Abstract
Introduction
Several advances have been made in Alzheimer's Disease (AD) modeling, however, there remains a need for a simulator that represents the full scope of disease progression and can be used to study new disease-modifying treatments for early-stage and even prodromal AD.
Methods
We developed AD Archimedes condition-event simulator, a patient-level simulator with a focus on simulating the effects of early interventions through changes in biomarkers of AD. The simulator incorporates interconnected predictive equations derived from longitudinal data sets.
Results
The results of external validations on AD Archimedes condition-event simulator showed that it provides reasonable estimates once compared to literature results on transition to dementia AD, institutionalization, and mortality. A case study comparing a disease-modifying treatment and a symptomatic treatment also showcases the benefits of early treatment.
Discussion
The AD Archimedes condition-event simulator is designed to perform economic evaluation on various interventions through close tracking of disease progression and the related clinical outcomes.
Keywords: Alzheimer's disease (AD), Prodromal AD, Disease-modifying treatments, Simulation, Cost-effectiveness analysis, Biomarkers, Predictive equations
1. Introduction
Quantifying the total value of an intervention requires an understanding of how its effects as measured in a clinical trial will translate to benefits for patients over relevant time horizons (often their remaining lifetimes) in a real-world setting. In many cases, it is necessary to use a mathematical framework—a model or simulation—to extrapolate from trial-reported outcomes to a real-world setting.
Many decision-analytic models have assessed the cost-effectiveness of treatments for Alzheimer's disease (AD) in the last two decades [1], [2], [3], [4], [5], [6]. Among economic models published in the last decade on AD treatment, virtually all of them have focused on symptomatic treatments (particularly acetylcholinesterase inhibitors or memantine) for patients with mild to severe AD. Most previous studies conceptualized the course of the disease in terms of health states defined by levels of disease severity according to categories of cognitive function, dependency level, or based on patient's location of care or need for full-time care [6], [7].
Several advances have been made in AD modeling in the recent years, such as including disease progression measures such as behavior, function, and dependence, modeling of disease progression as a continuous process rather than using discrete health states, and using individual patient simulation techniques [8], [9]. However, there remains a need for a disease simulation approach that integrates all these advances and represents the full scope of disease progression from the evolution of biomarkers of AD to cognitive and functional decline. This need is particularly acute to understand the value of the disease-modifying treatments (DMTs) currently in development for early-stage and even prodromal AD [10].
This article describes the AD Archimedes condition-event simulator (ACE), an individual patient simulation developed to predict the trajectory of cognitive decline in different stages of AD and the impact of treatment on that decline. We discuss the clinical and health economic inputs used in the simulator and show the results of external validations against results reported in the literature along with a case study comparing a DMT and a symptomatic treatment on disease progression.
2. Methods
2.1. AD ACE overview
The AD ACE is a patient-level simulator that captures the pathophysiology and management of AD, with a focus on simulating the effects of disease modification and early intervention on disease progression. The simulator incorporates interconnected predictive equations that have been derived mainly from longitudinal data sets; these equations describe disease progression through the evolution of AD biomarkers and various relevant patient-level scales of cognition, behavior, function, and dependence. The AD ACE also fully considers interrelated clinical, epidemiologic, and economic outcomes. The design of the AD ACE was based on a systematic literature review of AD economic modeling [11], International Society of Pharmacoeconomics and Outcomes Research good modeling practice guidelines [12], and a review of ongoing clinical trials for both symptomatic and DMTs of AD.
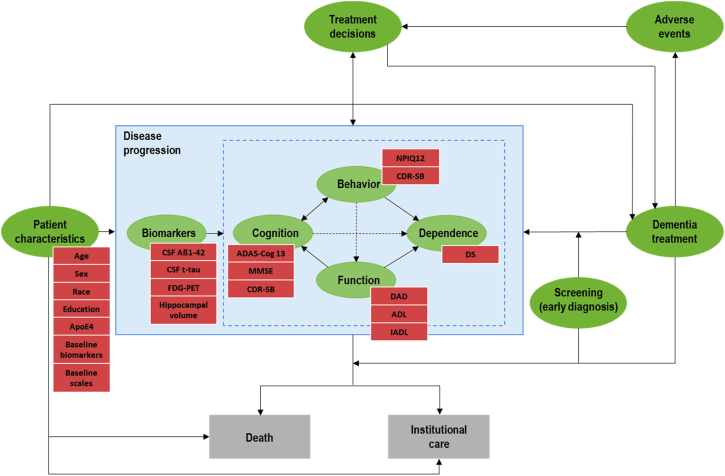
Fig. 1 is an influence diagram outlining the key relationships in this simulator. The hierarchy of biomarkers preceding cognitive and behavioral decline reflects the description from Jack et al. [13] of the cascade of disease progression in AD; however, the relationships between the components were only included where sufficient statistical evidence was present. Similarly, the relationships between cognitive and behavioral decline and subsequent loss of function and independence reflect the modeling approach used in prior economic models of mild to moderate dementia/AD [9], [14].
Fig. 1.
Influence diagram outlining the key relationships in the AD ACE simulator. Abbreviations: ADAS-Cog13, Alzheimer's Disease Assessment Scale-Cognitive Subscale 13; ADL, Activities of Daily Living; APOE ɛ4, Apolipoprotein E4; CDR-SB, Clinical Dementia Rating Sum of Boxes; CSF Aβ1–42, Cerebrospinal Fluid β amyloid; CSF t-tau, Cerebrospinal Fluid total-tau; DAD, Disability Assessment scale for Dementia; DS, Dependence Scale; FDG-PET, Fluorodeoxyglucose–positron emission tomography; IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; NPI-Q12, Neuropsychiatric Inventory Questionnaire 12.
In the AD ACE, prediction of biomarker progression is mainly determined by the patient's characteristics (age, race, sex, education, apolipoprotein E4 [APOE ɛ4] level) and relevant biomarkers. Cognitive, behavioral, functional, and dependence scores are, in turn, predicted based on patient characteristics, biomarkers, and other cognitive, behavioral, and functional scale values. In particular, cognition and behavior influence changes in function and dependence, and function contributes to predicted changes in dependence.
The AD ACE simulator represents the course of AD as a combination of evolving conditions and key events using the discretely integrated condition event simulation framework [14]. In the simulator, different aspects of patient characteristics, disease (e.g., relevant patient-level biomarkers and scales), and treatment are defined as conditions that are tracked throughout a patient simulation. At the start of a patient simulation, an initial value is assigned to each condition. These conditions may remain at their initial values or change over time as the simulation proceeds. Changes in the values of these conditions can affect the occurrence of various events. In the AD ACE, events are defined as instantaneous actions such as death, institutionalization, and treatment start/switch. Multiple events can occur simultaneously. At the start of the simulation, an initial time is assigned to each event along with a table that lists the consequences arising from that event (primarily updating the value of a condition and the time to future events). The simulation processes the events in the order of occurrence and executes the consequences of each event as indicated in each event table.
