loci with LOD scores above 3.0. CIM loci were required to have LOD scores of 2.50 or greater to be listed
The locations of suggestive and significant QTLs for each trait are summarized in Figures 3D and 4G. For social traits, D2 alleles increase traits means at all loci, with the exception of QTL CIM‐4 (Figure 3D). For the putative activity locus on Chr X, D2 alleles increase trait means for chamber entries, but B6 alleles increase traits means for habituation center time and latency, consistent with inverse relationship between these measures and activity (Table 1 and Figure 4G). For the putative activity locus on proximal Chr 12, B6 alleles increase trait means, while D2 alleles increase trait means for the adjacent (more distal) locus on Chr 12 identified for habituation center time (Figure 4G). Finally, for habituation center latency, B6 alleles increase trait means for the locus on Chr 11, while D2 alleles increase trait means for the putative fear/anxiety locus on Chr 13 (Figure 4G, see Knoll et al46).
Genes located within significant, as well as suggestive, QTLs were examined to identify putative QTGs using multiple criteria (Figures 3D and 4G; see section 2). Focusing on significant loci, a number of genes show sequence variation (nsSNPs), cis‐regulation and transcript expression in brain (noted in Figure 3D and 4G), making them good candidate QTGs for loci with large effect‐sizes. In particular, for the QTL on Chr 18 for USV count we identified several positional candidates including Gnal, which encodes the α subunit of the heterotrimeric G‐protein Gαolf. Gnal is cis‐regulated, possesses a nonsynonymous coding variant between the parental strains, and is expressed in sensory neurons of the olfactory epithelium and at high levels in the olfactory tubercle, striatum, hippocampus and cerebellum.95, 96 Heterozygous GNAL mutations underlie Dystonia‐2597 in humans and a similar dystonia phenotype in mice.98 Homozygous null Gnal mutations in mice result in anosmia and high levels of pup mortality because of failure to nurse, with abnormal maternal care exhibited by the rare surviving females.95 Thus, polymorphisms in Gnal potentially could affect USV count through effects on olfaction or social‐motivation. We identified several positional candidates for the activity QTL on Chr X, including protocadherin 19 (Pcdh19). Although no known coding variants or cis‐regulation of Pcdh19 have been identified in the parental strains, mutations in the PCDH19 gene underlie syndromic female‐restricted epilepsy and mental retardation (OMIM: 300088), which presents with comorbid autism and attention deficit hyperactivity disorder.99, 100 In mice, miR‐484‐mediated regulation of Pcdh19 is implicated in hyperactivity observed in a mouse model of Chr 16p13.11 microduplication.101 Two additional Chr X genes, nuclear RNA export factor 7 (Nxf7, 102) and brain‐expressed X‐linked gene 1 (Bex1,103), also have been implicated in activity‐based phenotypes in mice.
4. DISCUSSION
Combining detailed phenotyping of core domains of sociability with locus mapping in a mouse GRP, the present study shows broad heterogeneity with moderate to high heritability of social approach preference, USV communication and partner sniffing. Using correlation and factor analyses, we examined the behavioral architecture of social and nonsocial trait variation and provide new evidence that sociability comprises distinct behavioral domains (social approach, social novelty and DSI) and that activity and anxiety‐like behaviors are separate factors that vary independently of the social traits. Genetic mapping showed suggestive and significant loci for USV count, social approach, locomotor activity and anxiety‐like traits. A surprising finding was that genetic mapping of partner sniffing did not identify any suggestive or significant loci, despite the relatively high heritability of this trait (.51). This finding suggests that partner sniffing is highly polygenic and it is likely that natural variants in many genes, each with small effect size, contribute to trait variation. This interpretation is consistent with the often reported “missing heritability” and loci with small‐effect sizes for complex (and highly heritable) psychological and social traits in humans (see below), although shared and nonshared environmental effects may also play a role.104, 105 Genetic contributions to aggression and some affiliative social behaviors have been described using chromosome substitution strains and F2 intercross populations.54, 106, 107, 108, 109 The current study provides novel evidence of heritable variation in affiliative social behavior in a RI panel, including a new analysis of the behavioral and genetic architecture of social and nonsocial traits measured in 2 widely used tasks in mouse models of neurodevelopmental and psychiatric disorders. As such, the present work represents an important step toward determining the genetic architecture of sociability in order to advance the understanding of heterogeneity of typical and atypical sociability.
