Highlights
-
•
PANcreatic-DERived factor (PANDER) is a novel hormone regulating glucose levels.
-
•
Fasting PANDER levels were measured in T2D and non-T2D matched subjects from U.S.
-
•
Associations between PANDER and other hormones or metabolic parameters were examined.
-
•
PANDER was associated with increased HbA1c and fasting blood glucose in T2D subjects.
-
•
PANDER was not associated with adiponectin, HOMA-β and HOMA-IR.
Keywords: Fam3B, PANDER, Type 2 diabetes, HbA1c, Glucose
Abstract
Aim
PANcreatic-DERived factor (PANDER, FAM3B) is a novel hormone that regulates glucose levels via interaction with both the endocrine pancreas and liver. Prior studies examining PANDER were primarily conducted in murine models or in vitro but little is known regarding the circulating concentration of PANDER in humans, especially with regard to the association of type 2 diabetes (T2D) or overall glycemic regulation. To address this limitation, we performed a cross-sectional analysis of circulating serum PANDER concentration in association with other hormones that serve as either markers of insulin resistance (insulin and adiponectin) or to metabolic parameters of glycemic control such as fasting HbA1c and blood glucose (FBG).
Methods
Fasting serum was obtained from a commercial biorepository from 300 de-identified adult subjects with 150 T2D and non-T2D adult subjects collected from a population within the United States, respectively, matched on gender, age group and race/ethnicity. Concentration of PANDER, insulin and adiponectin were measured for all samples as determined by commercial ELISA. Metadata was provided for each subject including demography, anthropometry, and cigarette and alcohol use. In addition, fasting blood glucose (FBG) and HbA1c were available on T2D subjects.
Results
Multiple linear regression analyses were performed to examine the relationships between circulating log PANDER concentration on HbA1c, fasting glucose, log insulin, log HOMA-β and log HOMA-IR among T2D subjects and for insulin and adiponectin in non-T2D subjects. A significant linear association was identified between PANDER with fasting HbA1c (β 0.832 ± SE 0.22, p = 0.0003) and FBG (β 20.66 ± SE 7.43, p = 0.006) within T2D subjects. However, insulin, HOMA-β, HOMA-IR and adiponectin (p > 0.05) were not found to be linearly associated with PANDER concentration.
Conclusion
Within T2D subjects, PANDER is modestly linearly associated with increased HbA1c and FBG in a US population. In addition, highest circulating PANDER levels were measured in T2D subjects with HbA1c above 9.9. No association was identified with PANDER and insulin resistance or pancreatic β-cell function in T2D subjects.
Introduction
PANDER (PANcreatic-DERived factor or FAM3B) is a member within the family with sequence similarity 3 (FAM3) super family [1]. This secreted protein is comprised of 235 amino acids and is strongly expressed from the endocrine pancreas [2]. The initial discovery of PANDER was the result of a computational algorithm, ostensible recognition of folds (ORF), in an attempt to identify novel cytokines based on a predicted secondary structure of a four-helix bundle common to many cytokines [1]. From ORF, the four members of this family with sequence similarity 3 were identified, FAM3A-FAM3D, respectively. All members of the FAM3 family have 224–235 amino acids, including four conserved cysteines with a secretion signal sequence. FAM3B was later named PANcreatic-DERived factor (PANDER) in 2003 due to dominant expression in the endocrine pancreas [2]. Localization of PANDER to insulin granules within pancreatic β-cells revealed PANDER is co-secreted with insulin in response to glucose [3].