Disease progression determines a patient's quality of life, risk of institutionalization, societal costs of care, and mortality. The primary outputs of the AD ACE are incremental cost-effectiveness ratios, quality-adjusted life-years, total life-years, and disease management costs. Costs and quality-adjusted life-years are discounted at 3% in keeping with standard practice for U.S. cost-effectiveness modeling [12].
2.2. Disease model
The disease model in the AD ACE is a series of equations to predict the changes in various biomarkers and scales over time under different treatment effects in a broad range of patients, including those with diagnosed disease as well as cognitively normal (CN) patients. The model also captures changes over time based on an individual patient's evolving conditions.
2.2.1. Analytical data set
The data used in the analyses were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) [15]. The ADNI was launched in 2003 as a public-private partnership, led by Michael W. Weiner, MD. The primary goal of the ADNI has been to test whether biological markers and clinical and neuropsychological assessments can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. Details on data sets are included in the supplementary materials.
A total of 1735 patients from the three ADNI phases were included to derive the disease equations for individuals with normal cognition through mild dementia AD. Table 1 presents the baseline characteristics of the study sample. Nearly one-quarter of patients were CN, 20% had AD, 50% had late MCI, and the remainder were classified as significant memory concern/early MCI. The population across all groups was almost evenly split by sex, with an average age of 73.7 years and around 16 years of education before imputation. The majority was white and 75% were married; the percentage married was slightly higher among patients with AD, as was the presence of APOE ɛ4 alleles.
Table 1.
Baseline characteristics of patients in the ADNI population by disease stage
| Overall | AD | SMC/EMCI∗ | LMCI | CN | |
|---|---|---|---|---|---|
| N | 1735 | 340 (20%) | 106 (6%) | 873 (50%) | 416 (24%) |
| Male | 956 (55%) | 188 (55.3%) | 44 (41.5%) | 515 (59.0%) | 209 (50.2%) |
| Age | 73.7 | 75.0 | 72.2 | 72.9 | 74.8 |
| Years of education | 15.9 | 15.2 | 16.7 | 15.9 | 16.3 |
| Race: Caucasian | 1603 (92%) | 315 (92.6%) | 100 (94.3%) | 813 (93.1%) | 375 (90.1%) |
| Married | 1309 (75%) | 284 (83.5%) | 70 (66%) | 672 (77.0%) | 283 (68%) |
| APOE ɛ4 allele | |||||
| 0 copy | 912 (53%) | 113 (33.2%) | 70 (66%) | 430 (49.3%) | 299 (71.9%) |
| 1 copy | 635 (37%) | 158 (46.5%) | 32 (30.2%) | 342 (39.2%) | 103 (24.8%) |
| 2 copies | 171 (10%) | 65 (19.1%) | 1 (0.9%) | 94 (10.8%) | 11 (2.6%) |
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; AD, Alzheimer's disease; APOE ɛ4, apolipoprotein E4; CN, cognitively normal; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; SMC, significant memory concern.
Category only used in the ADNI 2 phase; EMCI was introduced in ADNI GO.
Longitudinal assessments for the following measures were extracted from the ADNI data set for analyses: cerebrospinal fluid (CSF) proteins (β amyloid 1–42; total-tau) linked to abnormal brain deposits; fluorodeoxyglucose–positron emission tomography (a functional imaging biomarker linked to reduced brain cell metabolic activity), and one magnetic resonance imaging measurement of hippocampal volume (structural imaging biomarker linked to brain shrinkage are included); as well as three cognition scales (Mini-Mental State Examination [MMSE], Alzheimer's Disease Assessment Scale–Cognitive Subscale 13 [ADAS-Cog13], Clinical Dementia Rating Scale Sum of Boxes [CDR-SB]) and one behavioral scale (Neuropsychiatric Inventory Questionnaire 12). A pooled data set was created with all measures of interest aligned at each assessment, interpolating to impute observations for missing values between assessments (see supplemental materials for additional information).
2.2.2. Derivation of equations from the ADNI data
The equations modeling change from the previous visit were derived for all biomarkers and cognitive/behavioral measures.
A linear mixed-modeling framework was used [16], allowing a random intercept to account for repeated measurements on patients in the data set. In addition to accounting for correlations within patients, the variance of random intercepts derived in the analyses quantified the degree of between-patient variability beyond what was accounted for by predictors; this was incorporated in the disease simulator to capture all relevant sources of variability.
In addition to patients' baseline characteristics, prior values of other biomarkers and cognitive/behavioral measures, as well as the prior values and rate of change of the measure being modeled were tested as potential predictors. To maintain a plausible causal structure in the equations, biomarkers were considered as potential predictors of each other and for the cognitive measures; similarly, the latter were tested as predictors for one another, but not as predictors for biomarkers. The equations also included the time since previous visit to account for the effect of duration on magnitude of change, but did not include disease status (i.e., AD, significant memory concern, MCI, and CN) to avoid restricting the simulator to use a single preset definition for these. Time since previous visit and APOE ɛ4 allele were forced in all equations as these were expected to be predictive of changes in measures.
Variable selection among other predictors was carried out in a two-step process; the association between potential predictors and outcomes was first assessed in a univariate way (i.e., without other predictors in the model). A multivariable equation was then constructed from the variables deemed statistically significant (at a P-value of .2). The internal validity of the equations was assessed by comparing predictions to observed values for patients in the ADNI data set, stratifying by disease status at entry into the study to assess accuracy within levels.
The accuracy of the equations was also tested in the simulator by attempting to replicate the observed patterns of change in ADNI. At each testing step, the equations were refined as needed to improve accuracy.
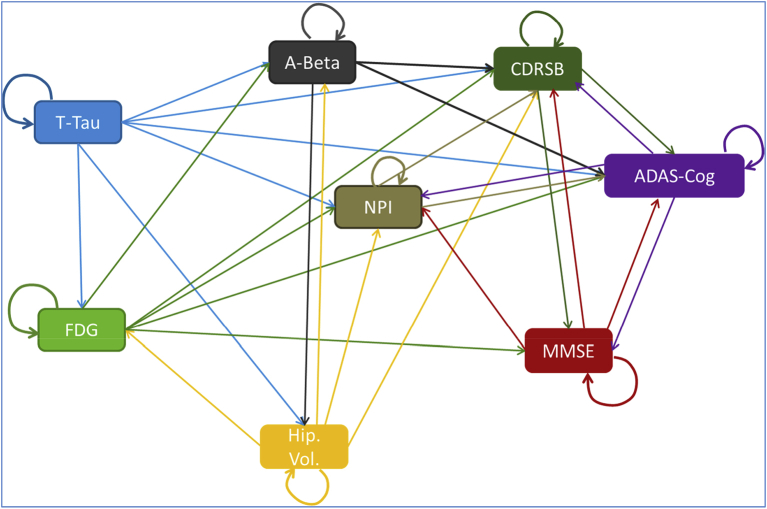
These analyses revealed a closely linked network of associations between biomarkers and cognitive ability over time, as illustrated in Fig. 2. Details of these equations are available in the supplementary materials.