One of the advantages of using the BXD panel is the opportunity to identify inbred strains with a broad range in sociability that well exceeds differences typically observed in a mutant mouse (compared with its wild‐type counterpart). The ranges reported here (~4‐6 to 1000‐fold) provide new opportunities to perform task‐specific differential neural circuit activation mapping, and pharmacological or behavioral interventions. One finding from the present study is that the B6 strain exhibits relatively low social behavior in both tasks compared with the D2 and the majority of BXD strains. The rank‐order differences in sociability between the B6 and D2 strains are independent of testing the B6 strain with a same‐ or different‐strain partner, yet are more robust in the DSI compared with the 3‐chamber task (Figure S1). Previous studies examining social behavior in adult dyads have provided mixed evidence of higher sociability in the D2 compared with the B6 strain.9, 51, 56, 91, 110, 111 Because these strains show differences in anxiety and aggression,56, 91, 110, 112, 113 we suggest that variation in strain differences in sociability across studies and tasks are likely due to features of the task and social partner (eg, familiarity of the testing environment, partner age) that differentially elicit affiliative, anxious, or aggressive behaviors. The B6 strain is popular for the construction of mutant mice for the assessment of genes involved in social behavior in our laboratory and others.75, 114 In the context of DSI, the present data suggest that placing mutations on the D2 (or BXD) background that has higher baseline affiliative social motivation might enhance the ability to detect genes involved in social behavior, which can readily be accomplished with new gene editing strategies.
The present study examined behavior only in male mice. We believe the results are relevant to understanding typical and atypical human sociability, as a number of NDDs that involve atypical social behavior are more prevalent in males than females.115, 116 Nevertheless, sexually dimorphic social behaviors are common across species117 and sex‐specific QTLs have been identified for numerous heritable physiological and complex behavioral traits.118, 119, 120, 121, 122 Thus, studies in females to identify common and unique social trait variation and loci are a priority for future work. Finally, in humans, both very low and excessively high levels of sociability may describe aspects of the behavioral continuum that are not conducive to successful social interactions. The data presented here do not address whether the low and high levels of sociability reflect the extremes of typical variation in social behavior or patterns of interaction that would be disruptive to successful group outcomes. These are important questions for future studies.
The BXD panel shows continuous variation in many complex behavioral traits, including emotional regulation, activity and learning ability.46, 49 This provides an opportunity to examine trait covariation, which can inform shared and nonshared QTLs (see below). The results of the factor analysis and the QTL mapping are highly consistent, with both analyses identifying distinct activity, social approach and direct interaction factors and QTLs (Factors 1‐3) as well as both indicating that center latency and center time are composite measures of activity and anxiety‐like behaviors (ie, mixed loadings on Factors 1 and 6). The 4 domains of sociability measured in the present study loaded on 3 separate factors, consistent with the existence of related, yet distinct behavioral domains. Social novelty was heterogeneous across the panel, but because of low heritability this trait was not mapped. We observed significant, yet generally modest, correlations among social traits both within and across tasks (Table 1). A positive correlation was observed between partner sniffing and vocal communication, which loaded together on Factor 3. These traits tended to occur synchronously during behavior, suggesting they are behaviorally coupled and reflect a shared motivation for affiliative sociability.123 Nevertheless, these traits had different heritabilities and distinct genetic maps (see below). Because the DSI and 3‐chamber tasks are designed to measure different aspects of sociability (eg, approach vs engagement), it is not entirely surprising that the behavioral measures from each task load on separate factors. Yet, because social approach precedes adult‐initiated partner sniffing, the lack of tighter correlation of these aspects of sociability across strains was somewhat unexpected. The expression of social behaviors depends importantly upon the interplay between the magnitude of internal social drives and the specific social (eg, age, sex, familiarity of the partner) and environmental (familiarity, safety, size) context.124, 125, 126, 127, 128, 129 Thus, we suggest that rank‐order variation across tasks is likely because of important task differences, including differences in partner attributes (juvenile, adult), behavioral requirements (chamber exploration, presence of novel objects), and ethological relevance of behaviors (direct interaction vs restricted partner access). Some strains showed especially concordant (eg, BXD15, 53, 100) or discordant (eg, BXD9, 21 or 28) sociability rank‐orders across tasks and these strains could be valuable for examining the influence of specific task features on behavioral profiles in future studies. In both tasks, there was no relationship between the social measures and activity (see below), consistent with previous reports that sociability can vary independently of activity.109, 130
Factor 1 explained 20% of the trait variance and included activity measures collected from both tasks. Activity measures were highly heritable and strain rank‐order in activity was remarkably similar across tasks, despite salient differences in the testing environments (chamber size, novel objects) and social contexts (social partner absence/presence) and time between tests. Genetic mapping provided evidence of consistent QTLs on Chr 12 (88.7‐99.4 Mb) and distal Chr X (130.5‐136.3) for multiple activity measures (Figure 4). Differences between the task requirements may explain the absence of the Chr X QTL for DSI activity.
