Abstract
Introduction
This study investigated concussion as a potential risk factor for increased alcohol consumption in university athletes.
Methods
Using a cross-sectional design, 41 university students (37% with a history of concussion) completed self-report measures, while electrodermal activation (EDA) was recorded for each participant to capture baseline physiological arousal.
Results
As expected, concussion status significantly predicted alcohol consumption over and above athletic status, b = 0.34, p = 0.034, 95% CI [0.195, 4.832], such that those with a prior concussion history engaged in greater alcohol consumption. Importantly, concussion status also significantly predicted baseline physiological arousal, b = −0.39, p = 0.014, 95% CI [−0.979, −0.120], such that those with a history of concussion exhibited lower EDA.
Conclusions
Elevated alcohol consumption among athletes is a pronounced associate of concussion in sports and may be a behavioral reflection of disruption to the orbitofrontal cortex – an area implicated in inhibition.
Abbreviations: EDA, electrodermal activation; SMH, Somatic Marker Hypothesis; vmPFC, ventromedial prefrontal cortex; TBI, traumatic brain injury
Keywords: Concussion, Arousal, Risk taking, Alcohol consumption, Athletes
Highlights
-
•
Alcohol consumption after concussion was assessed in university students
-
•
Specifically, attenuated physiological arousal after head injury was investigated as a mechanism of increased alcohol use
-
•
Those with a history of concussion were found to engage in greater alcohol consumption than their non-concussed peers
-
•
Those with a history of concussion also displayed dampened physiological arousal compared to their non-injured cohort
-
•
In the concussion group only, physiological underarousal approached significance as a predictor of elevated alcohol use
1. Introduction
According to Iverson and Lange (2009), head injury severity can be classified along a continuum spanning from mild to catastrophic. Injuries on the mild end of this spectrum, such as concussions, account for the majority of all reported injuries, reflecting approximately 70–90% (Cassidy et al., 2004). Concussions – impacts to the head or torso that generate acceleration/deceleration forces sufficient to alter one's state of consciousness (e.g., feeling confused or dazed; Kay et al., 1993) – are commonly sustained in high-risk sports (Gessel, Fields, Collins, Dick, & Comstock, 2007; Noble & Hesdorffer, 2013; Zuckerman et al., 2015). Indeed, over two academic years and 25 collegiate sports, Zuckerman et al. (2015) recorded 1670 sports-related concussions – the majority of which occurred during high-risk sports competitions, such as football (36.1% of reported concussions), ice hockey (13.4%) and women's soccer (8.1%). Similar rates have been recorded in high school and university student populations (Baker & Good, 2014; Halstead & Walter, 2010) implying that the nature of play demanded by these sports may place athletes at a greater risk of sustaining a head injury (McAllister et al., 2012). For example, in a high-risk sport like competitive cheerleading, concussions account for over 30% of all injuries sustained (Currie, Fields, Patterson, & Comstock, 2015) despite the non-contact nature of competition.
Given that the ventromedial prefrontal cortex (vmPFC) is highly susceptible to disturbance in any closed-head injury (Morales, Diaz-Daza, Hlatky, & Hayman, 2007), elevated levels of impulsivity and aggression are commonly reported post-concussion (Goswami et al., 2016), and concussion severity is negatively associated with baseline levels of physiological arousal (i.e., electrodermal activation [EDA] levels; Baker & Good, 2014; van Noordt & Good, 2011). Disruption to the vmPFC and subsequent post-injury underarousal limit one's ability to anticipate negative outcomes in unpredictable/risky situations (Damasio, Tranel, & Damasio, 1990), increasing the probability that an individual will engage in impulsive, aggressive, or risk-taking behavior. In particular, according to the Somatic Marker Hypothesis (SMH; Damasio, 1994), damage to the vmPFC compromises the regulation of physiological arousal cues, resulting in dampened autonomic functioning. In turn, this puts individuals in a physiologically unprepared and uninformed state, leading to greater addiction, risk-taking and impulsive behaviors without sufficient somatic signals to inform cognition and guide behavior (Verdejo-Garcia, Pérez-García, & Bechara, 2006). Indeed, patients with severe damage to the vmPFC have been reliably found to exhibit significant difficulties in decision-making under situations of uncertainty and greater risk-taking (Morales et al., 2005).
One risk-taking behavior that has been investigated post-injury is the increased engagement in substance use (e.g., binge drinking, cigarette smoking, etc.; McKinlay, Corrigan, Horwood, & Fergusson, 2014; Bjork & Grant, 2009). Although alcohol use tends to decrease during the first year after injury (Ponsford, Whelan-Goodinson, & Bahar-Fuchs, 2007), many studies show an increased likelihood of heavy drinking in traumatic brain injury (TBI) patients (Koponen et al., 2002) and a strong positive association between time since injury and alcohol consumption (Kreutzer, Witol, & Marwitz, 1996). Moreover, self-reported TBI has been linked to increased substance use during adolescence (Ilie et al., 2015) and, further, concussions sustained during childhood are predictive of problematic alcohol use in adolescence and early adulthood (Kennedy, Cohen, & Munafó, 2017). Given that dysregulated physiological arousal is common after concussion (Baker & Good, 2014; van Noordt & Good, 2011), it is hypothesized that attenuated arousal may play a key role in post-injury alcohol consumption. Specifically, since those with a history of concussion exhibit dampened somatic activation during the anticipatory stages of decision-making (van Noordt & Good, 2011), they may be unable to anticipate the negative consequences associated with drinking behavior and impulsively consume alcohol in excess. Alternatively, alcohol consumption may serve as a solution to chronic underarousal post-concussion and individuals may learn to consume alcohol as a means of boosting autonomic activity. In both cases, it is proposed that physiological underarousal may serve as a mechanism of increased drinking behavior after injury.