Fig. 2.
Relationships among markers in the disease model from ADNI Data. Abbreviations: A-Beta, amyloid β; ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; CDRSB, Clinical Dementia Rating Sum of Boxes; FDG, fluorodeoxyglucose; Hip. Vol., hippocampal volume; MMSE, Mini-Mental State Examination; NPI, The Neuropsychiatric Inventory Questionnaire; T-Tau, total-tau.
The disease equations do not rely on disease severity levels to predict disease progression in the scales and biomarkers of disease; that is the main advantage of AD ACE compared to previous models. However, AD severity levels were added to the model because they are commonly used as predictors of clinical outcomes in the literature.
2.2.3. Other equations and inputs used in the disease model
Published equations from the Assessment of Health Economics in Alzheimer's Disease II (AHEAD) model are further included in the AD ACE disease model to compute patient's functional and dependence scales and to better capture the more severe stages of dementia AD that ADNI study does not effectively represent [9], [17]. Seven AHEAD equations are built in the disease model. These include two cognition scales (MMSE, ADAS-Cog); one behavioral scale (Neuropsychiatric Inventory [NPI]); three functional scales (Disability Assessment scale for Dementia), (Activities of Daily Living [ADL], Instrumental Activities of Daily Living) and one dependence scale (Dependence scale) (see Table 2).
Table 2.
Model inputs and equations
| Equations | Coefficient and predictor |
|---|---|
| AHEAD disease equations | |
| Annual rate of change in MMSE | 5.47−0.43 PM1 −0.0042 PM2 + 0.14 PM3 − 0.079 PrevRate + 0.075 Age + Int |
| NPI change from baseline | (5.74 + 0.03 Weeks − 0.59 NPIbase − 0.0012 Weeks ∗ NPIbase + 0.24 NPIPrev − 1.74 White − 3.82 Black + 2.34 PsyMed + 0.12 MMSEbase − 0.22MMSErecent + Int) ∗ 1.44 |
| Total DAD score | 50.51 + 2.55MMSErecent − 0.21NPIrecent − 0.53 Age + 7.28 Female |
| Total DS score | 9.26 − 0.076 MMSErecent − 0.073 DADrecent + 0.035 Age + 0.72 Female |
| ADL change from baseline | 1.3473 + 0.06186 Weeks – 0.7923 ADLBase + 0.7128 ADLPrev + 0.1227 MMSEBase + 0.08959 Age + 0.8146 PsychMed – 3.0529 Black – 0.4922 MMSE |
| IADL change from baseline | 1.2749 + 0.1734 Weeks – 0.8433 IADLBase + 0.00153 IADLBase × Weeks + 0.8357 IADLPrev – 0.6701 Male + 0.1957 MMSEBase – 0.2783 MMSE – 0.157 ADLBase + 0.176 ADL |
| Mortality | |
|---|---|
| Guo et al. 2014 | Weibull shape: 1.85 |
| Weibull scale: 4.60 + 0.11 BsAge – 0.0009 BsAge2 + 0.33 Female + 0.023 BsMMSE |
| Institutionalization | |
|---|---|
| Guo et al. 2014 | |
| In institutional care by MMSE (%) | |
| Mild (25–30) | 0.0 |
| Mild-moderate (20–24) | 0.0 |
| Moderate (15–19) | 3.2 |
| Moderate-severe (10–14) | 17.1 |
| Severe (0–9) | 39.3 |
| Disease severity | MMSE thresholds [18] | CDR-SB thresholds [19] | ADAS-Cog13 thresholds [20] |
|---|---|---|---|
| CN | 29–30 | 0–0.5 | 0–4 |
| SMC | 28–29 | 0.5–1 | 4–7 |
| EMCI | 26–28 | 1–2.5 | 7–12 |
| LMCI | 25–26 | 2.5–4.5 | 12–15 |
| Mild AD | 23–25 | 4.5–7 | 15–21 |
| Mild-moderate AD | 20–23 | 7–9.5 | 21–28 |
| Moderate AD | 15–20 | 9.5–13 | 28–43 |
| Moderate-severe AD | 10–15 | 13–16 | 43–56 |
| Severe AD | 0–10 | 16–18 | 56–85 |
| Utilities | |||
| Patients [17] | 0.99-0.041 DS | ||
| Caregiver [17] | 0.84-0.0015 NPI 12 | ||
| Societal costs of care (monthly cost in U.S. dollar) | ||||||
|---|---|---|---|---|---|---|
| Gustavsson et al. 2011 |
Community care (home) |
Residential care (institutionalized) |
||||
| Predictors | Mild∗ | Moderate | Severe | Mild | Moderate | Severe |
| Intercept | 4184 | 4497 | 4159 | 9409 | 8591 | 8881 |
| CurDAD | −34 | −40 | −52 | 0 | 0 | 0 |
| CurMMSE | 22 | 26 | 34 | 0 | 0 | 0 |
| CurNPIQ12 | 24 | 29 | 37 | 164 | 164 | 166 |
Abbreviations: AHEAD, Assessment of Health Economics in Alzheimer's Disease II; ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; DAD, Disability Assessment scale for Dementia; DS, Dependence scale; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; NPIQ, Neuropsychiatric Inventory Questionnaire; ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; CN, cognitively normal; SMC, significant memory concern; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; Cur, current level; Bs, baseline level.
NOTE. PMs represent patients' previous MMSE measurements, partitioned over the scale of MMSE. PrevRate is the patient's last known rate of decline; age represents patient age at baseline; Weeks represents weeks of follow-up in the simulation; Months represents months of follow-up in the simulation; NPIbase is the patient's baseline NPI; NPI is the patient's last NPI. White and Black are dummy variables for race; PsychMed is a dummy variable for patients on psychiatric medications at baseline; MMSEbase represents the patient's MMSE at baseline; and MMSE represents the patient's current MMSE; male and female are dummy variables for gender; Int represents a random intercept parameter.
Mild: MMSE 20–25; moderate: MMSE 10–20; severe: MMSE < 10.
As a patient progresses to more severe stages of dementia AD, the simulator triggers a switch between the ADNI and AHEAD equations for MMSE, ADAS-Cog, and NPI measures based on a disease severity threshold (e.g., MMSE level), enabling simulation of the full spectrum of AD patients in various disease stages. Compatibility and consistency in reported measures were carefully assessed and tested once switching between ADNI and AHEAD equations.
2.2.4. Patient variability in disease equations
As an individual patient simulation using actual patient records at baseline, the AD ACE inherently predicts the effects of patient characteristics on disease progression. There is, however, substantial heterogeneity in potential patient trajectories that cannot be predicted using only the characteristics explicitly included in the AD ACE. The AD ACE equations include terms to capture between- and within-patient variability.
Between-patient variability is incorporated using the random-effect terms from the derivation of the disease equations. Thus, two patients with exactly identical baseline characteristics can potentially experience different courses of AD over time. The random-effect terms for different measures are correlated with one another. This ensures that measures that are related (e.g., different cognition scales) evolve in a consistent fashion within a single simulated patient.
