Abstract
Maize (Zea mays L.) contributes approximately 55% of China’s grain production. The effects of nitrogen (N) on maize grain morphology and starch granules remain elusive. In this study, a field experiment in clay loam soil was conducted using three maize hybrids (Suyu 30, Suyu 20, and Suyu 29) and four N levels (0, 360, 450, and 540 kg ha−1) in 2010 and 2012. The results indicated that increased grain length and width, starch granule number, surface area, and volume, was associated with the application of 450 kg ha−1 of N. Differences between superior (ear base) and inferior (apical) grains decreased under highest yield treatments. The effects of N levels on inferior grains was more than that on superior grains. The starch granules of superior grains showed more polygonal, and bigger shape than inferior grains. The results revealed that N levels affected size and morphology of starch granules and grains. The application of 450 kg N ha−1 resulted in larger-sized starch granules and less difference between superior and inferior grains.
Introduction
Maize has been associated with the largest plant area and highest total yield among various cereal crops, contributing to approximately 55% of China’s grain production. Similar to previous studies involving rice1,2 and wheat3, the later silk spikelets, which are usually located on the apical part of the ear, are either sterile or show poor grain filling activity4, thus rendering these as inferior. ‘Super’ rice, which possesses numerous spikelets in a panicle, are also unable to generate a high yield due to poor grain filling of its later-flowering inferior spikelets2,5. This problem involving inferior spikelets is further aggravated in the newly bred ‘super’ rice cultivars6. The maize grains in the lower and middle parts of the ear (superior grains) usually produce silk and fertilize early due to primigenic dominance7. However, the mechanism of grain filling in inferior and superior spikelets remains unknown and the relationship between grain filling in inferior spikelets and nitrogen (N) requirement remains elusive. It is therefore essential to investigate the physiological and ecological characteristics of superior and inferior grains and establish the mechanism of increased grain production.
Moisture regulates the length and surface area of grains, thus influencing grain yield8. It has been previously reported that panicles harboring smaller grain morphological features (length and width) significantly reduce rice yield in China9. In addition, it has been previously shown that shading, leaf cutting, spikelet removal, and increase in CO2 levels significantly improved the grain filling capacity of inferior grains10. It has been recently suggested that a metabolic interaction between polyamines and ethylene biosynthesis mediates grain filling in inferior rice spikelets11. Maize showed similar law. Shen et al.12 reported that apical kernel (inferior grains) of maize reacted differently to the nitrogen supply. That is, apical kernel developed well at an early grain filling stage and resulted in a higher kernel number, kernel weight and grain yield with better ear characteristics at maturity. Similar to response of rice starch13, Maize starch, whose morphology could be regulated by circumstances change (for example, high temperature)14, is stored as discrete semi-crystalline granules and consists of two main components, linear amylose and highly branched amylopectin15. Several studies have identified factors that influence the properties of starch granules, including long amylopectin branches, crystallinity, dense packing and restricted mobility of starch molecules, helix form, lamellar organization, and structural features of granules16–23. However, most of these studies mainly focused on the quality and physicochemical characteristics of starch, and information on the relationships between grain morphology and maize yield in superior and inferior grains is limited. Hence, this study was conducted to determine the relationship between yield and grain morphology in superior and inferior grains receiving various N levels and planted at a density of 82,500 plants ha−1.
Results
Effect of N rates on Grain Yield and Morphology
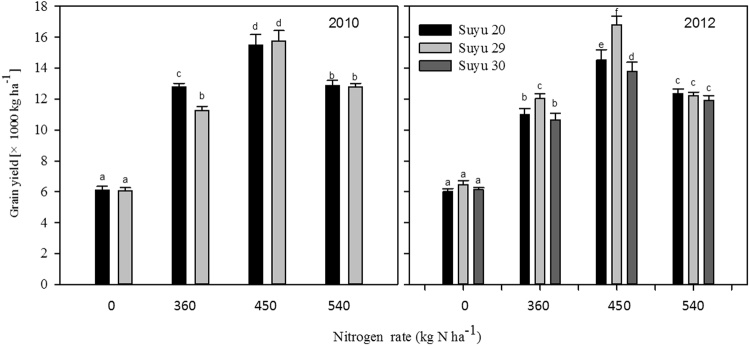
The application of various N levels resulted in a significant increase in yield of the three maize hybrids, Suyu 20, Suyu 29 and Suyu 30 (Fig. 1). As the N level increased, the yield of the three varieties presented single-peak curves, reaching peak values with 450 kg N ha−1 in both 2010 and 2012.
Figure 1.
Effect of nitrogen levels on the grain yield of summer maize.
Tables 1 and 2 illustrate that that 450 kg N ha−1 markedly improved the morphological characteristics of superior and inferior grains (except grain thickness) compared to 0 kg N ha−1. At 450 kg N ha−1, the variation in the grain density and 1,000-grain weight in both superior and inferior grains was minimal. The variance values of grain density and 1,000-grain weight of the three hybrids (Suyu 20, Suyu 29 and Suyu 30, respectively; the same as follows) were 0.5, 2.1, and 15.3 g L−1 and 82.8, 33.5, and 50.3 g, respectively. ANOVA results showed that the effect of N levels on inferior grains was greater than that on superior grains for grain density and 1,000-grain weight. For example, compared to 0 kg N ha−1, the application of 450 kg N ha−1 resulted in an improved grain density and 1,000-grain weight in inferior grains from the three hybrids, which measured 2.3, 1.5, and 1.0 and 1.9, 1.9, and 2.1 times higher than those of superior grains, respectively. On average of four nitrogen levels, the higher geometric volume of superior and inferior grains of the three hybrids were 1.6, 1.7, and 1.8 and 1.4, 1.5, and 1.6 times higher than those of volume of grain, respectively, suggesting that sinking strength was still an important factor in generating a high-yield maize population. In terms of the F-value, no significant differences in the effect of N levels on grain thickness were detected for superior grains over inferior grains. And, significant Cultivar × N treatment interactions were detected for any parameters of morphology structure of maize grains except thickness of superior grains The effect of various N levels on inferior grains was generally higher than on superior grains and the application of 450 kg N ha−1 decreased the variation between superior and inferior grains and increased grain length and width, thus improving maize sink and yield.
Table 1.
Effect of nitrogen levels on the morphology structure of superior grains.