The PANDER receptor is currently unknown. However, iodinated binding studies revealed the liver is a target tissue [4]. A plethora of publications reporting various biological aspects of PANDER have examined numerous animal models (reviewed in [5], [6], [7]). In summary, these studies indicated a hormonal biological role for PANDER for regulation of glycemic levels. Mechanism of PANDER action is through the regulation of hepatic insulin signaling and insulin secretion from the endocrine pancreas. Increased expression of circulating PANDER induces insulin resistance in murine models [8]. Concordantly, PANDER knockout models have increased hepatic insulin sensitivity and are resistant to high fat diet induced fasting hyperglycemia and hyperinsulinemia [9], [10]. Of clinical importance with regard to T2D is the demonstration that PANDER can induce selective hepatic insulin resistance. This differential signaling results in a metabolic state that mimics what is observed in T2D animal models and humans whereby gluconeogenesis and hepatic lipogenesis are both increased [11], [12]. Taken together, earlier evidence suggests that PANDER may serve as an etiological hormone during pathophysiological conditions driving selective hepatic insulin resistance (SHIR) within T2D [13].
One of the major gaps in understanding PANDER’s biological role and involvement with the pathogenesis of T2D is with regard to the circulating concentration in humans in association with glucose intolerance and the primary clinical measurement of glycemic control, HbA1c. At present, two reports have examined circulating PANDER concentration in association with metabolic syndrome and pancreatic β-cell dysfunction, however none have focused on the relationship of PANDER with glycemic control specifically within T2D subjects [14], [15]. Therefore, this limited clinical and physiological in vivo data presents a major limitation in the biological understanding of PANDER within T2D. To address this, we have performed a cross-sectional analysis of circulating PANDER concentration in the serum of 150 T2D subjects and matched non-T2D subjects in association with diabetes status, insulin and adiponectin in all subjects. Within T2D subjects, we examined the association of PANDER and fasting blood glucose (FBG), HbA1c, HOMA-β (measures of pancreatic β-cell function) and HOMA-IR (measure of insulin resistance).
Materials & methods
Participants and clinical information
Fasting serum samples from 300 (150 T2D and 150 non-T2D) subjects in combination with recorded metadata was obtained from a commercial biobank (BioServe Biotechnologies, Ltd). Comprehensive, de-identified, covariate data such as age, gender, race, anthropometric measures such as body mass index (BMI), and cigarette and alcohol use were provided. Subjects were matched on age, gender and race/ethnicity. For age, 145 were matched to a control subject within ±5 years, and 4 were matched within ±9 years. Due to limited availability of control subjects among minority groups, one T2D subject was matched to a control subject that was 13 years younger. Subject information and biospecimens were collected using IRB approved Consent Forms and Collections Protocols as described by BioServe Biotechnologies (www.bioserve.com). No identifying information was provided with the samples or the corresponding metadata. Study was determined as research exempt since samples were obtained from a de-identified commercial repository. The key between the BioServe ID and the patient HIPAA protected information is currently housed at the clinic of sample origin. Whole blood and serum were collected at time of visit and sent to Quest Diagnostics™ for measurement of HbA1c and fasting blood glucose, respectively. Additional serum aliquots were also obtained and immediately stored upon collection. All of the subjects in the Type 2 diabetes collection were identified through one of the IRB approved endocrinologists using data and sample collection protocols that have undergone IRB review. The eligibility criteria are listed below consisted of the following: (1) Patient has been diagnosed with Type II Diabetes, (2) Patient had a previous fasting glucose >126 mg on two occasions or post-prandial glucose of >200 mg/dl on one prior occasion, Patient is non-pregnant female or male >18 years of age, (3) Patient has sufficient understanding to agree to the study activities, (4) Patient has physician’s permission to participate and (5) Patient must not be related (by first degree) to any other study subjects. Our non-T2D group consisted of healthy subjects coming in for routine well-visits. The non-T2D group was initially screened using case report forms and questioned for presence of disease for three generations (on both sides of the family) for health history including diabetes, cancer, etc. These controls were used as healthy controls for a number of different types of studies based upon the extensive screening by the clinical studies nurses or a clinical research associate, and the case report form questions. Most samples were collected in 2000 or 2001 and stored at −70 °C since moment of collection. Freezers (maintained by Bioserve) were monitored electronically continuously, and checked once a day, manually, 365 days a year. All samples were collected using approved IRB protocols and stored in a CLIA approved laboratory.