Within-patient variability is incorporated in the disease model using the residuals from the fitting of the ADNI-based equations. These residual terms reflect the differences between the values predicted from the disease progression equations and the observed values for the patients on which the equations were based.
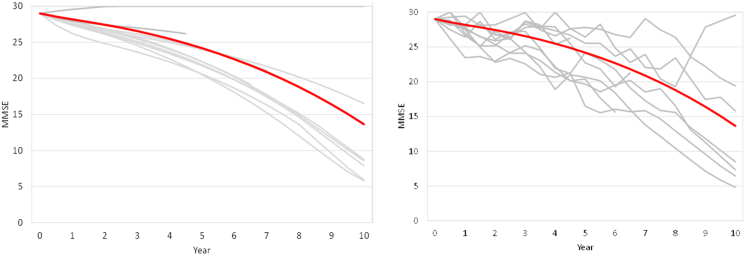
Fig. 3 (left) shows the individual trajectories for 10 simulated patients with the same baseline characteristics when between-patient heterogeneity is incorporated along with the overall mean trajectory (red line). Despite identical baseline characteristics, some of the simulated patients progressed faster and developed dementia AD while some did not progress much. Fig. 3 (right) shows the additional level of variation in simulated patients when within-patient heterogeneity is incorporated along with the between-patient effects. The overall range of outcomes is similar to that shown in Fig. 3 (left), but the paths become much more diverse, including some points where progression is apparently reversed. While these types of changes are apparent in individual patient data, they are both infrequent and balanced by corresponding rapid declines on average, so the average trajectory in a population shows monotonic progression. As such, within-patient variability has no effect where only population average behavior is of interest, such as in cost-effectiveness analyses, but does play a role where individual or small group trajectories are of interest, such as simulation of a clinical trial.
Fig. 3.
Between-patient (left figure) and within-patient (right figure) heterogeneity for predicted MMSE trajectories in an MCI patient. Abbreviations: MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
2.3. Additional clinical inputs
To populate the AD ACE clinical inputs that could not be informed directly by individual patient data from ADNI, targeted and systematic literature searches were conducted to identify relevant data sources. The searches were limited to studies published from 2000 to 2015 to emphasize the most up-to-date data and information available for predictors of location of care, mortality, costs of care, and quality of life. Publications were included based on the relevance of their populations, length of the study, whether their sample sizes were sufficiently large to be representative of the general AD population, and whether the predictors in the study were, or could be, included in the disease model (see Table 2).
Several different cognitive scales are commonly used in research studies and clinical trials in AD, including new composite measures that incorporate components of different cognitive scales. Including separate equations for ADAS-Cog, MMSE, and CDR-SB allows for a range of studies to be used as inputs and for comparisons of results.
2.3.1. Disease severity
The AD ACE disease model predicts disease progression for prodromal and dementia AD without relying on disease severity levels directly. However, in the literature, AD severity levels are commonly used as predictors of location of care, mortality, costs of care, and quality of life. Therefore, the AD ACE assigns disease severity based on each simulated patient's characteristics. In the base case, disease severity levels are defined solely based on cognition, specifically MMSE, following Perneczky et al. 2006 [18]. Alternative definitions based on other measures of cognition (i.e., CDR-SB and ADAS-Cog) or a combination of biomarkers and cognition were also tested and can be selected as alternatives in the model [19], [20].
2.3.2. Location of care
The risk of institutionalization increases as a patient progresses to the more severe stages of AD. In the AD ACE, rate of institutionalization is predicted using both U.S. and U.K. data sources. In Neumann et al. 2001 [21], in which three AD severity levels are defined based on patient's CDR-SB score, the risk of transition from community care to residential care is linked to the time a patient has spent at a particular AD severity level. The risk of institutionalization is further adjusted for each patient by applying a hazard ratio based on patient's current age and sex.
In the alternative approach, results derived from the Dependence in AD in England study are used to assign a probability of institutionalization to each patient based on disease severity level [17]. The user should take into account the differences in the structure of long-term care systems when picking between different approaches.
2.3.3. Mortality
The presence and severity of MCI and dementia AD are associated with reduced survival [22]. Studies with a target population of patients at dementia AD report a relatively higher mortality in patients in relation to the general population. In the AD ACE, patients with normal cognition and significant memory concern are assumed to have mortality reflective of the general population as presented in U.S. life tables [23], [24]. A 1.48 and 2.84 hazard ratio of death is then imposed for patients who have transitioned to MCI or dementia AD, respectively [22]. Where mortality is based on stage of dementia, the simulation uses the stage in the absence of any symptomatic treatment effect. This explicitly assumes that mortality in AD is related to the ongoing biological processes rather than a consequence of the clinical symptoms.
Given the challenge of measuring the rate and predictors of mortality and the sensitivity of many predictions to mortality, the AD ACE includes two alternative approaches to mortality for scenario analyses. The first alternative uses a Weibull parametric equation derived from the analysis of data from the Consortium to Establish a Registry for Alzheimer's Disease study to determine the patient's risk of death. This equation predicts survival using the patient's age, sex, and MMSE score at baseline [17]. The second alternative assigns a probability of death to patients based on a survival equation derived from an AD population by fitting power functions for different age and sex subgroups, without a direct role for disease severity [9].
2.4. Health economics inputs
2.4.1. Utilities
In the literature, the patient utility scores are estimated based on patients' disease severity level, need of full-time care, location of care, or dependency level to perform ADL. Some studies calculated the patient utility scores using a regression model based on patient's cognition, behavior levels, and location of care. The caregiver utility scores are calculated using a regression model based on both patient and caregiver characteristics, and patient's cognition, behavior, and function levels.
In the AD ACE, patient utilities are computed based on a combination of methods aggregated from studies of patients at different disease stages. For CN patients, age-based utilities are used from a widely cited study by Sullivan et al. [25]. For MCI patients, a static utility value is applied from a study by Neumann et al. [26], and for AD patients, a predictive equation is used that estimates patient utility as a function of MMSE, NPI, and location of care, based on a study of the donepezil trials by Getsios et al. [9]. The caregiver (spouse or non-spouse) utilities are computed based on the patient's NPI score, which was the only severity measure found to be significantly associated with this outcome in the analyses of data from Dependence in AD in England study [17]. The AD ACE also includes alternative approaches based on Dependence scale scores for patient utilities [17] and a predictive equation for caregiver utility based on patient's age, sex, MMSE, NPI, ADL, Instrumental Activities of Daily Living, and the use of anti-psychotic medications [9].
2.4.2. Costs
The costs of AD disease management and care costs are mainly determined by disease severity and dependency level to perform ADL, location of care (i.e., noninstitutionalization or institutionalization), and/or the need for full-time care.
