Abstract
Purpose
Melanocytomas are rare pigmented tumors that arise form melanocytes and have been reported in the central nervous system. Orbital melanocytomas “also known as blue nevus” are rarely reported. The occurrence of choroidal melanoma and orbital melanocytomas has never been described.
Observations
This is a case of orbital melanocytoma in a 34 year old female who presented with left proptosis and ecchymosis. She has the right eye enucleated to treat a large choroidal melanoma, 6 years earlier. Orbital metastasis was suspected. After orbital imaging and systemic evaluation, incisional biopsy was planned yet the mass could be totally excised and it turned out to be melanocytoma. The condition was not associated with nevus of Ota and the patient is not known to have any predisposing condition for melanocytic lesions.
Conclusion and importance
Melanocytoma and malignant melanoma share the same cell of origin. The benign course, the well differentiated cells, absence of anaplasia and the positive reaction to Human Melanoma Black-45 (HMB-45) and S-100 proteins established the diagnosis of the former. Such diagnosis was a relief for this one eyed patient.
(HMB-45:human melanoma black-45).
Keywords: Orbit, Melanocytoma, Choroidal melanoma, HMB-45, S-100
1. Introduction
Diffuse melanocytosis and neurocutaneous melanosis, melanocytoma and malignant melanoma represent a spectrum of lesions that originate from melanocytes.1 (see Table 1)
Table 1.
Summary of the clinical findings, associations, treatment modalities and follow up periods of all the reported isolated orbital melanocytomas in comparison to the currently reported case.
| Case | De Tella1 2003 | Mathai7 2008 | Tsugu6 2009 | Sato8 2009 | Ortiz9 2013 | Tregango3 2014 | Placilli4 2016 | Current case |
|---|---|---|---|---|---|---|---|---|
| Age | 35years | 40years | 51years | 49years | 68years | 28years | 26 years | 34 years |
| Gender | M | M | M | M | M | M | M | F |
| Side | Rt | Rt | Rt | Rt | Lt | Rt | Rt | Lt |
| Primary presentation | Proptosis | Proptosis | Proptosis Diplopia |
Diplopia | Proptosis | Proptosis | Proptosis Diplopia |
Proptosis Ecchymosis |
| Location | Intraconal, around ON | Intraconal Superior |
Intraconal | Intraconal Apex |
Intraconal Apex |
Rxtraconal | Intraconal around ON |
Intaconal Infero-medial |
| Intracranial extension | Yes | Yes | ||||||
| Associations | Recurrence after 17 y of previous removal | Ipsilateral Nevus of Ota | Lt cavernous hemangioma removed 13 y earlier | Rt Choroidal melanoma/enucleation 6 years earlier | ||||
| Treatment | Subtotal resection Radiotherapy Chemotherapy |
Resection | Resection | Subtotal resection Chemotherapy |
Resection | Resection | Resection | Resection |
| Follow up | 5 y | 6 m | 15 m | 12 y | 3 y | 7 m | 3 years | 1 y |
M, male; F, female; y, year; m, month; Rt, right; Lt, left, 1st; first.
Melanocytomas are rare pigmented primary tumors. They are usually discrete, solitary well differentiated and show slow growth yet there is a high probability of recurrence.2,3 Local and systemic dissemination as well as malignant transformation are rarely reported.4
Orbital malignant melanomas as well as melanocytomas have been reported. Primary malignant melanomas constituted less than 1% of orbital tumors5,6 while only 7 cases of orbital melanocytomas were described to date.1,3,4,6, 7, 8, 9
We report the eighth case of orbital melanocytoma and the first case associated with a contralateral previously treated choroidal malignant melanoma.
2. Case report
A thirty four year old female presented in 2016 with left proptosis of 6 months duration and recent ecchymosis and extensive subconjunctival hemorrhage following sudden rise of blood pressure (Fig. 1a). Further clinical evaluation revealed intact ocular motility, best corrected visual acuity (BCVA) was 0.9, normal anterior and posterior segments examination with no signs of optic nerve involvement.
