Abstract
Aim of the study
presentation of the uncommon paraneoplastic syndromes related to the gastrointestinal tract that may occur in children with neuroblastic tumors and their impact on the disease course.
Material and methods
Retrospective analysis of three cases of patients mainly with digestive tract-related symptoms, who were originally admitted to the gastroenterology department from 2013 to 2016 and were finally diagnosed with neuroblastic tumors.
Results
The clinical data analysis showed that the symptoms from gastrointestinal tract were dominant in analyzed subjects. The first case is a girl with weight loss, bloating and severe diarrhea, admitted to the hospital in a state of dehydration. The laboratory tests revealed severe hypokalemia. Finally, vasoactive intestinal peptide (VIP) secreting ganglioneuroblastoma was diagnosed and effective surgery was performed. The second case was also a girl who suffered from the incidents of watery diarrhea and flatulence. The tumor was detected by computerized tomography scan. The 3rd stage of ganglioneuroblastoma was diagnosed. The patient required chemotherapy, radiotherapy and surgery treatment. The third child was a boy, hospitalized due to abdominal pain, constipation and weakness. During the diagnostic process, the 4th stage of neuroblastoma was recognized. The chemotherapy, surgery, radiotherapy and immunotherapy were applied.
Conclusions
In children with common abdominal symptoms as chronic flatulence, diarrhea or severe constipation of unknown etiology, the neuroblastic tumors should be considered.
Keywords: neuroblastic tumors, VIP-secreting tumor, paraneoplastic syndrome, diarrhea, constipation
Introduction
Neuroblastic tumors are the most common extra-cranial malignant solid tumors in children. The abdominal tumor symptoms i.e. pain, flatulence or constipation in some cases, might result from the pressure of the pathological mass on the nearby organs or spinal cord lesions. Moreover, some types of neuroblastic tumors are capable for production of active peptides, which are especially responsible for manifestation considered to paraneoplastic neurologic disorders [1, 2]. However, clinical presentations of the paraneoplastic syndrome may be also associated with the gastrointestinal tract. It was previously shown that neuroblastic tumors are occasionally connected with watery diarrhea, hypokalemia and achlorhydria (WDHA) caused by hypersecretion of the vasoactive intestinal peptide (VIP) [1, 3]. Constipation, especially not responding to typical treatment is another symptom that may be associated with neuroblastoma diagnosis [1].
The aim of this study was to present 3 cases of children with symptoms from gastrointestinal tract which were later revealed as the uncommon digestive tract-related paraneoplastic syndromes related to neuroblastic tumors.
Material and methods
We retrospectively analyzed the medical records of three children diagnosed with neuroblastic tumors who were hospitalized between 2012 and 2016 in the gastroenterology department due to dominant symptoms from the gastrointestinal tract. The treatment processes including surgery, chemotherapy, radiotherapy as well as immunotherapy were conducted in pediatric oncology/hematology center.
Results
Case 1
A 17-month-old girl was admitted to the hospital with a 5-month history of periodic watery non-bloody diarrhea developed after antibiotic treatment. She also presented body weight loss (2 kg per 3 months) and flatulence, but no abdominal pain. The patient was admitted to the hospital in a poor condition with dehydration symptoms, her abdomen was distended, no peristalsis, but also no mass palpable was observed during the physical examination. On admission to the hospital, the child was feverish (38.5˚C). Serum electrolyte concentration indicated hyponatremia and severe hypokalemia (sodium 129 mmol/l, potassium 1,6 mmol/l). An intravenous potassium solution was used. It was required about 60 mmol of potassium per day to obtain a potassium serum concentration of 3 mmol/l. Because of the massive watery diarrhea and so severe hypokalemia, a VIP-secreting tumor was suspected. The computerized tomography scan (CT) showed a heterogeneous tumor (45 × 45 × 65 mm) with calcifications localized in retroperitoneal right space in front of the aitchbone. In 24-hour urine test, the increase of dopamine (356 µg) and normal value of vanillylmandelic acid (VMA) (1.9 mg) were observed. Serum neuron-specific enolase (NSE) and VIP concentration were above the upper limit of normal (31.61 ng/ml and 222 pmol/l respectively). The ferritin value (53.89 ng/ml) was in the normal range. A bone marrow examination as well as skeletal scintigraphy and metaiodobenzylguanidine (mIBG) scintigraphy did not detect any metastases. The patient underwent surgery, removing the tumor (65 × 43 × 42 mm) and local lymph nodes. The pathology examination revealed an atypical ganglioneuroblastoma (Fig. 1). The first molecular test showed a positive amplification of MYCN and chemotherapy was applied – carboplatin/etoposide, and subsequently cyclophosphamide, vincristine and doxorubicin (CADO). This treatment was completed after molecular verification (negative amplification of MYCN). 2.5 years after surgery patient remains free of disease (mIBG scintigraphy and magnetic resonance (MR) did not indicate the relapse of ganglioneuroblastoma). The VIP serum concentration was also normalized.
