Abstract
Purpose
To present the multimodal imaging characteristics including optical coherence tomography angiography (OCTA) as well as indocyanine green angiography (ICGA) of quiescent type 1 neovascularization (NV) in an asymptomatic eye with angioid streaks (AS).
Observations
A 67-year-old male patient was admitted to our clinic for routine eye examination. The presence of a quiescent type 1 NV was detected on both ICGA and OCTA in juxtafoveal localization under the retinal pigment epitheliumin the right eye. At the end of the one year of follow-up, the patient was asymptomatic and the type 1 NV was still quiescent.
Conclusions and Importance
In comparison to ICGA, OCTA seems to be an easily repeatable non-invasive imaging tool which enables us early detection and monitoring of type 1 NV lesions even in asymptomatic patients with AS.
Keywords: Angioidstreaks, Optical coherence tomography angiography, Type 1neovascularization
1. Introduction
Angioid streaks (AS) are characterized by breaks within calcified and thickened elastic fibers of Bruch's membrane (BM). They appear as reddish-brownish colored radiating lines from the optic nerve towards periphery at the posterior pole, bilaterally.1 AS are usually associated with some systemic diseases such as pseudoxanthoma elasticum (PE), however they can also be seen as isolated.
Any type of neovascularization (NV), the major cause of AS-associated vision loss, can develop in an eye with AS.2,3 Optical coherence tomography angiography (OCTA) is a noveltechnique that can detect NV, noninvasively.4 OCTA is thought to be useful tool for early detection of NV, as it is able to identify these lesions even in asymptomatic patients with AS, reported by Andreanos et al.5
Herein, we present the multimodal imaging characteristics including OCTA as well as indocyanine green angiography (ICGA) of quiescent type 1 NV in an asymptomatic eye with AS.
2. Case presentation