Both center latency and center time had primary and secondary loadings on Factors 1 and 6, consistent with the interpretation that these traits are composite measures of activity and anxiety‐like behaviors. We suggest that Factor 6 reflects anxiety‐like behavior based 2 lines of evidence. First, we suggest that there is face validity for strains with higher anxiety to be more inclined to spend time in the center chamber, which is ~one‐third of the size of the side chambers and is devoid of novel objects. Second, and more compelling, several of the loci identified for center time and center latency have been previously implicated in emotional regulation and anxiety. In particular, the center latency QTL on Chr 13 has been previously mapped by our laboratory and others for conditioned fear and anxiety.46, 49, 131 In addition, there is evidence that the center time QTL on distal Chr 12 is involved in emotional reactivity during fear learning.49 Finally, the center time QTL on distal Chr 1 is near a “QTL hotspot”94 identified repeatedly for neural and behavioral phenotypes including locomotor activity,132, 133, 134 emotional behavior,135, 136 and cerebellar size.137, 138 The same mice tested for social behavior in the present study also were tested for fear learning in a separate study.46 This afforded us the opportunity to examine whether putative anxiety measures would load with fear learning measures. We found that these measures loaded on separate factors, although, as described above, there are overlapping QTLs. Taken together the factor and genetic mapping data suggest that variation in center latency and center time relate to differences in both activity and anxiety‐like behavior.
Fifty strains provided sufficient power to detect significant QTLs for USV count (Chr 18) and activity (Chr X), as well as suggestive loci for USV count, social approach, activity and anxiety‐like behaviors. Currently, the field uses multiple criteria to identify high interest QTGs from significant QTLs, including sequence variation, cis‐regulation and correlation of transcript expression in relevant tissues with trait means, in addition to prior evidence for gene involvement in related behaviors. We used such an approach to stratify candidate QTGs (Figures 3 and 4), but additional studies are needed to examine sequence variation in coding and regulatory elements and to advance expression mapping and functional testing of 1 or more QTGs for heritable traits. As noted above, DSI and USV count were positively correlated and it is possible that genes that influence USV count could have pleiotropic effects on sniffing (or vice versa), but perhaps at effect sizes below that needed to generate a genome‐wide QTL of interest. This may be the case for the overlapping locus on Chr 19 that was detected for both USV count and social approach. Partner sniffing is a highly ethological, yet complex behavior that is comprised of multiple motivational, emotional, cognitive and behavioral components.123, 139, 140, 141 Although partner sniffing was highly heritable, we did not detect any suggestive or significant loci. There is increasing evidence that the genetic architecture of complex traits (number of loci, effect sizes) can vary considerably (eg, 1‐50%,142 and often hundreds of loci of small effect size contribute to complex traits in humans and mice.34, 143, 144, 145, 146 Allelic variation associated with heritable polygenic disorders defined by a constellation of symptoms, such as schizophrenia, correlates with relatively small changes in the expression of hundreds of genes,147 which together influence disorder risk. Sociability is a complex trait, but just one of many behavioral domains disrupted in certain psychiatric disorders. Thus, unpacking a complex trait such as partner sniffing using GRPs will increase power to elucidate underlying genetic mechanisms. For some traits, we found that there is a need for greater power by increasing strain number. We conducted power analyses assuming a single QTL accounting for a variable percentage of trait variance using the estimated trait variance from our existing data. Mapping in 47 RI strains typically enables detection of loci that account for ~30% of the trait variance, consistent with the effect sizes of the significant loci detected here. In contrast, mapping in 80 and 140 strains, respectively, is likely needed to detect loci accounting for 20% and 13% of trait variance (Figure S7). Power analyses based on more complex modeling approaches provide evidence of increased power in RI strains, where 100 strains are needed to detect a locus with 5% effect size.142, 148 While the genetic architecture and effect sizes of loci underlying partner sniffing are unknown, prior mapping of complex traits in mice suggests effect sizes ranging between ~2 and 15%.142 Thus, mapping in additional BXD strains is warranted both to identify loci for partner sniffing and confirm locations of suggestive loci for other traits.
5. CONCLUSIONS
Heterogeneity in social behavior is common. Yet compared with other behavioral domains, little is known about the heritable nature of variation in sociability, particularly as it may relate to the broad expression of symptom differences in neurodevelopmental and psychiatric disorders. The present findings show heritable variation in specific dimensions of sociability that is unrelated to variation in activity and anxiety‐like traits in male mice. The study also reports suggestive and significant QTLs that underlie measured trait variation in sociability, establishing a framework for the identification of specific genes and biological mechanisms that underlie typical and atypical social development.
Supporting information
Figure S1 Social behaviors in the parental strains with 129S1/SvImJ partners.
Figure S2 Additional behavioral measures from the direct social interaction task.
Figure S3 Additional behavioral measures from the 3‐chamber social interaction task.
Figure S4 Simple and composite interval maps for the behavioral traits.
Figure S5 Simple and composite interval maps for the behavioral traits, continued.
