Abstract
In the present study, application of Ecklonia maxima extract (Kelpak SL – a water soluble concentrate) was optimized and its impact on yield, nutraceutical and nutritional potential of Phaseolus vulgaris L. (var. Aura and Toska) was measured. The study was carried out in 2012 and 2013 in Poland. During the growing season, 0.2% and 0.4% solution of Kelpak SL was applied by single and double spraying of plants. These four treatments with Kelpak SL were compared with the control, where no biostimulator was applied. Kelpak SL treatments stimulated the yield of both cultivars studied. The application of E. maxima extract had no effect on the content of starch, free sugars or proteins in seeds of either of the tested cultivars. The highest level of phenolics was found for double sprayed Toska plants. All the tested variants of Kelpak SL application significantly increased the content of anthocyanins in the seeds. Also, both the reducing power and antiradical ability of Aura seeds were elevated in all the studied treatments. E. maxima extract is a natural, environmentally friendly and safe preparation increasing the yield and nutraceutical quality of beans without any negative effect on their nutritional quality.
Keywords: Antioxidant capacity, Ecklonia maxima, Kelpak SL, Nutrients, Phaseolus vulgaris L., Yield
1. Introduction
In organic manufacture, it is important to ensure that food production and processing methods are environmentally-friendly. There is a growing interest in using cultivation methods such as natural biostimulants, which improve yield without any negative effects on plant quality. Such a strategy allows for increasing biomass production, but also induces the natural resistance of plants, as well as improves nutraceutical quality of plant food (Vallad and Goodman, 2004, Zodape et al., 2010, Kavipriya et al., 2001, Złotek and Wójcik, 2014). Elicitation with biotic and abiotic factors, which is a method for the natural induction of plant resistance mechanisms, could be proposed as a new alternative, non-conventional and ecologically-friendly approach to plant protection (Vallad and Goodman, 2004, Złotek and Wójcik, 2014).
Products of natural origin, such as seaweed extract, are already used in plant production. In agricultural and horticultural crops, many species of seaweed (Ecklonia maxima, Kappaphycus alvarezii, Ascophyllum nodosum, Laminaria digitata, Laminaria hyperborea, Fucus vesiculosus, Durvillea potatorum, Fucus serratus) are used as a stimulator of the growth and development of plants (Verkleij, 1992, Sivasankari et al., 2006, Zodape et al., 2010, Kocira et al., 2013). Seaweed extracts usually occur in single or multi-compound preparation form. One of these is Kelpak SL – made from an extract of E. maxima. Kelpak SL contains cytokinins, auxins, polyamines, gibberellins, brassinosteroids (brassinolide and castasterone), phlorotannins (eckol and phloroglucinol) and low concentrations of abscisic acid (Papenfus et al., 2012, Stirk et al., 2014, Rengasamy et al., 2015a). Application of Kelpak SL can contribute to enhanced growth, yield and resistance against agricultural and horticultural plant pathogens, as has been confirmed by previous studies involving the production of small-sized tomatoes (Dobromilska et al., 2009), white beans (Kocira et al., 2013), and wheat (Matysiak et al., 2012). Also, foliar application of red algae (K. alvarezii) extract has improved the yield of Phaseolus radiata L. (Zodape et al., 2010). In the study by Kavipriya et al. (2001) on Vigna radiata (syn. P. radiata), an application of seaweed extract obtained from the marine green algae Ulva lactuca, Caulerpa scalpelliformis and the brown algae Sargassum plagiophyllum, Turbinaria conoides, Padina tetrastromatica, Dictyota dichotam stimulated germination and growth parameters, such as fresh and dry weight of shoots and roots.
Legume-based diets have long-term beneficial effects on human health including a preventive effect against hypertension, cardiovascular disease, diabetes and some types of cancer. Beans are rich in a number of important, high quality nutrients (protein, carbohydrate, dietary fiber), micronutrients (potassium, magnesium, folate, iron, and zinc) as well as bioactive compounds such as polyphenols and vitamins (Messina, 2014, Rebello et al., 2014). So far, only a few studies have been published about the impact of Kelpak SL on the chemical composition of the crop. Application of a seaweed extract changed the protein and starch content in wheat (Matysiak et al., 2012), as well as the protein and fiber content in alfalfa (Sosnowski et al., 2015). Induction of natural resistance in plants is very often linked with the overproduction of pathogen-related proteins and metabolites, such as hydroxylase inhibitors or phenolics (Świeca et al., 2013, Świeca and Baraniak, 2014, Złotek and Wójcik, 2014). These modifications in the biochemical composition may be translated to undesirable changes in the nutritional and pro-health quality of the crop. Thus the aim of the study was to determine the effect of Kelpak SL on plant yield and changes in the nutraceutical and nutritional quality of the common bean (var. Aura and Toska).
2. Materials and methods
2.1. Chemicals
ABTS (2,2-diphenyl-1-picrylhydrazyl), potassium ferricyanide, EDTA (ethylenediaminetetraacetic acid), PMSF (phenylmethanesulfonyl fluoride), BAPNA (α-N-benzoyl-dl-arginine-p-nitroanilidehydrochloride), HHL (Hippuryl-l-histidyl-l-leucine) and pepstatin A were purchased from Sigma–Aldrich (Poznan, Poland). All others chemicals were of analytical grade. Kelpak SL was purchased from Chemirol (Mogilno, Poland).