| Year | Hybrid | N Treatment | Grain density | 1000-grain weight | geometrical morphology of grain (cm) | Geometric volume of grain | Volume of grain | ||
|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | (g L−1) | (g) | Length | Width | Thickness | (cm3) | (cm3) | ||
| 2010 | Suyu20 | 0 | 1109 ± 35a | 312.5 ± 13.2a | 0.89 ± 0.04a | 0.81 ± 0.02a | 0.49 ± 0.02a | 0.35 ± 0.01a | 0.28 ± 0.01a |
| 360 | 1150 ± 27bc | 416.3 ± 14.5d | 1.03 ± 0.02c | 0.94 ± 0.02b | 0.52 ± 0.01a | 0.5 ± 0.01c | 0.36 ± 0.00c | ||
| 450 | 1198 ± 26c | 439.5 ± 9.3e | 1.24 ± 0.03e | 1.06 ± 0.01c | 0.53 ± 0.02a | 0.7 ± 0.02f | 0.37 ± 0.00c | ||
| 540 | 1124 ± 31ab | 422.4 ± 8.8de | 1.11 ± 0.01d | 0.93 ± 0.02b | 0.53 ± 0.01a | 0.55 ± 0.01d | 0.38 ± 0.00c | ||
| Suyu29 | 0 | 1101 ± 36a | 305.3 ± 10.8a | 0.94 ± 0.03b | 0.83 ± 0.01a | 0.52 ± 0.02a | 0.41 ± 0.01b | 0.28 ± 0.01a | |
| 360 | 1170 ± 27bc | 368.1 ± 14.9b | 1.03 ± 0.01c | 0.92 ± 0.02b | 0.51 ± 0.02a | 0.48 ± 0.01c | 0.31 ± 0.01b | ||
| 450 | 1196 ± 29c | 426.9 ± 7.7de | 1.21 ± 0.02e | 1.09 ± 0.01c | 0.52 ± 0.01a | 0.69 ± 0.02e | 0.36 ± 0.00c | ||
| 540 | 1131 ± 26ab | 376.2 ± 9.2c | 1.03 ± 0.03c | 0.91 ± 0.02b | 0.52 ± 0.02a | 0.49 ± 0.01c | 0.33 ± 0.01bc | ||
| 2012 | Suyu30 | 0 | 1123 ± 26a | 234.7 ± 9.1a | 0.96 ± 0.01b | 0.69 ± 0.01a | 0.51 ± 0.02b | 0.34 ± 0.02a | 0.21 ± 0.00a |
| 360 | 1198 ± 37bc | 278.7 ± 14.6b | 1.06 ± 0.03cd | 0.81 ± 0.02b | 0.47 ± 0.01ab | 0.4 ± 0.02c | 0.23 ± 0.00b | ||
| 450 | 1222 ± 38c | 299.4 ± 7.9d | 1.1 ± 0.03d | 0.91 ± 0.02cd | 0.48 ± 0.02ab | 0.48 ± 0.01e | 0.24 ± 0.01bc | ||
| 540 | 1195 ± 34bc | 281.2 ± 11.8bc | 1.09 ± 0.01cd | 0.87 ± 0.01c | 0.45 ± 0.02a | 0.43 ± 0.02d | 0.24 ± 0.01b | ||
| Suyu20 | 0 | 1113 ± 37a | 264.5 ± 12.1d | 0.92 ± 0.02a | 0.85 ± 0.02bc | 0.47 ± 0.02ab | 0.37 ± 0.01b | 0.24 ± 0.01d | |
| 360 | 1152 ± 34b | 392.4 ± 12.3f | 1.02 ± 0.04c | 0.96 ± 0.02d | 0.53 ± 0.01b | 0.52 ± 0.01f | 0.34 ± 0.00f | ||
| 450 | 1192 ± 34bc | 418.8 ± 11.0g | 1.23 ± 0.03e | 1.08 ± 0.03e | 0.52 ± 0.02b | 0.7 ± 0.01h | 0.35 ± 0.01g | ||
| 540 | 1131 ± 38ab | 390 ± 10.1f | 1.16 ± 0.03e | 0.92 ± 0.03cd | 0.53 ± 0.02b | 0.56 ± 0.02g | 0.34 ± 0.01g | ||
| Suyu29 | 0 | 1106 ± 32a | 264.3 ± 14.9c | 0.98 ± 0.04b | 0.82 ± 0.02b | 0.53 ± 0.02b | 0.42 ± 0.01cd | 0.24 ± 0.00c | |
| 360 | 1172 ± 36bc | 348.2 ± 9.2e | 1.05 ± 0.04c | 0.9 ± 0.01cd | 0.51 ± 0.01b | 0.48 ± 0.02e | 0.3 ± 0.00e | ||
| 450 | 1183 ± 32bc | 405.9 ± 9.2f | 1.19 ± 0.03e | 1.08 ± 0.01e | 0.52 ± 0.01b | 0.67 ± 0.01h | 0.34 ± 0.01f | ||
| 540 | 1143 ± 39ab | 353.7 ± 11.0e | 1.05 ± 0.03c | 0.93 ± 0.02cd | 0.52 ± 0.02b | 0.51 ± 0.02f | 0.31 ± 0.01e | ||
| F-value | Year | 0.06 NS | 245.81** | 10.19* | 0.51NS | 0.05NS | 2.3NS | 369.21NS | |
| Hybrid | 11.72** | 758.82** | 13.19** | 101.73** | 5.2* | 169.73** | 1539.3** | ||
| N treatment | 45.76** | 858.57** | 550.48** | 240.31** | 0.16NS | 492.42** | 913.87** | ||
| Year × Hybrid | 0.01NS | 1.97NS | 0.01NS | 3.16NS | 0.15NS | 0.52NS | 1.02NS | ||
| Year × N treatment | 0.48NS | 8.94* | 5.78** | 0.26NS | 0.07NS | 2.3NS | 21.93** | ||
| Cultivar × N treatment | 1.3* | 48.39** | 35.23** | 5.73** | 2.22NS | 21.6** | 107.99** | ||
| Year × Hybrid×N treatment | 0.04NS | 0.35NS | 1.87NS | 1.4NS | 0.25NS | 0.18NS | 1.63NS | ||
Means in the same column followed by the same letter do not differ statistically at the 0.05 probability level by an ANOVA protected Duncan’s test; *,**NS Significantly different at the 0.05 and 0. 01 probability levels and no significant difference, respectively.
Table 2.
Effect of nitrogen levels on the morphology structure of inferior grains.
| Year | Hybrid | N Treatment | Grain density | 1000-grain weight | geometrical morphology of grain (cm) | Geometric volume of grain | Volume of grain | ||
|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | (g L−1) | (g) | Length | Width | Thickness | (cm3) | (cm3) | ||
| 2010 | Suyu20 | 0 | 1013 ± 39a | 180.3 ± 10.6a | 0.72 ± 0.01a | 0.76 ± 0.03a | 0.51 ± 0.02a | 0.28 ± 0.01a | 0.18 ± 0.00a |
| 360 | 1065 ± 39c | 286.4 ± 10.7c | 1.01 ± 0.01d | 0.82 ± 0.01b | 0.52 ± 0.02ab | 0.43 ± 0.01d | 0.27 ± 0.01c | ||
| 450 | 1196 ± 27d | 328.7 ± 13.3e | 1.12 ± 0.03e | 0.94 ± 0.02c | 0.53 ± 0.01ab | 0.56 ± 0.02e | 0.27 ± 0.00c | ||
| 540 | 1089 ± 36c | 302.3 ± 8.5d | 1.03 ± 0.03d | 0.77 ± 0.02a | 0.54 ± 0.02b | 0.42 ± 0.01d | 0.28 ± 0.01cd | ||
