Abstract
Although radiation therapy is an effective treatment modality in many cancers, there is an urgent need to develop therapeutic drugs capable of overcoming radioresistance or minimizing normal tissue toxicity. A wide variety of marine-derived bioactive compounds have been screened for anti-cancer drug discovery, but little is known regarding radiation therapy applications. In this study, six different extracts of marine sponges collected from the Micronesian sea were screened for anti-cancer and radiosensitizing activity. Two extracts derived from Agelas sponges collected off the coast of Kosrae and Chuuk, the Federated States of Micronesia significantly decreased clonogenic survival of hepatocellular carcinoma (HCC) cells after exposure to ionizing radiation (IR). The Agelas extracts augmented IR-induced apoptosis and accumulation of reactive oxygen species (ROS). Endoplasmic reticulum (ER) stress was increased via unfolded protein response stimulation, which induced autophagy. N-acetylcysteine, a ROS scavenger, diminished ER stress and autophagy induction effects. This result indicated that Agelas extracts may sensitize HCC cells to IR via ROS overproduction in vitro. Our findings suggest that the Agelas sp. may have potential utility in radiosensitizer development.
Introduction
Cancer is a leading cause of death worldwide and development of anticancer drugs with improved sensitivity and specificity is urgently needed. Most anticancer drugs used clinically are based on chemically synthesized compounds, but bioactive molecules from natural organisms have been intensively investigated as novel drug sources. Bioactive compounds from marine organisms have received increased attention due to their diversity and complex habitats1,2. Bioactive molecules include proteins, peptides, amino acids, fatty acids, polysaccharides, phenolic compounds, photosynthetic pigments, and vitamins. A variety of marine bioactive molecules have been tested as candidates for lead compounds in anticancer drug discovery3–6.
Radiation therapy is a powerful treatment modality that is applied for approximately 50% of cancer patients. Recent advances in beam delivery techniques have led to an improvement of treatment outcome with better tumour control and minimal injury to normal tissues. Nonetheless, treating radiation resistance tumours is challenging due to tumour recurrence7,8. Much effort has been devoted to developing radiosensitizers, chemical compounds that potentiate the anti-cancer effect of radiation therapy, to increase local tumour control9–11. Major modes of radiation-induced tumour cell death include apoptosis, necrosis, and autophagy, which are mainly caused by accumulation of unrepairable DNA breaks12. Various chemotherapy drugs that possess DNA-damaging activity have been shown to exert a radiation-sensitizing effect, but they accompanied by significant adverse effects. Hypoxia-targeted agents are another mainstay of radiosensitizers that alleviate resistance to radiation therapy9. Molecular-targeted agents have also been shown to provide a synergistic effect with radiation therapy11.
Hepatocellular carcinoma (HCC), a primary malignancy of the liver, is the fifth most common cancer type and third most common cause of cancer-related death worldwide. Radiation therapy is recommended in combination with transarterial chemoembolization (TACE) for patients with advanced HCC who are not amenable to curative therapy options such as surgery, transplantation or radiofrequency ablation13. Sorafenib is a multi-targeted kinase inhibitor and the only Food and Drug Administration (FDA)-approved drug for the advanced HCC, but its efficacy is limited. Comprehensive genomic studies have provided clues for molecular targeted therapeutics or new treatment strategies, but little success has been reported14.
Investigations of bioactive compounds from terrestrial plants have demonstrated that compounds such as curcumin and quercetin could have potential as natural radiosensitizers15. However, applications of marine bioactive compounds have not been reported regarding radiation therapy. In this study, we obtained the extracts of marine species collected from the Micronesian ocean and evaluated the HCC cytotoxicity and radiation sensitization activity. We reported for the first time that marine sponges of the Agelas sp. may be a source of natural compounds with radiosensitizing activity.