2.2. Plant materials and growth conditions
The study was carried out in 2012 and 2013 in Perespa (50°66′N; 23°63′E), Poland. The soil type was characterized as Brown rendzina belonging to the Rendzinas soil group. It is alkaline (pH in 1 M KCl around 7.4–7.5) and rich in phosphorus, potassium, and magnesium. The experiment was established in a randomized block design in four replications with an elementary experimental plot area of 4.5 m2 (1.35 × 3.33 m). The study was carried out on two bean varieties (commonly cultivated in Poland and processed by food industry; Aura - white seed coatings and Toska – red seed coatings) to show the impact of Kelpak SL treatments on plants with a different phenylpropanoid metabolism. Bean seeds of the Aura and Toska cultivar were sown in the first 10-day period of May at a depth of 3–4 cm, with the spacing of drills set at 45 cm to achieve a density of 30 plants m2. The bean crop was harvested in the first – Aura cultivar – and second 10-day periods of August – Toska cultivar. During the growing season, 0.2% and 0.4% solution of Kelpak SL was applied by single spraying (at the 2–3 leaf stage) and double spraying of plants (first at the 2–3 leaf stage and second at the beginning of bean blooming). This was applied with a GARLAND FUM 12B battery field sprayer (Lechler LU 120-03) at a pressure of 0.30 MPa, using 300 l liquid per hectare. The four different variants of treatment with Kelpak SL were compared with the control, where plants were treated with the same volume of water (no biostimulator was applied). Tillage for bean was done using good agricultural practices. Mineral fertilization in kg of nutrient per hectare was as follows: 30 kg N ha−1, 60 kg P ha−1, 120 kg K ha−1. No pesticides were used (pests did not exceed the thresholds of harmfulness). Average rainfalls during the growing season of common beans are presented in Table 1.
Table 1.
Rainfall (mm) during the vegetative seasons of common bean.
| Months | Rainfall |
|
|---|---|---|
| 2012 | 2013 | |
| V | 90.2 | 116.6 |
| VI | 101.3 | 77.3 |
| VII | 79.6 | 88.3 |
| VIII | 85.7 | 112.6 |
| Average/total | 89.2/356.8 | 98.7/394.8 |
2.3. Plant yield and vigor determination
Upon harvesting, the number and weight of seeds, the number of pods and the weight of a thousand seeds for each plot (four replications of each combination) were recorded. The seeds were dried and ground. The flour was used for further analysis.
2.4. Nutrient analysis
2.4.1. Protein sequential fractionation based on solubility criteria
Albumins and globulins from common beans were isolated on the basis of solubility criteria according to the method of Ribeiro et al. (2004) with a slight modification (Durak et al., 2013). The proteins were subsequently sequentially extracted and purified using appropriate extraction solutions. The albumins were extracted in distilled water containing 10 mM CaCl2 and 10 mM MgCl2 (1 g·30 cm−3). The insoluble proteins were removed by centrifugation for 30 min at 9000 g, 4 °C. The resultant supernatant accounted for the albumin fraction. For globulin extraction, the pellet was resuspended in 30 cm3 100 mM Tris–HCl buffer, pH 7.5, containing 10% (w/v) NaCl, 10 mM EDTA. Solubilized globulins were obtained by centrifugation for 30 min at 9000 g, 4 °C. The resultant supernatant accounted for the globulin fraction.
2.4.2. Protein content
The protein content was determined with the Bradford method (Bradford, 1976), using bovine albumin as the standard protein.
2.4.3. Starch analysis
Total starch content was determined after dispersion of the starch granules in 2 M KOH (50 mg sample, 6 cm3 KOH) at room temperature (30 min, constant shaking) and hydrolysis of the solubilized starch with amyloglucosidase (1 mg cm−3; 14 U mg−1; EC 3.2.1.3) at 60 °C for 45 min. (Goni et al., 1997). Glucose content was determined using the standard dinitrosalicylic acid (DNSA) method (Miller, 1959). Total starch was calculated as glucose × 0.9. The free reducing sugar content of the samples was determined in order to correct the obtained total starch values. The sucrose content of the samples was also determined to correct the obtained total starch values. Samples dispersed in sodium acetate buffer, pH 5.0 were treated with 200 μl (10 mg in 1 cm3 of 0.4 M sodium acetate buffer, pH 5.0) invertase (EC 3.2.1.26; 300 U mg−1) for 30 min at 37 °C. After centrifugation, reducing sugars were analyzed in the supernatants using the DNSA reagent.
2.5. Nutraceutical potential
2.5.1. Phenolic and antioxidant capacity determination
2.5.1.1. Extract preparation
Bean flours (0.25 g in triplicate) were extracted three times with 4 cm3 of acetone/water/hydrochloric acid (70:29:1, v/v/v) (Xu and Chang, 2007). After centrifugation (10 min, 6800g) fractions were collected, combined and used for further analysis.
2.5.1.2. Phenolic determination
2.5.1.2.1. Determination of total phenolic compounds (TPC)
The amount of total phenolics was determined using Folin–Ciocalteau reagent (Singleton et al., 1974). To 0.5 cm3 of the sample, 0.5 cm3 H2O and 2 cm3 Folin–Ciocalteau reagent (1:5 H2O) were added, and after 3 min, 10 cm3 10% Na2CO3 and the contents were mixed and allowed to stand for 30 min. Absorbance at 725 nm was measured via a UV–Vis spectrophotometer. The amount of total phenolics was calculated as a gallic acid equivalent (GAE) in mg per g of dry mass (DM).
2.5.1.2.2. Determination of flavonoid content (TFC)
Total flavonoid content was determined according to the method described by Lamaison and Carnet (1990). One milliliter of extract was mixed with 1 cm3 2% AlCl3 × 6H2O solution (in methanol) and incubated at room temperature for 10 min. Thereafter, absorbance at 430 nm was measured. Total flavonoid content was calculated as a quercetin equivalent (QE) in mg per g of dry mass (DM).
2.5.1.2.3. Determination of anthocyanins (TAC)
Anthocyanin levels were measured by the pH differential method described by Fuleki and Francis (1968). Sample extracts were combined at a 1:20 ratio (v:v) with potassium chloride and with sodium acetate buffers at pH 1.0 and 4.5, respectively, in separate vessels. After an equilibration period (15 min), the raw absorbance of each solution was measured at 520 nm and 700 nm. A corrected absorbance value was calculated as [(A520 − A700) pH 1.0 − (A520 − A700) pH 4.5]. The anthocyanin content was calculated using the molar absorptivity (є) and molecular weights (MW) of cyanidin 3-glucoside (є = 26,900; MW = 449.2). Results were expressed as a cyanidin 3-glucoside equivalent (Cy3-GE) in mg per g of dry mass (DM).