At present, the proposed relationship between concussion and alcohol consumption through physiological underarousal remains theoretical and requires further investigation. Research shows that elevated alcohol consumption increases one's risk of sustaining a brain injury (Silver, Kramer, Greenwald, & Weissman, 2001), making it difficult to determine the extent to which premorbid risk-taking and alcohol use precipitate head injury. Indeed, athletes exhibit higher levels of sensation seeking (Hartman & Rawson, 1992; Mastroleo, Scaglione, Mallett, & Turrisi, 2013; Schroth, 1995), more frequent alcohol consumption, higher rates of heavy episodic drinking, a greater number of sexual partners, and a greater engagement in unsafe sex compared to non-athletes (Wetherill & Fromme, 2007). Thus, those with riskier personalities may be more likely to seek out risky activities, such as high-risk sports and binge drinking. For instance, athletic status is associated with a greater number of problem behaviors while under the influence, such as getting in trouble with the police (Nelson & Wechsler, 2001) and sustaining an injury (Leichliter, Meilman, Presley, & Cashin, 1998). Thus, athletes who engage in behavior that is risky enough to result in a concussive injury might also be more prone to engage in other risky behaviors such as excessive substance use.
Alternatively, others have proposed that alcohol may serve as a means of coping with sports-related stressors, reinforcing athletic performance, or fostering belongingness and involvement in sports culture (see Martens & Martin, 2010). For instance, athletes who have greater involvement in their teams (i.e., team captains) drink more per week and engage in more episodic drinking than those who are less involved (i.e., second-string players; Leichliter et al., 1998); thus, it may be that the added time commitment of athletics causes greater stress and maladaptive forms of coping (Damm & Murray, 1996; Marcello, Danish, & Stolberg, 1989). Few studies, however, have found support for this idea, as athletes do not report coping as a motivation for alcohol use (Green, Uryasz, Petr, & Bray, 2001; Herring et al., 2016). Similarly, the association between alcohol consumption and social and cultural motives remains unclear since many factors influence this relationship (Martens, Dams-O'Connor, & Beck, 2006).
Taken together, the above findings highlight the need to elucidate whether elevated alcohol consumption in athletics is reflective of pre-injury personality characteristics exclusively or whether the increased prevalence of concussion in sports, and subsequent dampened physiological feedback, may contribute. The aim of this study, therefore, was to investigate the potential role of concussion as a risk factor for increased alcohol consumption in university athletes. In particular, given the established relationship among head injury, decision-making processes, and physiological arousal, the current study sought to examine EDA as a potential mechanism of the association between concussion and alcohol use. First, it was predicted that both those with a history of concussion, and those classified as athletes, would engage in greater alcohol consumption than their non-concussed and non-athlete peers. Second, based on previous findings of physiological underarousal in concussed individuals (Baker & Good, 2014), we hypothesized that those with a history of concussion would exhibit lower baseline EDA compared to those with no concussion history. Lastly, we predicted that concussion history would be associated with increased alcohol use, over and above the effects of athletic status.
2. Methods
2.1. Participants
Forty-one Brock University students (Mage = 20.71, SD = 3.95; 19.5% male) attended a laboratory session in the Jack and Nora Walker Lifespan Development Centre testing facilities on campus in St. Catharines, Ontario, Canada. Poster advertisements, standardized recruitment PowerPoint slides (displayed in Psychology courses offering course credit for research participation), and the Brock University Psychology Department Research Pool (SONA) were used to recruit participants. Importantly, to eliminate the potential confound of diagnosis threat (Suhr & Gunstad, 2002; Suhr & Gunstad, 2005), participants were not recruited on the basis of head injury status and were only informed of the authors' added interests in concussion during the post-study debriefing.
In line with previous investigations (Gallant, Barry, & Good, in press), the primary sport listed for current participation in university athletics was used as a means of classifying athletic status, such that 26 individuals self-identified as non-athletes (63%), 7 as low-risk athletes (17%), and 8 as high-risk athletes (20%). Of the 15 self-reported athletes, 10 (67%) currently participated in a recreational sports league and 5 (33%) participated in a competitive sports league. Table 1 contains a list of all reported sports affiliations and their associated demographic frequencies. Fifteen participants (37%) self-reported having sustained a previous concussion, while 26 had no such history (63%). Of those who endorsed a concussion history, 8 (53%) were non-athletes, while 7 (47%) were athletes (2 low-risk and 5 high-risk). Given the low number of low-risk athletes with prior concussions, athletic status was collapsed to form two athlete categories (i.e., athlete, non-athlete). The average time since injury was 91.27 months (7.61 years) and ranged from 6 to 288 months. For more details regarding the severity of concussive injuries and the associated demographic frequencies see Table 2.
Table 1.
Self-reported sport-related activities currently played in University (n = 15).
| Sport-related activity | High-risk athletes (n = 8) |
Low-risk athletes (n = 7) |
|||
|---|---|---|---|---|---|
| n | % of total | Sport-related activity | n | % of total | |
| Ice Hockey | 2 | 13.3 | Basketball | 1 | 6.7 |
| Soccer | 2 | 13.3 | Volleyball | 2 | 13.3 |
| Figure skating | 2 | 13.3 | Rowing/Kayaking | 1 | 6.7 |
| Power/olympic Lifting | 2 | 13.3 | Dance | 1 | 6.7 |
| Swimming | 2 | 13.3 | |||
Table 2.