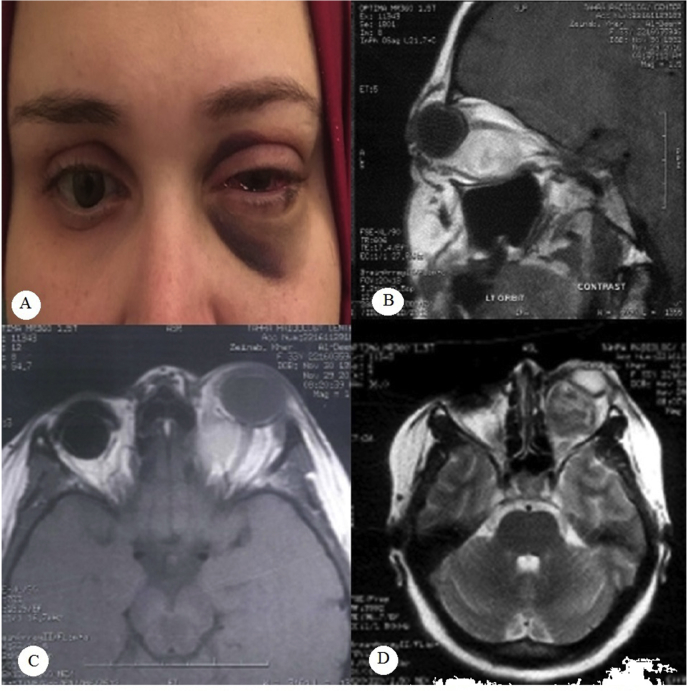
Fig. 1.
Clinical appearance and MRI images of the patient.
a-Clinical appearance at presentation showing Lt proptosis, ecchymosis and subconjunctival hemorrhage. The Rt eye is fitted with ocular prosthesis.
b- T1 weighted sagittal MRI image showing a hyperintense intraconal well circumscribed mass that lies inferiorly and surrounds the optic nerve with no clear line of separation.
c- T1 weighted axial image showing the masd pushing it temporally with no evidence of orbital apex involvement. The Rt orbit is anopthalmic with implant.
d- T2 weighted axial image showing the hypointense appearance of the previously described mass.
(Rt:right,Lt:left,MRI:magnetic resonance imaging).
The right orbit was anophthalmic following enucleation (by Nasr. HE) in 2010 for a large intraocular choroidal mass measuring 22 × 22 × 14 mm. The mass showed acoustic criteria of malignant melanoma with secondary exudative retinal detachment and no extraocular extension. This was further confirmed by histopathological studies and metastatic work up. By that time, examination of the Left eye and orbit was unremarkable. The patient received no adjuvant treatment and remained free from local recurrence and systemic spread for 2 years before she dropped her follow ups.
On her recent presentation, orbital computed tomography (CT) showed a well-defined isodense intraconal lesion measuring 2.6 × 2.5 × 2.4 mm with intrinsic areas of high density. The lesion was located infero-medially between the optic nerve and the inferior rectus pushing the former supero-laterally and the latter inferiorly as well as scalloping the lamina papyracia. The right orbit showed no signs of local recurrence.
Magnetic resonance imaging (MRI) confirmed the mass location and showed it to be hyper-intense in T1-weighted images (Fig. 1b&c) that became hypointense in T2-weighted images (Fig. 1d). General examination and metastatic work-up showed no evidence of metastases.
Incisional biopsy was planned anterior inferomedial transconjunctival orbitotomy with medial rectus muscle disinsertion by (Nasr HE). Intraoperatively, a dark brown round lesion was identified. It was surrounded by a thin capsule of fibrous tissue and had no firm adhesions to the optic nerve or the surrounding structures. Hence, the surgeon was able to deliver it as one intact mass measuring 2.5 × 2.5 × 2.7mm. The patient had an uneventful postoperative course with preserved preoperative vision and ocular motility.
Histopathological examination revealed a heavily pigmented lesion that was composed of polygonal cells sheets with few scattered lymphocytes, blood vessels and extravasated red blood cells (Fig. 2a–f). The cells had obscured cytological details due to heavily packed cytoplasm with melanin pigments yet they did not show anaplastic features (Fig. 2a–f). Immunohistochemistry revealed positive reaction for protein S-100 (Fig. 2a–f) and HMB-45monoclonal antibodies (Fig. 2a–f). Based on the previous findings, the diagnosis of melanocytoma was confirmed. The patient did not receive any adjuvant treatment and she is still tumor free after one year of follow up.