Results
A blind screen of anticancer and radiosensitizing activities in marine sponge extracts
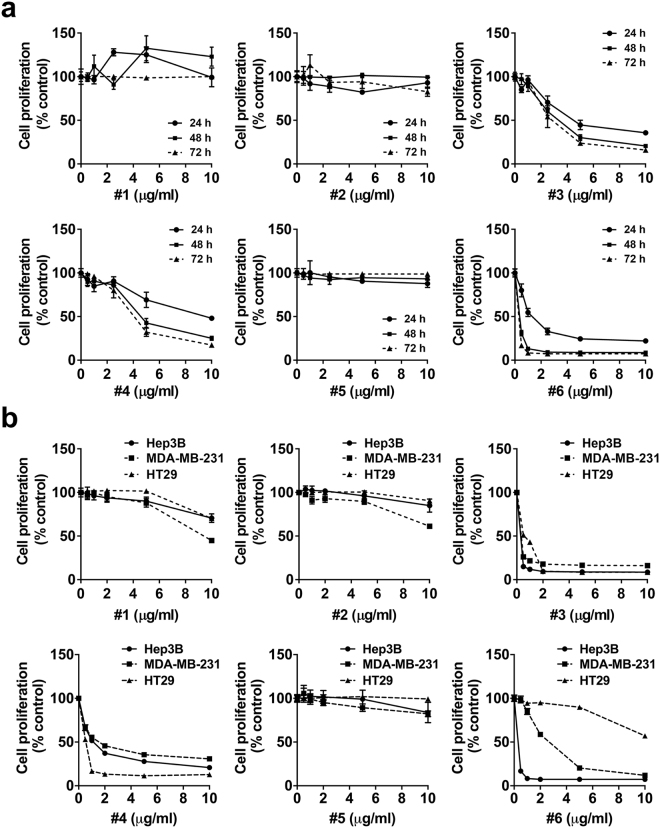
We selected six methanol extracts of marine sponges collected from the Micronesian ocean and identified marine-derived bioactivity with potential application for radiation therapy. We measured the time and concentration-dependent cytotoxicity of the extracts in human HCC Hep3B cells. A blind screen revealed that three of six samples (#3, #4, and #6) had a strong cytotoxicity effect in Hep3B cells, but the rest (#1, #2, and #5) had minimal effects on cell proliferation even at a concentration of 10 µg/mL (Fig. 1a). The cytotoxic extracts were prepared from Theonella sp. (#3 and #4) and Haliclona sp. (#6). Extracts with mild cytotoxic activity were prepared from Agelas sp. (#1 and #2) and Coscinoderma sp. (#5). Similar cytotoxic profiles were observed in other cancer types, including breast cancer MDA-MB-231 cells and colorectal cancer HT29 cells, except that #6 showed mild cytotoxicity against HT29 cells (Fig. 1b). The sponge extracts used in this study are summarized in Table 1.
Figure 1.
Screen of anticancer and radiosensitizing activity in the extracts of marine sponges collected from the Micronesian ocean. (a) Cytotoxicity of six marine sponge extracts were screened in human hepatocellular carcinoma Hep3B cells. Hep3B cells were incubated with either DMSO or the indicated concentrations of marine sponge extracts for 24, 48 and 72 h. Cytotoxicity was determined using a CCK-8 assay kit. Three of the samples had severe cytotoxicity in Hep3B cells. (b) Cytotoxicity of the six marine sponge extracts were compared in Hep3B, breast cancer MDA-MB-231, and colorectal cancer HT29 cells. The cells were incubated with the indicated concentrations of the sponge extracts for 72 h.
Table 1.
Marine sponge extracts used in this study.
| No | Sponge species | State | Country |
|---|---|---|---|
| 1 | Agelas sp. | Chuuk State | Federated States of Micronesia |
| 2 | Agelas sp. | Kosrae state | Federated States of Micronesia |
| 3 | Theonella sp. | Philippines | |
| 4 | Theonella sp. | Philippines | |
| 5 | Coscinoderma sp. | Kosrae state | Federated States of Micronesia |
| 6 | Haliclona sp. | Philippines |
The Agelas extracts have radiosensitizing activity
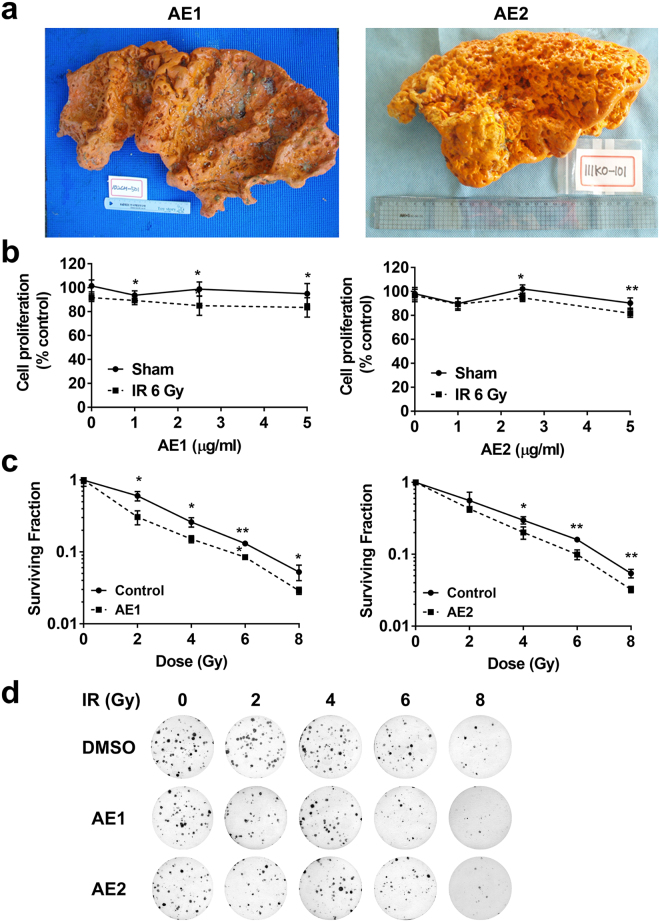
We screened radiosensitizing activity in three sponge extracts (#1, #2 and #5) which exhibited mild cytotoxicity. Two Agelas extracts, named AE1 (#1) and AE2 (#2) were prepared from the specimens collected from the oceans of Chuuk and Kosrae States, respectively, in Micronesia (Fig. 2a). A cytotoxicity assay showed that AE1 and AE2 further decreased cell proliferation at 5 µg/mL when combined with 6 Gy of IR (p < 0.05 and p < 