In the AD ACE, societal costs of care such as informal care, medical care, and community care are estimated for four countries (United States, United Kingdom, Spain, and Sweden) based on predictive equations extracted from Gustavsson et al. [27] with parameters on cognition (MMSE), functional (Disability Assessment scale for Dementia) and behavioral scales (Neuropsychiatric Inventory Questionnaire 12), and coefficients that are selected based on locale of care (community vs. residential) and patient's current disease severity (defined by the MMSE scale). Care costs are higher for patients in residential care settings than in community dwellings, but there are no significant differences between severity stages for patients in residential care. Disability Assessment scale for Dementia is the most important predictor of costs of care in the community, whereas for patients in residential care, the only disease severity measure with a significant effect on costs of care is Neuropsychiatric Inventory Questionnaire 12.
3. Results
3.1. External validation
The predictions of the AD ACE were verified by comparing simulator results to external data from different patient registries, clinical trials, and literature. After conducting a targeted literature review, we initially identified 22 studies with relevant outcomes on risk of mortality, institutionalization, and transition to dementia AD. Next, we looked at each selected study more carefully based on the following three criteria: (1) the availability of measures of interest, (2) patient populations that were within the scope of populations for which the AD ACE was developed, and (3) reporting of results in a manner that could be compared against AD ACE simulation results. In each validation example, the most appropriate clinical approaches available in AD ACE were picked based on the study country and the patient population, as described by the publication, was simulated by matching the characteristics presented in the article for those characteristics included in the AD ACE. In most cases, this meant matching baseline diagnosis, MMSE score, and age. Any characteristics in the AD ACE not presented in the publication were left unconstrained; that is, they were assumed to match the distributions in the ADNI population. External validations were performed without any model modifications or parameter tuning. An exception was made where patient follow-up differed markedly from that predicted in the simulation due to mortality—in those cases, a second comparison was performed with mortality excluded from the simulation.
3.1.1. Transition to dementia AD
In this validation test, after the study populations were matched and simulated for the study time horizons, the average time to develop dementia AD was compared between two published studies and the results from the AD ACE. Galluzzi et al. [28] studied an MCI population who converted to dementia AD in 1.7 ± 0.8 years on average. A population of similar baseline characteristics was selected from the ADNI population (i.e., prodromal AD, MMSE 25–28, and age 65–80 years) and simulated in the AD ACE. The predicted time to develop dementia AD was very close to the reported value, with a mean of 1.7 ± 1.2 years.
Wilson et al. [29] followed a population of patients with normal cognition at baseline who developed dementia AD at some point during the follow-up (minimum follow-up of 4 years). The study population on average developed dementia AD 7.5 years after initial signs of cognitive decline. Using AD ACE, the mean time to develop dementia AD for those patients who developed dementia AD was reported at 6.1 ± 3.2 years once the simulated patients matched to the study population characteristics (i.e., normal cognition, MMSE 26–30, and age 73–85 years), which is in reasonable agreement with the Wilson et al. results, although the predicted progression was somewhat faster in the simulated population. The simulated population, however, was found to have mortality substantially in excess of that reported by Wilson et al. In the absence of mortality, the simulated time to dementia AD was somewhat longer (7.2 ± 4.2 years), as a larger fraction of the patient population survived to develop dementia AD.
3.1.2. Institutionalization
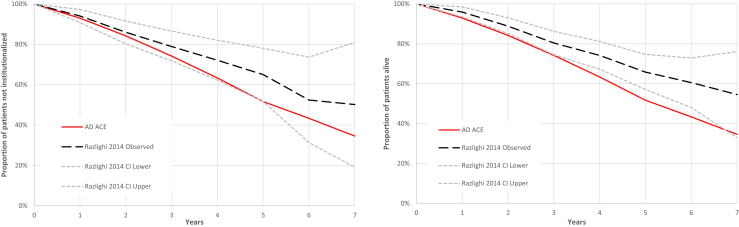
Simulated times to institutionalization were compared to published results in Razlighi et al. [30]. Razlighi et al. tracked a U.S. cohort with mild AD at baseline over a 10-year period and reported the mean rate of institutionalization [30]. A cohort of ADNI patients with dementia AD diagnosis, MMSE score 16 to 30, and age 66 to 83 years was matched and simulated in the AD ACE. The simulated rate of institutionalization proved to stay within the confidence bounds of the study rates over 7 years as shown in Fig. 4.
Fig. 4.
Simulated rates of institutionalization and survival versus Razlighi et al. [30]. Abbreviation: AD ACE, Alzheimer's disease Archimedes condition-event simulator.
3.1.3. Mortality
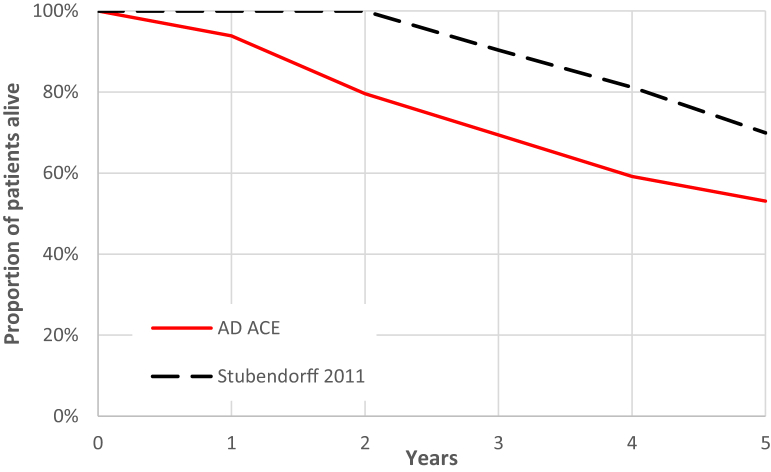
Mortality was validated explicitly against two sources—Stubendorff et al. and Razlighi et al. [30], [31]. Stubendorff et al. followed memory clinic patients with dementia AD (and those with Lewy Body Dementia, but only dementia AD patients were used in the validation) in the Swedish Alzheimer's Treatment Study. A cohort of ADNI patients with dementia AD diagnosis, MMSE 6 to 29, age 70 to 82 years, and cerebrospinal fluid total-tau 180 to 2144 were matched and simulated in the AD ACE. The AD ACE predicted somewhat higher rates of mortality than the publication, primarily in the first 2 years of the comparison, as shown in Fig. 5. The wide MMSE range (6–29) and the fact that the distribution of the AD severity is not given for the study population makes it hard to create a simulation population that well represents the actual study population.
Fig. 5.
Simulated survival versus Stubendorff et al. [31]. Abbreviation: AD ACE, Alzheimer's disease Archimedes condition-event simulator.
The Razlighi et al. [30] study population and simulation settings are described above in section 3.1.2. As in the case of institutionalization, the AD ACE agrees well with the publication, although with a slight trend to more rapid mortality (see Fig. 4).