Injury severity indicators of self-reported concussion as a function of athletic status.
| Injury Severity Indicators | Athletes (n = 7) |
Non-athletes (n = 8) |
Total (n = 15) |
|||
|---|---|---|---|---|---|---|
| n | % of total | n | % of total | n | % of total | |
| Loss of consciousness (LOC) | 3 | 20.0 | 2 | 13.3 | 5 | 33.3 |
| Duration of LOC | ||||||
| < 5 min | 2 | 13.3 | 2 | 13.3 | 4 | 26.7 |
| < 30 min | 1 | 6.7 | 0 | 0 | 1 | 6.7 |
| Diagnosed concussion | 5 | 33.3 | 5 | 33.3 | 10 | 66.7 |
| Required stitches | 0 | 0 | 1 | 6.7 | 1 | 6.7 |
| Received medical treatment | 3 | 20.0 | 4 | 26.7 | 7 | 46.7 |
| Overnight at medical facility | 0 | 0 | 0 | 0 | 0 | 0 |
| Symptoms for 20+ minutes | 5 | 33.3 | 4 | 26.7 | 9 | 60.0 |
| More than one injury | 3 | 20.0 | 1 | 6.7 | 4 | 26.7 |
2.2. Materials
2.2.1. Physiological arousal
Using Polygraph Professional Suite Software and Polygraph Professional equipment (Limestone Technologies, 2008) on a 16-inch Acer laptop computer, EDA was recorded as an index of physiological arousal via the Datapac USB 16-bit Data Acquisition Instrument and two silver‑silver chloride pads. In particular, the silver‑silver chloride pads were placed on the index and fourth fingers of participants' non-dominant hands. Electrodermal activation (EDA) amplitude was used as an index of physiological arousal in the current study, as it has been shown to be a reliable index of autonomic nervous system (ANS) functioning (Fowles & Schneider, 1974).
2.3. Self-report questionnaires
The Everyday Living Questionnaire (ELQ; Good, 2008) is a demographic questionnaire that collects information on participants' age, sex, education, medical history, leisure activities, and recreational and athletic involvements. In particular, the ELQ was used to determine head injury status and to assess alcohol use. Based on the ACRM criteria for concussion (Kay et al., 1993), participants were asked, “Have you ever hit your head with a force sufficient to alter your state of consciousness?” Participants who endorsed this item were classified as having a concussion history. Alcohol use was measured by a number of self-report questions embedded in the ELQ (e.g., weekly alcohol use and average number of alcoholic drinks per outing).
The HEXACO Personality Inventory – Revised (HEXACO-PI-R; Lee & Ashton, 2016) is a measure of the six major dimensions of personality, including: Honesty-Humility, Emotionality, Extraversion, Agreeableness, Conscientiousness, and Openness to Experience. For the current study, the 100-item version of the HEXACO was used to assess each of the six domain-level scales. Since pre-injury personality characteristics related to alcohol consumption are most relevant to the current research questions, the Extraversion, Agreeableness, Conscientiousness, and Openness to Experience domains were of particular interest to the current study, given their previously demonstrated associations with alcohol consumption (e.g., Hakulinen et al., 2015); the Prudence subscale from the Conscientiousness domain was also examined since it captures the tendency for risk-taking behaviour and impulsivity (i.e., high scores on this scale reflect an ability inhibit impulses and consider their options carefully prior to decision-making).
2.4. Procedure
Upon approval from the Brock University Research Ethics Board (#16-047), participants were tested individually for approximately 90 min. After providing informed consent, a 3-min recording of baseline EDA was obtained, following which, participants completed the self-report questionnaires and were debriefed.
2.5. Data analyses
The Statistical Package for the Social Sciences (SPSS) was used to compute all analyses and examine all assumptions. Unless stated otherwise, all statistical assumptions have been met. For all analyses, a statistical significance level of p < 0.05 was used; those approaching statistical significance, however, are also discussed. To examine categorical group differences, Chi-square tests of independence and independent samples t-tests were conducted. To predict alcohol consumption from concussion history, athletic status, and arousal, multiple regressions were conducted; all analyses were conducted with and without sex as a covariate and results did not differ.
3. Results
3.1. Demographic information
Given that previous research has demonstrated a relationship between drinking behavior and sex, age, and mental health status (Horner et al., 2005), Chi-square tests of independence were conducted to examine sex, age and mental health differences by concussion history and athletic status. Further Chi-square tests of independence were used to observe any differences in rates of concussion as a function of athletic status. No associations were found for any of the variables examined, as well as for any of the health-related variables (i.e., hospitalizations, diagnosed psychiatric condition, medication use, diagnosed learning disorder), use of services (i.e., physiotherapy, occupational therapy, learning resource teacher, or educational assistance), self-reported alertness, enjoyment of life, and life stressors (p's > 0.05).
Further group level differences between the concussion versus no-concussion group were examined via t-test statistics for each of the HEXACO personality dimensions. In particular, no differences were found between the concussion and no-concussion groups for the Extraversion, Agreeableness, Conscientiousness, and Openness to Experience domains, or for the Prudence subscale (p's > 0.2).