0.01, respectively; Fig. 2b). We performed a clonogenic survival assay, the gold standard method for assessing radiation sensitivity, to confirm the radiosensitizing activity of the Agelas extracts. The Hep3B cells were pre-treated with 1 µg/mL of AE1 or AE2 for 3 h, followed by exposure to various doses of IR. After 1–2 weeks, the surviving colonies were stained and counted. Dose response curves showed that the pre-treatment with either AE1 or AE2 significantly decreased clonogenic survival after exposure to IR (Fig. 2c and d). In contrast, Coscinoderma sp. extract (#5) showed no radiosensitizing activity in Hep3B cells (Supplementary Fig. S1a,b). As a positive control, treatment with suberoylanilide hydroxamic acid (SAHA), a well-known radiosensitizer16,17 led to enhancement of radiation-mediated inhibition of proliferation and clonogenic survival of Hep3B cells (Supplementary Fig. S1c,d). Therefore, these data suggest that the Agelas extracts may contain bioactive molecules that enhanced the radiation effect.
Figure 2.
The Agelas extracts decrease clonogenic survival after IR treatment in human hepatocellular carcinoma cells. (a) Photographs of the original Agelas sp. specimens collected from Chuuk State and Kosrae States from which AE1 and AE2 samples were extracted, respectively. (b) AE1 and AE2 increased cytotoxic effect when combined with 6 Gy of IR. Data are presented as mean ± SD (n=7). *p < 0.05, **p < 0.01. (c) Clonogenic survival assay showed AE1 and AE2 but not #5 significantly increased radiation sensitivity of Hep3B cells. Data are presented as mean ± SD (n=3). *p < 0.05, **p < 0.01. (d) Photos of Hep3B colonies survived after exposure of the indicated doses of X-rays. Hep3B cells were pre-treated with 1 µg/mL of AE1 or AE2 for 3 h, followed by X-ray irradiations.
The Agelas extracts enhance radiation-induced apoptosis
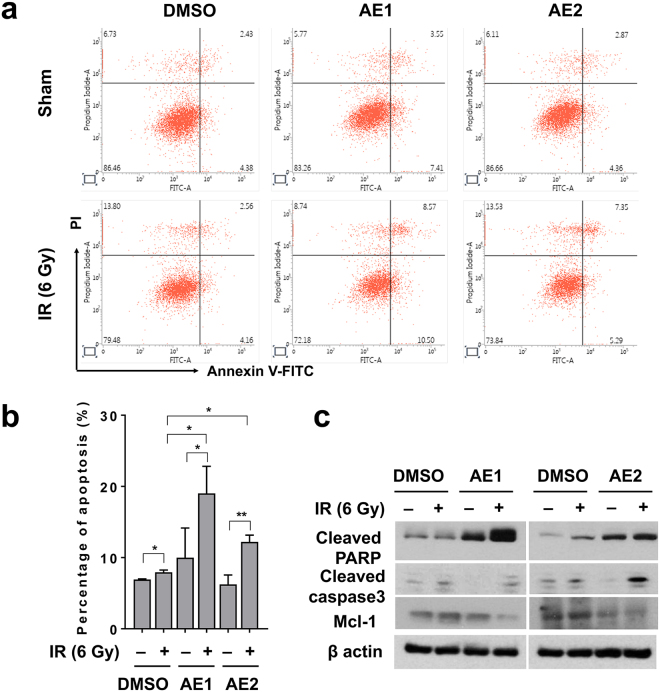
We determined the effects of the Agelas extracts on apoptosis, a mode of radiation-induced death, to determine the mechanism of increased radiation sensitization. Flow cytometry analysis with annexin V staining showed that AE1 and AE2 did not change the percentage of apoptotic cells, and 6 Gy of IR induced apoptotic cell death (p < 0.05; Fig. 3a,b). The pre-treatment with either AE1 or AE2 significantly enhanced IR-induced apoptosis (p < 0.05; Fig. 3a,b). Western blot analysis further confirmed that the Agelas extracts increased apoptosis (Fig. 3c). IR-induced expression of cleaved PARP, a surrogate marker of intrinsic apoptosis signalling, were further increased by pre-treatment with AE1 or AE2. The anti-apoptotic protein, Mcl-1, was decreased by the pre-treatment. Thus, enhanced radiosensitization caused by the Agelas extracts may be attributed to increased apoptotic cell death.
Figure 3.