PANDER, insulin and adiponectin measurements
Approximately 500 µl to 1 ml of serum was received from each subject from BioServe and stored at −80 °C prior to examination of PANDER, insulin and adiponectin concentration as determined by commercial ELISAs. Following receipt of serum samples, aliquots were compiled to ensure no variation in freeze/thaw exposure. PANDER concentration were examined in 100 µl of serum in duplicate following manufacturer’s instructions using the Human Pancreatic Derived Factor/FAM3B Antibody ELISA Kit (Catalog number E01P0025, Life Sciences Advanced Technologies, Inc.) with a reported sensitivity in this assay of 0.1 ng/mL and range of 0.5 to 20 ng/ml. Insulin levels were measured in 25 µl of serum in duplicate following manufacturer’s instructions using the Ultrasensitive Insulin ELISA Kit (Catalog number 80-INSHUU-E10, ALPCO) with a reported sensitivity of 0.135 µIU/mL and range of 0.15–20 µIU/mL. Adiponectin levels were determined in 5 µl of serum in duplicate following manufacturer’s instructions using the STELLUX® Chemi Human Total Adiponectin ELISA (Catalog number 80-ADPHUT-CH01, ALPCO) with a reported sensitivity of 0.08 ng/mL and range of 0.066–68.09 ng/mL.
Homeostatic model assessment
The homeostatic model assessment (HOMA) was utilized to measure both pancreatic β-cell function (HOMA-β) and insulin resistance (HOMA-IR). HOMA-β was calculated using the following formula: [360*fastinginsulin(μU/mL)/fastingglucose] − 63. HOMA-IR was determined by the following formula: [fasting glucose (mg/dL)∗fasting insulin (μU/mL)]/405. Due to the lack of availability of fasting measures of glucose for non-T2D subjects, HOMA-β and HOMA-IR were calculated only for T2D subjects.
Statistical analysis
Data are presented as means and standard deviations for continuous variables and as the number of subjects and percent for categorical data. Distributions of the PANDER, insulin, adiponectin, HOMA-β, HOMA-IR and hormonal concentrations were found to be skewed and were log transformed for all analyses. For these variables, the data are presented as the geometric mean and interquartile range. Due to the small number of minority subjects, race/ethnicity was categorized as white and other. Cigarette smoking status was defined as current, former, and never. Alcohol use was defined as any self-reported current alcohol use (>0 drinks per week or day). Exploratory and descriptive analyses were performed to examine the distributions of variables and identify bivariate associations using distribution appropriate statistical tests (e.g., t-test, Wilcoxon Rank-Sum, chi-square, etc.).
Separate linear regression models were constructed with HbA1c, FBG, insulin, adiponectin, HOMA-B and HOMA-IR as the dependent variables and log PANDER as the main independent variable of interest. The β and standard error (SE) for log PANDER is presented from unadjusted models and models adjusted for age, gender, race/ethnicity, duration of diabetes, and BMI. Additional covariates that were tested and not retained in the models were cigarette status and alcohol use since inclusion of these in the models did not meaningfully alter the estimate of association between PANDER and any of the dependent variables. As FBG and HbA1c was not available for non-T2D subjects, these analyses were restricted to T2D subjects. Linear regression analyses was also performed for insulin and adiponectin separately in those with and without diabetes. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Study design
The overall characteristics of the subject population are shown in Table 1. The mean age of subjects in this study population was approximately 60 years. The study population had slightly more males than females (52% vs 48%) and was predominantly Caucasian (71.3%). Current cigarette and alcohol use was higher in the non-T2D group (P ≤ 0.002). BMI was higher among those with T2D (32.6 ± 7.4 vs 25.2 ± 6.6, P < 0.001). Mean duration of T2D was 7 years, with a mean HbA1c of 8.3 (±2.2) and a mean FBG of 169.0 (±72.6). No significant differences were identified between the groups for insulin and PANDER. However, adiponectin was significantly lower among those with T2D (16.4 vs. 18.5, P < 0.05) as expected since decreased adiponectin levels are associated with insulin resistance.