2.5.1.3. Antioxidant activities
2.5.1.3.1. Antiradical activity (ABTS)
The experiments were carried out using an improved ABTS decolorization assay (Re et al., 1999). ABTS+• was generated by oxidation of ABTS with potassium persulfate. The ABTS radical cation (ABTS+•) was produced by reacting 7 mM stock solution of ABTS in distilled water with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in the dark for at least 6 h at room temperature prior to use. The ABTS+• solution was diluted to an absorbance of 0.7 ± 0.05 at 734 nm (Lambda 40 UV–Vis spectrophotometer, Perkin Elmer). The affinity of test material to quench ABTS free radicals was evaluated according to the following equation:
where AC – absorbance of control, AA – absorbance of sample. Free radical scavenging ability was expressed as a Trolox equivalent in mg per g of dry mass (DM).
2.5.1.3.2. Reducing power (RP)
Reducing power was determined by the method of Pulido et al. (2000). The sample (2.5 cm3) to be analyzed was mixed with phosphate buffer (2.5 cm3, 200 mM, pH 6.6) and potassium ferricyanide K3[Fe(CN6)] (2.5 cm3, 1%). The mixture was incubated at 50 °C for 20 min. Reactions were stopped with 0.5 cm3 10% TCA and centrifuged for 10 min, (6800g). The upper layer of the solution (2.5 cm3) was mixed with distilled water (2.5 cm3) and 0.5 cm3 0.1% FeCl3 and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated an increase in reducing power. Reducing power was expressed as a Trolox equivalent in mg per g of dry mass (DM).
2.5.2. Assay for ACE inhibitor activity
2.5.2.1. Preparation of ACE from pig lungs
An angiotensin converting enzyme was prepared according to the procedure of Hayakari et al. (1978) with slight modifications. Pig lungs were purchased in a local market and used as the starting material. Lung tissues were homogenized in 0.1 M borate buffer pH 8.3 containing pepstatin A (0.1 mM) and phenylmethanesulfonyl fluoride (0.1 mM) at 4 °C at a ratio of 1:2 (w/v). The homogenate was centrifuged for 20 min at 8000g, 4 °C. The purification of ACE was initiated by the addition of solid ammonium sulfate at 80% saturation and next dialyzed (MW cut-off 12 kDa) for 24 h at 4 °C against 20 volumes of 0.1 M borate buffer pH = 8.3. The dialysate was centrifuged for 20 min at 8000g, 4 °C. The activity of ACE was determined according to the Chang et al. (2001) spectrophotometric method with 5 mM HHL as a substrate. Supernatant containing an enzyme with 3 mU of activity (one unit of ACE activity was defined as an increase in absorbance of 0.001 per minute at 390 nm) was frozen and used for further analysis.
2.5.2.2. Assay for ACE inhibitor activity
ACE inhibitor activity (ACEI) was measured by the spectrophotometric method according to Chang et al. (2001) with slight modifications with 5 mM HHL as a substrate: the reaction was stopped by adding 0.7 cm3 of 0.1 M borate buffer with 0.2 M NaOH. The absorbance was measured at 390 nm and the ACE inhibition was determined as follows:
where A1 is the absorbance of sample with ACE and an inhibitor (albumin fraction, see Section 2.4.1.),
A2 is the absorbance of sample with an inhibitor, without ACE,
A3 is absorbance of sample with ACE and without an inhibitor.
ACEI was expressed as ACE inhibitor units per gram of DM sample. One ACE unit was defined as an increase of 0.001 units of absorbance per min under the assay conditions.
2.5.3. Activity of hydrolase inhibitors
2.5.3.1. Trypsin inhibitor activity
The trypsin inhibitor activity (TIA) was determined using α-N-benzoyl-DL-arginine-p-nitroanilidehydrochloride (BAPNA) as the substrate (Kakade et al., 1974). The amount of 0.25 cm3 of albumin fraction (see Section 2.4.1.) and 0.25 cm3 porcine pancreas trypsin was preincubated at 37 °C for 15 min. Then, 0.5 cm3 0.5 mM BAPNA was added and vortexed immediately to start the reaction. After incubating for 10 min, 0.5 cm3 30% acetic acid (v/v) was added to terminate the reaction. The reaction mixture was centrifuged for 5 min at 8000g. The residual activity of trypsin was measured by the absorbance at 410 nm due to p-nitroaniline. TIA was expressed as trypsin inhibitor units per gram of DM sample. One trypsin unit was defined as an increase of 0.001 units of absorbance per min under the assay conditions.
2.5.3.2. α-Amylase inhibitor activity
The α-amylase inhibitor activity was measured according to the modified method of Deshpande and Cheryan (1984). Porcine pancreatic α-amylase (220 U cm−3) was dissolved in 100 mM phosphate buffer (containing 20 mM CaCl2, pH 5.6). To measure the α-amylase inhibition activity, a mixture of 0.25 cm3 of α-amylase solution and 0.25 cm3 of extracted α-amylase inhibitor (albumin fraction) was first incubated in a water bath at 40 °C for 15 min. Then, 0.5 cm3 1% (w/v) soluble starch (dissolved in 20 mM sodium phosphate buffer containing 6 mM NaCl, pH 7) was added. After 10 min., the reaction was stopped by adding 1 cm3 of 3,5-dinitrosalicylic acid and heating in a boiling water bath for 10 min. The mixture was adjusted to 12 cm3 with double distilled water. The final results were compared with the activity of the same amount of the enzyme without the inhibitor. One inhibitory unit was defined as the amount of inhibitor which reduces the enzyme activity by one unit. One unit of α-amylase activity was defined as an increase of 0.001 units of absorbance per min under the assay conditions.