| Suyu29 | 0 | 1060 ± 37bc | 212.4 ± 13.7b | 0.84 ± 0.01b | 0.74 ± 0.02a | 0.51 ± 0.02a | 0.32 ± 0.01b | 0.2 ± 0.01b | |
| 360 | 1121 ± 326c | 328.7 ± 8.8e | 1.01 ± 0.02d | 0.82 ± 0.02b | 0.51 ± 0.01a | 0.43 ± 0.01d | 0.29 ± 0.01d | ||
| 450 | 1190 ± 38d | 403.6 ± 14.5f | 1.1 ± 0.02e | 0.94 ± 0.01c | 0.52 ± 0.02ab | 0.54 ± 0.01e | 0.34 ± 0.00e | ||
| 540 | 1104 ± 38c | 329.7 ± 14.8e | 0.93 ± 0.01c | 0.77 ± 0.03a | 0.51 ± 0.01a | 0.37 ± 0.02c | 0.3 ± 0.01d | ||
| 2012 | Suyu30 | 0 | 1110 ± 302b | 158.9 ± 3.7a | 0.68 ± 0.01a | 0.68 ± 0.01a | 0.39 ± 0.01a | 0.18 ± 0.01a | 0.14 ± 0.00a |
| 360 | 1146 ± 40bc | 194.4 ± 3.1c | 0.93 ± 0.02d | 0.71 ± 0.01ab | 0.48 ± 0.01bc | 0.31 ± 0.02c | 0.17 ± 0.01c | ||
| 450 | 1207 ± 37c | 249.1 ± 4.3e | 1.03 ± 0.02ef | 0.76 ± 0.03c | 0.47 ± 0.02bc | 0.37 ± 0.01d | 0.21 ± 0.01e | ||
| 540 | 1134 ± 26bc | 215.5 ± 5.7b | 0.9 ± 0.01d | 0.71 ± 0.01ab | 0.47 ± 0.02bc | 0.3 ± 0.02c | 0.19 ± 0.01b | ||
| Suyu20 | 0 | 1023 ± 31a | 181.8 ± 3.2b | 0.75 ± 0.02b | 0.75 ± 0.03bc | 0.5 ± 0.01c | 0.28 ± 0.01b | 0.18 ± 0.00d | |
| 360 | 1072 ± 38b | 324.5 ± 6.4f | 1.02 ± 0.03e | 0.85 ± 0.03e | 0.51 ± 0.01cd | 0.45 ± 0.01f | 0.3 ± 0.01f | ||
| 450 | 1193 ± 37c | 364.1 ± 6.5h | 1.1 ± 0.02f | 0.93 ± 0.01f | 0.55 ± 0.01d | 0.56 ± 0.01h | 0.31 ± 0.00f | ||
| 540 | 1089 ± 27b | 324.2 ± 7.9g | 1.06 ± 0.01ef | 0.79 ± 0.02cd | 0.54 ± 0.01d | 0.45 ± 0.01f | 0.3 ± 0.01g | ||
| Suyu29 | 0 | 1066 ± 33b | 198.3 ± 5.3d | 0.86 ± 0.02c | 0.75 ± 0.01bc | 0.5 ± 0.01c | 0.32 ± 0.01c | 0.19 ± 0.01e | |
| 360 | 1117 ± 38b | 288 ± 6.3h | 1.01 ± 0.02e | 0.81 ± 0.02de | 0.49 ± 0.02bc | 0.4 ± 0.02e | 0.26 ± 0.00h | ||
| 450 | 1185 ± 35c | 362.1 ± 7.7i | 1.07 ± 0.03f | 0.94 ± 0.01f | 0.51 ± 0.02cd | 0.52 ± 0.01g | 0.31 ± 0.01i | ||
| 540 | 1107 ± 28b | 302.7 ± 4.6h | 0.92 ± 0.01d | 0.78 ± 0.01cd | 0.5 ± 0.02c | 0.36 ± 0.01d | 0.27 ± 0.01h | ||
| F-value | Year | 0.11NS | 3.26NS | 0.21NS | 0.42NS | 0.65NS | 0.19NS | 8.44** | |
| Hybrid | 35.69** | 837.03** | 126.73** | 77.93** | 13.67** | 241.16** | 1486.75** | ||
| N treatment | 145.38** | 1422.6** | 1019** | 111.23** | 2.91* | 461.9* | 1551.04* | ||
| Year × Hybrid | 0.1NS | 229.43** | 4.35* | 0.28NS | 0.52NS | 7.24* | 338.44** | ||
| Year × N treatment | 0.23NS | 0.35NS | 5.05** | 0.53NS | 0.14NS | 1.33NS | 1.22NS | ||
| Cultivar × N treatment | 4.66* | 48.93** | 58.8** | 4.54** | 1.46** | 14.86** | 78.88** | ||
| Year × Hybrid × N treatment | 0.07NS | 16.67** | 1.00NS | 0.87NS | 0.13NS | 0.67NS | 23.99** | ||
Means in the same column followed by the same letter do not differ statistically at the 0.05 probability level by an ANOVA protected Duncan’s test; *,**NS Significantly different at the 0.05 and 0. 01 probability levels and no significant difference, respectively.
Effect of N on Starch Granules of Superior and Inferior Grains
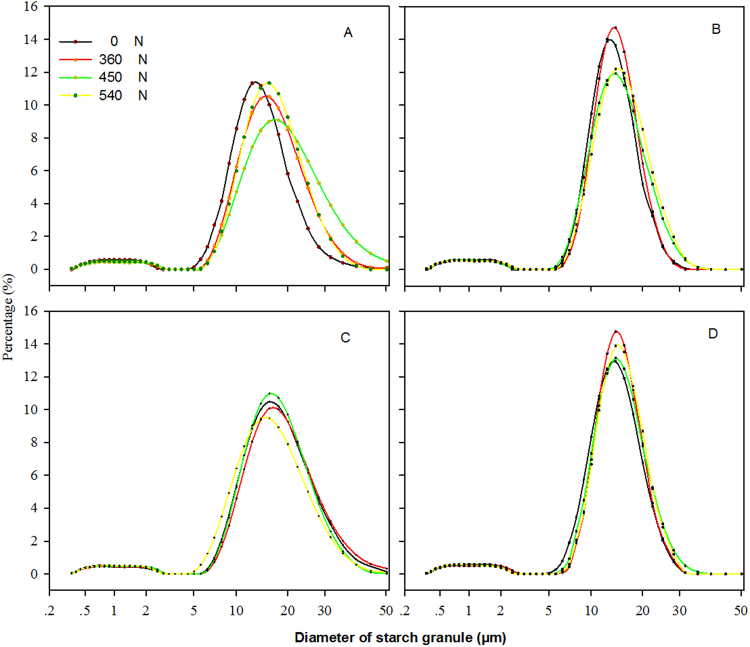
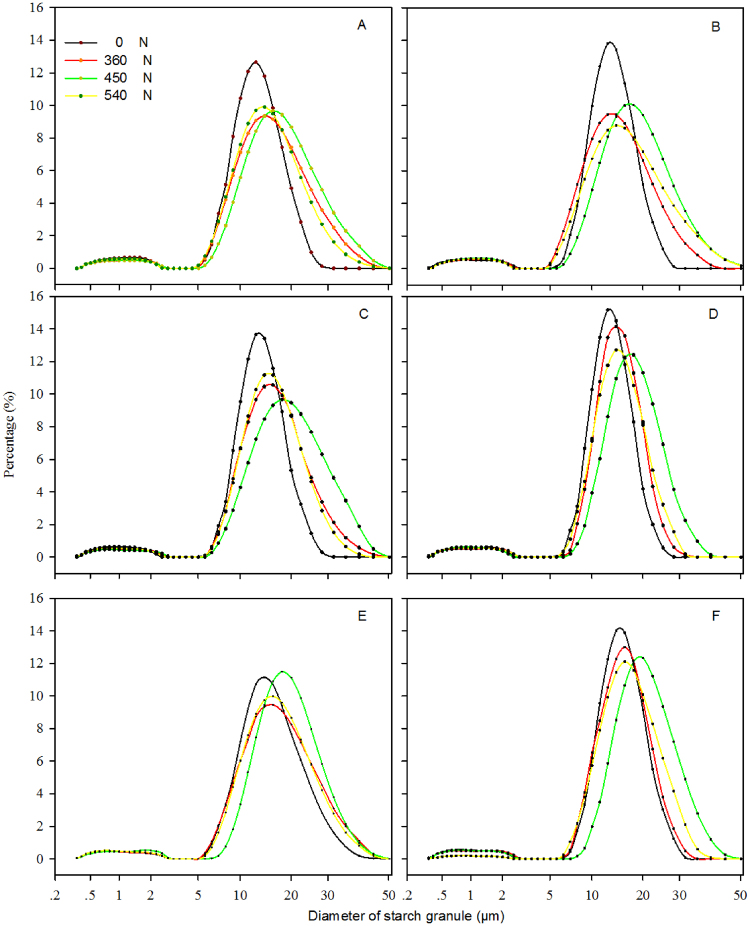
Figure 2 and Table 3 show the effects of N treatments on starch granules in superior and inferior grains. The effects on big starch granules were much greater than those on small starch granules. No significant differences in the diameter of starch granules <5 μm were observed among superior and inferior grains. The application of 450 kg N ha−1 resulted in a marked increase in the number of big starch granules. In the superior grains of Suyu 29, for example, the percentages starch granules with a diameter >15 μm using 0, 360, 450, and 540 kg N ha−1 were 49.13%, 57.93%, 64.16%, and 53.20% (Figs 2C and D and 3E and F). Figures 2 and 3 illustrate that the distribution of starch granules of superior grains was different from that of inferior grains; the starch granules of superior grains showed smaller peak diameters, but a wider distribution than that in inferior grains. In terms of F-value (Table 3), various N levels imparted significant differences in surface area and volume between superior and inferior grains from hybrids. And, significant hybrid × N treatment interactions were detected for surface area and volume of superior and inferior grains of maize. Overall, the application of 450 kg N ha−1 increased surface area and volume of starch granules, also, increased the number of big starch granules Furthermore, big starch granules improved maize yield compared to small starch granules. This may explain the observed improvement in grain density and 1,000-grain weight, which resulted in a higher maize yield.