Figure S6 Epistatic effects for USV count.
Figure S7 Power calculations to estimate ability to detect quantitative trait loci of varying effect sizes.
ACKNOWLEDGMENTS
This work was supported by the Simms/Mann Chair in Developmental Neurogenetics, the Program in Developmental Neurogenetics, and National Institute of Mental Health Grant R01 MH080759 (P.L.) and Autism Speaks Translational Postdoctoral Fellowship 7595 (A.T.K.). The authors declare no competing financial interests. We thank Dr. Robert Williams for helpful comments on the manuscript.
Knoll AT, Jiang K, Levitt P. Quantitative trait locus mapping and analysis of heritable variation in affiliative social behavior and co‐occurring traits. Genes, Brain and Behavior. 2018;17:e12431. https://doi.org/10.1111/gbb.12431
Funding information Autism Speaks, Grant/Award number: 7595; National Institute of Mental Health, Grant/Award number: R01 MH080759
REFERENCES
- 1. Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. [DOI] [PubMed] [Google Scholar]
- 2. Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65:831–844. [DOI] [PubMed] [Google Scholar]
- 3. Israel S, Hasenfratz L, Knafo‐Noam A. The genetics of morality and prosociality. Current Opinion in Psychology. 2015;6:55–59. [Google Scholar]
- 4. Reeb‐Sutherland BC, Levitt P, Fox NA. The predictive nature of individual differences in early associative learning and emerging social behavior. PLoS ONE. 2012;7:e30511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernald RD. Cognitive skills and the evolution of social systems. J Exp Biol. 2017;220:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fratkin JL, Sinn DL, Patall EA, Gosling SD. Personality consistency in dogs: a meta‐analysis. PLoS One. 2013;8:e54907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herrmann E, Hare B, Call J, Tomasello M. Differences in the cognitive skills of bonobos and chimpanzees. PLoS One. 2010;5:e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leonhardt SD, Menzel F, Nehring V, Schmitt T. Ecology and evolution of communication in social insects. Cell. 2016;164:1277–1287. [DOI] [PubMed] [Google Scholar]
- 9. Moy SS, Nadler JJ, Young NB, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shorter J, Couch C, Huang W, et al. Genetic architecture of natural variation in Drosophila Melanogaster aggressive behavior. Proc Natl Acad Sci U S A. 2015;112:E3555–E3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silk JB. The adaptive value of sociality in mammalian groups. Philos Trans R Soc Lond B Biol Sci. 2007;362:539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sokolowski MB. Social interactions in "simple" model systems. Neuron. 2010;65:780–794. [DOI] [PubMed] [Google Scholar]
- 13. Feinstein C, Singh S. Social phenotypes in neurogenetic syndromes. Child Adolesc Psychiatr Clin N Am. 2007;16:631–647. [DOI] [PubMed] [Google Scholar]
- 14. State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci. 2011;14:1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trull TJ, Widiger TA. Dimensional models of personality: the five‐factor model and the DSM‐5. Dialogues Clin Neurosci. 2013;15:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turkheimer E, Pettersson E, Horn EE. A phenotypic null hypothesis for the genetics of personality. Annu Rev Psychol. 2014;65:515–540. [DOI] [PubMed] [Google Scholar]
- 17. Hall NJ, Wynne CD. The canid genome: behavioral geneticists' best friend? Genes Brain Behav. 2012;11:889–902. [DOI] [PubMed] [Google Scholar]
- 18. Hare B, Plyusnina I, Ignacio N, et al. Social cognitive evolution in captive foxes is a correlated by‐product of experimental domestication. Curr Biol. 2005;15:226–230. [DOI] [PubMed] [Google Scholar]
- 19. Hare B, Tomasello M. Human‐like social skills in dogs? Trends Cogn Sci. 2005;9:439–444. [DOI] [PubMed] [Google Scholar]
- 20. Nelson RM, Temnykh SV, Johnson JL, et al. Genetics of interactive behavior in silver foxes (Vulpes vulpes). Behav Genet. 2017;47:88–101. [DOI] [PubMed] [Google Scholar]
- 21. Persson ME, Roth LS, Johnsson M, Wright D, Jensen P. Human‐directed social behaviour in dogs shows significant heritability. Genes Brain Behav. 2015;14:337–344. [DOI] [PubMed] [Google Scholar]
- 22. Trut L, Oskina I, Kharlamova A. Animal evolution during domestication: the domesticated fox as a model. Bioessays. 2009;31:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Webber ES, Harmon KM, Beckwith TJ, et al. Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough‐and‐tumble play. Behav Brain Res. 2012;229:138–144. [DOI] [PubMed] [Google Scholar]
- 24. O'Connell LA, Hofmann HA. Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Front Neuroendocrinol. 2011;32:320–335. [DOI] [PubMed] [Google Scholar]
- 25. Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skuse DH, Gallagher L. Genetic influences on social cognition. Pediatr Res. 2011;69:85R–91R. [DOI] [PubMed] [Google Scholar]