3.2. Alcohol consumption by head injury and athletic status
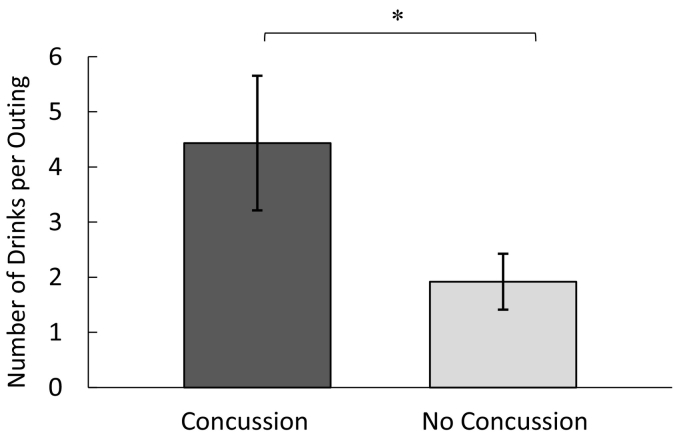
A linear regression was conducted, such that alcohol consumption (drinks per week) was regressed on athletic status (athlete, non-athlete) and head injury status. Neither athletic status, b = 0.04, p = 0.829, 95% CI [−2.459, 3.051], nor concussion history, b = 0.12, p = 0.486, 95% CI [−1.845, 3.807], significantly predicted weekly alcohol consumption. A separate linear regression was conducted, such that number of drinks per outing was regressed on athletic status and head injury status. Interestingly, and as expected, concussion was found to be a significant predictor of alcohol consumption per outing, b = 0.34, p = 0.034, 95% CI [0.195, 4.832], over and above the effects of athletic status (Table 3). In particular, concussion was associated with a greater number of alcoholic drinks per outing. Fig. 1 depicts this finding, showing the number of drinks per outing as a function of concussion status.
Table 3.
Hierarchical regression analysis for variables predicting alcohol consumption (n = 41).
| Variable | Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | |
| Concussion Status | 2.51 | 1.15 | 0.33⁎ | 2.57 | 1.18 | 0.34⁎ |
| Athletic Status | −0.28 | 1.20 | −0.04 | |||
| R2 | 0.11 | 0.11 | ||||
| F for change in R2 | 4.82⁎ | 2.38 | ||||
DV: Alcohol Consumption (Drinks per outing).
p < 0.05.
Fig. 1.
Mean number of alcoholic drinks consumed per outing as a function of head injury status (error bars represent standard errors of the mean).
3.3. Physiological arousal
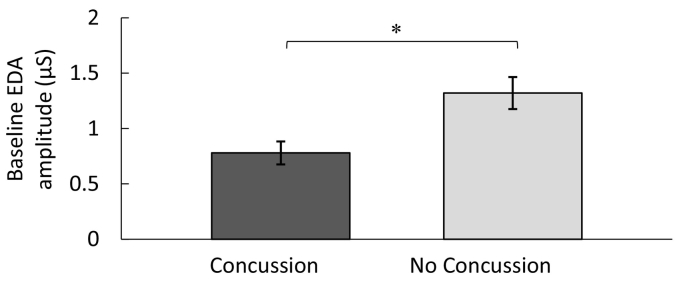
A linear regression was conducted, such that baseline physiological arousal (i.e., EDA peak amplitude) was regressed on athletic status (athlete, non-athlete) and head injury status. Concussion was found to be a significant predictor of baseline EDA, b = −0.39, p = 0.014, 95% CI [−0.979, −0.120], over and above the effects of athletic status, b = 0.04, p = 0.813, 95% CI [−0.379, 0.480]. Specifically, university students who self-reported a previous concussive injury had significantly lower levels of baseline arousal compared to those who did not endorse a history of concussion. Fig. 2 depicts this finding, showing baseline physiological arousal as a function of concussion status.
Fig. 2.
Baseline electrodermal activation (EDA) amplitude (μS) as a function of head injury status (Error bars represent standard errors of the mean).
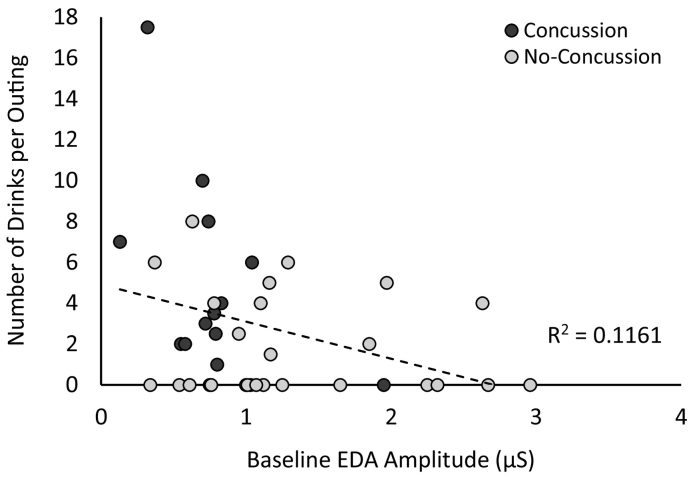
Further linear regressions were conducted, such that alcohol consumption was regressed on EDA peak amplitude and athletic status. Neither EDA amplitude, b = −0.27, p = 0.100, 95% CI [−3.537, 0.322], nor athletic status, b = 0.02, p = 0.895, 95% CI [−2.519, 2.871], were found to be significant predictors of weekly alcohol consumption. However, subsequent analyses revealed that EDA amplitude was a significant predictor of the average number of alcoholic drinks consumed per outing, b = −0.34, p = 0.034, 95% CI [−3.475, −0.144], over and above the effects of athletic status, b = 0.02, p = 0.884, 95% CI [−2.209, 2.554], such that those who had lower baseline levels of physiological arousal consumed more per outing. See Fig. 3 for a depiction of this relationship in terms of drinks per outing.