3.2. Structural sensitivity analyses
The AD ACE includes several options for varying model structure. The disease progression model allows the simulator to switch between the ADNI and AHEAD equations at a fixed threshold of disease severity. In addition, multiple options from the literature for predicting mortality and location of care are implemented and may be selected in the AD ACE. In this section, the results of structural sensitivity analyses on selection of disease equations and clinical inputs are separately discussed.
3.2.1. Disease equation selection
As patients progress to more severe stages of dementia AD that the ADNI study does not effectively represent, the AD ACE switches to AHEAD equations for cognition and behavioral scales to make the model more representative and accurate across all stages of AD.
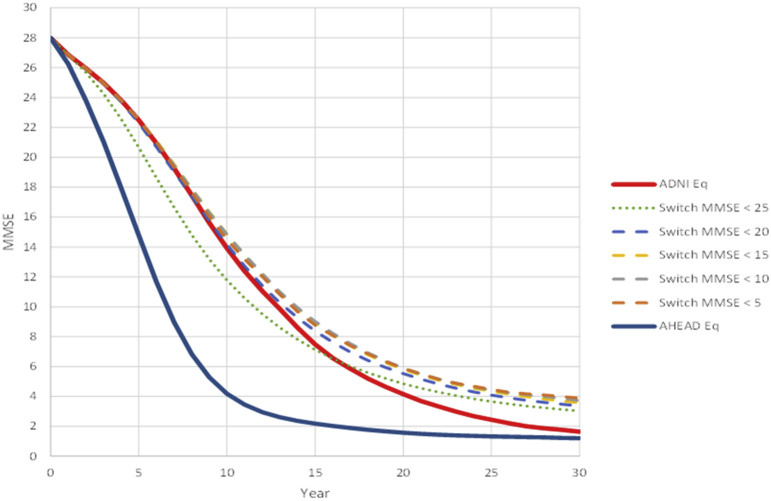
Fig. 6 shows the impact of switching from ADNI-based equations to those in the AHEAD model at various MMSE thresholds on predicted trajectories in the AD ACE. Fig. 6 indicates that overall, a slower decline in MMSE is achieved by switching between the ADNI and AHEAD data, as compared to following the trajectories of each cohort separately. This is mainly due to slower progression in ADNI-based equations compared to when extrapolating from AHEAD to early stages of disease and vice versa when extrapolating from ADNI to the late stages. Importantly, however, this figure shows that, for MCI patients over a range of MMSE score thresholds from 5 to 20 (very severe AD to mild AD; dashed lines in figure), there is little impact on simulated cognitive decline of which equations were used in this range.
Fig. 6.
Simulated trajectories of MMSE for varying combinations of ADNI and AHEAD equations over a range of MMSE score thresholds. Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; AHEAD, Assessment of Health Economics in Alzheimer's Disease II; MMSE, Mini-Mental State Examination.
The ADNI and AHEAD equations were derived independently from completely separate data sets, but the results of this sensitivity analysis shows that they offer similar predictions of disease progression rates over the range in which they overlap (i.e., mild to moderate AD). This sensitivity analysis shows that the ADNI and AHEAD equations will be used where they are strongest, once a switch at a fixed threshold of disease severity is enforced for patients in their mild to moderate AD.
3.2.2. Predictors of mortality and location of care
The interaction between predictors of mortality and location of care may have substantial effect on the predicted economic outcomes of a simulation. A structural sensitivity analysis was performed to evaluate the impact of using different published analyses of mortality and location of care in a simulation of AD.
The alternative location of care and mortality approaches proposed in sections 2.3.2 and 2.3.3 were examined separately in this sensitivity analyses and compared to the results of the AD ACE with default selections. For these runs, all ADNI patients were simulated for a 10-year time horizon. Both alternative mortality approaches resulted in slightly longer survival (5.87 and 6.14 years) than did the default approach (5.57 years). The alternative approach for location of care also reduced the fraction of patients who were institutionalized from 15% to 10%. These results indicate how structural sensitivity analyses are essential for understanding the uncertainty in a simulation of AD and the clinical outcomes.
3.3. Case study
A case study is provided in this section to compare the potential economic and clinical impacts of an early DMT versus a symptomatic treatment from a U.S. societal perspective. A symptomatic treatment only improves the symptoms of AD, whereas a DMT alters the evolution of the disease by affecting the AD biomarkers.
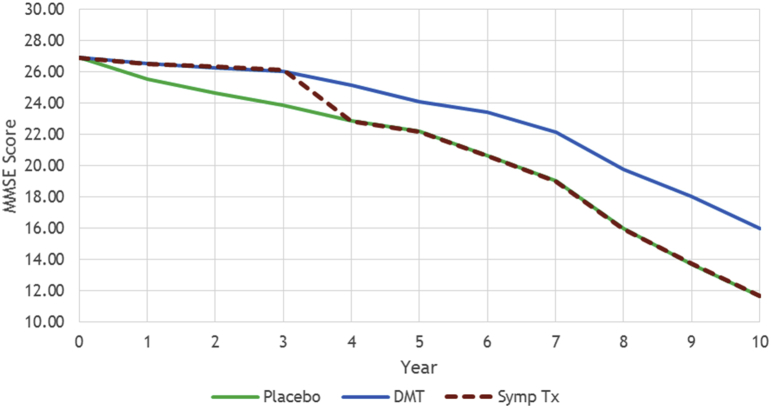
In this case study, the disease progression of 500 late MCI patients from ADNI population was studied under two hypothetical treatments over a 10-year time horizon. Five hundred simulated patients were used as this is sufficient for convergence of the simulation results in comparisons between treatments. For both treatments, similar effects were assumed on all AD scales (i.e., cognition, behavior, function, and dependence) for 3 years. In particular, the average MMSE score of patients on the two treatment arms was assumed to improve around two points compared to the no treatment arm over 3 years (see Fig. 7). The desired effects for the DMT were achieved by imposing early treatment effects on the biomarkers of AD such as fluorodeoxyglucose–positron emission tomography and amyloid markers.
Fig. 7.
MMSE trajectories for DMT, symptomatic treatment, and placebo. Abbreviations: DMT, disease-modifying treatment; MMSE, Mini-Mental State Examination; Symp Tx, Symptomatic treatment.
After 3 years, for the symptomatic treatment, all effects were gradually flattened in 1 year and the AD scale trajectories were reverted to the natural history trajectories that they would have followed in the absence of any treatment. For the DMT, on the other hand, we assumed the patients will follow their natural history after the treatment shows no additional effect. Fig. 7 illustrates the MMSE trajectories for no treatment arm (green line for placebo) and the two hypothetical treatment arms (dashed red line for symptomatic treatment and blue line for DMT).
Table 3 shows the results of the case study for the no treatment, symptomatic treatment, and DMT arms over 10 years. The slower rate of disease progression in the DMT patients resulted in 0.28 additional life years and quality-adjusted life-years compared to placebo. The DMT also reduced the fraction of patients who developed dementia AD, institutionalized, and died by 11%, 8%, and 2% accordingly and delayed the average time to develop dementia AD by almost 2 years. A DMT impacts institutional care and mortality by delaying disease progression to more severe stages of AD, where the risk of death and institutionalization is higher. The cost of care also reduced for DMT patients despite longer life years due to slower progression of disease and lower rate of institutionalization.