2.6. Statistical analysis
The materials for the studied parameters were collected over the two seasons (2012–2013). Unless stated otherwise, the experimental results were mean ± SD of three parallel experiments (n = 18). To statistically analyze particular yield parameters data of four replicates of each combination were taken (n = 40). The Shapiro–Wilk test was performed for the normal distribution of data. The results were analyzed statistically using a one-way analysis of variance, ANOVA. The significance of differences between evaluated mean values was estimated by means of Duncan’s test intervals of confidence at a significance level of p < 0.05. Statistical analysis was performed with Statistica 12 (StatSoft, Inc.).
3. Results
Kelpak SL treatments stimulated the yields of both studied cultivars. Single spraying of Aura bean with 0.4% Kelpak SL solution caused a significant increase in the number and weight of seeds, number of pods (by 8.6%, 9.2% and 9.1% in respect to control) (Table 2). Double foliar applications on plants with a higher concentration of Kelpak SL (DS 0.4%) resulted in a 3.4% increase in the average weight of a thousand seeds in comparison with control. Generally, the studied treatments improved Aura yield. Similar observations were also found for the Toska cultivar; however, in respect to the control only in terms of the number of seeds were the changes statistically significant (Table 2). An insignificant but noteworthy increase in the weight of Toska seeds was observed (up to about 12% for SS 0.2%), regardless of methods of application.
Table 2.
Yield and weight of thousand seeds of Phaseolus vulgaris L. (Aura and Toska cultivars) effected by Kelpak SL application.
| Parameters | Application method | Bean cultivar |
|
|---|---|---|---|
| Aura | Toska | ||
| Number of seeds [No. m−2] | C | 773.9a | 637.2 a |
| SS 0.2% | 789.7ab | 710.4 b | |
| DS 0.2% | 736.4a | 701.9 ab | |
| SS 0.4% | 841.0b | 687.8 ab | |
| DS 0.4% | 769.3a | 692.5 ab | |
| Weight of seeds [g m−2] | C | 322.7ab | 314.1a |
| SS 0.2% | 327.8ab | 353.5a | |
| DS 0.2% | 310.9a | 345.7a | |
| SS 0.4% | 352.5b | 339.1a | |
| DS 0.4% | 331.5ab | 346.5a | |
| Number of pods [No. m−2] | C | 231.3a | 183.7a |
| SS 0.2% | 235.2a | 198.9a | |
| DS 0.2% | 231.1a | 201.0a | |
| SS 0.4% | 252.5b | 189.0a | |
| DS 0.4% | 243.0ab | 200.8a | |
| Weight of thousand seeds [g] | C | 415.7a | 492.2a |
| SS 0.2% | 420.5ab | 495.9a | |
| DS 0.2% | 421.3ab | 495.2a | |
| SS 0.4% | 419.1ab | 492.7a | |
| DS 0.4% | 429.8b | 500.4a | |
C, control; SS 0.2%, single spraying by 0.2% solution of Kelpak SL; DS 0.2%, double spraying by 0.2% solution of Kelpak SL; SS 0.4%, single spraying by 0.4% solution of Kelpak SL; DS 0.4%, double spraying by 0.4% solution of Kelpak SL.
Means (n = 40) in the columns, on the selected features, followed by different small letters are significantly different at p < 0.05.
To evaluate the influence of Kelpak SL treatments on nutrient content in the studied beans the protein (albumins and globulins) and starch were investigated. The treatments with Kelpak SL did not change the content of starch or free sugars in the tested seeds (Table 3). Similar observations were found for protein content, where only in Aura beans after SS 0.2% treatment was a significant decrease in the sum of albumins and globulins (not exceeding 10%) observed (Table 3).
Table 3.
Change of main nutrient contents of Phaseolus vulgaris L. affected by the application of Kelpak SL.
| Application method | Starch [mg g−1 d.m.] | Free sugars [mg g−1 d.m.] | Albumins [mg g−1 d.m.] | Globulins [mg g−1 d.m.] | Albumin + globulins [mg g−1 d.m.] | |
|---|---|---|---|---|---|---|
| Aura | C | 530.6 ± 37.8a | 0.40 ± 0.08a | 122.1 ± 6.84a | 87.07 ± 4.59ab | 209.1 ± 5.71b |
| SS 0.2% | 513.0 ± 42.4a | 0.33 ± 0.32a | 111.7 ± 5.59a | 79.80 ± 5.60a | 191.5 ± 5.59a | |
| DS0.2% | 508.1 ± 67.1a | 0.35 ± 0.09a | 112.3 ± 3.92a | 82.61 ± 6.01a | 194.9 ± 4.96a | |
| SS 0.4% | 489.8 ± 94.1a | 0.31 ± 0.14a | 112.7 ± 1.77a | 88.10 ± 4.62ab | 200.8 ± 3.19ab | |
| DS 0.4% | 487.5 ± 57.7a | 0.37 ± 0.11a | 116.5 ± 5.50a | 87.02 ± 3.94ab | 203.5 ± 4.72ab | |
| Toska | C | 442.5 ± 69.2a | 0.32 ± 0.05a | 122.2 ± 7.41a | 90.72 ± 3.03bc | 212.9 ± 5.22b |
| SS 0.2% | 442.5 ± 54.4a | 0.43 ± 0.17a | 122.8 ± 12.15a | 88.19 ± 4.32ab | 211.0 ± 8.24b | |
| DS 0.2% | 435.0 ± 51.1a | 0.41 ± 0.14a | 119.2 ± 14.69a | 98.97 ± 3.91c | 218.1 ± 9.30b | |
| SS 0.4% | 441.8 ± 81.9a | 0.39 ± 0.06a | 108.9 ± 20.83a | 90.86 ± 5.58abc | 199.7 ± 13.21ab | |
| DS 0.4% | 447.4 ± 69.2a | 0.32 ± 0.08a | 115.6 ± 6.80a | 95.81 ± 9.17abc | 211.4 ± 7.99b | |
C, control; SS 0.2%, single spraying by 0.2% solution of Kelpak SL; DS 0.2%, double spraying by 0.2% solution of Kelpak SL; SS 0.4%, single spraying by 0.4% solution of Kelpak SL; DS 0.4%, double spraying by 0.4% solution of Kelpak SL.