Fig. 3.
Number of alcoholic drinks consumed per outing as a function of baseline electrodermal activation (EDA) amplitude (μS).
Lastly, those with and without a history of concussion were examined independently; in the no-concussion group, there was a trend for athletic status (athlete, non-athlete) to be related to alcohol consumption, b = 0.35, p = 0.096, 95% CI [−0.366, 4.179], such that athletes reported engaging in heavier alcohol consumption per outing than non-athletes. Moreover, EDA amplitude did not significantly predict drinking behavior in those without a history of concussion. In contrast, in the concussion group, EDA amplitude was found to approach significance when predicting alcohol consumption, b = −0.49, p = 0.056, 95% CI [−11.739, 0.177], such that lower EDA amplitude was associated with heavier alcohol consumption per outing. In addition, athletic status (athlete, non-athlete) did not significantly predict drinking behavior in participants with a history of concussion. Table 4, Table 5 contain further details regarding this relationship.
Table 4.
Hierarchical regression analysis for variables predicting alcohol consumption in the no-concussion group (n = 26).
| Variable | Model 1 |
Model 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| B | SE B | β | p | B | SE B | β | p | |
| EDA amplitude | −0.57 | 0.70 | −0.17 | 0.424 | −0.68 | 0.67 | −0.20 | 0.321 |
| Athletic status | 1.91 | 1.10 | 0.35 | 0.096 | ||||
| R2 | 0.03 | 0.15 | ||||||
| F for change in R2 | 0.66 | 3.03 | ||||||
DV: Alcohol Consumption (Drinks per outing).
Table 5.
Hierarchical regression analysis for variables predicting alcohol consumption in the concussion group (n = 15).
| Variable | Model 1 |
Model 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| B | SE B | β | p | B | SE B | β | p | |
| EDA amplitude | −5.75 | 2.86 | −0.49 | 0.065 | −5.78 | 2.74 | −0.49 | 0.056 |
| Athletic status | −3.13 | 2.12 | −0.34 | 0.167 | ||||
| R2 | 0.24 | 0.35 | ||||||
| F for change in R2 | 4.05 | 2.17 | ||||||
DV: Alcohol Consumption (Drinks per outing).
4. Discussion
The purpose of this research was to examine concussion as a possible risk factor for elevated alcohol consumption in university athletes, as well as to investigate physiological underarousal as a potential mechanism underlying this relationship. Although a number of studies have identified factors that influence heavy drinking in athletes, to the authors' knowledge, this is the first investigation to examine the effects of concussion on drinking behavior, over and above the effects of premorbid athletic status. Results indicated that individuals with a history of concussion report higher levels of alcohol consumption per outing than their non-injured cohort, over and above the effects of athletic status. Further, those with a history of concussion exhibited significantly lower levels of arousal than those without a prior concussion.
Contrary to our first hypothesis, it was demonstrated that athletes and non-athletes did not differ in terms of drinking behaviors, despite previous research indicating that athletes engage in greater substance use (Damm & Murray, 1996; Wetherill & Fromme, 2007). This non-significant finding may be explained in part by the similar rates of concussion observed across non-athletes and athletes. If head injury is a primary factor in drinking behavior, then differences in alcohol consumption would be expected when rates of concussion vary according to athletic status, which was not found to be the case. Nonetheless, it is also possible that due to the small sample size of the current study, there may have been insufficient power to detect differences in drinking behaviour as a function of athletic status.
As expected however, it was found that participants with a history of concussion reported significantly greater alcohol consumption on a per outing basis compared to those with no such history, but this was not found for frequency of alcohol use. This is consistent with previous research showing that individuals with TBI are unlikely to be classified as light or social drinkers (Kolakowsky-Hayner et al., 2002). Indeed, Kolakowsky-Hayner et al. (2002) found that while approximately 50% of individuals with brain injury refrained from using alcohol post-injury, 43% were classified as moderate or heavy drinkers. In another study (Corrigan, Rust, & Lamb-Hart, 1995), it was found that while individuals with a brain injury engage in significantly greater pre-injury alcohol use when compared to the normative population, approximately 20% of those initially classified as ‘light’ drinkers or abstained from alcohol use before injury engaged in heavy drinking behavior after injury.
Further, concussion status predicted drinks per outing over and above athletic status. This finding is consistent with the idea that risk-taking behavior (including elevated alcohol consumption) observed following concussion may be a function of injury and physiological underarousal, rather than exclusively preinjury characteristics (e.g., riskier personalities such as sensation seeking or impulsivity). Indeed, no significant differences in personality factors (including those assessing impulsive tendencies) were observed across the concussion and no-concussion groups in the current study.
Among the somatic symptoms observed following concussion, dysregulated physiological arousal levels are common (e.g., Baker & Good, 2014; van Noordt & Good, 2011); the current study replicated these findings, showing that those with a history of concussion exhibit significantly lower baseline EDA than their non-injured cohort. Moreover, our results provide preliminary evidence of a link between physiological arousal and alcohol consumption. For those with a history of concussion, binge drinking may reflect an attempt to increase arousal levels, as opposed to representing thrill or excitement seeking.