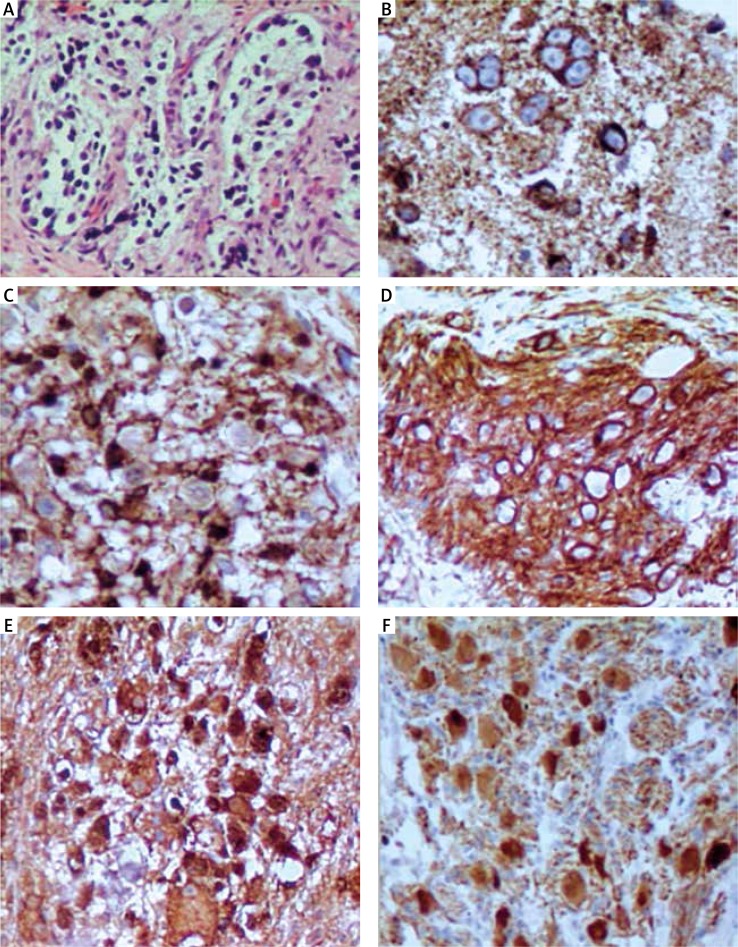
Fig. 1.