The Agelas extracts enhance IR-induced apoptosis. (a) Apoptosis was assessed by flow cytometry with FITC-conjugated annexin V/PI staining. Hep3B cells were pre-treated with 10 µg/mL of AE1 or AE2 for 3 h, followed by exposure to 6 Gy of IR. After 24 h of incubation, Hep3B cells were co-stained with annexin V-FITC and PI and was subjected to flow cytometer. (b) Quantification of apoptotic cell percentage showed the Agelas extracts enhanced IR-induced apoptosis. The data represent the means ± SD of three independent experiments (n=3; *p < 0.05, **p < 0.01). (c) Western blot analysis confirmed enhanced apoptosis by the Agelas extracts. Hep3B cell lysates were immunoblotted for the detection of cleaved PARP1, and Mcl-1. β-actin was used as a loading control. Uncropped images are shown in Supplementary Fig. S3.
The Agelas extracts induce autophagy by stimulating endoplasmic reticulum stress
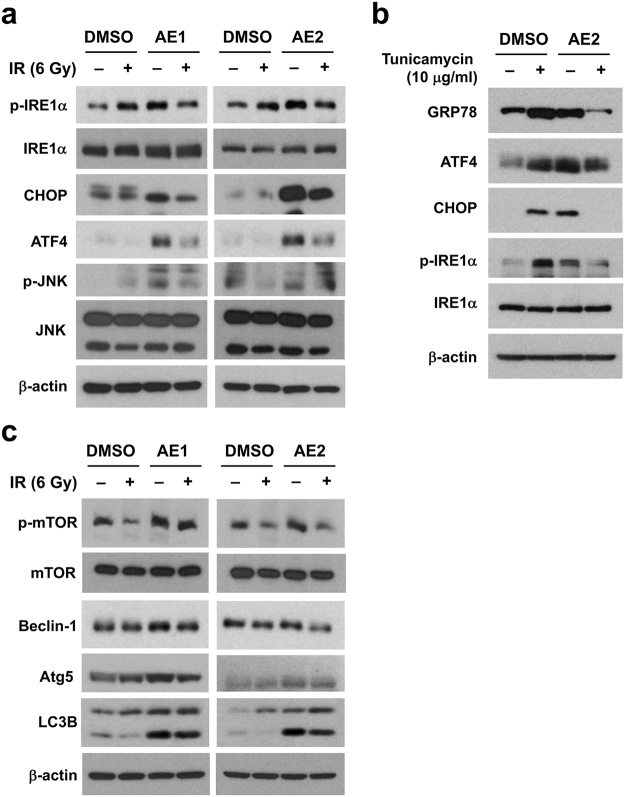
Autophagy induction is a survival mechanism activated by exposure to IR, so autophagy is considered a potential therapeutic target18,19. The effect of the Agelas extracts on endoplasmic reticulum (ER) stress, which triggers autophagy, was determined by protein expression of the unfolded protein response (UPR) pathways20. Major UPR pathways are comprised of UPR sensors such as PERK, IRE1α, and ATF6 with their downstream effectors. Both AE1 and AE2 activated the UPR pathways, as indicated by increased expression of phospho-IRE1α, CHOP, ATF4, and phospho-JNK (Fig. 4a). In contrast, Coscinoderma sp. extract (#5) did not induce ER stress (Supplementary Fig. S2a). IR alone dose-dependently increased phospho-IRE1α, GRP78 and ATF4 (Supplementary Fig. S2b). The addition of IR suppressed the Agelas extract-mediated UPR activation (Fig. 4a). Similarly, tunicamycin, a strong ER stress inducer21, suppressed expression of UPR proteins including GRP78, ATF4, CHOP and phospho-IRE1α when co-treated with AE2 (Fig. 4b). Next, we determined the effect of the Agelas extracts on autophagy since excessive ER stress induces autophagy. Both AE1 and AE2 induced the expression of the autophagy-related proteins including phospho-mTOR, beclin-1, Atg5, and LC3B-II in Hep3B cells (Fig. 4c). Combined treatment with 6 Gy of IR diminished the Agelas extract-induced autophagy (Fig. 4c), which was apparent in the AE2-treated samples. A similar pattern was observed in the autophagy and UPR responses, so the Agelas extracts may trigger autophagy signalling via ER stress.
Figure 4.
The Agelas extracts induce autophagy by stimulating ER stress. (a) Pre-treatment with the Agelas extracts led to induction of ER stress. Hep3B cells were pre-incubated with 10 µg/mL of AE1 or AE2 for 3 h, followed by 6 Gy of IR. At 24 h post-irradiation, the samples were prepared and subjected to Western blot analysis. Expression of unfolded protein response-related proteins, including phospho-IRE1α, total IRE1α, CHOP, ATF4, phospho-JNK and total JNK were upregulated by pre-treatment with AE1 and AE2 but diminished by co-treatment with 6 Gy of IR. β-actin was used as a loading control. (b) Agelas extract-induced ER stress signaling was suppressed by tunicamycin, a well-known unfold protein response inducer. Expression of GRP78, ATF4, CHOP, phospho-IRE1α was induced by either AE2 or tunicamycin, but was suppressed by the combination treatment. (c) Pre-treatment with the Agelas extracts led to induction of autophagy. Treatment with 10 µg/mL of AE1 or AE2 increased expression of autophagy-related proteins including phospho-mTOR, beclin-1, Atg5 and LC3B-II. Co-treatment with IR suppressed the Agelas extract-induced autophagy. Uncropped images are shown in Supplementary Fig. S3.