Those with a history of concussion have been found to exhibit dampened arousal in anticipation of decision-making, leading to disadvantageous and risky decisions without the somatic “markers” to bias their behaviour in a socially-acceptable manner (e.g., Damasio, 1994; van Noordt & Good, 2011). Therefore, the increased alcohol consumption after injury may be reflective of a reduced capacity to anticipate outcomes. After injury, individuals may be insufficiently alerted to the potential dangers of a given situation (e.g., the negative consequences of excessive alcohol consumption) and unable to implicitly monitor their environment via physiological cues. Indeed, previous investigations have shown that concussion is associated with increased risk-taking (Buckley & Chapman, 2016) and substance use (O'Jile, Ryan, Parks-Levy, Betz, & Gouvier, 2004), despite the fact that these individuals are capable of accurately assessing the risk level of a situation (O'Jile et al., 2004).
4.1. Limitations
It is important to acknowledge several limitations of the current study. The correlational nature of this research restricts our ability to make causal inferences regarding the relationship between alcohol consumption and concussion. No reports of pre-injury alcohol use were collected and group differences (i.e., such as greater alcohol consumption and lower physiological arousal) may have existed prior to concussion incidents. Longitudinal research designs, establishing pre-injury behaviors prior to assessment, and more thorough access to historical behavioral profiles could assist future investigations.
Another limitation is the use of retrospective self-reports of head injury and alcohol use, self-report is more reliable when supported by corroborating information (Paulhus & Vazire, 2007). Nonetheless, since many head injuries go undocumented (McCrea, 2008; McCrea, Hammeke, Olsen, Leo, & Guskiewicz, 2004), supplementary information is often unavailable. Further, when asked to estimate alcohol consumption, individuals tend to underestimate usage (Stockwell et al., 2004), thus, a better understanding of alcohol consumption in individuals with a head injury could include more precise monitoring of alcohol intake (e.g., proactive reporting, observation, record keeping).
Another consideration would be a larger sample size which would provide greater power for statistical analyses and an opportunity to examine possible mediation effects associated with physiological arousal. It would be interesting to examine whether physiological arousal mediates the relationship between concussion and increased alcohol consumption and to investigate potential moderating effects such as drinking motives.
Furthermore, the current study consisted of university students and did not include individuals with a prior concussion who did not pursue post-secondary education. This population may not reflect the general population in that they may have different coping or adjustment skills that have permitted them to continue to university. Moreover, although university students have been found to engage in greater alcohol use compared to the general population (O'Malley & Johnston, 2002), the current findings of greater consumption in those with a history of concussion compared to their non-injured peers are consistent with non-student populations (Corrigan et al., 1995).
5. Conclusions
This study demonstrated that university students with a history of concussion consume more alcohol per outing than their non-injured cohort and that concussion status is predictive of alcohol use, over and above the effects of athletic status. Importantly, it was also found that those with a history of concussion display significantly lower physiological arousal than their non-injured peers. Moreover, despite previous literature indicating that athletes engage in greater alcohol consumption (Wetherill & Fromme, 2007), athletes and non-athletes did not differ in terms of drinking. Further, physiological underarousal was associated with greater alcohol consumption. Taken together, these findings imply that drinking behaviors are associated with concussion status (i.e., physiological underarousal), over and above athletic status; individuals may consume more alcohol post-injury due to physiological unpreparedness.
Role of funding sources
Bradey Alcock and Caitlyn Gallant held funding from the Canadian Institutes of Health Research (CIHR) Frederik Banting and Charles Best Canada Graduate Scholarship during the preparation of this research, and Dr. Dawn Good is affiliated with the Ontario Brain Injury Association (OBIA) and Ontario Neurotrauma Foundation (ONF). The CIHR, OBIA, and ONF had no role in the study design, collection, analysis, or interpretation of the data, writing of the manuscript or the decision to submit the paper for publication.
Contributors
Bradey Alcock participated in the data acquisition, while all listed authors (Bradey Alcock, Caitlyn Gallant, and Dr. Dawn Good) designed the study, and conducted the statistical analysis and interpretation of the data, the literature review, and the writing of the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of interest
All authors declare that they have no conflicts of interest.