Examination of specific markers expression of ganglioneuroblastoma (case 1). A) Hematoxylin and eosin staining of the tumor sample (HE; 200×). B) NB84 expression in the tumor (immunohistochemistry NB84; 400×). C) Protein S100 immunoreactivity on the cells of the tumor (in this case ganglioneuroblastoma, S100; 400×). D) CD56 also called neural cell adhesion molecule (NCAM) immunoreactivity on the ganglioneuroblastoma cells (CD56; 200×). E) Positive expression of neuron specific enolase in the tumor sample (NSE) (NSE; 200×). F) PGP9.5 expression in the tumor sample (PGP9.5; 200×)
Case 2
A 2-year-old girl was admitted to the hospital in relatively good condition due to flatulence symptoms and a 7-month history of periodic watery diarrhea (7 times per day). Physical examination indicated distended abdomen and hypertension. The malabsorption syndrome was suspected. Gastrofiberoscopy and colonoscopy were performed and no abnormalities were detected. The abdominal USG and CT (Fig. 2) showed a heterogeneous tumor with calcifications localized in retroperitoneal space. A VIP-secreting neuroblastoma was considered. The increased serum NSE level (67.66 ng/ml) was discovered. A catecholamine urine test indicated a significant increase of dopamine (1974 µg/24 h), noradrenaline (80.4 µg/24 h) and VMA (44 mg/24 h). No metastases to bone morrow, lungs or bones were detected. The investigation of the MYCN gene amplification was negative. Based on the result of the 2nd abdominal CT, the 3rd stage of non-operative ganglioneuroblastoma was recognized and chemoteraphy after biopsy was implemented (carboplatin, etoposide according to the European Low and Intermediate Risk Neuroblastoma – SIOPEN Study). However, after obtaining the result of elevated serum VIP concentration (73 pmol/l), the surgery treatment was performed, as a result of which the tumor (105 × 75 × 70 mm) and lymph nodes were removed. The histological examination revealed intermixed ganglioneuroblastoma, neoplastic cells were detected also in lymph nodes. In the control abdominal CT the residual disease and residual lymph nodes were detected, although serum VIP concentration normalized and the diarrhea symptoms were stopped. Postoperative chemotherapy (1 cycle of CADO, 2 cycles of carboplatin/etoposide) was applied and surgery to remove the residual disease was performed. The pathology examination revealed the presence of malignant cells in removed tissues. In this situation, the 4th cycle of chemotherapy (carboplatin/etoposide) and radiotherapy were administrated. 2 years after completion of therapy the MR examination and mIBG did indicate neither the residual nor recurrence of the disease.
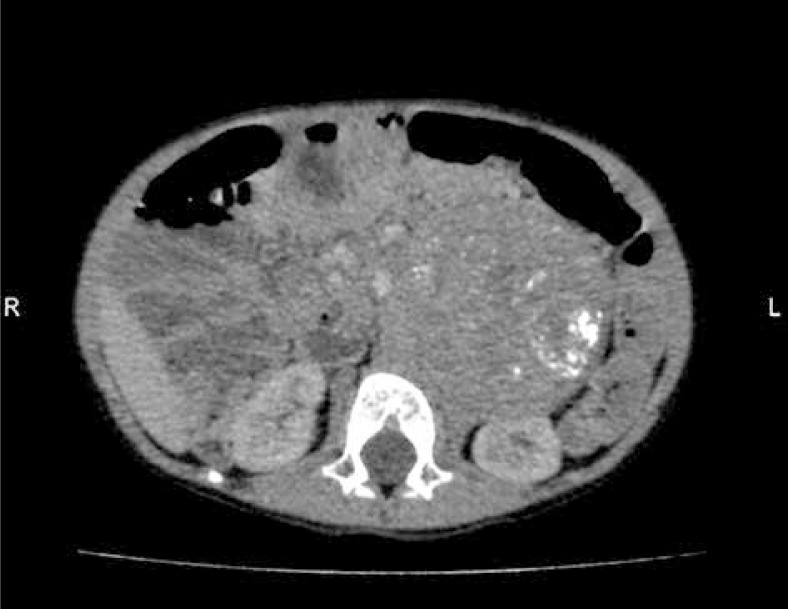
Fig. 2.