The Agelas extracts augment radiation-induced reactive oxygen species production
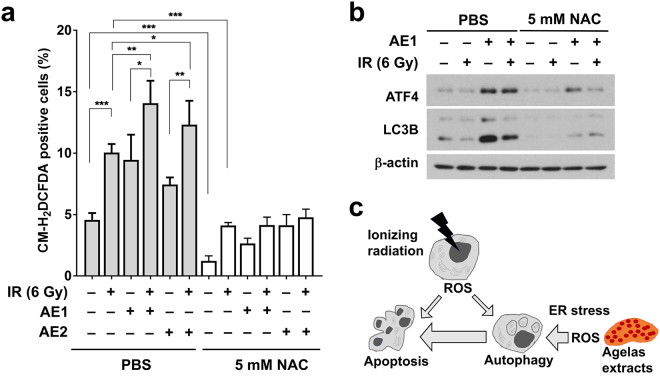
Reactive oxygen species (ROS) are a common source of IR-induced DNA damage and ER stress. We determined the effect of the Agelas extracts on ROS production by using the redox sensitive fluorescence dye, CM-H2DCFDA. Treatment with 6 Gy of IR significantly increased intracellular ROS levels, compared to the sham-treated control (p < 0.001, Fig. 5a). Pre-treatment with either AE1 or AE2 also induced intracellular ROS production and further augmented ROS levels when combined with IR (Fig. 5a). We determined the effect of N-acetylcysteine (NAC), a common ROS scavenger, on Agelas extract-mediated ER stress and autophagy. Pre-treatment with 5 mM NAC decreased intracellular ROS levels and abolished ROS augmentation by either IR or the Agelas extracts (p < 0.001, Fig. 5a). The NAC pre-treatment strongly suppressed the AE1-induced expression of ATF4 and LC3B-II (Fig. 5b). The suppressive effect of NAC was also observed with AE2 (data not shown). These results suggest that the increased ROS levels from Agelas extract treatment may induce ER stress and autophagy, leading to the radiosensitization effect (Fig. 5c).
Figure 5.
The Agelas extracts augment IR-induced ROS production. (a) Hep3B cells were pre-incubated with 10 µg/mL of AE1 or AE2 and CM-H2DCFDA for 3 h, followed by exposure to 6 Gy of IR. After 48 h of incubation, intracellular ROS levels were determined by DCF fluorescence using Flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001. (b) N-acetylcysteine (NAC) abolished the induction of ER-stress and autophagy by the Agelas extracts. Pre-treatment with 5 mM NAC decreased AE1-induced expression of ATF4 and LC3B-II. PBS, phosphate buffered saline. Uncropped images are shown in Supplementary Fig. S3. (c) Schematic diagram of how the Agelas extracts increase radiosensitization in Hep3B cells.
Discussion
Due to highly diverse marine habitats, metabolites isolated from marine organisms are considered a rich resource of bioactive natural products. Numerous efforts to screen bioactive molecules have resulted in identification of various metabolites that possess potential anti-cancer activity2,5,22. Several marine-derived anti-cancer drugs are already FDA-approved or being tested in clinical trials2,4. For example, cytarabine, a synthetic pyrimidine nucleoside originally isolated from the Caribbean sponge Tethya crypta has been approved for leukaemia. Trabectedin is an FDA-approved marine metabolite of Ecteinascidia turbinata, a tunicate from the Caribbean and Mediterranean Sea, for soft tissue sarcoma treatment2,4. While the list of marine-derived drugs tested in clinical trials is rapidly growing, none have been tested as radiation therapy adjuvants.
HCC is a leading cause of cancer-related mortality because patients with HCC are often diagnosed at advanced stages where curative approaches are not feasible. Radiation therapy is an effective option in this situation and sensitizers that improve the efficacy of radiation therapy have been investigated9,15. We obtained extracts of marine sponges collected from the deep sea near Micronesia and screened their radiosensitizing activity. These novel marine-derived extracts could have potentially applications with radiation therapy for HCC treatment.
Initial blind tests were performed with six marine-derived extracts based on a cell proliferation assay. Among them, two samples slightly increased radiation-mediated cytotoxicity, which were obtained from Agelas sp. separately collected from oceans near the Chuuk and Kosrae states. The radiosensitizing effect of the Agelas extracts was confirmed by clonogenic survival assay, the gold standard method for measuring radiation sensitivity. The Agelas extracts alone did not demonstrate cytotoxicity, but extracts from Theonella sp. and Haliclona sp. had strong cytotoxic effects on HCC cells. Coscinoderma extract showed the similar cytotoxic profile with Agelas extracts but no radiosensitizing activity, indicating a distinctive feature of Agelas extracts from others.