Figure 2.
Effects of nitrogen levels on starch granule volume distribution of superior (A and C) and inferior (B and D) grains from Suyu 20 (A, and B), and Suyu 29 (C and D) in 2010.
Table 3.
Effects of nitrogen levels on weighted average of surface area and volume of superior and inferior grains of maize.
| Year | Hybrid | N Treatment | Surface area (μm2) | Volume (μm3) | ||
|---|---|---|---|---|---|---|
| kg ha−1 | Superior | Inferior | Superior | Inferior | ||
| 2010 | Suyu20 | 0 | 6.45 ± 0.12g | 6.34 ± 0.12i | 17.5 ± 0.13lm | 12.68 ± 0.49n |
| 360 | 7.68 ± 0.13cde | 7.02 ± 0.10cde | 19.36 ± 0.10i | 14.43 ± 0.29j | ||
| 450 | 8.46 ± 0.11a | 6.65 ± 0.06fghi | 24.74 ± 0.17c | 15.85 ± 0.37cd | ||
| 540 | 6.08 ± 0.10h | 6.41 ± 0.06hi | 16.56 ± 0.13n | 15.38 ± 0.12f | ||
| Suyu29 | 0 | 7.78 ± 0.12cde | 7.64 ± 0.09a | 20.42 ± 0.39g | 15.68 ± 0.14e | |
| 360 | 7.45 ± 0.10e | 6.57 ± 0.13ghi | 17.36 ± 0.11m | 14.39 ± 0.08j | ||
| 450 | 8.57 ± 0.15a | 7.15 ± 0.11bcd | 25.15 ± 0.09b | 15.91 ± 0.09c | ||
| 540 | 7.55 ± 0.1de | 7.27 ± 0.09bc | 16.54 ± 0.12n | 15.26 ± 0.07g | ||
| 2012 | Suyu30 | 0 | 6.99 ± 0.20f | 6.44 ± 0.23ghi | 14.96 ± 0.28o | 12.75 ± 0.35n |
| 360 | 7.48 ± 0.12e | 7.28 ± 0.18bc | 20.12 ± 0.24h | 16.71 ± 0.27b | ||
| 450 | 7.83 ± 0.29cd | 6.74 ± 0.17efg | 23.74 ± 0.37d | 14.46 ± 0.35j | ||
| 540 | 7.04 ± 0.26f | 7.01 ± 0.22cde | 18.37 ± 0.32k | 17.54 ± 0.29a | ||
| Suyu20 | 0 | 6.50 ± 0.20g | 6.63 ± 0.2fghi | 16.5 ± 0.29n | 13.15 ± 0.25m | |
| 360 | 7.71 ± 0.15cde | 6.90 ± 0.23def | 19.42 ± 0.26i | 14.25 ± 0.36k | ||
| 450 | 8.28 ± 0.14ab | 6.66 ± 0.15fgh | 22.35 ± 0.29e | 13.99 ± 0.16l | ||
| 540 | 7.07 ± 0.19f | 6.75 ± 0.17efg | 14.84 ± 0.3o | 14.8 ± 0.42i | ||
| Suyu29 | 0 | 7.96 ± 0.13bc | 6.9 ± 0.28def | 19.14 ± 0.23j | 15.17 ± 0.32gh | |
| 360 | 7.75 ± 0.15cde | 7.08 ± 0.26cd | 22.19 ± 0.27f | 15.11 ± 0.24h | ||
| 450 | 8.35 ± 0.17a | 7.18 ± 0.27bcd | 26.92 ± 0.41a | 15.84 ± 0.41cd | ||
| 540 | 7.48 ± 0.14e | 7.42 ± 0.22ab | 17.58 ± 0.38l | 15.79 ± 0.28de | ||
| F-value | R2 | 0.684** | 0.753** | 0.997** | 0.979** | |
| Year | 1.24NS | 1.15NS | 8.14NS | 12.91NS | ||
| Hybrid | 13.52** | 38.76** | 502.52* | 269.54* | ||
| N treatment | 29.13** | 30.61** | 4721.69* | 483.25* | ||
| Year × Hybrid | 0.51NS | 1.65NS | 621.02** | 47.74** | ||
| Year × N treatment | 1.44NS | 7.09** | 27.79** | 42.29** | ||
| Cultivar × N treatment | 3.04* | 3.84** | 121.08** | 122.38** | ||
| Year × Hybrid × N treatment | 1.71NS | 1.03NS | 19.31** | 21.74** | ||
Means in the same column followed by the same letter do not differ statistically at the 0.05 probability level by an ANOVA protected Duncan’s test; *,**NS Significantly different at the 0.05 and 0. 01 probability levels and no significant difference, respectively.
Figure 3.
Effects of nitrogen levels on starch granule volume distribution of superior (A,C and E) and inferior (B,D and F) grains from Suyu 30 (A and B), Suyu 20 (C and D), and Suyu 29 (C and D) in 2012.
Correlation analysis
Table 4 illustrate that grain yield of maize positively and significantly correlated with any parameters of grain and starch granule of grain except surface area and volume of inferior grains. Compared with 1,000-grain weight of superior grains, there were more significant correlation relations with inferior grains. Based on the correlation coefficients, maize yield was most closely related to any morphology parameters of both superior and inferior grains, followed by 1000-grain weight and grain density.
Table 4.