A 67-year-old male patient was admitted to our clinic for routine eye examination. Best corrected visual acuity was 20/20 and intraocular pressure was 12 mmHg in both eyes. Slit-lamp examination of the anterior segment was unremarkable, fundus examination showed the presence of AS and drusen-like pigmentary changes in the macula bilaterally. Systemic evaluation of the patient regarding underlying diseases for AS was negative. Color (CP) and red-free photography (RP) (Topcon3D-OCT 2000 Corporation, Tokyo, Japan), infrared photography (IRP), fundus autofluorescence (FAF), spectral-domain optical coherence tomography (SD-OCT), fluorescein angiography (FA), indocyanine green angiography (ICGA) (Heidelberg Spectralis HRA + OCT, Heidelberg, Germany) and OCTA (RTVue-XR Avanti OCT system, Optovue, Fremont, CA) were performed.
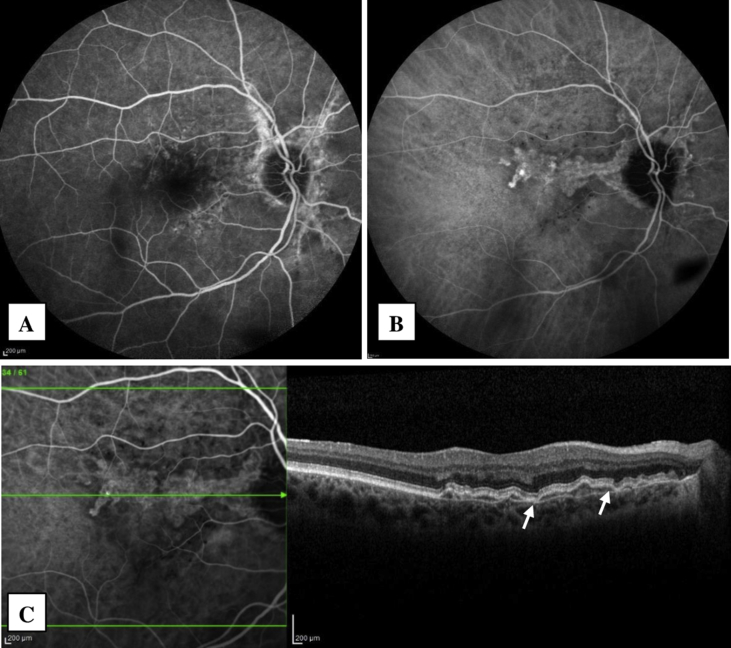
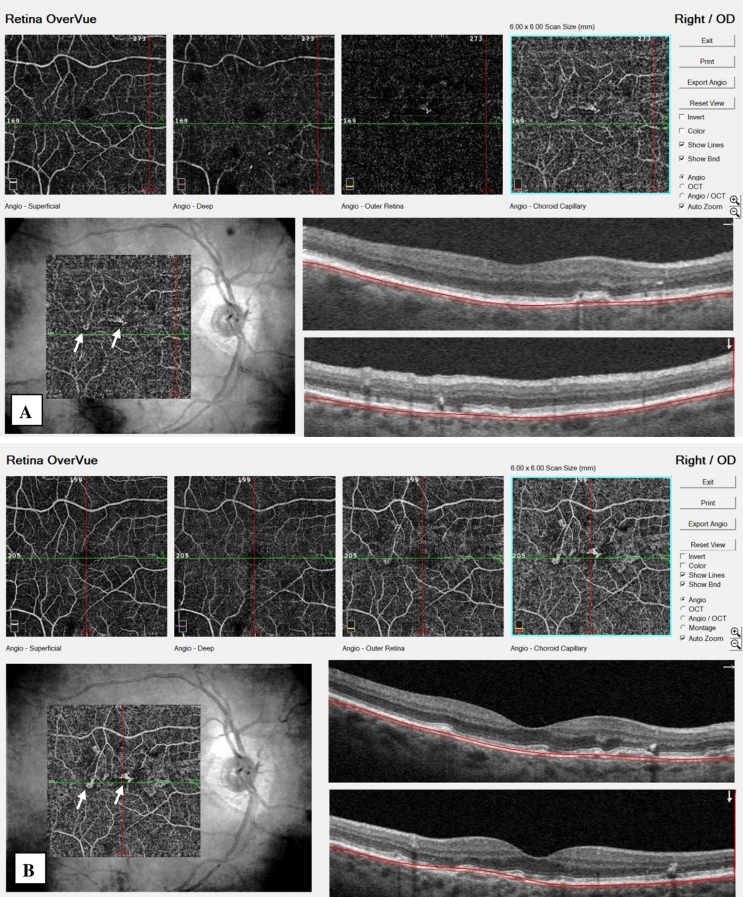
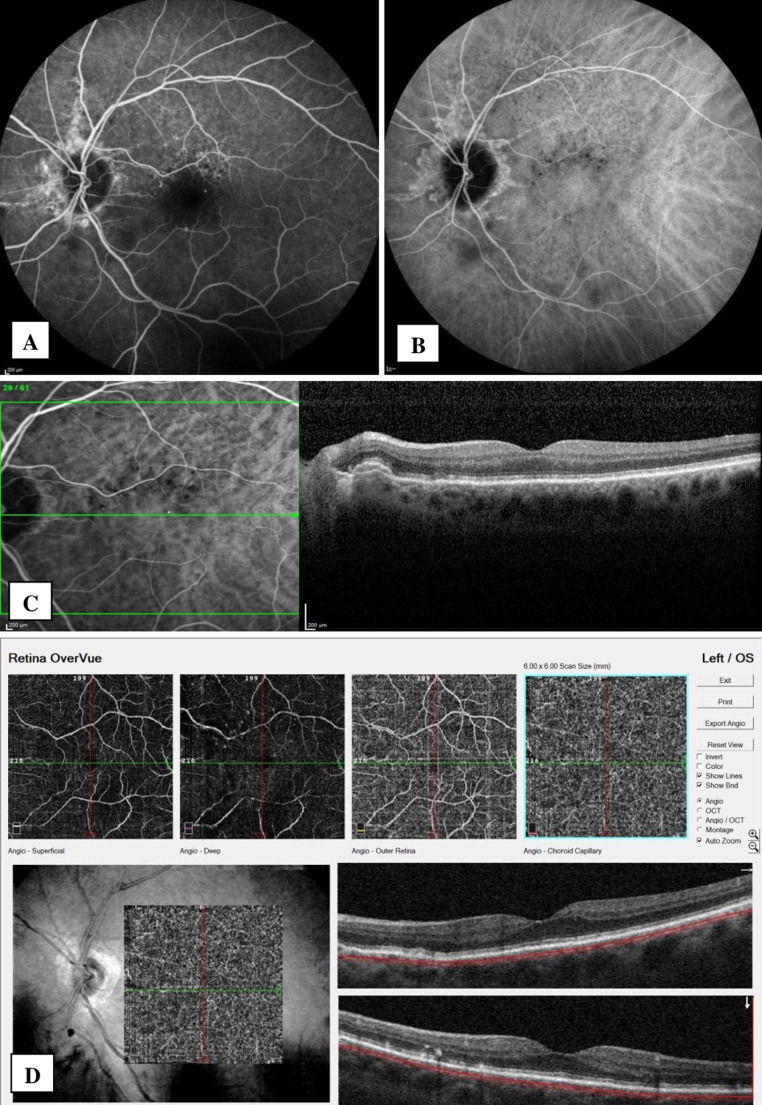
CP was consistent with AS. RP and IRP showed small spots particularly on the superior part of the macula. FAF demonstrated areas of hypoautofluorescence corresponding to AS, in addition to small hyperautofluorescent spots around the macula in both eyes. In the right eye, FA showed the irregular staining pattern of AS, without any leakage throughout all phases (Fig. 1A). ICGA demonstrated slightly hyperreflective plaques along the AS. Focal staining areas were present at the end of the plaque extending towards the macula in the late phase (Fig. 1B). SD-OCT revealed an irregularly and slightly elevated retinal pigment epithelium (RPE) due to moderately reflective material between the RPE and BM (Fig. 1C). BM contained multiple breaks. There were no any exudative changes on SD-OCT. OCTA detected the presence of small vascular networks in juxtafoveal localization under the RPE in this eye (type 1 NV) (Fig. 2). This localization was in correlation with the focal staining areas, seen on ICGA. At the end of the one year of follow-up, the patient was asymptomatic. Type 1 NV was still quiescent and there was no change in its size (Fig. 2). The images of the left eye were given in Fig. 3.