We investigated the effects of Agelas extracts on apoptosis and autophagy to elucidate the underlying mechanisms of enhanced radiation-mediated cytotoxicity. Flow cytometry and biochemical analyses revealed that neither extract increased apoptotic death in Hep3B cells. Apoptosis was enhanced when the extracts were combined with IR, suggesting that radiosensitization may be related to increased apoptotic death. IR activates survival signalling pathways, and autophagy is a cellular mechanism that confers radioresistance18,19,23,24. Preclinical studies have shown that inhibiting autophagy via siRNA against beclin 1 and ATG5, or pharmacological inhibitors such as 3-MA, increase the efficacy of radiotherapy in breast cancer, glioma, colorectal, and oesophageal cancer cells25–27. This suggests that autophagy may be a potential therapeutic target in cancer patients that receive radiation therapy. Autophagy induction via AKT or mTOR inhibitors also leads to radiosensitization in lung cancer and glioma28,29. Since autophagy is implicated in cancer cell survival and death, treatment strategies must be carefully designed. We found that the Agelas extracts alone greatly induced autophagy, but they suppressed autophagy and induced apoptosis when they were combined with IR.
Induction of ER stress and UPR activation stimulated autophagy, which is implicated in cancer progression and therapeutic response. IR induces the unfolded protein response and ER stress in this study and others18,19. Agelas extracts dramatically induced ER stress, but Coscinoderma extract did not, suggesting that the excessive ER stress may be closely related to the radiosensitization effect. Moreover, combination of Agelas extracts with tunicamycin, a well-known ER stress inducer, suppressed UPR activation as seen in their combination with IR. The ROS accumulated by water radiolysis during radiation treatment is a source of DNA oxidative damage and a critical inducer of ER stress. Both Agelas extracts increased expression of the proteins related to ER stress and autophagy as well as ROS production. Co-treatment with the ROS scavenger, NAC, suppressed the Agelas extract-mediated autophagy induction. This result suggested that ROS production by the Agelas extracts may induce ER stress and autophagy. Taken together, our data suggest that combination of the Agelas extracts with IR may lead to excessive ROS production, thereby shifting the balance from survival to death (Fig. 5c).
Sponges are a primitive marine invertebrate that produce over 5,300 different metabolites, including alkaloids, glycosides, macrolides, and terpenoids. Sponges have been investigated and utilized as a rich source of natural products for anti-cancer drug discovery3,6. Agelas sp. contain several well-characterized metabolites, such as agelasines and oroidin. A variety of structural modifications have been attempted to increase the efficacy and specificity of their bioactivity30,31. To our knowledge, this is the first report describing the radiosensitizing activity of marine sponge extracts and providing mechanistic insights enhanced radiation efficacy by Agelas extracts. Further studies are warranted to identify and develop natural radiosensitizers from the marine sponges Agelas extracts and evaluate in vivo efficacy in animal models.
Materials and Methods
Preparation of marine sponge extracts
The Agelas sponges were collected by hand using SCUBA at a 10-meter depth off the shores of Chuuk and Kosrae, the Federated States of Micronesia. The lyophilized sponge was extracted with MeOH and CH2Cl2 at room temperature for 12 h. The combined extract was partitioned between n-BuOH and H2O to remove salts, and the organic layer was concentrated under vacuum and dissolved in DMSO for the evaluation of biological activity.
Cell culture
Human hepatocellular carcinoma Hep3B, human breast adenocarcinoma MDA-MB-231, human colorectal adenocarcinoma HT29 cells were purchased from the Korean Cell Line Bank (Seoul National University, Seoul, Korea) or from the American Type Culture Collection (ATCC, Manassas, VA, USA). Hep3B and MDA-MB-231 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS), 1% Antibiotic-Antimycotic (AA, Thermo Fisher Scientific, Waltham, MA, USA), and 25 mM HEPES (Gibco, Carlsbad, CA, USA). HT29 cells were grown in RPMI 1640 medium supplemented with 10% FBS, 1% AA. Cells were maintained in a humidified 37 °C incubator with 5% CO2.
Cell viability assay
Cell viability was determined by Cell Counting Kit-8 assay (CCK-8, Dojindo Laboratories, Kumamoto, Japan). Hep3B cells were seeded at 1 × 103 cells/well into a 96-well plate and incubated with various concentrations of the extract samples for 24, 48, and 72 h. After 2 h of incubation with CCK-8 solution, the absorbance at 450 nm was determined using a SpectraMax i3 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The relative cell viability was calculated as percentage of untreated control.