Table 1.
Subject Characteristics.
| Characteristic | T2D N = 150 | Non-T2D N = 150 | p-value |
|---|---|---|---|
| Age (years)* | 60.7 ± 12.6 | 60.3 ± 12.5 | |
| Male (n)† | 78 (52.0) | 78 (52.0) | |
| Race/ethnicity (n) † | |||
| White | 107 (71.3) | 107 (71.3) | |
| Other | 43 (28.7) | 43 (28.7) | |
| Cigarettes (n)† | 0.001 | ||
| Current | 17 (12.6) | 44 (30.6) | |
| Former | 46 (34.1) | 36 (25.0) | |
| Never | 72 (53.3) | 64 (44.4) | |
| Alcohol use (n)† | 0.002 | ||
| Yes | 32 (21.5) | 56 (37.6) | |
| No | 117 (78.5) | 93 (62.4) | |
| Duration of diabetes (years)‡ | 7 (3–13) | NA | |
| BMI (kg/m2)* | 32.6 ± 7.4 | 25.2 ± 6.6 | <0.001 |
| HbA1c (%)* | 8.3 ± 2.3 | NA | |
| Fasting blood glucose (mg/dl)* | 169.0 ± 72.6 | NA | |
| PANDER (ng/ml)§ | 0.41 (0.33–0.64) | 0.40 (0.28–0.62) | |
| Insulin (μIU/ml)§ | 12.0 (7.1–17.8) | 10.7 (5.9–18.5) | |
| Adiponectin (ng/ml)§ | 16.4 (11.6–21.8) | 18.5 (14.1–26.7) | 0.048 |
| HOMA-β§ | 48.8 (24.0–85.3) | NA | |
| HOMA-IR§ | 4.6 (2.5–7.0) | NA | |
| Types of T2D treatment | |||
| Su only | 28 (18.7%) | NA | |
| Bi only | 15 (10%) | ||
| TZ only | 10 (6.7%) | ||
| I only | 20 (13.3%) | ||
| Su/Bi/Tz/I Combination | 68 (45.3%) | ||
| No Treatment | 9 (6%) |
*Data presented as mean ± SD, p-value from t-test
†Data presented as number (%), p-value from Chi-square test
‡Data presented as median (25th-75th percentile)
§Data presented as geometric mean (25th-75th percentile), p-value from t-test
Types of T2D treatment- Su – sulfonylurea, Bi – biguanides, TZ – Thiazolidinediones, I – insulin, Su/Bi/TZD/I – combination therapy of 2 or more medications, N – No treatment. Data presented as number (%),
Multiple linear regression models
Table 2 presents the results of the multiple linear regression models of log PANDER on HbA1c, FBG, log insulin, log HOMA-β, log HOMA-IR adjusted for age, gender, race, BMI, and duration of diabetes (unadjusted are also shown). In this model, a significant linear association was identified between PANDER with HbA1c (β 0.75 ± 0.23, P = 0.002) and FBG (β 20.4 ± 7.7, P = 0.010). Log insulin, log HOMA-β, log HOMA-IR, and adiponectin were not found to be linearly associated with PANDER concentration (p > 0.05). In addition, further linear regression analysis was performed evaluating the controls and T2D subjects separately for the adiponectin and insulin regressions. There was no association between adiponectin and PANDER in controls, and the interaction term by diabetes status was not significant (data not shown). However, in the non-T2D subjects, PANDER is significantly negatively associated with insulin (β −0.22 ± 0.11, p = 0.044) and the interaction term by diabetes status reached near significance (p = 0.079).
Table 2.