CT of the abdomen – heterogeneous tumor with calcifications (70 × 70 × 100 mm) localized in retroperitoneal left space near left kidney (case 2)
Case 3
A 4-year-old boy presented with several weeks history of abdominal pain, severe constipation and weakness was admitted to the hospital in relatively good condition. In the clinical examination no palpable abdominal mass was detected. The abdominal USG and CT revealed 131 × 82 × 111 mm mass localized in the left hypochondriac and mesogastric regions partially displacing left kidney, pancreas, spleen and intestines. In the laboratory tests the leukopenia (3100/µl) and lymphocytopenia (930/µl) were noted. Moreover, hypoproteinemia (total protein 4.0 g/dl), increased LDH (4876 IU/l) as well as NSE (371 ng/ml) were detected. The serum protein electrophoresis test showed a decreased ratio of γ-globulin (albumin 68.6%, globulins: α1 6.6%, α2 12.5%, β1 5.0%, β2 3.9%, γ 3.4%). Immunological analysis showed IgG deficiency (3.61 g/l) while IgM and IgA concentration were normal. A catecholamine urine test indicated a slight increase of VMA (6.7 mg/24 h). mIBG scintigraphy showed an abnormal uptake of radionuclides into the tumor mass as well as cranium, spine, pelvis, femur bones and under clavicle area (lymph node). A bone marrow biopsy confirmed metastases. A biopsy of the tumor showed features of undifferentiated type neuroblastoma. The 4th stage of neuroblastoma without MYCN amplification was recognized and the treatment according to High Risk Neuroblastoma Study 1.7 of SIOP-Europe (SIOPEN) was administrated. The rapid induction therapy – cisplatin, vincristine, carboplatin, etoposidee, and cyclophosphamide (COJEC) was applied that lead to regression of the tumor mass and removal of metastases. After granulocyte colony-stimulating factor (G-CSF) therapy the apheresis of peripheral blood stem cell (PBSC) by femoral cannulation was performed. The early post apheresis period was complicated by the femoral artery pseudoaneurysm removed by surgical method. 10 days later, laparotomy for the left adrenal gland tumor resection was conducted. Histopathology showed the regressive changes (90% of the tumor) and features of stroma-poor neuroblastoma. Afterwards, mega-dose chemotherapy (Busulfan/Melphalan) and autologous stem cell transplantation (5.86 × 106 CD34+/kg bw) were performed. In the control mIBG scintigraphy the uptake of radionuclides was not detected that allowed for the administration of radiotherapy to the side of the primary tumor. One week later the treatment of residual disease was started with retinoid (13-cis-retinoic acid) therapy, afterwards immunotherapy with antibodies against the disialoganglioside GD2 was applied (anti-GD2). First anti-GD2 administration was complicated with capillary leak syndrome and cytokine release syndrome (massive swelling, decrease capillary saturation, bronchial obturation, diarrhea, itchy skin). After side effect regression the treatment was continued. At presence the relapse was not detected. Finally, the boy obtained 4 cycles of 13-cis-retinoid acid and 4 cycles of anti-GD2 immunotherapy.
Discussion
On the basis of the retrospective analysis, it can be assumed that even common symptoms such as diarrhea or constipation and bloating can be treated as the first symptoms of the malignant process in children. However, palpable abdominal mass is not always detected during the physical examination. It was recognized that less than 1% of all neuroblastic tumors display clinical symptoms of VIP secretion, while paraneoplastic syndrome connected with constipation is extremely rare [1, 4, 5]. Moreover, it should be emphasized that not gastrointestinal but neurological disorders, mainly opsoclonus-myoclonus syndrome, are the most common paraneoplastic syndromes in children with neuroblastoma [17].
Excessive VIP production may cause widespread effects mainly in the gastrointestinal tract. The reduction of sodium, chlorine and water absorption as well as potassium secretion in the intestine leads to watery diarrhea and electrolyte disorders [6]. Hypokalemia, like in patients 1 is very difficult to control [3]. Moreover, the weight loss is also typical [6, 7]. Verner and Morrison first described these symptoms in 1958 [8]. In children, WDHA syndrome is usually caused by neurogenic tumors, which most frequently occur in the adrenal glands or retroperitoneum [1, 6].
To our knowledge, less than 100 VIP-secreting neuroblastic tumors have been described over the last 40 years. The clinical experience is based on case reports. Most frequently diarrhea is the first disturbance and it appears about 5 months before tumor diagnosis [3, 5]. In our patients the principal and initial symptom was flatulence. Diarrhea was observed periodically. In the literature, other paraneoplastic syndromes like neurological disorders in this group of patients were described [1]. This phenomenon is probably caused by anti-neuroblastoma immune response and antibodies production that contribute to limiting tumor growth and metastatic potential but their antineuronal activity is also confirmed [1, 9].
The VIP secretion by neuroblastic tumors is associated with a favorable outcome provided it is resectable, rare MYCN amplification and metastases [3, 5, 10]. Analysis of the presented series of cases confirm this data. The favorable prognosis is probably the result of the modulatory role of VIP that has been shown to induce both the growth inhibition and morphological differentiation of neuroblastoma cells by autocrine regulation [11]. Moreover, it was recognized that high levels of the TrkA receptor are expressed in low-stage neuroblastomas, which are characterized by a good patient prognosis. TrkA is a transmembrane receptor tyrosine kinase for nerve growth factor (NGF). The activation of this receptor promotes neuronal differentiation and regression in the neuroblastoma. In embryonic mouse model, it was shown that VIP is a regulator of NGF and stimulates increased a molecular weight isoform of NGF [12–14].