Table 3.
Case study results for no treatment, symptomatic treatment, and DMT arms
| Model outputs | No treatment (placebo) | Symptomatic treatment | DMT |
|---|---|---|---|
| Patient LYs | 5.29 | 5.29 | 5.57 |
| Patient QALYs | 3.77 | 3.80 | 4.05 |
| Cost of care | $88,772 | $86,581 | $82,462 |
| Average time to develop dementia AD (years)∗ | 2.64 | 3.72 | 4.62 |
| Average time in institutional care (years)∗ | 1.84 | 2.03 | 1.63 |
| Percentage of patients died | 72 | 72 | 70 |
| Percentage of patients developed dementia AD | 70 | 60 | 59 |
| Percentage of patients institutionalized | 15 | 13 | 7 |
Abbreviations: LYs, life-years; QALYs, quality-adjusted life-years; AD, Alzheimer's disease; DMT, disease-modifying treatment.
The average time in institutional care and to develop dementia AD is only computed for patients who were institutionalized or developed dementia AD accordingly.
The symptomatic treatment showed no improvement in total life-years and mortality as it has no effect on the underlying AD biomarkers. A lower number of patients are also diagnosed with dementia AD under symptomatic arm because some patients died and did not exhibit AD symptoms during the 3-year period that the symptoms were temporarily improved. The average total years spent in institutional care for symptomatic patients were slightly improved compared to the placebo arm, whereas this improvement was much more significant for the DMT arm. The results of this case study clearly support the benefits of early treatment for AD patients.
4. Limitations
As with any model, there are important limitations that must be noted when interpreting the results of this model. Most importantly, the disease progression equations in the AD ACE were derived primarily from ADNI, a noninterventional observational study. As such, the relationships between biomarkers of disease and clinical outcomes are based on correlations in the data. The true causal relationships will need to be elucidated from randomized, interventional studies.
While there is a rich body of studies describing the burden of illness associated with AD, there are comparatively few published studies that describe how the burden, in terms of institutionalization care, mortality, cost, and quality of life, varies with disease progression; those that are available typically include relatively broad categories of disease stage (e.g., mild, moderate, and severe AD). Thus, while disease progression is reflected continuously in the model, costs, risk of mortality, institutional care, and quality of life may change discretely when crossing key thresholds from the source studies. In some cases, the clinical sources used in the model were based on studies from multiple countries despite the important differences in the structure of their care systems or on studies conducted years prior. The structure of the model was designed to accommodate new studies as they become available; however, for the continued relevance of the model, it is essential that those studies are incorporated as they are published and that local studies are selected where possible.
Some of the clinical outcomes in the AD ACE, such as risk of institutional care, are merely linked to cognitive pathways. As a result, the model is not able to capture the implications of symptomatic interventions that only intervene on noncognitive pathways like behavior or function on such outcomes.
While the model includes three measures of cognition to capture a relatively wide range of potential input data and applications, different versions of each scale (e.g., Alzheimer's Disease Assessment Scale–Cognitive Subscale 11 and Alzheimer's Disease Assessment Scale–Cognitive Subscale 13) were used in some of the clinical sources incorporated in the model. In such instances, a conversion factor is used between the full and brief instrument scores in the model, which may not capture all of the differences between the versions of each scale. Further, the AD ACE currently only assigns risk of institutional placement to patients with AD and assumes that those patients without AD do not enter nursing homes and have a caregiver (spouse or non-spouse) until they move to institutional care.
Different scales and cutoffs were used to define severity in the source studies for some clinical inputs used in the model (e.g., mortality, location of care, utilities, and costs). Therefore, severity levels specific to different clinical approaches are defined and tracked independently in the model.
5. Discussion
Despite the significant societal burden of AD and the challenge of developing effective treatments for this disease, patient access to new treatments will depend on quantifying the ability of those treatments to alleviate the clinical and economic burden of the disease [10]. Because much of that burden is concentrated in the advanced stages of disease, valuing treatments will require evidence to be extrapolated from clinical trials to patients' remaining lives. However, predicting the long-term effects of treatment, particularly one initiated at the earliest stages of disease, is complicated by the absence of a scientific consensus on the causes and predictors of disease progression.
The model described here was designed to facilitate the abovementioned quantification, while recognizing the uncertainties inherent in the process. In particular, when relying on a noninterventional study to represent disease progression, a model embeds the assumption that modifying the components of the disease pathophysiology that are predictive of future disease progression in natural history is causative and that their causal role is reversible. The model presented here only considers those elements of disease pathophysiology that are hypothesized to be part of the cascade of AD progression [13] to minimize the risk of incorporating spurious correlations in the disease equations. However, the reliance on natural history is a key potential limitation of the model that can only be evaluated as findings from DMTs are reported.
Along with the need for research on the causal relationships in the pathophysiology of AD, there remain substantial uncertainties regarding the clinical progression of the disease in terms of how cognitive, behavioral, and functional declines translate to the economic and social burdens of AD. A specific strength of the model developed here is the decoupling of disease progression modeling from disease staging and in turn from the economic implications. Because of this, the range of published literature on the translation of clinical disease progression to diagnosed stage and economic outcomes can be evaluated in the model using structural sensitivity analyses, as recommended by the International Society of Pharmacoeconomics and Outcomes Research good modeling practice guidelines [12].
Supporting the value of future treatments for AD will require a model that predicts the long-term clinical and economic outcomes of AD and the effects of treatments [10], [11]. While ongoing testing and advancement will be required as research toward an effective DMT for AD continues to be reported, the model presented here integrates currently available data and literature so those predictions can be made using current evidence.
Research in Context.
-
1.
Systematic review: While economic and decision models of AD have advanced in recent years, there remains a need for a comprehensive model that covers the full course of the disease, particularly the earliest stages of decline.
-
2.
Interpretation: The model described here was developed to reflect progression from normal cognition to advanced AD across multiple pathophysiological and clinical facets of the disease. This permits modeling of treatments ranging from very early disease modification to symptomatic treatments in advanced disease.
-
3.
Future directions: Research in the progression and economic impacts is a large and active field and the model will need to be tested and updated to incorporate new findings from that research. In particular, the model presented here relies heavily on extrapolation of natural history data.
Acknowledgments
The authors wish to thank Rodrigo Dos Santos, Yogi Kulkarni, Asli Ozen, and Luis Hernandez for their contributions toward the development of the model, as well as Janet Dooley for her assistance with the preparation of this article.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; MesoScale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Evidera funded the developed model described in this article.
