Abstract
To study the effect of raspberry total flavonoids on perimenopausal model in mice. Blank group, sham operation, and the rest of the mice made the menopausal model. Choose 72 mice castrated completely random divided into 6 groups for the experiment, respectively: model group, gengnianan (GNA) capsule group, soybean isoflavone soft (SIS) capsule group, high, mid and low dose group of total flavonoids of raspberry (TFR). Animals in each group were given the corresponding drugs tenth days after operation, and were given intragastrical administration of once a day for continuous administration of 21 days. Each group of mice in the administration of 18 days to determine the number of autonomic activities within 5 min, in the administration of 19–20 days to determine the incubation of the mice first entry into the darkroom and the number of shocks into the darkroom within 5 min. At 2 h after the last administration (fasting for 12 h), mice were sacrificed and serum was collected. Serum levels of E2, T, LH and FSH were measured. Dissect the uterus, uterus, thymus and spleen. Weigh the wet weight and calculate the organ index, the morphological changes of uterus, thymus and spleen were observed. The results showed that the TFR had a good therapeutic effect on the perimenopausal model of mice after giving a high, mid and low dose of raspberry flavonoids for some time.
Keywords: Total flavonoids of raspberry (TFR), Perimenopausal model, Autonomic activity, Memory, Sex hormone levels
1. Introduction
Perimenopausal period, also known as menopause, is the transition from the reproductive age of women to the elderly (about 45–55 years old), due to a series of changes in ovarian dysfunction. Clinical manifestations of menstrual disorders to menopause, memory loss, irritability, tidal sweat, insomnia, joint pain, etc., is a necessary stage for every woman's life, have seriously affected women's physical and mental health, work and life (Li et al., 2016). Chinese medicine using the overall concept and syndrome differentiation theory, according to different physical, syndromes treatment of perimenopausal syndrome, efficacy and less adverse reactions. What we need to be concerned about is that women's perioperative prevention is particularly important, in the treatment of perimenopausal syndrome at the same time, should fully reflect the traditional Chinese medicine “treatment did not occur disease” thinking, take preventive measures. To avoid low-level Chinese medicine treatment of perimenopausal syndrome experimental and clinical research, under the guidance of traditional Chinese medicine theory, combined with traditional Chinese medicine technology and methods to strengthen scientific research and experimental design, unified standardized dialectical system, to find the objective of the disease differentiation indicators, the establishment of standardized index system, improve the scientific, objectivity and reliability of Chinese medicine for perimenopausal syndrome (Gohar et al., 2017, Jamal et al., 2017). Raspberry as a traditional nourishing kidney medicine, in China has thousands of years of use history, researchers from the raspberry separation of terpenoids, flavonoids, alkaloids, coumarins and other ingredients, and the preliminary pharmacological screening was carried out for the isolated compounds, in the anti-tumor, anti-aging, scavenging free radicals and other aspects of a clear activity. Perimenopausal care is a hot issue that is of great concern both at home and abroad, China’s perimenopausal women ranks first in the world, therefore, the search for perimenopausal drugs and research is more urgent. In this study, ovarian castration was used to induce the perimenopausal model of mice to observe the effect of TFR on perimenopausal effects in mice (Fan et al., 2014).
2. Materials and methods
2.1. Animals
KM mice, female, 20–25 g, provided by the Wuhan Institute of Biological Products (Animal permit number: 42000400000611). Laboratory Certificate of Conformity: SYXK (Henan) 2010-001.
2.2. Experimental reagents and drugs
Injection of penicillin sodium, North China Pharmaceutical Co., Ltd., specifications: 4 million units, lot: c1206807; Raspberry, Anhui Xinxing Chinese Herbal Medicine Pieces Co., Ltd., lot: 20130901; raspberry total flavonoids, ethanol extracted AB-8 microporous resin, the content of 57.86%; Gengnianan capsule, Shanxi Star Pharmaceutical Co., Ltd. production, lot: 121104; Soybean isoflavones E soft capsule, Weihai Purple Biotechnology Development Co., Ltd. production, lot: 13070302.