Correlations analysis among maize yield and the parameters of grain and starch granule of superior and inferior grain.
| Grain morphology | Yield | S-Grain density | S-1000-grain weight | S-Length | S-Width | S-Thickness | S-geometric volume of grain | S-volume of grain | S-Surface area | S-Volume | I-Grain density | I-1000-grain weight | I-Length | I-Width | I-Thickness | I-geometric volume of grain | I-volume of grain | I-Surface area |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-Grain density | 0.77** | 1 | ||||||||||||||||
| S-1000-grain weight | 0.76** | 0.29 | 1 | |||||||||||||||
| S-Length | 0.90** | 0.71** | 0.69** | 1 | ||||||||||||||
| S-Width | 0.85** | 0.55* | 0.86** | 0.82** | 1 | |||||||||||||
| S-Thickness | 0.19 | −0.28 | 0.63** | 0.28 | 0.4 | 1 | ||||||||||||
| S-geometric volume of grain | 0.85** | 0.53* | 0.86** | 0.92** | 0.96** | 0.52* | 1 | |||||||||||
| S-volume of grain | 0.65** | 0.12 | 0.98** | 0.59** | 0.79** | 0.69** | 0.80** | 1 | ||||||||||
| S-Surface area | 0.51* | 0.55** | 0.38 | 0.56** | 0.62** | 0.35 | 0.64** | 0.28 | 1 | |||||||||
| S-Volume | 0.54* | 0.68** | 0.38 | 0.59** | 0.68** | 0.09 | 0.64** | 0.27 | 0.80** | 1 | ||||||||
| I-Grain density | 0.77** | 0.88** | 0.35 | 0.83** | 0.60** | 0.01 | 0.68** | 0.2 | 0.67** | 0.71** | 1 | |||||||
| I-1000-grain weight | 0.82** | 0.43 | 0.91** | 0.76** | 0.90** | 0.57** | 0.90** | 0.86** | 0.55* | 0.45* | 0.53* | 1 | ||||||
| I-Length | 0.90** | 0.65** | 0.83** | 0.87** | 0.86** | 0.41 | 0.88** | 0.74** | 0.58** | 0.58** | 0.67** | 0.87** | 1 | |||||
| I-Width | 0.69** | 0.45* | 0.82** | 0.72** | 0.94** | 0.50* | 0.91** | 0.75** | 0.69** | 0.71** | 0.53* | 0.84** | 0.76** | 1 | ||||
| I-Thickness | 0.45* | 0 | 0.76** | 0.42 | 0.69** | 0.48* | 0.62** | 0.79** | 0.22 | 0.24 | 0 | 0.64** | 0.61** | 0.64** | 1 | |||
| I-geometric volume of grain | 0.82** | 0.51* | 0.91** | 0.84** | 0.96** | 0.53* | 0.96** | 0.84** | 0.63** | 0.63** | 0.57** | 0.91** | 0.92** | 0.93** | 0.74** | 1 | ||
| I-volume of grain | 0.74** | 0.29 | 0.92** | 0.66** | 0.85** | 0.62** | 0.83** | 0.89** | 0.45* | 0.33 | 0.37 | 0.98** | 0.82** | 0.79** | 0.69** | 0.87** | 1 | |
| I-Surface area | 0.07 | 0.08 | 0.01 | 0.03 | 0.12 | 0.05 | 0.07 | 0.01 | 0.46* | 0.29 | 0.11 | 0.15 | 0.13 | 0.02 | 0.06 | 0.05 | 0.15 | 1 |
| I-Volume | 0.4 | 0.44* | 0.17 | 0.46* | 0.29 | −0.1 | 0.31 | 0.13 | 0.34 | 0.36 | 0.43 | 0.25 | 0.42 | 0.09 | 0.14 | 0.25 | 0.2 | 0.63** |
S: Super grain; I: Inferior grain; *,**Correlations are significant at the 0.05 and 0.01 levels, respectively.
Starch Granule Morphology of Superior and Inferior Grains
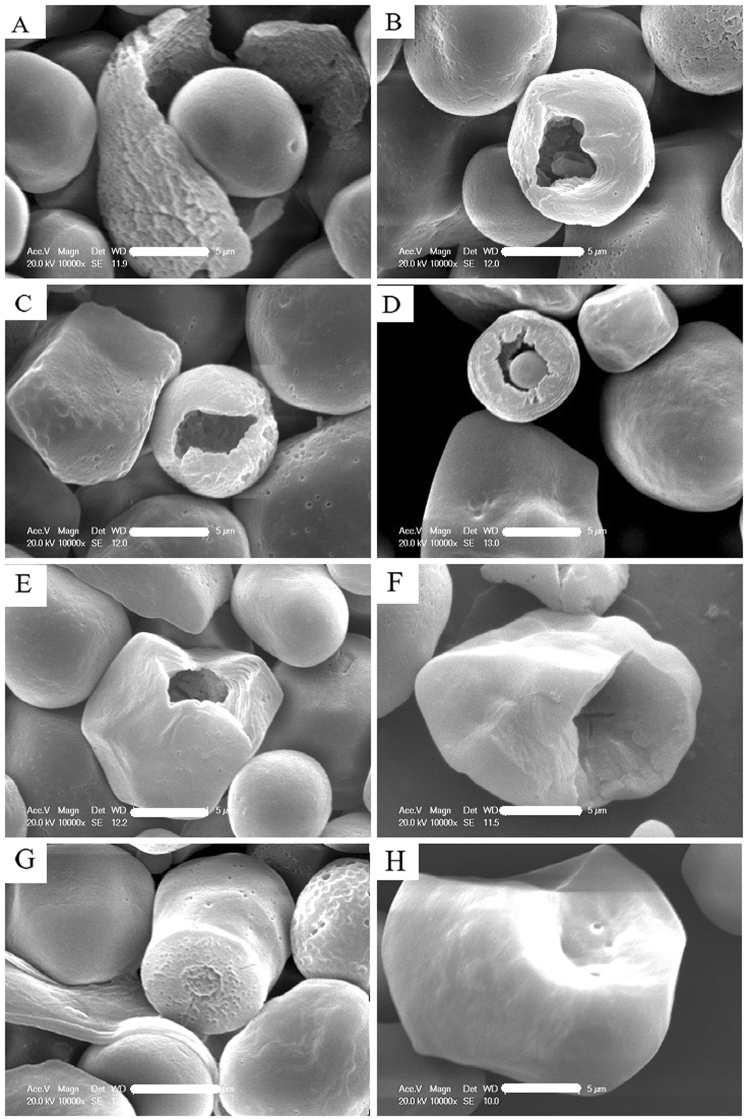
The starch granule morphology of the three maize hybrids using 0 and 450 kg N ha−1 was examined using SEM. The granules showed polygonal and spherical shapes (Fig. 4), similar to the finding of Wei et al.15 using rice starch. However, several striking differences were observed between the superior and inferior grains. For example, the polygonal starch granules of superior grains (Fig. 4A,B,E,F,I and J) were bigger than inferior grains (Fig. 4C,D,G,H,K and L). Conversely, at 0 kg N ha−1, isolated starch granules from inferior grains with more smooth surfaces were spherical in shape (Fig. 4C,G and K). The SEM figures (10,000×) of the starch granules also showed mechanical damage (Fig. 5), ‘Hollow’ and ‘solid’ inner structures were also observed in the starch granules. The wall of spherical starch granules was thin and its chambers were large. The walls of the polyhedral starch granules were thick and layered and its chambers were small. These features (except starch granule damage) may explain the observed improvement in grain density and 1,000-grain weight, which improved maize production grain density.
Figure 4.