Linear regression models of log PANDER (independent) on HbA1c, fasting glucose, log insulin, log HOMA-β, log HOMA-IR, and adiponectin (dependent) among T2D subjects.
| Outcome (dependent variable) | Unadjusted |
Adjusted* |
||
|---|---|---|---|---|
| β ±SE | p-value | β ±SE | p-value | |
| HbA1c | 0.74 ± 0.24 | 0.002 | 0.75 ± 0.23 | 0.002 |
| Fasting blood glucose | 17.8 ± 7.7 | 0.023 | 20.4 ± 7.7 | 0.010 |
| Log Insulin | 0.03 ± 0.09 | 0.728 | 0.05 ± 0.09 | 0.564 |
| Log HOMA-β | −0.15 ± 0.11 | 0.190 | −0.15 ± 0.12 | 0.226 |
| Log HOMA-IR | 0.12 ± 0.10 | 0.232 | 0.16 ± 0.10 | 0.127 |
| Adiponectin | 0.02 ± 0.06 | 0.797 | −0.001 ± 0.06 | 0.987 |
Adjusted for age, sex, race, BMI, and duration of diabetes. Models are separate models with HbA1c, fasting blood glucose, log insulin, log HOMA-β, log HOMA-IR, and adiponectin as the dependent variables, respectively, and log PANDER as the independent variable. β and standard error estimates presented are for log PANDER in unadjusted and in adjusted models.
Serum PANDER concentration in association with HbA1c
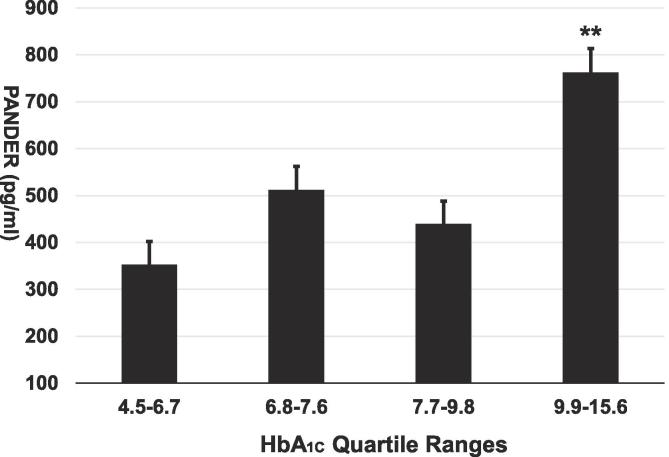
To further examine the associations of PANDER with long term glucose intolerance, least squares adjusted means of PANDER were examined by quartiles of HbA1c within T2D subjects. Increased PANDER concentration were significantly associated with increased HbA1c quartile ranges within the T2D group (1st quartile 352.4 pg/ml vs 4th quartile 762.9 pg /ml, P = 0.0085) (Fig. 1).
Fig. 1.
Circulating PANDER concentration is associated with increased HbA1c. Unadjusted means of PANDER by quartiles of HbA1c (n = 37–38 per quartile) are shown in pg/ml. ** denotes P value < 0.01 as compared to 1st quartile.
Discussion
This study indicates that increased circulating PANDER concentration is associated with elevated FBG and HbA1c among those with T2D. This is one of the largest cross-sectional studies examining the association of PANDER with measures of glycemic control among those with T2D to show with linear regression that PANDER concentration is linearly associated with increased HbA1c and FBG. Limited clinical data regarding circulating PANDER has inhibited the complete understanding of this novel hormone’s involvement in the onset or progression of T2D. In combination with prior PANDER animal model data showing overexpression can promote fasting and fed glucose intolerance [8], [16], our results provide additional evidence for this association. The primary source of the circulating PANDER is assumed to be primarily derived from both pancreatic α and β-cells. PANDER is found in high abundance within the endocrine pancreas with glucose serving as a potent secretagogue [2], [17]. Therefore, higher glucose levels may increase PANDER secretion from the endocrine pancreas resulting in increased circulating PANDER concentration. However, PANDER is also strongly expressed and secreted from the liver with glucose also serving as a potent PANDER secretagogue [16], [18]. Taken together, the origin of the circulating PANDER may be derived from multiple organ sources including both the endocrine pancreas and the liver. Increased circulating glucose concentration, as reflected by a higher HbA1c, may indeed promote increased production and release from multiple tissues such as pancreatic islets and hepatocytes. Other tissues that have been shown to also produce PANDER (i.e. brain, stomach and intestines) are most likely contributing to the increased PANDER secretion modestly observed under high glucose conditions as observed in our study but this may require further characterization.