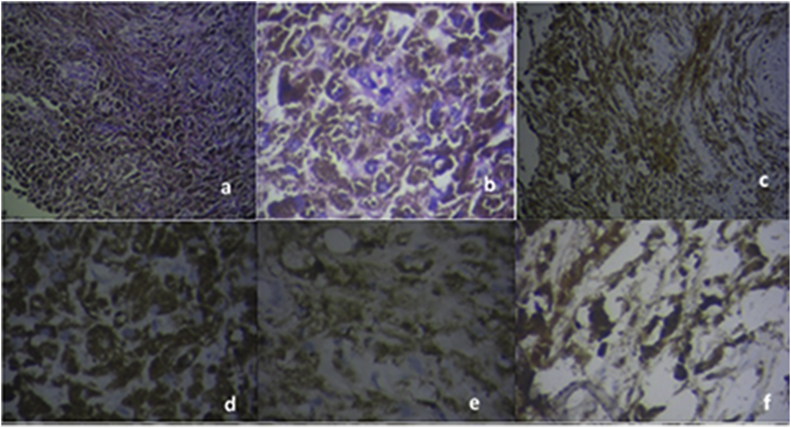
Fig. 2.
Histopathological appearance of the removed tissue.
a- Low magnification (H& E) showing polygonal cell sheets with heavy pigmentation with no anaplasia.
b- High Magnification (H& E ×200) showing obscured cytoplasm details due to heavy pigmentations with no anaplastic features or necrosis.
c–f: Immunohistochemical staining.
c- HMB-45, low magnification.
d- HMB-45, high magnification.
e− S-100, low magnification.
f- S-100, high magnification.
(MRI:magnetic resonance imaging, Rt; right, Lt; left, H&E;hematoxylin and eosin, HMB; human melanoma black).
3. Discussion
Melanocytomas are borderline tumors between cellular blue nevus and spindle melanomas.1,7 They originate from melanocytes that are derived from the neural crest.3 They are usually well-differentiated with a benign nature, therefore, they show no tissue invasion and they present as space occupying lesion with corresponding signs and symptoms according to their site.5,6,10
In literature, around 110 cases of meningeal melanocytomas have been reported with female predilection especially in the 5th decade of life.1,6,7 They were reported in masses related to leptomeninges where melanocytes show high concentration.1,7
Intraocular melanocytomas have been described in all parts of the uveal tract, sclera and conjunctiva yet few reports are available in literature about orbital presentation.4 Our report is the first to describe the occurrence of orbital melanocytoma and malignant melanoma in the same patient.
During the patient's preliminary evaluation metastases was the primary presumption due to the history of enucleation done 6 years earlier to treat large choroidal melanoma. Systemic evaluation revealed no evidence of distant metastasis. We came to the conclusion that the presenting isolated intraconal orbital mass still could be a solitary metastasis or a new lesion.
Malignant melanoma has variable clinical and histological appearance, hence it is difficult to clinically differentiate it from melanocytoma as well as other neuroectodermal tumors with melanin content such as melanotic meningioma, schwannoma and neurofibroma. All of them should be considered in the differential diagnosis.2,7
Metastases from choroidal melanoma to the contralateral orbit are rarely reported.11 Although, orbital metastases have been described following systemic spread from choroidal melanoma,12 there are few reports about orbital secondary lesions to be the first sign of distant spread.11,13,14
Radiological studies can be of use in diagnosing melanin-containing lesions but they are not conclusive. Computed Tomography (CT) scans show melanocytic tumors as well defined isodense to slightly hyperdense lesions with homogeneous contrast-enhancement.8 This appearance is very similar to that of meningioma yet intralesional calcifications or adjacent bone hyperostosis are rarely found with meningeal melanocytomas.3,15
The signal intensity of magnetic resonance imaging (MRI) images depends on melanin amount, fibrous component and presence of intralesional hemorrhage8 The lesion in the reported case was iso to hyperintense on T1-weighted MRI images then became isointense to hypointense on T2-weighted images, a feature that was found in all the reported cases of orbital melanocytoma.3,15 Intralesional hemorrhages are more common to occur in melanomas and this could help differentiating it from melanocytoma.16
To the best of our knowledge, there are 7 described cases of orbital melanocytomas1,3,4,6, 7, 8, 9 and 1 case of meningeal sphenoid melanocytoma that showed extension in the frontal lobe white matter and was associated with two retrobulbar masses. This patient had been treated from ipsilateral nevus of Ota 20 years earlier.17
The data of the reported isolated orbital cases as well as the current case are summarized in (Table). The duration of the symptoms in all the reported cases ranged from 2 months to 4 years. Our patient is the first reported female to have such an isolated orbital mass. She did not suffer from diplopia being one eyed and visual acuity was intact.