Footnotes
The authors have no conflicts of interest to declare.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.trci.2018.01.001
Supplementary Data
References
- 1.Cohen J.T., Neumann P.J. Decision analytic models for Alzheimer's disease: state of the art and future directions. Alzheimers Dement. 2008;4:212–222. doi: 10.1016/j.jalz.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Green C. Modelling disease progression in Alzheimer's disease: a review of modelling methods used for cost-effectiveness analysis. Pharmacoeconomics. 2007;25:735–750. doi: 10.2165/00019053-200725090-00003. [DOI] [PubMed] [Google Scholar]
- 3.Green C., Shearer J., Ritchie C.W., Zajicek J.P. Model-based economic evaluation in Alzheimer's disease: a review of the methods available to model Alzheimer's disease progression. Value Health. 2011;14:621–630. doi: 10.1016/j.jval.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Loveman E., Green C., Kirby J., Takeda A., Picot J., Payne E. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer's disease. Health Technol Assess. 2006;10 doi: 10.3310/hta10010. [DOI] [PubMed] [Google Scholar]
- 5.Oremus M. Systematic review of economic evaluations of Alzheimer's disease medications. Expert Rev Pharmacoecon Outcomes Res. 2008;8:273–289. doi: 10.1586/14737167.8.3.273. [DOI] [PubMed] [Google Scholar]
- 6.Pouryamout L., Dams J., Wasem J., Dodel R., Neumann A. Economic evaluation of treatment options in patients with Alzheimer's disease: a systematic review of cost-effectiveness analyses. Drugs. 2012;72:789–802. doi: 10.2165/11631830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Bond M., Rogers G., Peters J., Anderson R., Hoyle M., Miners A. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess. 2012;16:1–470. doi: 10.3310/hta16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caro J.J., Getsios D., Migliaccio-Walle K., Raggio G., Ward A., Group A.S. Assessment of health economics in Alzheimer's disease (AHEAD) based on need for full-time care. Neurology. 2001;57:964–971. doi: 10.1212/wnl.57.6.964. [DOI] [PubMed] [Google Scholar]
- 9.Getsios D., Blume S., Ishak K.J., Maclaine G.D. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer's disease: a UK evaluation using discrete-event simulation. Pharmacoeconomics. 2010;28:411–427. doi: 10.2165/11531870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson A., Green C., Jones R.W., Forstl H., Simsek D., de Reydet de Vulpillieres F. Current issues and future research priorities for health economic modelling across the full continuum of Alzheimer's disease. Alzheimers Dement. 2017;13:312–321. doi: 10.1016/j.jalz.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez L., Ozen A., DosSantos R., Getsios D. Systematic review of model-based economic evaluations of treatments for Alzheimer's disease. Pharmacoeconomics. 2016;34:681–707. doi: 10.1007/s40273-016-0392-1. [DOI] [PubMed] [Google Scholar]
- 12.Caro J.J., Briggs A.H., Siebert U., Kuntz K.M., Force I-SMGRPT Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Value Health. 2012;15:796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Jack C.R., Jr., Petersen R.C., Xu Y., O'Brien P.C., Smith G.E., Ivnik R.J. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caro J.J. Discretely Integrated Condition Event (DICE) Simulation for Pharmacoeconomics. Pharmacoeconomics. 2016;34:665–672. doi: 10.1007/s40273-016-0394-z. [DOI] [PubMed] [Google Scholar]
- 15.Alzheimer’s Disease Neuroimaging Initiative (ADNI). ADNI Dataset, 2015. Alzheimer’s Disease Neuroimaging Initiative; 2014.
- 16.McCulloch C.E., Neuhaus J.M. John Wiley & Sons, Ltd; Hoboken, New Jersey: 2001. Generalized linear mixed models. [Google Scholar]
- 17.Guo S., Getsios D., Revankar N., Xu P., Thompson G., Bobula J. Evaluating disease-modifying agents: a simulation framework for Alzheimer's disease. Pharmacoeconomics. 2014;32:1129–1139. doi: 10.1007/s40273-014-0203-5. [DOI] [PubMed] [Google Scholar]
- 18.Perneczky R., Wagenpfeil S., Komossa K., Grimmer T., Diehl J., Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14:139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 19.O’Bryant S.E., Lacritz L.H., Hall J., Waring S.C., Chan W., Khodr Z.G. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the National Alzheimer’s Coordinating Center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S., Hernandez L., Wasiak R., Gaudig M. Modelling the clinical and economic implications of galantamine in the treatment of mild-to-moderate Alzheimer's disease in Germany. J Med Econ. 2010;13:641–654. doi: 10.3111/13696998.2010.528101. [DOI] [PubMed] [Google Scholar]
- 21.Neumann P.J., Araki S.S., Arcelus A., Longo A., Papadopoulos G., Kosik K.S. Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology. 2001;57:957–964. doi: 10.1212/wnl.57.6.957. [DOI] [PubMed] [Google Scholar]
- 22.Wilson R.S., Aggarwal N.T., Barnes L.L., Bienias J.L., Mendes de Leon C.F., Evans D.A. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66:767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), National Vital Statistics System . Centers for Disease Control and Prevention; Atlanta, GA: 2006. Worktable 210F. Deaths from 113 selected causes, alcohol-induced causes, drug-induced causes, and injury by firearms, by 5-year age groups, race and sex: United States. [Google Scholar]
- 24.Social Security Administration. Social Security Actuarial Life Table. Period Life Table, 2011. Social Security Administration; 2014. Available at: https://www.ssa.gov/oact/STATS/table4c6.html.
- 25.Sullivan P.W., Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann P.J., Sandberg E.A., Araki S.S., Kuntz K.M., Feeny D., Weinstein M.C. A comparison of HU12 and HU13 utility scores in Alzheimer's disease. Med Decis Making. 2000;20:413–422. doi: 10.1177/0272989X0002000405. [DOI] [PubMed] [Google Scholar]
- 27.Gustavsson A., Brinck P., Bergvall N., Kolasa K., Wimo A., Winblad B. Predictors of costs of care in Alzheimer's disease: a multinational sample of 1222 patients. Alzheimers Dement. 2011;7:318–327. doi: 10.1016/j.jalz.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Galluzzi S., Geroldi C., Ghidoni R., Paghera B., Amicucci G., Bonetti M. The new Alzheimer's criteria in a naturalistic series of patients with mild cognitive impairment. J Neurol. 2010;257:2004–2014. doi: 10.1007/s00415-010-5650-0. [DOI] [PubMed] [Google Scholar]
- 29.Wilson R.S., Segawa E., Boyle P.A., Anagnos S.E., Hizel L.P., Bennett D.A. The natural history of cognitive decline in Alzheimer's disease. Psychol Aging. 2012;27:1008–1017. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razlighi Q.R., Stallard E., Brandt J., Blacker D., Albert M., Scarmeas N. A new algorithm for predicting time to disease endpoints in Alzheimer's disease patients. J Alzheimers Dis. 2014;38:661–668. doi: 10.3233/JAD-131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stubendorff K., Hansson O., Minthon L., Londos E. Differences in survival between patients with dementia with Lewy bodies and patients with Alzheimer's disease–measured from a fixed cognitive level. Dement Geriatr Cogn Disord. 2011;32:408–416. doi: 10.1159/000335364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.