Irradiation experiments
X-ray irradiation was performed as previously described32. Briefly, Hep3B cells were irradiated with 6-MV X-rays at a dose rate of 3.96 Gy per min using a linear accelerator Varian Clinac 6EX machine (Varian Medical Systems, Palo Alto, CA, USA). The cell dishes were placed under 2 cm thickness solid water phantom with source surface distance of 100 cm and field size of 30 × 30 cm. The X-ray absolute dose was calibrated according to TG-51 and verified with Gafchromic film to 1% accuracy.
Clonogenic survival assay
Clonogenic assay was performed as previously described32,33. Hep3B cells were seeded into a six well plate in triplicate and then incubated overnight (200 cells for 0 Gy and 2 Gy; 400 cells for 4 Gy; 800 cells for 6 Gy; 1000 cells for 8 Gy). The cells were pre-treated with 5 µg/mL of sponge extracts or 1 μM suberoylanilide hydroxamic acid (SAHA; Sigma, St. Louse, MO, USA) for 3 h and were subsequently irradiated with increasing doses of 0, 2, 4, 6, and 8 Gy of IR. After incubation for 7–14 days, surviving cells were fixed and stained with 1% crystal violet and the colonies containing 50 or more cells were manually counted. Plating efficiency was calculated as % of colonies from seeded cells and cell survival fraction at each irradiation dose was determined by dividing the plating efficiency of the irradiated cells by that of the sham-treated control. The survival curves were drawn using GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA, USA).
Apoptosis assay
Apoptosis was assessed by flow cytometry after annexin V/propidium iodide (PI) staining. 1 × 105 Hep3B cells were plated in 6-well plates and next day the cells were pre-treated with 10 µg/mL of AE1 or AE2 for 3 h, followed by exposure to 6 Gy of IR. After 72 h incubation, cells were treated with trypsin, washed with PBS (pH 7.4), and stained with annexin V-FITC (BD Pharmingen, San Diego, CA, USA) and 2 μg/mL PI in annexin V binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) for 15 min at 37 °C in the dark. Samples were analyzed by using a BD FACSVerse flow cytometer (Bectron-Dickinson, CA, USA) and data acquisition software, BD FACSuite.
ROS measurement
Hep3B cells were pre-treated with 5 mM N-acetylcysteine for 1 h before the incubation with the marine sponge extracts. Cells were pre-incubated with 10 µg/mL of AE1 or AE2 and 2 μM Carboxy-H2DCFDA for 3 h. Intracellular ROS level was analyzed by flow cytometry 48 h post-irradiation (FACSVerse, Becton-Dickinson, CA, USA).
Western blot analysis
Hep3B cells were lysed in a lysis buffer (20 mM Tris, pH 8.0) containing 137 mM NaCl, 10% glycerol, 1% nonidet P-40, 10 mM EDTA, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 10 mg/mL leupeptin. The supernatant was collected after centrifugation at 13,000 rpm for 15 min. Protein concentration in each lysate was determined using Bio-Rad protein assay reagent (Bio-Rad, Richmond, CA, USA). Equal amounts of proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). Blots were blocked overnight with 5% skim milk in PBS at 4 °C, followed by probing with primary antibodies overnight. After incubation with secondary antibodies for 1 h, bands of interest were visualized with Amersham enhanced chemiluminescence detection reagents (GE healthcare, Piscataway, NJ, USA).
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 7.02. Statistical significance of differences between experimental groups was calculated with an unpaired, two-tailed, Student’s t-test. P values < 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This work is supported by grants from Marine Biotechnology Program (20150220) funded by Ministry of Oceans and Fisheries and the Samsung Biomedical Research Institute (SMX1180141).