Fig. 1.
Fluorescein angiography (FA) (A), Indocyanine green angiography (ICGA) (B) and spectral domain optical coherence tomography (SD-OCT) (C) images of the right eye. SD-OCT shows irregularly elevated retinal pigment epithelium and Bruch's membrane breaks (white arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Small vascular networks (white arrows) are clearly seen in the right eye at choriocapillaris slab of optical coherence tomography angiography (OCTA) at the initial exam (A) and did not show any change in its size at the last exam (B).
Fig. 3.
Fluorescein angiography (FA) (A), Indocyanin green angiography (ICGA) (B), spectral domain optical coherence tomography (SD-OCT) (C) images and choriocapillaris slab of optical coherence tomography angiography (OCTA) (D) of the left eye. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
We have demonstrated the presence of quiescent type 1 NV in only one eye of a patient with AS before exudative changes occur and documented all multimodal imaging characteristics including OCTA as well as ICGA of this NV lesion.
Just recently, Andreanos et al.5 reported a case of nonexudative choroidal NV in the right eye and active choroidal NV in the other eye secondary to AS detected with the use of OCTA. They defined nonexudative choroidal NV as there was no leakage on FA and no exudation on OCT despite the presence of pigment epithelial detachment (PED). It was localized between the outer plexiform layer and BM on OCTA. Patient was monitored over an eight-month period and there was slight increase in the size of choroidal NV.
In our case, there was no sign of exudation, including PED, on SD-OCT. FA showed the irregular staining pattern of AS, without any leakage throughout all phases. The quiescent type 1 NV lesions have been detected on both OCTA and ICGA. We observed that these two imaging modalities were fully compatible with each other, in terms of both detecting the presence and the localization of NV which was clearly demonstrated between RPE and BM. We did not notice any change in its size over one-year of follow-up.
Gal-Or et al.6 previously reported the presence of active NV using multimodal images including OCTA, in the follow-up of a patient with AS who had previously treated for NV in his fellow-eye. In addition, they indicated that NV is an important reason for blindness in 42–84% of eyes with AS. Cebeci et al.3 showed that any type of NV, including polypoidal choroidal vasculopathy, can develop in eyes with AS.
Even though ICGA is known as a gold standard to diagnose type 1 NV correctly, it is an invasive procedure. Moreover, it is expensive, time-consuming and not repeatable at each follow-up visit. However, OCTA is a fast, safe and non-invasive imaging strategy that can be performed at each visit.
4. Conclusions
Due to these limitations of ICGA, OCTA seems to be an easily repeatable imaging tool which enables us early detection and monitoring of type 1 NV lesions even in asymptomatic patients with AS.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Financial support
No financial support was received for this submission.
Conflicts of interest
Dr. Mentes has served as a consultant for Allergan, Bayer and Novartis, Thea Pharma. Dr. Sermet has served as a consultant for Novartis, Allergan and Bayer. Dr. Karaca has no financial disclosures related to this manuscript.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Contributor Information
Jale Mentes, Email: jalementes@gmail.com.
Irmak Karaca, Email: irmakkaracamd@gmail.com.
Figen Sermet, Email: fbatioglu@gmail.com.
References
- 1.Georgalas I., Tservakis I., Papaconstaninou D. Pseudoxanthoma elasticum, ocular manifestations, complications and treatment. Clin Exp Optom. 2011;94:169–180. doi: 10.1111/j.1444-0938.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 2.Gliem M., Finger R.P., Fimmers R. Treatment of choroidal neovascularization due to angioid streaks: a comprehensive review. Retina. 2013;33:1300–1314. doi: 10.1097/IAE.0b013e3182914d2b. [DOI] [PubMed] [Google Scholar]
- 3.Cebeci Z., Bayraktar S., Oray M. Silent polypoidal choroidal vasculopathy in a patient with angioid streaks. Arq Bras Oftalmol. 2016;79:200–201. doi: 10.5935/0004-2749.20160058. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal R., Xin W., Keane P.A. Optical coherence tomography angiography: a non-invasive tool to image end-arterial system. Expert Rev Med Devices. 2016;13:519–521. doi: 10.1080/17434440.2016.1186540. [DOI] [PubMed] [Google Scholar]
- 5.Andreanos K.D., Rotsos T., Koutsandrea C. Detection of nonexudative choroidal neovascularization secondary to angioid streaks using optical coherence tomography angiography. Eur J Ophthalmol. 2017;27(5):e140–e143. doi: 10.5301/ejo.5000995. [DOI] [PubMed] [Google Scholar]
- 6.Gal-Or O., Balaratnasingam C., Freund K.B. Optical coherence tomography angiography findings of choroidal neovascularization in pseudoxanthoma elasticum. Int J Retina Vitreous. 2015;1:1–5. doi: 10.1186/s40942-015-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]