The lesion was resected through transconjuntival approach due to its large size and anterior extension. As it was encapsulated with no attachments to the surrounding structures, total resection was feasible instead of the original plan of taking a biopsy. The origin of the tumor could not be detected during the surgery, yet it is still presumed that it originated from the meninges of the optic nerve1,8
Grossly, the removed lesion was dark brown, solid, well-circumscribed mass. All the previous reports had a similar description for melanocytoma apart from one case where it was amelanotic and diagnosis was reached through immunohistochemistry.9
In our case, the tumor cells were uniform, polygonal, arranged in sheets with dense melanin and no anaplasia. These criteria can be crucial in differentiating melanocytoma from malignant melanoma.2,8 Prominent vascular network, calcification as well as cystic spaces filled with fibrinous material were also described in melanocytomas.18
Immunohistochemical positivity for HMB-45 and S-100 indicated that this tumor is derived from melanocytes.1, 2, 3, 4 Vimentin8 and Melan-A9 were also used in other studies.
Our patient did not receive any adjuvant treatment and she is still free of recurrence after one year of follow up. Two out of the seven reported patients, needed adjuvant treatment (radiotherapy alone or with additional chemotherapy) as the lesion was subtotally removed due to intracranial extension.1,8
The follow-up time ranged from 6 months to 12 years and all the reported patients were alive without evidence of disease recurrence.1,3,4,6, 7, 8, 9 However, the patient who received both chemotherapy and radiotherapy presented 2 years after surgery with visual loss and a subfrontal mass that was excised and found to be due to radionecrosis.1
There is no standard protocol for treating orbital melanocytoma due to its rarity. The therapeutic decision is usually based on the available data from treating the meningeal cases. Total resection with or without adjuvant therapy is considered the gold-standard treatment.3 Adjuvant radio and chemotherapy should be considered in treating incompletely resected masses as well as tumors that show aggressive behavior with local invasion.1,7
The prognosis in most cases of meningeal melanocytoma is good and local recurrence can be significantly reduced with total tumor excision.19 Studies showed that 5- year survival rate reached 100% with complete or incomplete resection associated with radiotherapy and it dropped to 46% with incomplete resection only.3,20
Careful follow up for cases diagnosed to have melanocytoma is mandatory as there is a rare incidence of malignant transformation into melanoma as well as reported meningeal dissemination.4,8
4. Conclusions
Meningeal melanocytomas are well-differentiated benign lesions that can be totally cured by surgical resection. Their presence in the orbital area is rarely reported, hence they are considered as a diagnostic and therapeutic challenge to both surgeons and pathologists.
Melanocytoma and malignant melanoma share the same cell of origin. This is the first report of these two lesions to occur in the same patient. The factors that control this remain undefined but metastasis was on the top of the differential diagnosis list. Clinical and systemic evaluation must rule out primary cutaneous, mucosal or ocular melanomas. Radiological data could also be helpful but they are usually not conclusive.
The benign histopathology, immunohiostochemistry and the clinical behavior are the milestones in management. A proper complete evaluation can help preserve the eyeball in cases of orbital involvement.
Complete surgical resection is recommended due to the potential of recurrence and the rare possibility of malignant transformation. Aggressive management is suggested an in case of incomplete excision, recurrences or intermediate-grade tumors.
Patient consent
Written consent for publication of personal identifying information including medical record details and photographs was obtained from the patient.
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures:
Haytham E Nasr.
Mohamed A Nouh.
Rania A Ahmed.
Abdelrahman M. Elhusseiny.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Acknowledgments
None.