Our findings are consistent in certain aspects with 2 other studies examining circulating PANDER concentration [14], [15]. Cao et al. published that elevated circulating PANDER levels are associated with metabolic syndrome in a Chinese population of 212 individuals between 40 and 65 years old [14]. In general, PANDER concentration were positively associated with metabolic score, fasting plasma glucose, and 2 h glucose. With regard to negative associations, PANDER concentration were inversely related to HOMA-β and high-density lipoprotein cholesterol. Their study employed logistic regression analysis to demonstrate that circulating PANDER was associated with an increased odds ratio of impaired glucose tolerance or T2D following adjustment of other cofounders. In addition, increased plasma PANDER concentration were associated with dyslipidemia (high triglyceride concentration and low HDL). Our previously published in vivo proteomic examination of the PANDER transgenic model identified that hepatic lipogenesis was the most strongly impacted biological process and strongly supports the potential that increased PANDER concentration may be related to dyslipidemia [19], [20]. Unfortunately, levels of circulating triglycerides or cholesterol were not measured or available for this study.
Shehata et al. examined circulating PANDER concentration to evaluate the association of PANDER with impaired pancreatic β-cell function in T2D [15]. In a cohort of 63 T2D subjects with varied clinical duration, concentration of PANDER, proinsulin (PI), C-peptide and insulin (I) were measured. Serum PANDER concentration was significantly elevated in T2D subjects with longer disease duration. However, this relationship was not identified in our study (Data not shown, Spearman correlation (r) = 0.03 (p = 0.69). Furthermore, PANDER was negatively correlated to HOMA2-β and positively associated with proinsulin, PI/I and PI/C-pep ratios. Comparing our results to those earlier PANDER studies, we have also identified an association with PANDER concentration and measures of glycemic control. Our study failed to identify a significant relationship between PANDER and HOMA-β as reported in the prior study. However, the differences in PANDER associations found in our study versus others could be attributed to variations in geographical collection sites as each investigation was performed either in China, Egypt or U.S, respectively.
Increased PANDER concentration has been demonstrated to promote hepatic insulin resistance in various animal models. However, this relationship in humans has not been sufficiently examined. Adiponectin is considered to be an insulin sensitizer with pleiotropic effects such as suppression of hepatic gluconeogenesis, stimulation of fatty acid oxidation, pancreatic β-cell insulin secretion, and skeletal muscle glucose uptake [21]. Overall, adiponectin is negatively correlated with insulin resistance with increased circulating concentration associated with a lower risk of T2D [22], [23]. The association of adiponectin with insulin sensitivity justified our examination of this hormone in relation to circulating PANDER. Although we observed the expected significant decrease in adiponectin concentration in T2D as compared to non-T2D subjects there was no measured association with PANDER concentration in either T2D or non-T2D subjects. Since only total adiponectin levels were measured in this study this represents a potential limitation. Adiponectin is initially expressed as a monomer of 28–30 kD that is assembled in homooligomers of: low molecular weight trimeric form, medium molecular weight hexameric, and high molecular weight (HMW) [24]. HMW oligomers appear to be the major forms in insulin sensitivity activities of adiponectin [25] and therefore future studies examining the relationship of PANDER and adiponectin should include measurements of the HMW form and lack of this measurement was a limitation of our investigation.
