Abstract
Background
MEGDEL syndrome (3-MethylGlutaconic aciduria, Deafness, Encephalopathy, Leigh-like syndrome) is a severe neurometabolic disease with infantile onset.
Phenomenology Shown
Progressive and marked dystonia over a 6-year period in an adult male with MEGDEL syndrome.
Educational Value
Generalized dystonia may be the main manifestation of a milder form of MEGDEL syndrome, which begins during adulthood.
Keywords: MEGDEL syndrome, SERAC1, adult, dystonia, Leigh syndrome
A 31-year-old male with a history of mild psychomotor delay was referred for generalized dystonia with chorea-like movements (Video 1). At 24 years old, he had a few episodes of subacute encephalopathy triggered by fever and then developed cervical dystonia. Dystonia gradually worsened to become generalized dystonia. He also developed progressive lower limb spasticity and hyperkinetic dysarthria. There was no sign of cerebellar dysfunction or parkinsonism.
Video 1. MEDGEL Syndrome in Adulthood Revealed by Generalized Dystonia and Spasticity. 2010: cervical dystonia, mild lower limb spasticity, dysarthria. 2012: generalized dystonia, worsening of lower limb spasticity. 2016: worsening of generalized dystonia, spasticity and dysarthria.
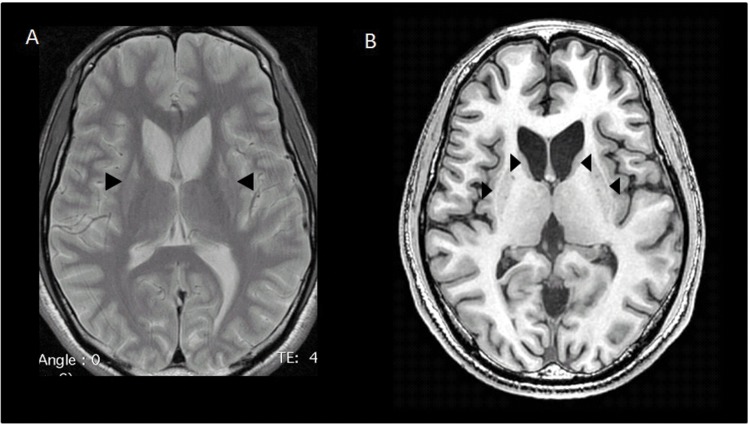
Because symptoms began and worsened after fever episodes, an inborn error of energy metabolism was suspected. However, investigations of intermediary metabolism (plasma ammonia, lactate, amino acids, acylcarnitines, phytanic acid, pristanic acid, and urine organic acids) were normal. Brain magnetic resonance imaging (MRI) showed bilateral shrunken striata, suggestive of Leigh syndrome (Figure 1). Electromyography showed severe axonal neuropathy and a visual evoked potential revealed a bilateral optic neuropathy. Audiograms were normal. Mitochondrial investigations on the patient’s muscle biopsy (immunohistochemistry, oxidative phosphorylation (OXPHOS) activities, quantity, size, and sequencing of the entire mitochondrial DNA) were normal. Nonetheless, a gene panel dedicated to mitochondrial diseases identified compound heterozygous variants in SERAC1. The first variant (c.1347-1350dupATCT, p.Val451fs) had already been reported in MEGDEL syndrome (3-MethylGlutaconic aciduria, Deafness, Encephalopathy, Leigh-like syndrome). The second variant (c.1598C>T, p.Pro533Leu) was predicted to be pathogenic by three in silico prediction software programs (SIFT, Polyphen-2, and GVGD). The parents’ blood DNA was not available for testing. A control of urine organic acids showed mild 3-methylglutaconic aciduria.
Figure 1. Brain Magnetic Resonance Imaging. Basal ganglia with hyperintense signal (arrows) on axial T2-weighted image (A), corresponding to hypointense signal and atrophy (arrows) on axial T1-weighted image (B).
MEGDEL syndrome is a rare disorder caused by bi-allelic mutations in SERAC1, which encodes a protein of the mitochondrial membrane.1 Typically, the phenotype is one of major motor (dystonia and spasticity) and intellectual disability that starts early in life and is associated with deafness; most patients never learn to speak or walk.2 3-Methylglutaconic aciduria is a cardinal biological marker of the disease and the MRI pattern evolves from the pathognomonic “putaminal eyes” to shrinking and atrophy of the basal ganglia over the disease course.3
Our observation illustrates that adult-onset generalized dystonia can be the main manifestation in milder atypical forms of MEGDEL syndrome. Brain abnormalities suggested by MRI could be a good clue to the diagnosis whereas the 3-methylglutaconic aciduria level may fluctuate and can be transiently undetectable.
Acknowledgments
We thank Dr Thierry Gendre for the care he provided to the patient.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflict of interest.
Ethics Statements: All patients that appear on video have provided written informed consent; authorization for the videotaping and for publication of the videotape was provided.
References
- 1.Wortmann SB, Vaz FM, Gardeitchik T, Vissers LELM, Renkema GH, Schuurs-Hoeijmakers JHM, et al. Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat Genet. 2012;44:797–802. doi: 10.1038/ng.2325. doi: http://dx.doi.org/10.1038/ng.2325. [DOI] [PubMed] [Google Scholar]
- 2.Maas RR, Iwanicka-Pronicka K, Ucar SK, Alhaddad B, AlSayed M, Wortmann SB, et al. Progressive deafness-dystonia due to serac1 mutations: a study of 67 cases. Ann Neurol. 2017;82:1004–1015. doi: 10.1002/ana.25110. doi: http://dx.doi.org/10.1002/ana.25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wortmann SB, van Hasselt PM, Baric I. Eyes on MEGDEL: distinctive basal ganglia involvement in dystonia deafness syndrome. Neuropediatrics. 2015;46:98–103. doi: 10.1055/s-0034-1399755. doi: http://dx.doi.org/10.1055/s-0034-1399755. [DOI] [PubMed] [Google Scholar]