Abstract
Purpose
To report the transient resurgence of symptomatic silicone oil droplets following intravitreal bevacizumab injections.
Observations
We report seven patients presenting with silicone oil droplets following intravitreal bevacizumab injections. These were the first cases noted in 10 years using the same supplier of preloaded syringes in an estimated 90,413 injections performed by 7 physicians. They occurred during a 4 month period (06–10/2016), suggesting they may have been related to a batch or batches of syringes. Symptomatic floaters attributed to the droplets were noted on an average of 6.7 ± 1.5 days following the injection and followed an average of 10.4 ± 3.75 injections over a period of 3.4 ± 1.9 years and resolved in 5 of our 7 patients within 9 months.
Conclusions and importance
Symptomatic intravitreal silicone oil droplets are a rare complication of intravitreal injections. Symptoms are generally transient and not clinically significant and hence the benefits of treating potentially blinding eye diseases in this fashion appear to outweigh the limited risk of the rare, temporary floaters. The current series may be related to a batch or batches of syringes.
Keywords: Silicone oil droplets, Intravitreal bevacizumab, Vitreous
1. Introduction
Intravitreal injections of anti-vascular endothelial growth factor (VEGF drugs) have revolutionized the management of blinding eye diseases including macular degeneration, diabetic retinopathy and retinal vein occlusions.1 They are now the leading treatment option for each of these diseases. Early in the development of intravitreal injections, there were some reports of the accumulation of symptomatic oil droplets in the vitreous cavity. These were associated with the injection of pegaptanib, ranibizumab and bevacizumab and attributed to the silicone oil used as lubricating agents in the needles and syringes.2 The droplets were first reported in 20063 with the last report published in 2008.2 We also first noted symptomatic droplets during the first year after pegaptanib was approved but did not notice symptomatic oil droplets from 2007 until mid-2016. Here we report our recent cases of symptomatic oil droplets following intravitreal bevacizumab injections in our clinic using plastic syringes and discuss their possible cause and medical implications and solutions to this problem.
2. Methods
Retrospective chart review of patients undergoing intravitreal injections of bevacizumab and reporting new onset of floaters that persist through the interval.
3. Findings
3.1. Case 1
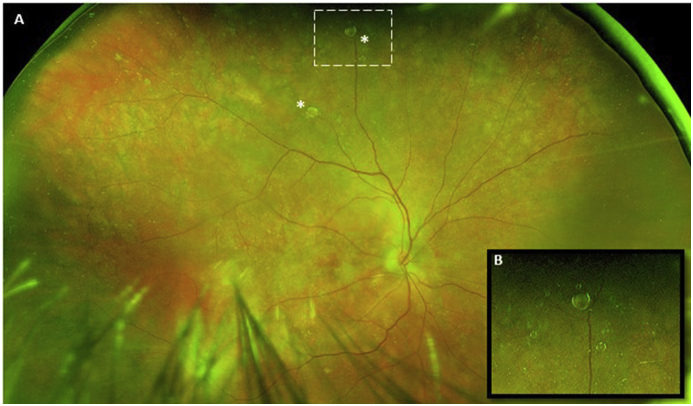
An 89 year old Caucasian female with a history of wet macular degeneration presented 7 weeks after her last intravitreal bevacizumab injection complaining of the onset of mobile, iridescent floaters beginning 7 days after her injection. Her visual acuity (VA) measured 20/20 with intraocular pressure (IOP) measurements of 17 mmHg, in her right eye, on the day of her injection. On the day she presented with symptoms, her visual acuity measured 20/20 with IOP measurements of 19 mmHg. The oil droplet was documented with Optos® fundus images (See Fig. 1). By her last follow up, her floater had resolved.
Fig. 1.
A. Optos® Fundus image of silicone oil droplets* following intravitreal bevacizumab. B. Magnified image of upper silicone oil droplet. Note, multiple, smaller, adjacent droplets in the background.
3.2. Case 2
A 47 year old Caucasian female with a history of wet macular degeneration presented 4 weeks after her intravitreal bevacizumab injection complaining of the onset of a mobile floater in her right eye beginning a week after her injection. Her VA measured 20/Count Fingers (CF) at 4 feet with IOP measurements of 13 mmHg, in her right eye, on the day of her injection. On the day she presented with symptoms, her visual acuity measured 20/200 with IOP measurements of 16 mmHg. By her most recent follow-up, the floater had resolved and the droplet was no longer present on clinical exam.
3.3. Case 3
A 71 year old Asian male with a history of wet macular degeneration presented 4 weeks after his intravitreal bevacizumab injection complaining of the onset of new “dust-like” floaters in his left eye beginning 3 days after his injection. His VA measured 20/50 with IOP measurements of 13 mmHg, in his left eye, on the day of his injection. On the day he presented with symptoms, his visual acuity measured 20/200 with IOP measurements of 16 mmHg in the affected eye. On his last follow-up, there was no evidence of a silicone oil droplet on exam.