A limitation of our study is the lack of HbA1c and FBG measurements for our non-T2D group. Unfortunately, this prevented further examination of PANDER concentration in association with glycemic control within non-T2D subjects. There was no significant difference in PANDER concentration between those with and without T2D in our study. However, this may be attributed to several reasons. Based on our HbA1c quartile examination, PANDER concentration appears to exhibit a threshold effect where PANDER concentration is not significantly increased until HbA1c is 9.9% or above. Since 50% of our T2D group consisted of having a HbA1c of 7.6 or less, this may have confounded the discrimination of PANDER concentration between the T2D and non-T2D groups. Nonetheless, we still identified a very significant linear relationship between PANDER and HbA1c within T2D subjects. Within non-T2D subjects, an interesting association was observed in that PANDER was negatively associated with insulin levels. This may be explained biologically due to the differences between physiological and pathophysiological conditions between non-T2D and T2D insulin resistance. During normal physiological conditions (non-diabetic), PANDER is mediating insulin action and promoting insulin clearance. Our previous findings from the PANDER knockout model indicated that hepatic insulin clearance is decreased during absence of PANDER [26]. If PANDER is regulating insulin clearance, this may explain why increased PANDER is negatively associated with insulin levels in non-T2D subjects but no association is found during insulin resistant conditions when the action of PANDER may be inhibited.
In conclusion, PANDER is modestly associated with increased HbA1c and FBG within T2D subjects particularly in those with uncontrolled hyperglycemia. Our limited findings did not provide evidence to support that PANDER could serve as a useful biomarker for insulin resistance or pancreatic β-cell function. Nonetheless, our results do provide initial translational data regarding the fasting circulating levels of PANDER in T2D subjects.
Acknowledgments
This study was supported by grant R56-DK105173-01A1 (to B.R.B. and A.C.A.) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. The authors report no conflict of interest.
Contributor Information
Amy C. Alman, Email: aalman@health.usf.edu.
Brant R. Burkhardt, Email: bburkhardt@usf.edu.
References
- 1.Zhu Y., Xu G., Patel A. Cloning, expression, and initial characterization of a novel cytokine-like gene family. Genomics. 2002;80(2):144–150. doi: 10.1006/geno.2002.6816. [DOI] [PubMed] [Google Scholar]
- 2.Cao X., Gao Z., Robert C.E. Pancreatic-derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin-secreting beta-cells. Diabetes. 2003;52(9):2296–2303. doi: 10.2337/diabetes.52.9.2296. [DOI] [PubMed] [Google Scholar]
- 3.Xu W., Gao Z., Wu J., Wolf B.A. Interferon-gamma-induced regulation of the pancreatic derived cytokine FAM3B in islets and insulin-secreting betaTC3 cells. Mol Cell Endocrinol. 2005;240(1–2):74–81. doi: 10.1016/j.mce.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Yang J., Wang C., Li J. PANDER binds to the liver cell membrane and inhibits insulin signaling in HepG2 cells. FEBS Lett. 2009;583(18):3009–3015. doi: 10.1016/j.febslet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Wilson C.G., Robert-Cooperman C.E., Burkhardt B.R. PANcreatic-DERived factor: Novel hormone PANDERing to glucose regulation. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Burkhardt B.R., Guan Y., Yang J. Role of pancreatic-derived factor in type 2 diabetes: evidence from pancreatic beta cells and liver. Nutr Rev. 2012;70(2):100–106. doi: 10.1111/j.1753-4887.2011.00457.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang J.C., Guan Y.F. Family with sequence similarity 3 gene family and nonalcoholic fatty liver disease. J Gastroen Hepatol. 2013;28:105–111. doi: 10.1111/jgh.12033. [DOI] [PubMed] [Google Scholar]