- 27. Chailangkarn T, Trujillo CA, Freitas BC, et al. A human neurodevelopmental model for Williams syndrome. Nature. 2016;536:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015;350:aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lencz T, Knowles E, Davies G, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry. 2014;19:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry. 2015;20:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–878. [DOI] [PubMed] [Google Scholar]
- 33. Rung J, Cauchi S, Albrechtsen A, et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–1115. [DOI] [PubMed] [Google Scholar]
- 34. Robinson EB, Pourcain BS, Anttila V, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Constantino JN. The quantitative nature of autistic social impairment. Pediatr Res. 2011;69:55R–62R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. [DOI] [PubMed] [Google Scholar]
- 37. Constantino JN, Todorov A, Hilton C, et al. Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD. Mol Psychiatry. 2013;18:137–138. [DOI] [PubMed] [Google Scholar]
- 38. Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 39. Lundstrom S, Chang Z, Rastam M, et al. Autism spectrum disorders and autistic like traits: similar etiology in the extreme end and the normal variation. Arch Gen Psychiatry. 2012;69:46–52. [DOI] [PubMed] [Google Scholar]
- 40. St Pourcain B, Whitehouse AJ, Ang WQ, et al. Common variation contributes to the genetic architecture of social communication traits. Mol Autism. 2013;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andreux PA, Williams EG, Koutnikova H, et al. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell. 2012;150:1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chesler EJ, Lu L, Shou S, et al. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet. 2005;37:233–242. [DOI] [PubMed] [Google Scholar]
- 44. Collaborative Cross Consortium . The genome architecture of the collaborative cross mouse genetic reference population. Genetics. 2012;190:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Houtkooper RH, Mouchiroud L, Ryu D, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Knoll AT, Halladay LR, Holmes A, Levitt P. Quantitative trait loci and a novel genetic candidate for fear learning. J Neurosci. 2016;36:6258–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mackay TF. Mutations and quantitative genetic variation: lessons from drosophila. Philos Trans R Soc Lond B Biol Sci. 2010;365:1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neuner SM, Garfinkel BP, Wilmott LA, et al. Systems genetics identifies Hp1bp3 as a novel modulator of cognitive aging. Neurobiol Aging. 2016;46:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Philip VM, Duvvuru S, Gomero B, et al. High‐throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010;9:129–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–2180. [DOI] [PubMed] [Google Scholar]
- 51. Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic‐like behavior in mice. Genes Brain Behav. 2004;3:287–302. [DOI] [PubMed] [Google Scholar]
- 52. Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2010;10:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brodkin ES, Goforth SA, Keene AH, Fossella JA, Silver LM. Identification of quantitative trait loci that affect aggressive behavior in mice. J Neurosci. 2002;22:1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach‐avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. [DOI] [PubMed] [Google Scholar]
- 56. Faure A, Pittaras E, Nosjean A, Chabout J, Cressant A, Granon S. Social behaviors and acoustic vocalizations in different strains of mice. Behav Brain Res. 2017;320:383–390. [DOI] [PubMed] [Google Scholar]
- 57. Aldinger KA, Plummer JT, Qiu S, Levitt P. SnapShot: genetics of autism. Neuron. 2011;72:418–8.e1. [DOI] [PubMed] [Google Scholar]
- 58. Kazdoba TM, Leach PT, Yang M, Silverman JL, Solomon M, Crawley JN. Translational mouse models of autism: advancing toward pharmacological therapeutics. Curr Top Behav Neurosci. 2016;28:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 2004;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taylor BA, Wnek C, Kotlus BS, Roemer N, MacTaggart T, Phillips SJ. Genotyping new BXD recombinant inbred mouse strains and comparison of BXD and consensus maps. Mamm Genome. 1999;10:335–348. [DOI] [PubMed] [Google Scholar]
- 61. Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–292. [DOI] [PubMed] [Google Scholar]
- 62. Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. [DOI] [PubMed] [Google Scholar]
- 63. NESCent Working Group on Integrative Models of Vertebrate Sociality: Evolution, Mechanisms, and Emergent Properties , Hofmann HA, Beery AK, et al. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol Evol. 2014;29:581–589. [DOI] [PubMed] [Google Scholar]
- 64. O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision‐making network. Science. 2012;336:1154–1157. [DOI] [PubMed] [Google Scholar]