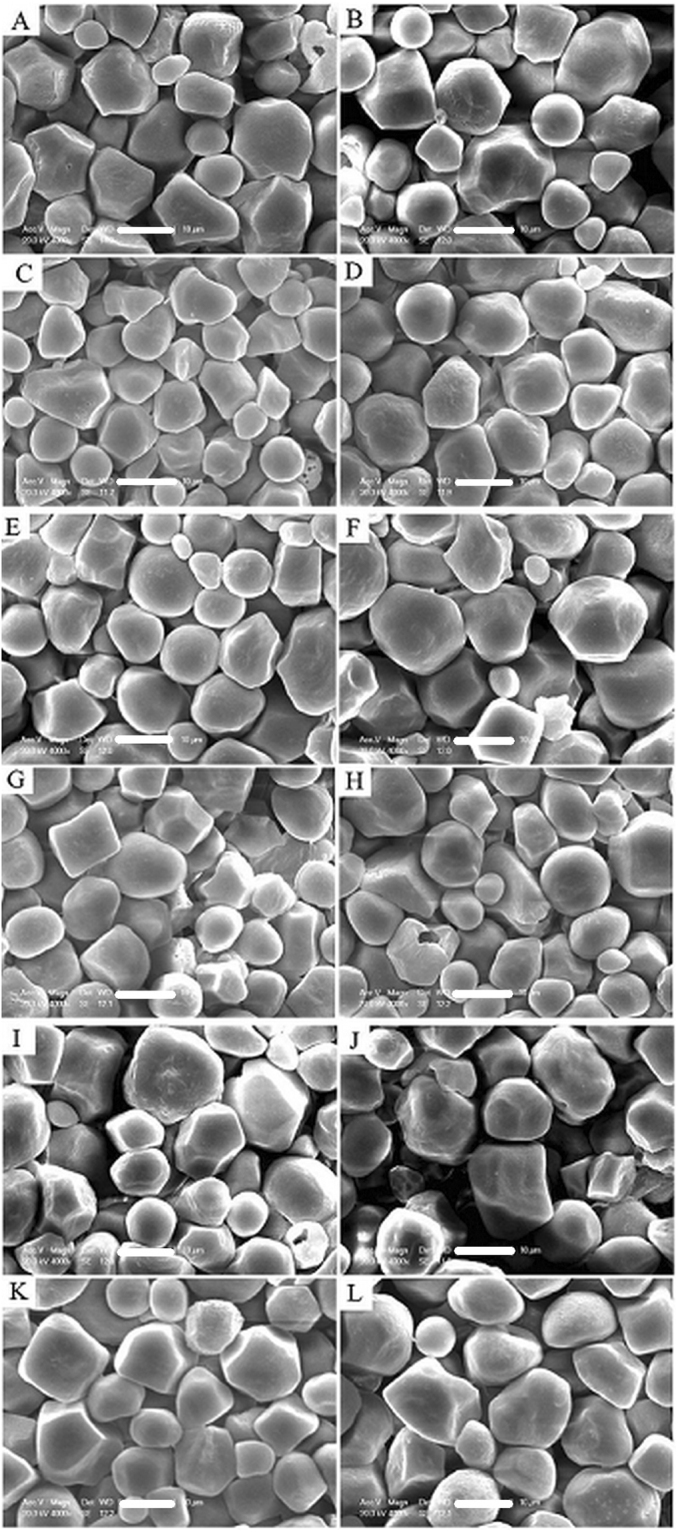
Scanning electron microscopy of the starch granules arrangement of superior(A,B,E,F,I and J) and inferior (C,D,G,H,K, and L) grains from Suyu 30(A–D), Suyu 20 (E–H) and Suyu 29 (I–L) and effected by 0 (B,D,F,H,J and L) and 450 (A,C,E,G,I and K) kg N ha−1 (4000×, Scale bar = 10 μm).
Figure 5.
Scanning electron microscopy of the starch granules from mechanical damage of Suyu 30 (A,C, and F), Suyu 20 (B,D and G) and Suyu 29 (E and H) (10000×, Scale bar = 5 μm.).
Discussion
Grain and Starch Granule Morphology and its Relationship with Maize Yield
Improved sink activity, sources, and assimilate and nutrient flow are essential in increasing yield. Increasing the grain number currently serves as the main approach in improving sink activity in maize, although evidence on the relationship between morphology structure of grain and yield is limited. Previous studies have shown that grain morphology (length, width, and surface area) could be regulated by moisture, as evidenced by studies using rice9 or starch granule morphology of rice could be regulate by N level13. In cereals, maximum seed volume is established before reaching its maximum dry weight and this is generally estimated as the developmental stage when the kernel maximum water content is achieved24. The 1,000-grain weight of maize and length of grain are quantitative traits that are controlled by multiple genes and are often influenced by environmental factors25. Because the ovaries at the tip of the ear have not reached their maximum size at the silking stage7, the apical kernels of maize ears have smaller size and dry matter accumulation rates than basal kernels. However, this difference could be regulated by changing the temperature during growth26 and N levels12. Our research indicated that under high-density conditions (82,500 plants ha−1), the application of 450 kg N ha−1 also mitigated this difference in grain density and 1,000-grain weight between superior and inferior grains and significantly increased grain density, length, width, and volume of grains, with minimal effects on grain thickness. It appears that smaller differences in superior and inferior grains improved maize yield. N largely influenced grain morphology in inferior grains than that in superior grains. Our this result is very similar to one of previous reports12.
The starch content was positively correlated with the maize starch granule volume ranges of 0.8 to 2 μm, 2 to 10 μm, and 10 to 15 μm, but negatively correlated with other size ranges18. Starch granules with a diameter of ≥15 μm were predominant19. Temperature could also regulate starch granule size. Interestingly, temperature affected granule size, pasting temperature, and transition temperature, in which a lower temperature prevailed during the filling stage, resulting in larger starch granules and lower pasting and transition temperatures20. Our results demonstrated a positive correlation between large starch granules and maize yield. Furthermore, the predominance of large starch granules could be strongly influenced by N levels, although several striking differences between superior and inferior grains have been observed. For example, superior grains showed a wider distribution than inferior grains. In the superior grains of Suyu 29, the percentages of starch granules with a diameter of >15 μm using 0, 360, 450, and 540 kg N ha−1 were 49.13%, 57.93%, 64.16%, and 53.20% (Figs 2C and D and 3E and F) respectively.
The Microstructure of Starch Granules
Recent developments in microscopy have allowed the examination of starch granule microstructures, which provide information on the physicochemical characteristics of maize starch, as well as grain filling mechanisms and yield. Most of the previous studies on the microstructure of starch granules mainly focused on three aspects of starch granules: internal structures, external structures, and physicochemical characteristics. A semi-compound granule generally contains one exterior surface and two or more hila, as described by Wei et al.15 and Nordmark et al.21. However, some starch granules have small cavities with a diameter range of 0.07 to 0.10 μm, as well as channels and pores randomly distributed across their surfaces, often in clusters and showing variations22,23. Evidences from SEM and chemical analyses have shown that the pores are randomly distributed, varying in number per granule, and the channels were irregularly bent27. These granules provide the architecture of blocklets, and a normal blocklet is mainly formed by crystalline and amorphous lamellae28,29 previously reported that the semi-crystalline blocklets generally consist of two types, ‘normal’ and ‘defective’, which occur within the same starch molecule and are the basic units that construct starch granules. The normal blocklets comprise the hard shells, whereas soft shells consist of the defective ones; both hard shells and soft shells are discontinuous structures30. Nordmark and Ziegler21 observed radially oriented crystalline lamellae using atomic force microscopy (AFM) and observed linear or lightly branched starch polymers composed of spherulites. Amylopectin plays a more important role in blocklet architecture, whereas the other component provides strength and flexibility to starch granules31. The length of the side chain of amylopectin varies with the size, as well as the inner and surface layers of starch granules32,33. We observed ‘hollow’ and ‘solid’ inner structures in the starch granules (Fig. 5); the wall of the spherical starch granules was thin and the chamber was big. The wall of the polyhedral starch granules was thick and layered and the chamber was small. Li et al.29 suggested that the hydrolysis of α-amylase or other chemicals that flowed into starch granules along channels were responsible for this ‘hollow’ formation. However, Wei et al.13 and our current study suspect that the physicochemical characteristics of granule walls, as well as the ‘hollow’ and ‘solid’ inner structures of starch granules, may generally occur in nature and are controlled by the conditions of the environment (e.g., N and temperature) during the filling stage, thus affecting maize yield. More extensive research on this area is therefore warranted.