References
- Baker J.M., Good D.E. Physiological emotional under-arousal in individuals with mild head injury. Brain Injury. 2014;28(1):51–65. doi: 10.3109/02699052.2013.857787. (857787) [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Grant S.J. Does traumatic brain injury increase risk for substance abuse? Journal of Neurotrauma. 2009;26(7):1077–1082. doi: 10.1089/neu.2008.0849. (0849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley L., Chapman R.L. Associations between self-reported concussion with later violence injury among Australian early adolescents. Journal of Public Health. 2016;39(1):52–57. doi: 10.1093/pubmed/fdw009. [DOI] [PubMed] [Google Scholar]
- Cassidy J.D., Carroll L., Peloso P., Borg J., Von Holst H., Holm L.…Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating Centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine. 2004;36(0):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Corrigan J.D., Rust E., Lamb-Hart G.L. The nature and extent of substance abuse problems in persons with traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1995;10(3):29–46. [Google Scholar]
- Currie D.W., Fields S.K., Patterson M.J., Comstock R.D. Cheerleading injuries in United States high schools. Pediatrics. 2015;137(1) doi: 10.1542/peds.2015-2447. [DOI] [PubMed] [Google Scholar]
- Damasio A. G.P. Putnam; New York: 1994. Descartes' error: Emotion, reason and the human brain. [Google Scholar]
- Damasio A.R., Tranel D., Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioral Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damm J., Murray P. Alcohol and other drug use among college student-athletes. Counseling College Student-Athletes: Issues and Interventions. 1996;2:185–220. [Google Scholar]
- Fowles D.C., Schneider R.E. Effects of epidermal hydration on skin conductance responses and levels. Biological Psychology. 1974;2(1):67–77. doi: 10.1016/0301-0511(74)90032-5. [DOI] [PubMed] [Google Scholar]
- Gallant C., Barry N., Good D. Physiological arousal in athletes following repeated subconcussive impact exposure. Current Psychology. 2018 (in press) [Google Scholar]
- Gessel L.M., Fields S.K., Collins C.L., Dick R.W., Comstock R.D. Concussions among United States high school and collegiate athletes. Journal of Athletic Training. 2007;42(4):495. [PMC free article] [PubMed] [Google Scholar]
- Good D. Neuropsychology cognitive research lab. Brock University, St. Catharines; Ontario: 2008. Everyday living questionnaire. [Google Scholar]
- Goswami R., Dufort P., Tartaglia M.C., Green R.E., Crawley A., Tator C.H.…Davis K.D. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Structure and Function. 2016;221(4):1911–1925. doi: 10.1007/s00429-015-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G.A., Uryasz F.D., Petr T.A., Bray C.D. NCAA study of substance use and abuse habits of college student-athletes. Clinical Journal of Sport Medicine. 2001;11:51–56. doi: 10.1097/00042752-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Hakulinen C., Elovainio M., Batty G.D., Virtanen M., Kivimäki M., Jokela M. Personality and alcohol consumption: Pooled analysis of 72,949 adults from eight cohort studies. Drug and Alcohol Dependence. 2015;151:110–114. doi: 10.1016/j.drugalcdep.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead M.E., Walter K.D. Sport-related concussion in children and adolescents. Pediatrics. 2010;126(3):597–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- Hartman M.L., Rawson H.E. Differences in and correlates of sensation seeking in male and female athletes and nonathletes. Personality and Individual Differences. 1992;13(7):805–812. [Google Scholar]
- Herring T.E., Zamboanga B.L., Olthuis J.V., Pesigan I.J.A., Martin J.L., McAfee N.W., Martens M.P. Utility of the athlete drinking scale for assessing drinking motives among high school athletes. Addictive Behaviors. 2016;60:18–23. doi: 10.1016/j.addbeh.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Horner M.D., Ferguson P.L., Selassie A.W., Labbate L.A., Kniele K., Corrigan J.D. Patterns of alcohol use 1 year after traumatic brain injury: A population-based, epidemiological study. Journal of the International Neuropsychological Society. 2005;11(3):322–330. doi: 10.1017/S135561770505037X. (10.10170S135561770505037X) [DOI] [PubMed] [Google Scholar]
- Ilie G., Mann R.E., Hamilton H., Adlaf E.M., Boak A., Asbridge M.…Cusimano M.D. Substance use and related harms among adolescents with and without traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2015;30(5):293–301. doi: 10.1097/HTR.0000000000000101. [DOI] [PubMed] [Google Scholar]
- Iverson G.L., Lange R.T. Moderate and severe traumatic brain injury. In: Schoenberg M.R., Scott J.G., editors. The black book of neuropsychology: A syndrome based approach. Springer; New York: 2009. [Google Scholar]
- Kay T., Harrington D.E., Adams R., Anderson T., Berrol S., Cicerone K.…Harley P. Mild traumatic brain injury committee, American congress of rehabilitation medicine, head injury interdisciplinary special interest group. Definition of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1993;8(3):86–87. [Google Scholar]
- Kennedy E., Cohen M., Munafó M. Childhood traumatic brain injury and the associations with risk behavior in adolescence and young adulthood: A systematic review. The Journal of Head Trauma Rehabilitation. 2017 doi: 10.1097/HTR.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowsky-Hayner S.A., Gourley E.V., III, Kreutzer J.S., Marwitz J.H., Meade M.A., Cifu D.X. Post-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Injury. 2002;16(7):583–592. doi: 10.1080/02699050110119475. [DOI] [PubMed] [Google Scholar]
- Koponen S., Taiminen T., Portin R., Himanen L., Isoniemi H., Heinonen H.…Tenovuo O. Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. American Journal of Psychiatry. 2002;159(8):1315–1321. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- Kreutzer J.S., Witol A.D., Marwitz J.H. Alcohol and drug use among young persons with traumatic brain injury. Journal of Learning Disabilities. 1996;29:643–651. doi: 10.1177/002221949602900608. [DOI] [PubMed] [Google Scholar]
- Lee K., Ashton M.C. Psychometric properties of the HEXACO-100. Assessment. 2016:1–15. doi: 10.1177/1073191116659134. [DOI] [PubMed] [Google Scholar]
- Leichliter J.S., Meilman P.W., Presley C.A., Cashin J.R. Alcohol use and related consequences among students with varying levels of involvement in college athletics. Journal of American College Health. 1998;46(6):257–262. doi: 10.1080/07448489809596001. [DOI] [PubMed] [Google Scholar]
- Marcello R.J., Danish S.J., Stolberg A.L. An evaluation of strategies developed to prevent substance abuse among student-athletes. Sport Psychologist. 1989;3:196–211. [Google Scholar]
- Martens M.P., Dams-O'Connor K., Beck N.C. A systematic review of college student-athlete drinking: Prevalence rates, sport-related factors, and interventions. Journal of Substance Abuse Treatment. 2006;31(3):305–316. doi: 10.1016/j.jsat.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Martens M.P., Martin J.L. College athletes' drinking motives and competitive status: Additional examination of the athlete drinking scale. Addiction Research and Theory. 2010;18:23–32. [Google Scholar]
- Mastroleo N.R., Scaglione N., Mallett K.A., Turrisi R. Can personality account for differences in drinking between college athletes and non-athletes? Explaining the role of sensation seeking, risk-taking, and impulsivity. Journal of Drug Education. 2013;43(1):81–95. doi: 10.2190/DE.43.1.f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Tosteson T.D.…Grove M.R. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78(22):1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea M. Oxford University Press; New York: 2008. Mild traumatic brain injury and postconcussion syndrome: The new evidence base for diagnosis and treatment. [Google Scholar]
- McCrea M., Hammeke T., Olsen G., Leo P., Guskiewicz K. Unreported concussion in high school football players: Implications for prevention. Clinical Journal of Sport Medicine. 2004;14(1):13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- McKinlay A., Corrigan J., Horwood L.J., Fergusson D.M. Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood. The Journal of Head Trauma Rehabilitation. 2014;29(6):498–506. doi: 10.1097/HTR.0000000000000001. [DOI] [PubMed] [Google Scholar]
- Morales D., Diaz-Daza O., Hlatky R., Hayman L.A. Brain, contusion. 2007. http://emedicine.medscape.comlartic1e/337782- overview Retrieved from.