- 65. Szekely T, Moore AJ, Komdeur J. Social Behavior. Genes, Ecology and Evolution. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 66. Brigman JL, Mathur P, Lu L, Williams RW, Holmes A. Genetic relationship between anxiety‐related and fear‐related behaviors in BXD recombinant inbred mice. Behav Pharmacol. 2009;20:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biol Psychiatry. 2011;69:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Malkki HA, Donga LA, de Groot SE, Battaglia FP, Pennartz CM. Appetitive operant conditioning in mice: heritability and dissociability of training stages. Front Behav Neurosci. 2010;4:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Owen EH, Christensen SC, Paylor R, Wehner JM. Identification of quantitative trait loci involved in contextual and auditory‐cued fear conditioning in BXD recombinant inbred strains. Behav Neurosci. 1997;111:292–300. [DOI] [PubMed] [Google Scholar]
- 70. Taylor BA, Bedigian HG, Meier H. Genetic studies of the Fv‐1 locus of mice: linkage with Gpd‐1 in recombinant inbred lines. J Virol. 1977;23:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mozhui K, Hamre KM, Holmes A, Lu L, Williams RW. Genetic and structural analysis of the basolateral amygdala complex in BXD recombinant inbred mice. Behav Genet. 2007;37:223–243. [DOI] [PubMed] [Google Scholar]
- 72. Heinrichs SC, Koob GF. Application of experimental stressors in laboratory rodents. Curr Protoc Neurosci. 2006. , Chapter 8, Unit 8.4 Supplement 34:1–17. [DOI] [PubMed] [Google Scholar]
- 73. Matthews GA, Nieh EH, Vander Weele CM, et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell. 2016;164:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. [DOI] [PubMed] [Google Scholar]
- 75. Thompson BL, Levitt P. Complete or partial reduction of the Met receptor tyrosine kinase in distinct circuits differentially impacts mouse behavior. J Neurodev Disord. 2015;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang M, Silverman JL, Crawley JN. Automated three‐chambered social approach task for mice. Curr Protoc Neurosci. 2011, Chapter 8, Unit 8.26 Supplement 56:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Segbroeck M, Knoll AT, Levitt P, Narayanan S. MUPET‐mouse ultrasonic profile ExTraction: a signal processing tool for rapid and unsupervised analysis of ultrasonic vocalizations. Neuron. 2017;94:465–485.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hammerschmidt K, Reisinger E, Westekemper K, Ehrenreich L, Strenzke N, Fischer J. Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC Neurosci. 2012;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kikusui T, Nakanishi K, Nakagawa R, Nagasawa M, Mogi K, Okanoya K. Cross fostering experiments suggest that mice songs are innate. PLoS One. 2011;6:e17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mahrt EJ, Perkel DJ, Tong L, Rubel EW, Portfors CV. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J Neurosci. 2013;33:5573–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shifman S, Bell JT, Copley RR, et al. A high‐resolution single nucleotide polymorphism genetic map of the mouse genome. PLoS Biol. 2006;4:e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. [DOI] [PubMed] [Google Scholar]
- 83. Alberts R, Schughart K. QTLminer: identifying genes regulating quantitative traits. BMC Bioinform. 2010;11:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Overall RW, Kempermann G, Peirce J, et al. Genetics of the hippocampal transcriptome in mouse: a systematic survey and online neurogenomics resource. Front Neurosci. 2009;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mozhui K, Lu L, Armstrong WE, Williams RW. Sex‐specific modulation of gene expression networks in murine hypothalamus. Front Neurosci. 2012;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sen S, Satagopan JM, Broman KW, Churchill GA. R/qtlDesign: inbred line cross experimental design. Mamm Genome. 2007;18:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moles A, Costantini F, Garbugino L, Zanettini C, D'Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182:223–230. [DOI] [PubMed] [Google Scholar]
- 88. Panksepp JB, Jochman KA, Kim JU, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nadler JJ, Moy SS, Dold G, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. [DOI] [PubMed] [Google Scholar]
- 91. Moy SS, Nadler JJ, Young NB, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Williams RW, Bennett B, Lu L, et al. Genetic structure of the LXS panel of recombinant inbred mouse strains: a powerful resource for complex trait analysis. Mamm Genome. 2004;15:637–647. [DOI] [PubMed] [Google Scholar]
- 93. Demarest K, Koyner J, McCaughran J Jr, Cipp L, Hitzemann R. Further characterization and high‐resolution mapping of quantitative trait loci for ethanol‐induced locomotor activity. Behav Genet. 2001;31:79–91. [DOI] [PubMed] [Google Scholar]
- 94. Mozhui K, Ciobanu DC, Schikorski T, Wang X, Lu L, Williams RW. Dissection of a QTL hotspot on mouse distal chromosome 1 that modulates neurobehavioral phenotypes and gene expression. PLoS Genet. 2008;4:e1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. [DOI] [PubMed] [Google Scholar]
- 96. Jones DT, Reed RR. Golf: an olfactory neuron specific‐G protein involved in odorant signal transduction. Science. 1989;244:790–795. [DOI] [PubMed] [Google Scholar]