Materials and Methods
Plant Materials and Growth Conditions
The experiment was conducted from June to October in 2010 and 2012 in clay loam soil plots at the Key Laboratory of Crop Genetics and Physiology of Yangzhou University (119°26′E, 32°24′N), Jiangsu Province, China. This site generally experiences a sub-tropical humid, monsoon climate. Three maize hybrids were analyzed, Suyu 20, Suyu 29, and Suyu 30. The first two hybrids have been extensively used in recent years in China, whereas Suyu 30 was authorized for propagation by the National Crop Variety Examination and Approving Committee in 2011, based on its high heterocyst and yield potential, especially in southeast China. The field of the test plots showed similar chemical property, agronomic histories and favorable conditions for irrigation and drainage Soil from a nearby clay loam field (0 to 20 cm depth) was collected, air-dried, and sieved through a 5-mm screen. The soil contained 96.71 mg kg−1 N (sum of NO3− and NH4+-N), 33.3 mg kg−1 phosphorus (P), 129 mg kg−1 potassium (K), 28.5 g kg−1 organic matter, and 1.80 g kg−1 total N at pH 6.81.
Experimental Design
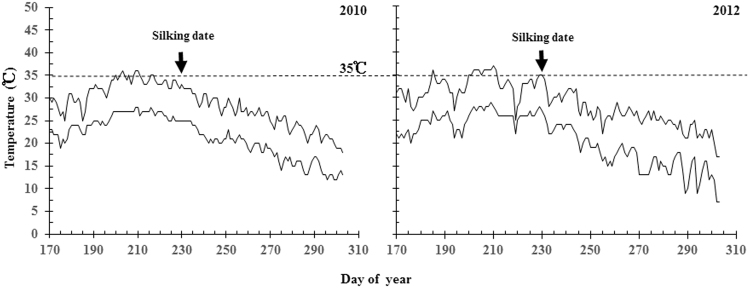
A split-plot experimental design consisting of three replicates was used in the study, with hybrids planted in the main plots and N treatments conducted in the sub-plots. Seeds were sowed on June 27 each year at a density of 82,500 plants ha−1 in test plot area of 110 m2. Planting followed a 0.3 m by 0.7 m row spacing pattern. Four N levels (Granule urea, N concentration = 46%) were utilized (0, 360, 450, and 540 kg ha−1) based on the previous experimental results in which the yield of maize reached its peak at 450 kg N ha−1. Vegetative and reproductive N treatments (Granule urea, N concentration = 46%) was incorporated to the soil at the 10-leaf and anthesis stages. Table 5 shows the N application scheme in detail. Base fertilizers of 150 kg ha−1 P2O5 and 225 kg ha−1 K2O were applied once upon planting. Figure 6 shows the temperature condition during the period from sowing to final harvest for the 2010 and 2012 growing seasons. Other field management measurements were carried out according to the maize requirements for high yield.
Table 5.
Nitrogen fertilization scheme (kg ha−1).
| N levels | Base-fertilizer | Ear-fertilizer | Grain- fertilizer |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 360 | 135 | 225 | 0 |
| 450 | 135 | 270 | 45 |
| 540 | 135 | 315 | 90 |
Figure 6.
Temperature condition during the period from sowing to final harvest for the 2010 and 2012 growing seasons of maize. The minimum and maximum temperature are given for each day. Arrows indicate the start of silking date.
Grain Morphology
Ten ears from each treatment were harvested 50 days after anthesis and fully dried. The grains of four rows at the base of the ear were removed and divided into 4 equal portions. The grains from the lower one-fourth portion were designated as superior, whereas those from the top one-fourth portion were defined as inferior. The length (a), width (b), thickness (c), and 1,000-grain weight of the superior and inferior grains were measured. The volume of the grains (approximately 100 g) was assessed using the 95% alcohol displacement method. The following calculations were performed: geometric volume of the grain = a × b × c; and grain density = weight of the grain (approximately 100 g)/volume of the grains.
Isolation of Starch Granules
Starch granules were isolated according to the method of Wei et al.15 and Lu35, with minor modifications. The maize grains were steeped in 0.2% NaOH for 48 h at room temperature. The samples were then rinsed in ultrapure water and then ground using a blender for 3 min. The suspensions were filtered through a 100-mesh sieve. The materials collected on the screen were again homogenized for 2 min, and then passed through the same sieve. The filtrates were adjusted to a pH level of 9.5 with 0.2% NaOH and a pH controller, mixed with excess alkaline protease (Enzyme Commission (EC) Number 3.4.21.62, Beijing Solarbio Science & Technology Co., Ltd., China), and stirred at 42 °C the pH was adjusted when it dropped below pH 8.5) . Approximately 18 h later, the starch was washed with 0.2% NaOH and centrifuged (4,000 g for 10 min) until no biuret reaction occurred (or protein content was less than 0.5%), and finally washed with water. The starch was further treated with anhydrous ethanol and a mixture of methanol and chloroform (v/v = 1:1) three times. Finally, the starch was collected and dried under atmosphere, ground into powder, and then passed through a 200-mesh sieve.
Starch Granule Size
The analysis of starch granule size was conducted using a laser particle size analyzer (Mastersizer 2000, Malvern, England) as described elsewhere34, with minor modifications. Granule sizing experiments were carried out through laser diffractometry using a Mastersizer 2000 instrument, equipped with HydroMu dispersing unit (Malvern). Measurements were taken under the following conditions: anhydrous alcohol mobile phase, particle refractive index of 0.54, particle absorption coefficient of 4, anhydrous alcohol refractive index of 1.366, and a general calculation model for irregular particles. Ten measurement cycles of 10 s each were taken, and the data obtained were averaged using the equipment software (Mastersizer 2000, ver. 5.20 from Malvern).
Granule Morphology
Granule morphology was examined using the method described by Lu et al.35, with minor modifications. Starch granules that were steeped in 95% ethanol for 4 h were mounted on circular aluminum stubs and air-dried. Subsequently, these were coated with gold, examined under a scanning electron microscope (SEM, XL-30 ESEM, Philips, Netherlands), and then photographed at an accelerating potential of 20 kV.
Grain Yield and Other Components
The harvested plot size was 22 m2 (four 11-m rows at the center of each plot). Mean grain yield and other components were estimated for each treatment at each location.
Statistical Analysis. Analysis of variance (ANOVA) using a linear model was performed using the software Statistical Product and Service Solutions (SPSS Inc., Chicago, IL, USA) to detect the main effects of hybrids, nitrogen, years and their interaction. Treatments were compared by Duncan’s test and differences were declared as statistically significant if P < 0.05. Pearson’s correlations were calculated to determine the relationship between different parameters of maize. All graphics were drawn using Sigmaplot 12.5. The results from 2010 and 2012 followed the same trend. So, all of the calculations in the present study based on the averages of 2010 and 2012.
Acknowledgements
This work was financially supported by grants from the National Natural Science of Foundation (30971731, 31000684, and 31271640), Project of Graduate Science and Technology Innovation of Jiangsu Province(CXZZ13-0906), the China Agriculture Research System on Maize Industry (grant No. CARS-02-68), and Major Agriculture Science Foundation of Upland Grain Crops Breeding of Zhejiang Province (grant No. 2016C02050-9-1).
Author Contributions
G.W. and W.P. planed and supervised the experiments. F.Z., L.J., D.W. and F.B. conducted the experiment and analyzed the results. F.Z. and L.J. wrote the first draft of the manuscript. All authors contributed to interpretation of results and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-25859-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/14/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Contributor Information
Fucheng Zhao, Email: Encliff@163.com.
Guiyue Wang, Email: Zjdygy@163.com.