3.4. Case 4
A 33 year old Hispanic female with a history of macular edema (ME) secondary to her history of central retinal vein occlusion (CRVO) presented 4 weeks after her intravitreal bevacizumab injections complaining of the onset of new mobile, “dot-like” floaters in her right eye beginning a day after her injection. Her VA measured 20/30 with IOP measurements of 18 mmHg, in the right eye, on the day of her injection. On the day she presented with symptoms, her VA measured 20/40 and her IOP measured 18 mmHg. The floaters have not resolved on her most recent follow-up, and the droplet was still present but was not interfering in any way with her daily activities of living.
3.5. Case 5
A 44 year old Caucasian female with a history of diabetic macular edema (DME) presented 7 days after her intravitreal bevacizumab injection complaining of the onset of new mobile, translucent floaters in her left eye beginning 6 days after her injection. Her VA measured 20/50 with IOP measurements of 18 mmHg, in the left eye, on the day of her injection. On the day she presented with symptoms, her VA measured 20/25 and her IOP measured 18 mmHg. In her most recent follow-up, she states that she still sees floaters but the silicone oil droplets were not noted. She denies any effect on activities of daily living.
3.6. Case 6
A 58 year old African American male with a history of DME and proliferative diabetic retinopathy (PDR) presented 13 days after his last intravitreal bevacizumab injection in his right eye complaining of 2 “air-bubble” like floaters in his right eye. His VA measured 20/25-1 with IOP measurements of 26 mmHg, in the right eye, on the day of the injection. On the day he presented with symptoms, his VA measured 20/20 and his IOP measured 20 mmHg. He was lost to follow up ever since his last follow up date.
3.7. Case 7
A 81 year old Caucasian female with a history of wet macular degeneration, presented 15 days after her intravitreal bevacizumab injection in her right eye complaining of new floaters in her right eye. Her VA measured 20/20-1 with IOP measurements of 18 mmHg, in the right eye, on the day of the injection. On the day she presented with symptoms, her VA measured 20/25 and her IOP measured 14 mmHg. At her most recent follow-up, she denied floaters and no longer had oil droplets visible on examination or fundus imaging studies.
4. Discussion
Silicone oil droplets have been reported following the injection of bevacizumab in the past with the last published report appearing 9 years ago.2 The problem appears largely isolated to insulin syringes which are loaded in the pharmacy under sterile conditions and then delivered to the ophthalmologists' office for use to treat patients. The silicone lining the syringe and the needle which serve as lubricating agents to facilitate movement appears to be the culprit. Some change in the manufacturing process with the resulting increase in net silicone in the bevacizumab solution within the syringe (perhaps more oil or less adherence of the oil to the barrel e.g. due to a decrease in syringe hydrophobicity) may be the cause of the recent rise in oil droplets which has been reported in an American Society of Retina Specialist (ASRS) alert, late in 2016. A specific lot of syringes may have been the culprit as we noticed the symptoms in only a 4 month window in the past 10 years, between June 24, 2016 and October 27, 2016. Another possibility is a change in the container used to carry the bevacizumab. Since the syringes are known to contain silicone, they seem the more probable source. Here we have reported 7 cases of symptomatic oil droplets that occurred after intravitreal injections numbering between 1 and > 16. Our experience suggests a recent change in the behavior of the syringes used to carry the bevacizumab, likely related to the lot or lots of syringes utilized during that period of time.
We did have a handful of patients who complained of oil droplet related floaters prior to instituting Electronic Medical Records (EMR) charting, 10 years ago – these patients were documented in paper charts and we are unable to search them. That was also during a period when pegaptanib injections were still being given and reports of droplets with the pre-loaded syringes did appear in the literature.2 Data from the last 3 years in our most recent EMR system indicates a total of 27,124 injections in 4597 patients performed by 7 different physicians, indicating a rate of 0.026% per injection or 0.15% per patient. It has been our impression that patients' complaints regarding the oil droplets diminish over time and that they become less symptomatic. Indeed, we've noted that 5 of our 7 patients became asymptomatic during their follow up here (6–9 months of follow up). One of the 7 patients was lost to follow-up. The resolution is likely due to the lower density of the silicone oil droplets compared to vitreous and hence the droplets' rising to the area of the pars plicata and ciliary body. Polydimethylsiloxane (PDMS) is the most commonly used silicone to siliconize syringes and it has a density of 965 kg/m3 compared with 1000 kg/m3 of water which approximates the vitreous fluid.