- 97. Fuchs T, Saunders‐Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pelosi A, Menardy F, Popa D, Girault JA, Herve D. Heterozygous Gnal mice are a novel animal model with which to study dystonia pathophysiology. J Neurosci. 2017;37:6253–6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Depienne C, LeGuern E. PCDH19‐related infantile epileptic encephalopathy: an unusual X‐linked inheritance disorder. Hum Mutat. 2012;33:627–634. [DOI] [PubMed] [Google Scholar]
- 100. Higurashi N, Nakamura M, Sugai M, et al. PCDH19‐related female‐limited epilepsy: further details regarding early clinical features and therapeutic efficacy. Epilepsy Res. 2013;106:191–199. [DOI] [PubMed] [Google Scholar]
- 101. Fujitani M, Zhang S, Fujiki R, Fujihara Y, Yamashita T. A chromosome 16p13.11 microduplication causes hyperactivity through dysregulation of miR‐484/protocadherin‐19 signaling. Mol Psychiatry. 2017;22:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Callaerts‐Vegh Z, Ahmed T, Vermaercke B, et al. Nxf7 deficiency impairs social exploration and spatio‐cognitive abilities as well as hippocampal synaptic plasticity in mice. Front Behav Neurosci. 2015;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Koo JH, Smiley MA, Lovering RM, Margolis FL. Bex1 knock out mice show altered skeletal muscle regeneration. Biochem Biophys Res Commun. 2007;363:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mangino M, Roederer M, Beddall MH, Nestle FO, Spector TD. Innate and adaptive immune traits are differentially affected by genetic and environmental factors. Nat Commun. 2017;8:13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Matteson LK, McGue M, Iacono WG. Shared environmental influences on personality: a combined twin and adoption approach. Behav Genet. 2013;43:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jones‐Davis DM, Yang M, Rider E, et al. Quantitative trait loci for interhemispheric commissure development and social behaviors in the BTBR T(+) tf/J mouse model of autism. PLoS One. 2013;8:e61829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nehrenberg DL, Wang S, Buus RJ, Perkins J, de Villena FP‐M, Pomp D. Genomic mapping of social behavior traits in a F2 cross derived from mice selectively bred for high aggression. BMC Genet. 2010;11:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Roubertoux PL, Guillot PV, Mortaud S, et al. Attack behaviors in mice: from factorial structure to quantitative trait loci mapping. Eur J Pharmacol. 2005;526:172–185. [DOI] [PubMed] [Google Scholar]
- 109. Takahashi A, Tomihara K, Shiroishi T, Koide T. Genetic mapping of social interaction behavior in B6/MSM consomic mouse strains. Behav Genet. 2010;40:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ma L, Piirainen S, Kulesskaya N, Rauvala H, Tian L. Association of brain immune genes with social behavior of inbred mouse strains. J Neuroinflammation. 2015;12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mozhui K, Karlsson RM, Kash TL, et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate‐mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Selmanoff MK, Maxson SC, Ginsburg BE. Chromosomal determinants of intermale aggressive behavior in inbred mice. Behav Genet. 1976;6:53–69. [DOI] [PubMed] [Google Scholar]
- 114. International Mouse Knockout Consortium , Collins FS, Rossant J, Wurst W. A mouse for all reasons. Cell. 2007;128:9–13. [DOI] [PubMed] [Google Scholar]
- 115. Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bayless DW, Shah NM. Genetic dissection of neural circuits underlying sexually dimorphic social behaviours. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fullerton J, Cubin M, Tiwari H, et al. Linkage analysis of extremely discordant and concordant sibling pairs identifies quantitative‐trait loci that influence variation in the human personality trait neuroticism. Am J Hum Genet. 2003;72:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mogil JS, Richards SP, O'Toole LA, et al. Identification of a sex‐specific quantitative trait locus mediating nonopioid stress‐induced analgesia in female mice. J Neurosci. 1997;17:7995–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nuzhdin SV, Pasyukova EG, Dilda CL, Zeng ZB, Mackay TF. Sex‐specific quantitative trait loci affecting longevity in drosophila melanogaster. Proc Natl Acad Sci U S A. 1997;94:9734–9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wang S, Yehya N, Schadt EE, Wang H, Drake TA, Lusis AJ. Genetic and genomic analysis of a fat mass trait with complex inheritance reveals marked sex specificity. PLoS Genet. 2006;2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zubenko GS, Hughes HB, Stiffler JS, Zubenko WN, Kaplan BB. Genome survey for susceptibility loci for recurrent, early‐onset major depression: results at 10cM resolution. Am J Med Genet. 2002;114:413–422. [DOI] [PubMed] [Google Scholar]