Means (±SD, n = 18) in the columns followed by different small letters are significantly different at p < 0.05.
No significant effects on α-amylase inhibitor activity were determined in either of the tested bean cultivars after spraying with seaweed preparation (Table 4). Kelpak SL treatment also had no effect on trypsin inhibitor activity in Aura beans; however, in the case of the Toska cultivar, apart from the SS 0.4% treatment, spraying with Kelpak caused a significant decrease in the studied activity (Table 4).
Table 4.
Change in selected hydroxylase inhibitors activity of Phaseolus vulgaris L. affected by the application of Kelpak SL.
| Bean cultivar | Application method | α-amylase inhibitors activity [kU g−1 d.m.] | Trypsin inhibitors activity [kU g−1 d.m.] |
|---|---|---|---|
| Aura | C | 5.17 ± 0.81abc | 8.19 ± 1.18ab |
| SS 0.2% | 3.89 ± 1.09ab | 8.09 ± 1.04ab | |
| DS 0.2% | 5.32 ± 0.77bc | 8.51 ± 0.44b | |
| SS 0.4% | 5.13 ± 0.07b | 8.42 ± 0.44b | |
| DS 0.4% | 3.43 ± 0.96a | 7.98 ± 1.14ab | |
| Toska | C | 5.62 ± 0.37bc | 8.84 ± 0.22b |
| SS 0.2% | 6.10 ± 0.42c | 7.60 ± 0.22a | |
| DS 0.2% | 6.16 ± 0.33c | 7.53 ± 0.20a | |
| SS 0.4% | 6.09 ± 0.52c | 7.81 ± 0.61ab | |
| DS 0.4% | 5.90 ± 4.48c | 7.27 ± 0.55a | |
C, control; SS 0.2%, single spraying by 0.2% solution of Kelpak SL; DS 0.2%, double spraying by 0.2% solution of Kelpak SL; SS 0.4%, single spraying by 0.4% solution of Kelpak SL; DS 0.4%, double spraying by 0.4% solution of Kelpak SL.
Means (±SD, n = 18) in the columns followed by different small letters are significantly different at p < 0.05.
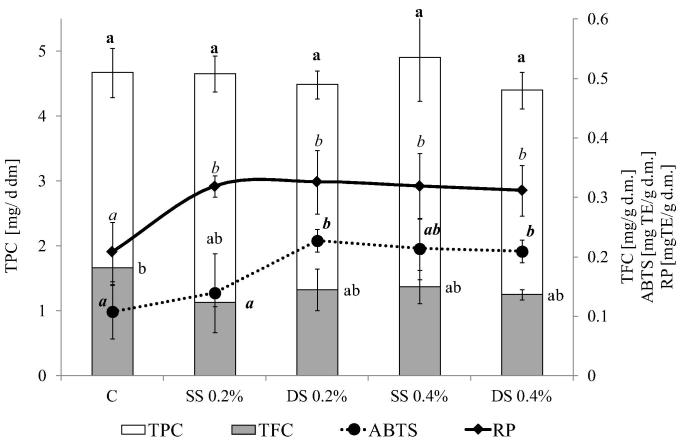
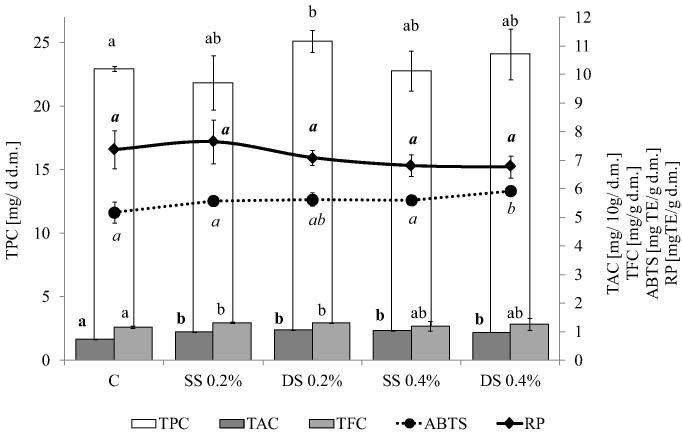
Kelpak SL applications significantly influenced the phenolic content and antioxidant potential of the studied bean seeds. The contents of phenolics in seeds obtained in the treated plants were comparable to or higher than those determined for controls. A significant positive effect of the stimulation of phenolic compounds content was observed for Toska varieties. The highest TPC content was found for double sprayed plants (25.12 mg g−1 dry mass, an increase of about 14.7% in respect to controls). All the tested variants of Kelpak SL application resulted in a significant increase in anthocyanin content in seeds. Additionally, the flavonoid contents in SS (0.2%) and DS (0.2%) were 12.8% and 13.6% higher than those of the controls, respectively. The Toska seeds were characterized by an antioxidant capacity comparable with that determined for the control. A significant increase in reducing power was observed only for the DS 0.4% treatment (Fig. 1).
Figure 1.
Change in phenolic and antioxidant capacity bean (var. Toska) effected by Kelpak SL application. C, control; SS 0.2%, single spraying by 0.2% solution of Kelpak SL; DS 0.2%, double spraying by 0.2% solution of Kelpak SL; SS 0.4%, single spraying by 0.4% solution of Kelpak SL; DS 0.4%, double spraying by 0.4% solution of Kelpak SL. TPC, total phenols; TFC, total flavonoids; ABTS, antiradical activity; RP, reducing power. Means (n = 18) in the columns followed by different small letters are significantly different at p < 0.05.