References
- 1.Yang JC, Zhang JH. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010;61:1–5. doi: 10.1093/jxb/erp348. [DOI] [PubMed] [Google Scholar]
- 2.Yang JC. Mechanism and regulation in the filling of inferior spikelets of rice. Acta Agron. Sin. 2010;36:2011–2019. [Google Scholar]
- 3.Zhang CH, et al. Starch granules size distribution in superior and inferior grains of wheat is related to enzyme activities and their gene expressions during grain filling. J.Cereal.Sci. 2010;51:226–233. doi: 10.1016/j.jcs.2009.12.002. [DOI] [Google Scholar]
- 4.Ou-Lee TM, Setter TL. Enzyme Activities of starch and sucrose pathways and growth of apical and basal maize kernels. Plant Physiol. 1985;79:848–851. doi: 10.1104/pp.79.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu GH, et al. Regulation of expression of starch synthesis genes by ethylene and ABA in relation to the development of rice inferior and superior spikelets. J.Exp.Bot. 2011;62:3907–3916. doi: 10.1093/jxb/err088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng SH, et al. Progress in research and development on hybrid rice: A super-domesticate in China. Ann.,Bot. 2007;100:959–966. doi: 10.1093/aob/mcm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cárcova J, Otegui ME. Ovary growth and maize kernel Set. Crop Sci. 2007;47:1104–1110. doi: 10.2135/cropsci2006.09.0590. [DOI] [Google Scholar]
- 8.Zhang WX, et al. Grain morphological traits measured based on vision detection technology and their relation to grain weight in rice under different water condition. Acta Agron. Sin. 2008;34:1826–1835. doi: 10.3724/SP.J.1006.2008.01826. [DOI] [Google Scholar]
- 9.Zhang ZJ, et al. Symptomatic of the shrunk-grain panicle and the change characteristics in its grain. Sci. Agric. Sin. 2006;39:1536–1544. [Google Scholar]
- 10.Murty PSS, Murty K. S. Spikelet sterility in relation to nitrogen and carbohydrate contents in rice. Indian J. Plant Physiol. 1982;25:40–48. [Google Scholar]
- 11.Chen TT, et al. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 2013;64:2523–2538. doi: 10.1093/jxb/ert115. [DOI] [PubMed] [Google Scholar]
- 12.Shen LX, et al. Top-grain filling characteristics at an early stage of maize(Zea mays L.) with different nitrogen use efficiencies. J. Integr. Agri. 2017;16:626–639. doi: 10.1016/S2095-3119(16)61457-0. [DOI] [Google Scholar]
- 13.Zhu D, et al. Effects of nitrogen level on structure and physicochemical properties of rice starch. Food Hydrocoll. 2016;63:525–532. doi: 10.1016/j.foodhyd.2016.09.042. [DOI] [Google Scholar]
- 14.Lu D, et al. Effects of heat stress during grain filling on the structure and thermal properties of waxy maize starch. Food Chem. 2014;143:313–318. doi: 10.1016/j.foodchem.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 15.Wei CX, et al. Comparison of the crystalline properties and structural changes of starches from high-amylose transgenic rice and its wild type during heating. Food Chem. 2011;128:645–652. doi: 10.1016/j.foodchem.2011.03.080. [DOI] [Google Scholar]
- 16.Ji Y, et al. Structure and function of starch from advanced generation of new corn lines. Carbohydr.Polym. 2003;54:305–319. doi: 10.1016/S0144-8617(03)00181-4. [DOI] [Google Scholar]
- 17.Ji Y, et al. Thermal and structure properties of unusual starches from developmental corn lines. Carbohydr.Polym. 2003;51:439–450. doi: 10.1016/S0144-8617(02)00216-3. [DOI] [Google Scholar]
- 18.Zhang L, et al. Starch granule size distribution in grains of maize with different starch contents. Acta Agron. Sin. 2011;44:1596–1602. [Google Scholar]
- 19.Cui LN, et al. Starch granule size distribution in maize kernel with different endosperm types. Acta Agron. Sin. 2012;38:1723–1727. doi: 10.3724/SP.J.1006.2012.01723. [DOI] [Google Scholar]
- 20.Kaur A, et al. Physicochemical, thermal and pasting properties of starches separated from different potato cultivars grown at different locations. Food Chem. 2007;101:643–651. doi: 10.1016/j.foodchem.2006.01.054. [DOI] [Google Scholar]
- 21.Nordmark TS, Ziegler GR. Structural features of non-granular spherulitic maize starch. Carbohydr. Res. 2002;37:1467–1475. doi: 10.1016/S0008-6215(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 22.Fannon JE, Hauber RJ, BeMiller J. N.Surface pores of starch granules. Cereal Chem. 1992;69:284–288. [Google Scholar]
- 23.Fannon JE, Hauber RJ, BeMiller JN. Interior channels of starch granules. Cereal Chem. 1992;70:611–613. [Google Scholar]
- 24.Salaa RG, Westgate ME, Fernando H. Andrade source/sink ratio and the relationship between maximum water content, maximum volume, and final dry weight of maize kernels. Field Crops Res. 2007;101:19–25. doi: 10.1016/j.fcr.2006.09.004. [DOI] [Google Scholar]
- 25.Zhang WQ, et al. QTL Analysis of kernel ratio, kernel depth, and 100-Kernel weight in maize (Zea mays L.) Acta Agron. Sin. 2013;39:455–463. doi: 10.3724/SP.J.1006.2013.00455. [DOI] [Google Scholar]
- 26.Ou-Lee TM, Setter TL. Effect of increased temperature in apical regions of Maize Ears on Starch-Synthesis Enzymes and accumulation of sugars and starch. Plant Physiol. 1985;79:852–855. doi: 10.1104/pp.79.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray JA, BeMiller J. N. Development and utilization of reflectance confocal laser scanning microscopy to locate reaction sites in modified starch granules. Cereal Chem. 2004;81:278–286. doi: 10.1094/CCHEM.2004.81.2.278. [DOI] [Google Scholar]
- 28.Tang H, Mitsunaga T, Kawamura Y. Molecular arrangement in blocklets and starch granule architecture. Carbohydr.Polym. 2006;63:555–560. doi: 10.1016/j.carbpol.2005.10.016. [DOI] [Google Scholar]
- 29.Li JH, et al. Starch from hull-less barley: ultrastructure and distribution of granule-bound proteins. Cereal Chem. 2003;80:524–532. doi: 10.1094/CCHEM.2003.80.5.524. [DOI] [Google Scholar]
- 30.Wang X, et al. Proteomics profiling reveals carbohydrate metabolic enzymes and 14-3-3 proteins play important roles for starch accumulation during cassava root uberization. Scientific Reports. 2016;6:19643. doi: 10.1038/srep19643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, et al. Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Scientific Reports. 2016;6:28271. doi: 10.1038/srep28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez Z, Perez E. Effect of acetylation on some properties of rice starch. Starch –Stärke. 2002;54:148–154. doi: 10.1002/1521-379X(200204)54:3/4<148::AID-STAR148>3.0.CO;2-N. [DOI] [Google Scholar]
- 33.Jane J, et al. Location of amylose in normal starch granules: susceptibility of amylose and amylopectin to cross-linking reagents. Cereal Chem. 1992;69:405–409. [Google Scholar]
- 34.Sadowski Z, et al. Synthesis of silver nanoparticles using microorganisms. Materials Sci.-Poland. 2008;6:419–424. [Google Scholar]
- 35.Lu DL, Lu WP. Effects of protein removal on the physicochemical properties of waxy maize flours. Starch /Stärk. 2012;64:874–881. doi: 10.1002/star.201200038. [DOI] [Google Scholar]