- Morales D.M., Marklund N., Lebold D., Thompson H.J., Pitkanen A., Maxwell W.L.…Graham D.I. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience. 2005;136(4):971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Nelson T.F., Wechsler H. Alcohol and college athletes. Medicine & Science in Sports & Exercise. 2001;33(1):43–47. doi: 10.1097/00005768-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Noble J.M., Hesdorffer D.C. Sport-related concussions: A review of epidemiology, challenges in diagnosis, and potential risk factors. Neuropsychology Review. 2013;23(4):273–284. doi: 10.1007/s11065-013-9239-0. [DOI] [PubMed] [Google Scholar]
- van Noordt S., Good D. Mild head injury and sympathetic arousal: Investigating relationships with decision-making and neuropsychological performance in university students. Brain Injury. 2011;25(7–8):707–716. doi: 10.3109/02699052.2011.580312. [DOI] [PubMed] [Google Scholar]
- O'Jile J.R., Ryan L.M., Parks-Levy J., Betz B., Gouvier W.D. Sensation seeking and risk behaviors in young adults with and without a history of head injury. Applied Neuropsychology. 2004;11(2):107–112. doi: 10.1207/s15324826an1102_7. [DOI] [PubMed] [Google Scholar]
- O'Malley P.M., Johnston L.D. Epidemiology of alcohol and other drug use among American college students. Journal of Studies on Alcohol. 2002;14:23–39. doi: 10.15288/jsas.2002.s14.23. [DOI] [PubMed] [Google Scholar]
- Paulhus D.L., Vazire S. The self-report method. Handbook of Research Methods in Personality Psychology. 2007;1:224–239. [Google Scholar]
- Ponsford J., Whelan-Goodinson R., Bahar-Fuchs A. Alcohol and drug use following traumatic brain injury: A prospective study. Brain Injury. 2007;21(13–14):1385–1392. doi: 10.1080/02699050701796960. [DOI] [PubMed] [Google Scholar]
- Schroth M.L. A comparison of sensation seeking among different groups of athletes and nonathletes. Personality and Individual Differences. 1995;18(2):219–222. [Google Scholar]
- Silver J.M., Kramer R., Greenwald S., Weissman M. The association between head injuries and psychiatric disorders: Findings from the New Haven NIMH epidemiologic catchment area study. Brain Injury. 2001;15(11):935–945. doi: 10.1080/02699050110065295. [DOI] [PubMed] [Google Scholar]
- Stockwell T., Donath S., Cooper-Stanbury M., Chikritzhs T., Catalano P., Mateo C. Under-reporting of alcohol consumption in household surveys: A comparison of quantity–frequency, graduated–frequency and recent recall. Addiction. 2004;99(8):1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- Suhr J.A., Gunstad J. “Diagnosis threat”: The effect of negative expectations on cognitive performance in head injury. Journal of Clinical and Experimental Neuropsychology. 2002;24(4):448–457. doi: 10.1076/jcen.24.4.448.1039. [DOI] [PubMed] [Google Scholar]
- Suhr J.A., Gunstad J. Further exploration of the effect of “diagnosis threat” on cognitive performance in individuals with mild head injury. Journal of the International Neuropsychological Society. 2005;11(1):23–29. doi: 10.1017/S1355617705050010. [DOI] [PubMed] [Google Scholar]
- Technologies L. Limestone Technologies, Inc.; Odessa, ON, CA: 2008. Polygraph professional suite. [Google Scholar]
- Verdejo-Garcia A.P.G.M., Pérez-García M., Bechara A. Emotion, decision-making and substance dependence: A somatic-marker model of addiction. Current Neuropharmacology. 2006;4(1):17–31. doi: 10.2174/157015906775203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R.R., Fromme K. Alcohol use, sexual activity, and perceived risk in high school athletes and non-athletes. Journal of Adolescent Health. 2007;41(3):294–301. doi: 10.1016/j.jadohealth.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S.L., Kerr Z.Y., Yengo-Kahn A., Wasserman E., Covassin T., Solomon G.S. Epidemiology of sports-related concussion in NCAA athletes from 2009–2010 to 2013–2014: Incidence, recurrence, and mechanisms. The American Journal of Sports Medicine. 2015;43(11):2654–2662. doi: 10.1177/0363546515599634. [DOI] [PubMed] [Google Scholar]