In the case of the Aura cultivar, the application of Kelpak SL did not affect total phenolic content. The antioxidant capacity of Aura seeds was significantly improved by all the studied treatments. Both reducing power and antiradical ability were increased. The results for reducing potential were about 50% higher for all variants of application in respect to the control. A similar observation, apart from the SS 0.2% treatment, was found for the ability to quench free radicals (Fig. 2).
Figure 2.
Change in phenolic and antioxidant capacity of beans (var. Aura.) effected by Kelpak SL application. C, control; SS 0.2%, single spraying by 0.2% solution of Kelpak SL; DS 0.2%, double spraying by 0.2% solution of Kelpak SL; SS 0.4%, single spraying by 0.4% solution of Kelpak SL; DS 0.4%, double spraying by 0.4% solution of Kelpak SL. TPC, total phenols; TFC, total flavonoids; TAC, total anthocyanins; ABTS, antiradical activity; RP, reducing power. Means (n = 18) in the columns followed by different small letters are significantly different at p < 0.05.
The ability to inhibit ACE activity was found only for Aura cultivar seeds. All treatments decreased this activity; however, the changes were statistically significant only in SS 0.2% and DS 0.4% treatments (Table 5).
Table 5.
Change in selected hydroxylase inhibitors activity of Phaseolus vulgaris L. effected by the application of Kelpak SL.
| Bean cultivar | Application method |
|||||
|---|---|---|---|---|---|---|
| C | SS 0.2% | DS 0.2% | SS 0.4% | DS 0.4% | ||
| ACE inhibitor activity [U/g DM] | Aura | 155.3 ± 39.64b | 91.11 ± 11.72a | 138.61 ± 36.37ab | 142.78 ± 6.8b | 96.11 ± 6.70a |
| Toska | ND | ND | ND | ND | ND | |
C, control; SS 0.2%, single spraying by 0.2% solution of Kelpak SL; DS 0.2%, double spraying by 0.2% solution of Kelpak SL; SS 0.4%, single spraying by 0.4% solution of Kelpak SL; DS 0.4%, double spraying by 0.4% solution of Kelpak SL.
ND – not detected.
Means (±SD, n = 18) followed by different small letters are significantly different at p < 0.05.
4. Discussion
The results obtained in the present study showed the stimulating effect of E. maxima extract (Kelpak SL) on the yield and nutraceutical quality of the common bean. The increase in the yield observed in this study confirms previous reports describing the positive impact of seaweed extracts on the yield of plants. Treatments with E. maxima extracts have improved tepary bean (Phaseolus acutifolius) production, but with a rather average weight of seeds than their number in pots (Beckett et al., 1994). Foliar spraying of Kelpak SL aimed at improving yield has already been used for crops such as cereals, maize, blue lupine and potatoes (Matysiak and Kaczmarek, 2008). Also, Thirumaran et al. (2009) observed that SLF (Seaweed Liquid Fertilizer) prepared from Rosenvingea intricata has a positive impact on yield in okra (Abelmoschus esculentus L.).
Despite the numerous studies on the effects of seaweed extract application aimed to improve growth and yield in crop plants, there are only few reports concerning the changes of nutritional and nutraceutical quality. In the present study, there was no significant influence of Kelpak SL application on the contents of the main nutrients in the tested bean varieties. However, other studies have indicated that seaweed extract can improve the nutritional quality of some plants. For example, seaweeds extract treatment had increased the protein content in okra (A. esculentus L.), green gram (Phaseolus radiate L.) and fenugreek (Trigonella foenum-graecum L.) by 28.04%, 19.43% and over three-fold, respectively. In all the above-mentioned plants seaweed extract treatments also increased carbohydrate content and in okra also dietary fiber content (by about 36% in respect to untreated plants) (Zodape et al., 2008, Zodape et al., 2010, Pise and Sabale, 2010).
There is a lack of information concerning the influence of seaweed extracts on protease inhibitors activity, but there are some data on the impact of abiotic elicitation on protease inhibitor activities. In response to stress, plants overproduce pathogen-related proteins, inter alia, protease and amylase inhibitors. In the work conducted by Świeca and Baraniak (2014), elicitation with H2O2 caused a decrease in trypsin and chymotrypsin inhibitors activity. In contrast, an increase in α-amylase inhibitors activity in response to osmotic stresses was described by Świeca et al. (2013). Results obtained in the present work indicated that Kelpak SL applications influenced trypsin inhibitors’ activity, but did not affect the activity of α-amylase inhibitors. On the one hand, an increased activity of hydrolyse inhibitors may limit nutrients’ digestibility; however on the other hand, these inhibitors exhibit a number of important health-promoting properties. For example, the ACE inhibitor is responsible for antihypertensive activity, and the trypsin inhibitor, which belongs to the Bowman–Birk inhibitor family, has been shown to have antiproliferative and anti-inflammatory effects (Rebello et al., 2014).
The nutraceutical potential of legumes is linked with a high content of bioactive constituents, such as phenolics, vitamins, and bioactive peptides (Jakubczyk and Baraniak, 2014). There is much evidence that an induction of the phenylopropanoid metabolism by different environmental factors and stress conditions results in a significant increase in phenolic compound levels (Świeca and Baraniak, 2014, Złotek et al., 2014, Szymanowska et al., 2015, Świeca, 2015). Plant phenolics are very desirable compounds in the human diet because of their antioxidant properties and different preventive roles against diseases associated with oxidative stress, such as cancer and cardiovascular and neurodegenerative (Riedel et al., 2012). So, changes in phenolic concentrations may influence the antioxidant activities of plant foods.