The serum VIP concentration is the most useful marker used to detect this type of tumors and observe possible relapse of malignancy. The cases evaluation proved that the successful treatment caused VIP normalization, what is consistent with the observations of other authors [3, 5].
The papers review showed that 50% of patients with neuroblastic tumors indicated increased levels of urinary catecholamines [1]. We observed the increase urinary level of dopamine, noradrenaline and VMA. The USG and CT are necessary to recognize tumor localization. Aggressive surgery is the appropriate type of treatment to eliminate the tumor and control watery-diarrhea syndrome. Neoadjuvant chemotheraphy is applied in metastatic and unresectable tumors. In 2nd case chemotherapy and surgery were necessary for elimination of residual disease, however, only the stabilization of the disease was achieved and the radiotherapy was implemented. Somatostatin and steroids are recommended only in a situation when the tumor has not been localized [3, 5].
Severe or chronic constipation is frequently observed in children. The etiology of pediatric constipation may be multifactorial but organic pathology is occasionally recognized [15, 16]. As previously mentioned constipation as a paraneoplastic syndrome of neuroblastic tumors has been extremely rare described [1, 17]. Wildhaber et al. in 2002 reported a girl with ganglioneuroblastoma who presented with severe constipation due to pseudoobstruction. It was shown that symptoms were caused by destruction of the ganglion cells by antineuronal nuclear antibodies (ANNA) [18]. Another authors confirmed a correlation between serum presence of ANNA-1 or ANNA-2 antibodies and gastro-intestinal disturbances, ranging from constipation to a paralytic ileus in patients with neuroblastic tumors [1, 17, 19]. In the 3rd case the examination of mentioned nuclear antibodies has not been performed, however during the treatment process while the mass of the tumor decreased systematically the constipation was still noticed. In this group of patients persistent constipation may be caused by various mechanisms, e.g. bowel obstruction resulting from abdominal mass pressure, spine/nerves compression due to the dumb-bell tumor extension into the vertebral column that affect sphincters as well as antineuronal nuclear antibody activity [17, 18].
The most common reason of hypoproteinemia in neuroblastoma cases is hypoalbuminemia that may be multifactorial (increased protein loss by kidney, decrease of hepatic synthetic function or protein-loosing enteropathy). However, in 3rd case decreased ratio of gamma-globulin was recognized that may result from lymphocytopenia and probably altered distribution and function of peripheral blood mononuclear cells described by other authors [20].
The main therapeutic option for neuroblastic tumors with autoimmune ganglion cell destruction is surgery that was one of the steps in the treatment process in described case. Corticosteroids may be considered in cases with high ANNA levels and presence of various neurological symptoms [1].
In conclusions, neuroblastic tumors may present non-specific gastrointestinal symptoms in addition to the typical manifestation. The watery chronic diarrhea may be a sole symptom of malignant disease but flatulence might be even the first non-characteristic feature put ahead of diarrhea. In all children with WDHA syndrome, the secreting VIP tumor has to be suspected. Similarly, in case of severe constipation especially in children without gastrointestinal disorders in the past, the suspicion of the possible presence of undiagnosed tumor should be considered.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wildhaber B, Niggli F, Bergsträsser E, Stallmach T, Sacher P. Paraneoplastic syndromes in ganglioneuroblastoma: contrasting symptoms of constipation and diarrhoea. Eur J Pediatr. 2003;162:511–3. doi: 10.1007/s00431-003-1212-0. [DOI] [PubMed] [Google Scholar]
- 2.Schuler D, Koós R, Krauze I, Péter A. Paraneoplastic syndrome in childhood. Acta Paediatr Acad Sci Hung. 1977;18:31–40. [PubMed] [Google Scholar]