One concern regarding retained oil droplets is the development of glaucoma. Some droplets may pass into the anterior chamber (AC) and get caught in the trabecular meshwork – we have not seen this on gonioscopy and it is unlikely that the silicone oil droplets have any impact on the IOP. In fact, an analysis of patients' IOP on the day of the intravitreal injection associated with symptomatic oil droplets compared to the pressures following the injections revealed no significant difference (p = 0.343). In addition, the most commonly utilized silicone oil for vitreoretinal surgery is of comparable viscosity (1000 centistokes) and is well tolerated as a long-term tamponade.4
While silicone-free syringes are now available, the use of silicone does serve a purpose and those that make the switch should keep in mind that the resistance of the syringe may be different. Physicians should be prepared to adjust their injection technique, accordingly, though in practice, silicone-free syringes seem to be efficient, just more expensive. The silicone may also reduce adherence and leaching of drugs in the barrel of the syringe and this must be kept in mind, as well, though the large molecular weight of bevacizumab makes leaking unlikely.5 Interestingly, there is evidence that Silicone-Oil-Free (SOF) polymers for syringes can help the stability of protein based drugs during shipping and handling6 and such polymers should be considered for use in syringes for intravitreal injections. Further studies of the potential risks and benefits of silicone free syringes are warranted. Of note, preloaded glass syringes that can be preloaded with anti-VEGF drugs are now available that have the silicone “baked” into the glass and appear to minimize silicone droplets entering the solution.7
Because of the new, recent anecdotal reports of oil droplets noted following intravitreal bevacizumab injections this past 6 months, OMIC released new consent forms for use when performing intravitreal injections with bevacizumab, as well as other anti-VEGF drugs. The new consent forms address all types of floaters.
In summary, we noticed a recurrence of symptomatic intravitreal silicone oil droplets in our practice during 2016 after a 10 + year hiatus. It is an unforeseeable complication of intravitreal injections with an incidence of 0.026% out of 27,124 injections in the past 3 years (extrapolated to 0.008% in the past 10 years) but should now be considered as a possible occurrence. Oil droplets are not always symptomatic and when symptoms arise, they have been transient in the patients we have been able to monitor over time. The risk of oil droplets should not outweigh the risk of vision loss in patients with potential blinding retinal diseases. The use of silicone-free syringes should also be considered. The cause of the droplets remains unclear and the manufacturing companies of both the syringes as well as the drugs used in the injections should investigate this phenomena.
5. Patient consent
The Western University Institutional Review Board deemed protocol approval not necessary for such a case series. Patients in this series provided signed voluntary and informed consent to the described treatment. Patients in this series displayed appropriate capacity to provide consent. Patients understood the risks, benefits, and alternatives for the intravitreal injections administered and understood they were entitled to withdraw previous consent at any time during the treatment.
Consent to publish the case series was not obtained from the patients. The case series does not contain any identifying information.
Funding
No grant support or funding was given.
Conflicts of interest
The following authors have no financial disclosures: JY, RPG, EG, JC, KK, RP.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Acknowledgments
None.
References
- 1.Brand C.S. Management of retinal vascular diseases: a patient-centric approach. Eye. 2012; Apr;26(Suppl 2):S1–S16. doi: 10.1038/eye.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakri S.J., Ekdawi N.S. Intravitreal silicone oil droplets after intravitreal drug injections. Retina. 2008 Jul-Aug;28(7):996–1001. doi: 10.1097/IAE.0b013e31816c6868. [DOI] [PubMed] [Google Scholar]
- 3.Freund K.B., Laud K., Eandi C.M., Spaide R.F. Silicone oil droplets following intravitreal injection. Retina. 2006;26:701–703. doi: 10.1097/01.iae.0000223177.08438.2b. [DOI] [PubMed] [Google Scholar]
- 4.Gallemore R.P., McCuen B.W., II . Silicone oil in vitreoretinal surgery. In: Ryan S.J., editor. Retina. fourth ed. Elsevier Mosby; Philadelphia: 2006. pp. 2211–2234. [Google Scholar]
- 5.Reuter B., Peterson C. Syringe Siliconization Trends, methods, analysis procedures. TechnoPharm. 2012;2(4):238–244. [Google Scholar]
- 6.Krayukhina E., Tsumoto K., Uchiyama S., Fukui K. Effects of syringe material and silicone oil lubrication on the stability of pharmaceutical proteins. J Pharm Sci. 2015;104(2):527–535. doi: 10.1002/jps.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sealing Solutions D.A.T.W.Y.L.E.R. SCHOTT pharmaceutical systems. Optimisation of syringe performance for ocular injections & beyond: impact of silicone oil. ONdrugDelivery. 2015; Oct;61 [Google Scholar]