Some studies indicate that seaweed extract can also influence plant metabolism, enhancing the content of some bioactive compounds. Pise and Sabale (2010), studying the effect of three seaweed extracts (Ulva fasciata, Sargassum ilicifolium and Gracilaria corticata) on the yield and quality of T. foenum-graecum L., found significantly higher chlorophyll, carotenoid and phenolic contents in plant sprayed with the tested extracts. Also, Keitte mango trees treated with algae extracts reacted by the increased production of vitamin C in fruits (Abd El-Motty et al., 2010).
In the present study, elicitation of bean plants with Kelpak SL resulted in an increase in phenolic contents, especially in the Toska cultivar (Fig. 1). The increased phenolic content, observed after Kelpak SL application, was translated into the antioxidant potential of the studied bean seeds (Figure 1, Figure 2). These results closely correspond with data from other studies. In the research by Fan et al. (2011), commercial extracts of brown seaweed (A. nodosum) at a concentration of 1.0 g/l significantly enhanced the total phenolic and flavonoid content and antioxidant activity in spinach leaves.
Kelpak SL is used as a natural, environmentally-friendly and safe preparation, which increases the yield and nutrient quality of plants in many crops (Matysiak and Kaczmarek, 2008, Kocira et al., 2013, Sosnowski et al., 2015). Although there are not enough studies into the specific compounds within E. maxima that are responsible for the desirable effects observed after application, based on the chemical composition of commercial extracts some conclusions may be stated. Rengasamy et al. (2015b) suggested that one of the most important factors for plant physiological response is eckol – a phlorotannin identified in Kelpak SL, which possibly acts through the synergistic effects with other plant growth hormones. Eckol treatment enhanced α-amylase and malate dehydrogenase activities, level of proteins, total phenolics and iridoid glycosides of maize roots.
5. Conclusions
In conclusion, foliar application of Kelpak SL (E. maxima extracts) stimulates the yield and nutraceutical potential of the common bean. The final effect was strongly determined by the cultivar, numbers of treatments and biopreparation concentration. The highest increase in yield in Aura beans was noted by single spraying with a 0.4% solution of Kelpak SL. For the Toska cultivar, the best results were recorded after double treatments with 0.2% Kelpak SL. Application of E. maxima extracts did not significantly affect the main nutrient contents, but had a positive impact on the content and the activity of certain bioactive compounds.
Acknowledgements
This research was supported by the Ministry of Science and Higher Education of Poland as part of the statutory activities of the Department of Machinery Exploitation and Production Process Management, University of Life Sciences in Lublin.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Anna Kocira, Email: akocira@pwsz.chelm.pl.
Michał Świeca, Email: michal.swieca@up.lublin.pl.
Sławomir Kocira, Email: slawomir.kocira@up.lublin.pl.
Urszula Złotek, Email: urszula.zlotek@up.lublin.pl.
Anna Jakubczyk, Email: anna.jakubczyk@up.lublin.pl.
References
- Abd El-Motty E.Z., Shahin M.F.M., El-Shiekh M.H., Abd-El-Migeed M.M.M. Effect of Algae extract and yeast application on growth, nutritional status, yield and fruit quality of Keitte mango trees. Agric. Biol. J. North Am. 2010;1(3):421–429. [Google Scholar]
- Beckett R.P., Mathegka A.D.M., Van Staden J. Effect of seaweed concentrate on yield of nutrient-stressed tepary bean (Phaseolus acutifolius Gray) J. Appl. Phycol. 1994;6:429–430. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang B., Chen R., Huang I., Chang H. Assays for angiotensin converting enzyme inhibitory activity. Anal. Biochem. 2001;291:84–88. doi: 10.1006/abio.2001.5005. [DOI] [PubMed] [Google Scholar]
- Deshpande S.S., Cheryan M. Preparation and anti-nutritional characteristics of dry bean (Phaseolus vulgaris L.) protein concentrates. Qual. Plant Foods Hum. Nutr. 1984;34:185–196. [Google Scholar]
- Dobromilska R., Gubarewicz K., Konieczny M. Influence of seaweed preparations on yield and quality of the small-sized tomato planted under covers. Zesz. Probl. Post. Nauk Roln. 2009;539:143–149. [Google Scholar]
- Durak A., Baraniak B., Jakubczyk A., Świeca M. Biologically active peptides obtained by enzymatic hydrolysis of Adzuki bean seeds. Food Chem. 2013;141:2177–2183. doi: 10.1016/j.foodchem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Fan D., Hodges M., Zhang J., Kirby C.W., Ji X., Locke S.J., Critchley A.T., Prithiviraj B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011;124:195–202. [Google Scholar]
- Fuleki T., Francis F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968;33:72–77. [Google Scholar]
- Goni I., Garcia-Alonso A., Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997;17:427–437. [Google Scholar]
- Hayakari M., Kondo Y., Izumi H. A rapid and simple spectrophotometric assay of angiotensin-converting enzyme. Anal. Biochem. 1978;84:361–369. doi: 10.1016/0003-2697(78)90053-2. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A., Baraniak B. Angiotensin I converting enzyme inhibitory peptides obtained after in vitro hydrolysis of pea (Pisum sativum var. Bajka) globulins. BioMed Res. Int. 2014 doi: 10.1155/2014/438459. Article ID: 438459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakade M.L., Rackis J.L., McGhee J.E., Puski G. Determination of trypsin inhibitor activity of soy bean products: a collaborative analysis of an improved procedure. Cereal Chem. 1974;51:376–382. [Google Scholar]