- 123. Sirotin YB, Costa ME, Laplagne DA. Rodent ultrasonic vocalizations are bound to active sniffing behavior. Front Behav Neurosci. 2014;8:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Babineau BA, Bliss‐Moreau E, Machado CJ, Toscano JE, Mason WA, Amaral DG. Context‐specific social behavior is altered by orbitofrontal cortex lesions in adult rhesus macaques. Neuroscience. 2011;179:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chabout J, Serreau P, Ey E, et al. Adult male mice emit context‐specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One. 2012;7:e29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hanson JL, Hurley LM. Context‐dependent fluctuation of serotonin in the auditory midbrain: the influence of sex, reproductive state and experience. J Exp Biol. 2014;217:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hoier S, Pfeifle C, von Merten S, Linnenbrink M. Communication at the garden fence—context dependent vocalization in female house mice. PLoS One. 2016;11:e0152255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Williamson CM, Lee W, Romeo RD, Curley JP. Social context‐dependent relationships between mouse dominance rank and plasma hormone levels. Physiol Behav. 2017;171:110–119. [DOI] [PubMed] [Google Scholar]
- 130. Berton O, Ramos A, Chaouloff F, Mormde P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behav Genet. 1997;27:155–166. [DOI] [PubMed] [Google Scholar]
- 131. Carhuatanta KA, Shea CJ, Herman JP, Jankord R. Unique genetic loci identified for emotional behavior in control and chronic stress conditions. Front Behav Neurosci. 2014;8:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. DeFries JC, Gervais MC, Thomas EA. Response to 30 generations of selection for open‐field activity in laboratory mice. Behav Genet. 1978;8:3–13. [DOI] [PubMed] [Google Scholar]
- 133. Gershenfeld HK, Neumann PE, Mathis C, Crawley JN, Li X, Paul SM. Mapping quantitative trait loci for open‐field behavior in mice. Behav Genet. 1997;27:201–210. [DOI] [PubMed] [Google Scholar]
- 134. Kelly MA, Low MJ, Phillips TJ, Wakeland EK, Yanagisawa M. The mapping of quantitative trait loci underlying strain differences in locomotor activity between 129S6 and C57BL/6J mice. Mamm Genome. 2003;14:692–702. [DOI] [PubMed] [Google Scholar]
- 135. Milhaud JM, Halley H, Lassalle JM. Two QTLs located on chromosomes 1 and 5 modulate different aspects of the performance of mice of the B x D ty RI strain series in the Morris navigation task. Behav Genet. 2002;32:69–78. [DOI] [PubMed] [Google Scholar]
- 136. Turri MG, Talbot CJ, Radcliffe RA, Wehner JM, Flint J. High‐resolution mapping of quantitative trait loci for emotionality in selected strains of mice. Mamm Genome. 1999;10:1098–1101. [DOI] [PubMed] [Google Scholar]
- 137. Airey DC, Lu L, Williams RW. Genetic control of the mouse cerebellum: identification of quantitative trait loci modulating size and architecture. J Neurosci. 2001;21:5099–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hager R, Lu L, Rosen GD, Williams RW. Genetic architecture supports mosaic brain evolution and independent brain‐body size regulation. Nat Commun. 2012;3:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kazdoba TM, Leach PT, Crawley JN. Behavioral phenotypes of genetic mouse models of autism. Genes, Brain and Behavior. 2016;15:7–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes Brain Behav. 2011;10:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Matsuo T, Hattori T, Asaba A, et al. Genetic dissection of pheromone processing reveals main olfactory system‐mediated social behaviors in mice. Proc Natl Acad Sci U S A. 2015;112:E311–E320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev Genet. 2005;6:271–286. [DOI] [PubMed] [Google Scholar]
- 143. Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Parker CC, Palmer AA. Dark matter: are mice the solution to missing heritability? Front Genet. 2011;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schizophrenia Working Group of the Psychiatric Genomics Consortium , Ripke S, Neale BM, et al. Biological insights from 108 schizophrenia‐associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yang J, Benyamin B, McEvoy BP, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Fromer M, Roussos P, Sieberts SK, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Valdar W, Flint J, Mott R. Simulating the collaborative cross: power of quantitative trait loci detection and mapping resolution in large sets of recombinant inbred strains of mice. Genetics. 2006;172:1783–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Social behaviors in the parental strains with 129S1/SvImJ partners.
Figure S2 Additional behavioral measures from the direct social interaction task.
Figure S3 Additional behavioral measures from the 3‐chamber social interaction task.
Figure S4 Simple and composite interval maps for the behavioral traits.
Figure S5 Simple and composite interval maps for the behavioral traits, continued.
Figure S6 Epistatic effects for USV count.
Figure S7 Power calculations to estimate ability to detect quantitative trait loci of varying effect sizes.