Author Contributions
C.C. and H.C.P. conceived and designed the study. C.C. and A.S. performed the experiments. H.-S. L. and Y.-J. Lee prepared and provided the marine extract samples. C.C. and H.C.P. interpreted the data and wrote the paper. H.C.P. supervised the study. All authors reviewed the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24745-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamed I, Özogul F, Özogul Y, Regenstein JM. Marine Bioactive Compounds and Their Health Benefits: A Review. Comprehensive Reviews in Food Science and Food Safety. 2015;14:446–465. doi: 10.1111/1541-4337.12136. [DOI] [Google Scholar]
- 2.Mayer AM, et al. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Ye J, Zhou F, Al-Kareef AM, Wang H. Anticancer agents from marine sponges. J Asian Nat Prod Res. 2015;17:64–88. doi: 10.1080/10286020.2014.970535. [DOI] [PubMed] [Google Scholar]
- 4.Schwartsmann G, da Rocha AB, Mattei J, Lopes R. Marine-derived anticancer agents in clinical trials. Expert Opinion on Investigational Drugs. 2003;12:1367–1383. doi: 10.1517/13543784.12.8.1367. [DOI] [PubMed] [Google Scholar]
- 5.Sawadogo WR, et al. A Survey of Marine Natural Compounds and Their Derivatives with Anti-cancer Activity Reported in 2012. Molecules. 2015;20:7097–7142. doi: 10.3390/molecules20047097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calcabrini, C., Catanzaro, E., Bishayee, A., Turrini, E. & Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar Drugs15, 10.3390/md15100310 (2017). [DOI] [PMC free article] [PubMed]
- 7.Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12:527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) 2007;19:397–417. doi: 10.1016/j.clon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Linam, J. & Yang, L.-X. Recent Developments in Radiosensitization. Vol. 35 (2015). [PubMed]
- 11.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 12.Kim BM, et al. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int J Mol Sci. 2015;16:26880–26913. doi: 10.3390/ijms161125991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohri N, et al. Radiotherapy for Hepatocellular Carcinoma: New Indications and Directions for Future Study. JNCI: Journal of the National Cancer Institute. 2016;108:djw133–djw133. doi: 10.1093/jnci/djw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thillai K, Ross P, Sarker D. Molecularly targeted therapy for advanced hepatocellular carcinoma - a drug development crisis? World J Gastrointest Oncol. 2016;8:173–185. doi: 10.4251/wjgo.v8.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik A, et al. Role of Natural Radiosensitizers and Cancer Cell Radioresistance: An Update. Anal Cell Pathol (Amst) 2016;2016:6146595. doi: 10.1155/2016/6146595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerelchuluun, A. et al. Histone Deacetylase Inhibitor Induced Radiation Sensitization Effects on Human Cancer Cells after Photon and Hadron Radiation Exposure. Int J Mol Sci19, 10.3390/ijms19020496 (2018). [DOI] [PMC free article] [PubMed]
- 17.Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang SM, Harari PM. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62:223–229. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo S, Comincini S. Autophagy and ionizing radiation in tumors: the “survive or not survive” dilemma. J Cell Physiol. 2013;228:1–8. doi: 10.1002/jcp.24118. [DOI] [PubMed] [Google Scholar]
- 19.Chaurasia M, Bhatt AN, Das A, Dwarakanath BS, Sharma K. Radiation-induced autophagy: mechanisms and consequences. Free Radic Res. 2016;50:273–290. doi: 10.3109/10715762.2015.1129534. [DOI] [PubMed] [Google Scholar]
- 20.Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic Reticulum Stress and the Hallmarks of Cancer. Trends Cancer. 2016;2:252–262. doi: 10.1016/j.trecan.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Cheng X, et al. Connecting endoplasmic reticulum stress to autophagy through IRE1/JNK/beclin-1 in breast cancer cells. Int J Mol Med. 2014;34:772–781. doi: 10.3892/ijmm.2014.1822. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Torres, V. et al. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules22, 10.3390/molecules22071037 (2017). [DOI] [PMC free article] [PubMed]
- 23.Tam SY, Wu VW, Law HK. Influence of autophagy on the efficacy of radiotherapy. Radiat Oncol. 2017;12:57. doi: 10.1186/s13014-017-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ondrej M, Cechakova L, Durisova K, Pejchal J, Tichy A. To live or let die: Unclear task of autophagy in the radiosensitization battle. Radiother Oncol. 2016;119:265–275. doi: 10.1016/j.radonc.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked Autophagy Sensitizes Resistant Carcinoma Cells to Radiation Therapy. Cancer Research. 2008;68:1485–1494. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, et al. Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol Med Rep. 2015;12:1645–1652. doi: 10.3892/mmr.2015.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy JMM, Thorburn A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacology & Therapeutics. 2011;131:130–141. doi: 10.1016/j.pharmthera.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Combined Bcl-2/Mammalian Target of Rapamycin Inhibition Leads to Enhanced Radiosensitization via Induction of Apoptosis and Autophagy in Non–Small Cell Lung Tumor Xenograft Model. Clinical Cancer Research. 2009;15:6096–6105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara K, et al. Akt inhibitor shows anticancer and radiosensitizing effects in malignant glioma cells by inducing autophagy. Int J Oncol. 2007;31:753–760. [PubMed] [Google Scholar]
- 30.Dyson L, Wright AD, Young KA, Sakoff JA, McCluskey A. Synthesis and anticancer activity of focused compound libraries from the natural product lead, oroidin. Bioorg Med Chem. 2014;22:1690–1699. doi: 10.1016/j.bmc.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Roggen H, et al. Antimicrobial and Antineoplastic Activities of Agelasine Analogs Modified in the Purine 2-Position. Archiv der Pharmazie. 2011;344:50–55. doi: 10.1002/ardp.201000148. [DOI] [PubMed] [Google Scholar]
- 32.Shin SW, et al. Mechanism of the Antitumor and Radiosensitizing Effects of a Manganese Porphyrin, MnHex-2-PyP. Antioxid Redox Signal. 2017;27:1067–1082. doi: 10.1089/ars.2016.6889. [DOI] [PubMed] [Google Scholar]
- 33.Il YJ, et al. Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation. Sci Rep. 2017;7:14986. doi: 10.1038/s41598-017-15165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.