- Kavipriya R., Dhanalakshmi P.K., Jayashree S., Thangaraju N. Seaweed extract as a biostimulant for legume crop, green gram. J. Ecobiotechnol. 2001;3:16–19. [Google Scholar]
- Kocira A., Kornas R., Kocira S. Effect assessment of Kelpak SL on the bean yield (Phaseolus vulgaris L.) J. Cent. Eur. Agric. 2013;14:67–76. [Google Scholar]
- Lamaison J.L.C., Carnet A. Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D.C.) en fonction de la vegetation. [Contents the main flavonoids in the flowers Crataegeus monogyna Jacq and Crataegeus laevigata (Poiret D.C.) depending on the vegetation] Pharm. Acta Helv. 1990;65:315–320. [Google Scholar]
- Matysiak K., Kaczmarek S. Potential advantages of Kelpak bioregulator applied to some field crops. In: Dąbrowski Z.T., editor. Biostimulators in Modern Agriculture. Filds Crop. Editorial House Wieś Jutra; Warsaw: 2008. pp. 99–106. [Google Scholar]
- Matysiak K., Kaczmarek S., Leszczyńska D. Influence of liquid seaweed extract of Ecklonia maxima on winter wheat cv. Tonacja. J. Res. Appl. Agric. Eng. 2012;57:44–47. [Google Scholar]
- Messina V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014;100:437S–442S. doi: 10.3945/ajcn.113.071472. [DOI] [PubMed] [Google Scholar]
- Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. [Google Scholar]
- Papenfus H.B., Stirk W.A., Finnie J.F., Van Staden J. Seasonal variation in the polyamines of Ecklonia maxima. Bot. Marina. 2012;55:539–546. [Google Scholar]
- Pise N.M., Sabale A.B. Effect of seaweed concentrates on the growth and biochemical constituents of Trigonella foenum-graecum L. J. Phytol. 2010;2(4):50–56. [Google Scholar]
- Pulido R., Bravo L., Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rebello C.J., Greenway F.L., Finley J.W. Whole grains and pulses: a comparison of the nutritional and health benefits. J. Agric. Food Chem. 2014;62:7029–7049. doi: 10.1021/jf500932z. [DOI] [PubMed] [Google Scholar]
- Rengasamy K.R.R., Kulkarni M.G., Stirk W.A., Van Staden J. Eckol – a new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015;27:581–587. [Google Scholar]
- Rengasamy K.R.R., Kulkarni M.G., Stirk W.A., Van Staden J. Eckol improves growth, enzyme activities, and secondary metabolite content in maize (Zea mays cv. Border King) J. Plant Growth Regul. 2015;34(2):410–416. [Google Scholar]
- Ribeiro A.C., Teixeira A.R., Ferreira R.B. Characterization of globulins from common vetch (Vicia sativa L.) J. Agric. Food Chem. 2004;52:4913–4920. doi: 10.1021/jf049833p. [DOI] [PubMed] [Google Scholar]
- Riedel H., Akumo D.N., Saw N.M.M.T., Kütük O., Neubauer P., Smetanska I. Elicitation and precursor feeding influence phenolic acids composition in Vitis vinifera suspension culture. Afr. J. Biotechnol. 2012;11(12):3000–3008. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1974;299:152–178. [Google Scholar]
- Sivasankari S., Venkatesalu V., Anantharaj M., Chandrasekaran M. Effect of seaweed extracts on the growth and biochemical constants of Vigna sinensis. Bioresour. Technol. 2006;97:1745–1751. doi: 10.1016/j.biortech.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Sosnowski J., Jankowski K., Wiśniewska-Kadżajan B., Malinowska E., Kolczarek R., Czelusiński W., Radzka E. Shaping of chemical composition and digestibility of Medicago x varia T. Martyn under the influence of Ecklonia maxima seaweed extract. Fresenius Environ. Bull. 2015;24(3a):881–887. [Google Scholar]
- Stirk W.A., Tarkowská D., Turečová V., Strnad M., Van Staden J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014;26:561–567. [Google Scholar]
- Świeca M. Elicitation with abiotic stresses improves pro-health constituents, antioxidant potential and nutritional quality of lentil sprouts. Saudi J. Biol. Sci. 2015;22(4):409–416. doi: 10.1016/j.sjbs.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świeca M., Baraniak B. Influence of elicitation with H2O2 on phenolics content, antioxidant potential and nutritional quality of Lens culinaris sprouts. J. Sci. Food Agric. 2014;94(3):489–496. doi: 10.1002/jsfa.6274. [DOI] [PubMed] [Google Scholar]
- Świeca M., Baraniak B., Gawlik-Dziki U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013;138:1414–1420. doi: 10.1016/j.foodchem.2012.09.122. [DOI] [PubMed] [Google Scholar]
- Szymanowska U., Złotek U., Karaś M., Baraniak B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015;172:71–77. doi: 10.1016/j.foodchem.2014.09.043. [DOI] [PubMed] [Google Scholar]
- Thirumaran G., Arumugam M., Arumugam R., Anantharaman P. Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus medikus. Am-Euras. J. Agron. 2009;2(2):57–66. [Google Scholar]
- Vallad G.E., Goodman R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004;44:1920–1934. [Google Scholar]
- Verkleij F.N. Seaweed extracts in agriculture and horticulture. Biol. Agric. Hortic. 1992;8:309–324. [Google Scholar]
- Xu B.J., Chang S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007;72(2):159–166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Złotek U., Wójcik W. Effect of arachidonic acid elicitation on lettuce resistance towards Botrytis cinerea. Sci. Hortic. 2014;179:16–20. [Google Scholar]
- Złotek U., Świeca M., Jakubczyk A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.) Food Chem. 2014;148:253–260. doi: 10.1016/j.foodchem.2013.10.031. [DOI] [PubMed] [Google Scholar]
- Zodape S.T., Kawarkhe V.J., Patolia J.S., Warade A.D. Effect of liquid seaweed fertilizer on yield and quality of okra (Abelmoschus escelentus L.) J. Sci. Ind. Res. 2008;67:1115–1117. [Google Scholar]
- Zodape S.T., Mukhopadhyay S., Eswaran K., Reddy M.P., Chikara J. Enhanced yield and nutritional quality in green gram (Phaseolus radiata L) treated with seaweed (Kappaphycus alvarezii) extract. J. Sci. Ind. Res. 2010;69:468–471. [Google Scholar]