References
- 1.De Tella O.I., Jr., Agner C., Aguiar P.H. Aggressive management of orbital meningeal melanocytoma. Acta Neurochir (Wien) 2003;145:1121–1126. doi: 10.1007/s00701-003-0121-3. [DOI] [PubMed] [Google Scholar]
- 2.Brat D.J., Giannini C., Scheithauer B.W., Burger P.C. Primary melanocytic neoplasms of the central nervous system. Am J Surg Pathol. 1999;23:745–754. doi: 10.1097/00000478-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Tregnago A.C., Furlan M.V., Bezerra S.M. Orbital melanocytoma completely resected with conservative surgery in association with ipsilateral nevus of Ota: report of a case and review of the literature. Head Neck. 2015 Apr;37(4):E49–E55. doi: 10.1002/hed.23828. [DOI] [PubMed] [Google Scholar]
- 4.Palicelli A., Distano M.G., Panzarasa G. Orbital meningeal melanocytoma: histological, immunohistochemical and molecular characterization of a case and review of the literature. Pathol Res Pract. 2016 doi: 10.1016/j.prp.2016.07.009. https://doi.org/10.1016/j.prp.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 5.Spencer W.H. fourth ed. vol. 4. 1996. pp. 2458–2459. (Orbit. Ophthalmic Pathology). Philadelphia,Saunders. [Google Scholar]
- 6.Tsugu H., Nabeshima K., Matsumoto S. A case of a heavily pigmented orbital melanocytoma. Brain Tumor Pathol. 2009;26:25–29. doi: 10.1007/s10014-008-0242-8. [DOI] [PubMed] [Google Scholar]
- 7.Mathai A.M., Naik R., Pai M.R. Orbital melanocytoma. Orbit. 2008;27:383–387. doi: 10.1080/01676830802333626. [DOI] [PubMed] [Google Scholar]
- 8.Sato K., Kubota T., Kodera T. Melanocytoma in the orbital apex. J Neurooncol. 2009;92:107–110. doi: 10.1007/s11060-008-9723-1. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz J., Del Carmen S., Goncalves J.M., Carrascal E. Loss of visual acuity on the left eye and ipsilateral exophthalmos in a 68-year-old male patient. Neuropathology. 2014;34:214–216. doi: 10.1111/neup.12079. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien D.F., Crooks D., Mallucci C. Meningeal melanocytoma. Child's Nerv Syst. 2006;22:556–561. doi: 10.1007/s00381-005-0019-x. [DOI] [PubMed] [Google Scholar]
- 11.George S., Cooke C.A., Mc Ginnity G.F. Treated Choroidal melanoma with late metastases to the contralateral orbit. Clin Med Pathol. 2009 Apr 3;2:5–8. doi: 10.4137/cpath.s767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zografos L., Ducrey N., Beati D. Metastatic melanoma in the eye and orbit. Ophthalmology. 2003;110(11):2245–2255. doi: 10.1016/j.ophtha.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Bowling B.S., Damato B.E., Foy P.M. Choroidal melanoma metastatic to the contralateral orbit: implications for patient management. Eye. 1994;8:144–145. doi: 10.1038/eye.1994.29. [DOI] [PubMed] [Google Scholar]
- 14.Shields J.A., Perez N., Shields C.L. Orbital melanoma metastatic from contralateral choroid: management by complete surgical resection. Ophthalmic Surg Laser. 2002;33(5):416–420. [PubMed] [Google Scholar]
- 15.Smith A.B., Horkanyne–Szakaly I., Schroeder J.W., Rushing E.J. From the radiologic pathology archives: mass lesions of the dura: beyond meningioma-radiologic-pathologic correlation. Radiographics. 2014;34:295–312. doi: 10.1148/rg.342130075. [DOI] [PubMed] [Google Scholar]
- 16.Tailor T.D., Gupta D., Dalley R.W. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics. 2013;33:1739–1758. doi: 10.1148/rg.336135502. [DOI] [PubMed] [Google Scholar]
- 17.Hino K., Nagane M., Fujioka Y., Shiokawa Y. Meningeal melanocytoma associated with ipsilateral nevus of Ota presenting as intracerebral hemor- rhage: case report. Neurosurgery. 2005;56 doi: 10.1227/01.neu.0000159716.45457.bc. E1376; discussion E1376. [DOI] [PubMed] [Google Scholar]
- 18.Lo Russo F.J., Boniuk M., Font R.L. Melanocytoma (magnocellular nevus) of the ciliary body: report of 10 cases and review of the literature. Ophthalmology. 2000;107:795–800. doi: 10.1016/s0161-6420(99)00151-7. [DOI] [PubMed] [Google Scholar]
- 19.Rades D., Heidenreich F., Tatagiba M. Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg. 2001;95(2 Suppl):225–231. doi: 10.3171/spi.2001.95.2.0225. [DOI] [PubMed] [Google Scholar]
- 20.Rades D., Schild S.E., Tatagiba M. Therapy of meningeal melanocytomas. Cancer. 2004;100:2442–2447. doi: 10.1002/cncr.20296. [DOI] [PubMed] [Google Scholar]