- 3.Zhang WQ, Liu JF, Zhao J, Zhao SY, Xue Y. Tumor with watery diarrhoea, hypokalaemia in a 3-year-old girl. Eur J Pediatr. 2009;168:859–62. doi: 10.1007/s00431-008-0898-4. [DOI] [PubMed] [Google Scholar]
- 4.Parodi S, Merlo DF, Ranucci A, Miligi L, Benvenuti A, Rondelli R, Magnani C, Haupt R. SETIL Working Group Risk of neuroblastoma, maternal characteristics and perinatal exposures: the SETIL study. Cancer Epidemiol. 2014;38:686–94. doi: 10.1016/j.canep.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Bourdeaut F, de Carli E, Timsit S, et al. Neuroblastoma Committee of the Société Française des Cancers et Leucémies de l’Enfant et de l’Adolescent. VIP hypersecretion as primary or secondary syndrome in neuroblastoma: A retrospective study by the Société Française des Cancers de l’Enfant (SFCE) Pediatr Blood Cancer. 2009;52:585–90. doi: 10.1002/pbc.21912. [DOI] [PubMed] [Google Scholar]
- 6.Kanık A, Baran M, Çayan Ö, Eliaçık K, Özdemir T, Helvacı M, Çeçen E. Vasoactive intestinal peptide releasing tumor which caused to chronic watery diarrhea and hypokalemia. Turk Pediatri Ars. 2014;49:160–2. doi: 10.5152/tpa.2014.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sintusek P, Thammarakcharoen T, Shuangshoti S, Vivatvakin B. Unremitting watery diarrhoea in early childhood period. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-217532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med. 1958;25:374–80. doi: 10.1016/0002-9343(58)90075-5. [DOI] [PubMed] [Google Scholar]
- 9.Gesundheit B, Smith CR, Gerstle JT, Weitzman SS, Chan HS. Ataxia and secretory diarrhea: two unusual paraneoplastic syndromes occurring concurrently in the same patient with ganglioneuroblastoma. J Pediatr Hematol Oncol. 2004;26:549–52. doi: 10.1097/01.mph.0000139414.66455.a4. [DOI] [PubMed] [Google Scholar]
- 10.Lacej SR, Gribble TJ, Kosloske AM. Favorable prognosis of vasoactive intestinal peptide-secreting ganglioneuroblastoma. Pediatr Surg Int. 1989;4:217–9. [Google Scholar]
- 11.Pence JC, Shorter NA. The autocrine function of vasoactive intestinal peptide on human neuroblastoma cell growth and differentiation. Arch Surg. 1993;128:591–5. doi: 10.1001/archsurg.1993.01420170127020. [DOI] [PubMed] [Google Scholar]
- 12.Hill JM, Mehnert J, McCune SK, Brenneman DE. Vasoactive intestinal peptide regulation of nerve growth factor in the embryonic mouse. Peptides. 2002;23:1803–8. doi: 10.1016/s0196-9781(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 13.Schramm A, Schulte JH, Astrahantseff K, et al. Biological effects of TrkA and TrkB receptor signaling in neuroblastoma. Cancer Lett. 2005;228:143–53. doi: 10.1016/j.canlet.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Brodeur GM, Minturn JE, Ho R, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244–50. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401–9. doi: 10.1111/j.1572-0241.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 16.Rowan-Legg A. Canadian Paediatric Society, Community Paediatrics Committee Managing functional constipation in children. Paediatrics Child Health. 2011;16:661–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YT, Feng LH, Zhang Z, Zhong XD, Chang J. Different Kinds of Paraneoplastic Syndromes in Childhood Neuroblastoma. Iran J Pediatr. 2015;25:e266. doi: 10.5812/ijp.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildhaber B, Niggli F, Stallmach T, Willi U, Stauffer UG, Sacher P. Intestinal pseudoobstruction as a paraneoplastic syndrome in ganglioneuroblastoma. Eur J Pediatr Surg. 2002;12:429–31. doi: 10.1055/s-2002-36853. [DOI] [PubMed] [Google Scholar]
- 19.Drukker CA, Heij HA, Wijnaendts LC, Verbeke JI, Kaspers GJ. Paraneoplastic gastro-intestinal anti-Hu syndrome in neuroblastoma. Pediatr Blood Cancer. 2009;52:396–8. doi: 10.1002/pbc.21807. [DOI] [PubMed] [Google Scholar]
- 20.Pranzatelli MR, Travelstead AL, Tate ED, Allison TJ, Lee ND, Fisher J, Jasty R. Immunophenotype of blood lymphocytes in neuroblastoma-associated opsoclonus myoclonus. J Pediatr Hematol Oncol. 2004;26:718–23. doi: 10.1097/00043426-200411000-00006. [DOI] [PubMed] [Google Scholar]