- 8.Robert-Cooperman C.E., Dougan G.C., Moak S.L. PANDER transgenic mice display fasting hyperglycemia and hepatic insulin resistance. J Endocrinol. 2014;220(3):219–231. doi: 10.1530/JOE-13-0338. [DOI] [PubMed] [Google Scholar]
- 9.Robert-Cooperman C.E., Wilson C.G., Burkhardt B.R. PANDER KO mice on high-fat diet are glucose intolerant yet resistant to fasting hyperglycemia and hyperinsulinemia. FEBS Lett. 2011;585(9):1345–1349. doi: 10.1016/j.febslet.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moak S.L., Dougan G.C., MarElia C.B. Enhanced glucose tolerance in pancreatic-derived factor (PANDER) knockout C57BL/6 mice. Dis Models Mech. 2014;7(11):1307–1315. doi: 10.1242/dmm.016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown M.S., Goldstein J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Biddinger S.B., Hernandez-Ono A., Rask-Madsen C. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7(2):125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson C.G., Robert-Cooperman C.E., Burkhardt B.R. PANcreatic-DERived factor: novel hormone PANDERing to glucose regulation. FEBS Lett. 2011;585(14):2137–2143. doi: 10.1016/j.febslet.2011.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao X., Yang C., Lai F. Elevated circulating level of a cytokine, pancreatic-derived factor, is associated with metabolic syndrome components in a Chinese population. J Diabetes Investig. 2016;7(4):581–586. doi: 10.1111/jdi.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shehata M.M., Kamal M.M., El-Hefnawy M.H., El-Mesallamy H.O. Association of serum pancreatic derived factor (PANDER) with beta-cell dysfunction in type 2 diabetes mellitus. J Diabetes Complications. 2017;31(4):748–752. doi: 10.1016/j.jdiacomp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Chi Y., Wang C. Pancreatic-derived factor promotes lipogenesis in the mouse liver: role of the Forkhead box 1 signaling pathway. Hepatology. 2011;53(6):1906–1916. doi: 10.1002/hep.24295. [DOI] [PubMed] [Google Scholar]
- 17.Burkhardt B.R., Yang M.C., Robert C.E. Tissue-specific and glucose-responsive expression of the pancreatic derived factor (PANDER) promoter. Biochim Biophys Acta. 2005;1730(3):215–225. doi: 10.1016/j.bbaexp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Ratliff W.A., Athanason M.G., Chechele A.C. Hepatic nutrient and hormonal regulation of the PANcreatic-DERived factor (PANDER) promoter. Mol Cell Endocrinol. 2015;413:101–112. doi: 10.1016/j.mce.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Athanason M.G., Stevens S.M., Jr., Burkhardt B.R. Hepatic SILAC proteomic data from PANDER transgenic model. Data Brief. 2016;9:159–162. doi: 10.1016/j.dib.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Athanason M.G., Ratliff W.A., Chaput D. Quantitative proteomic profiling reveals hepatic lipogenesis and liver X receptor activation in the PANDER transgenic model. Mol Cell Endocrinol. 2016;436:41–49. doi: 10.1016/j.mce.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brochu-Gaudreau K., Rehfeldt C., Blouin R., Bordignon V., Murphy B.D., Palin M.F. Adiponectin action from head to toe. Endocrine. 2010;37(1):11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- 22.Diez J.J., Iglesias P. The role of the novel adipocyte-derived protein adiponectin in human disease: an update. Mini Rev Med Chem. 2010;10(9):856–869. doi: 10.2174/138955710791608325. [DOI] [PubMed] [Google Scholar]
- 23.Li S., Shin H.J., Ding E.L., van Dam R.M. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki T., Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 25.Calton E.K., Miller V.S., Soares M.J. Factors determining the risk of the metabolic syndrome: is there a central role for adiponectin? Eur J Clin Nutr. 2013;67(5):485–491. doi: 10.1038/ejcn.2013.1. [DOI] [PubMed] [Google Scholar]
- 26.Robert-Cooperman C.E., Carnegie J.R., Wilson C.G. Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function. Diabetes. 2010;59(9):2209–2218. doi: 10.2337/db09-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]