2.3. Experimental instruments
Autonomic activities test instrument, Chengdu taimeng Technology Co. Ltd., model: ZZ-6; mouse step through instrument, Chengdu taimeng Technology Co. Ltd., model: BA-200; high speed refrigerated centrifuge, science innovation Limited by Share Ltd Zhongjia branch, model: KDC-160HR; eliasa, BIO-RAD, model: 680.
2.4. Modeling and administration
100 mice weighing 23–25 g female KM mice were randomly selected 12 as the blank group, the sham operation was treated, and all the other mice were made of menopausal model. Mice were intraperitoneally injected with 10% chloral hydrate (0.03 ml/10 g) anesthesia after abdominal fixation, first cut the ovarian fallopian tube (including fat) with a thin line ligation, and then remove the bilateral ovaries. After intensive care, intramuscular injection of penicillin 200,000 u/kg (0.1 mL each) to prevent infection, continuous 3 days, once a day. 5 days after the start of the mouse vaginal smear, once a day for 5 days to determine whether the complete removal of ovaries (Rashid et al., 2017). The smear showed the Estrus reaction of the mouse to abandon. Choose 72 mice castrated completely random divided into 6 groups for the experiment, respectively: model group, GNA capsule group, SIS capsule group, high, mid and low dose group of TFR. Animals in each group were given corresponding drugs tenth days after operation, GNA capsule group fed GNA capsule suspension 675 mg/kg, SIS capsule group fed SIS capsule suspension 250 mg/kg, high, mid and low dose group of TFR respectively by gavage, high, mid and low dose of TFR 200 mg/kg, 100 mg/kg, 50 mg/kg, blank group and model group were fed with the same volume of 0.5% CMC solution. 1 times a day, 0.1 ml/10 g, continuous administration of 21 days. Each group of mice in the administration of 18 days to determine the number of autonomic activities within 5 min, in the administration of 19–20 days to determine the incubation of the mice first entry into the darkroom and the number of shocks into the darkroom within 5 min. At 2 h after the last administration (fasting for 12 h), mice were sacrificed and serum was collected. Serum levels of E2, T, LH and FSH were measured. Dissect the uterus, uterus, thymus and spleen. Weigh the wet weight and calculate the organ index, the path morphology changes of uterus, thymus and spleen were observed.
2.5. Method statistical analysis
SPSS 17.0 for windows has been used for statistical processing. The measurement data is represented by mean ± variance ( ± s), and group comparison has adopted analysis of variance; and the Rid it tests has been used to rank the data.
3. Results
3.1. Effect of autonomic activity on the perimenopausal period in mice
From the Table 1, compared with the blank group, the number of autonomic activities and stands were significantly reduced in the model group, (P < 0.01), indicating that the mice in the model group reduced the curiosity of the fresh environment. Compared with the model group, the GNA capsule group, SIS capsule group, high and mid dose group of TFR could significantly increase the number of activities and standing (P < 0.01), low dose group of TFR could increase the number of activities and standing (P < 0.05).
Table 1.
Effect of autonomic activity on the perimenopausal period in mice ( ± s, n = 12).
| Group | Dose (mg/kg) | Number of activities (frequency) | Number of standing (frequency) |
|---|---|---|---|
| Blank group | – | 134.333 ± 18.102** | 59.583 ± 8.959** |
| Model group | – | 85.667 ± 11.555 | 27.333 ± 5.662 |
| GNA capsule group | 675 | 130.500 ± 19.870** | 52.083 ± 11.365** |
| SIS capsule group | 250 | 125.917 ± 11.547** | 50.167 ± 14.640** |
| TFR high-dose group | 200 | 123.083 ± 14.902** | 45.500 ± 10.068** |
| TFR mid-dose group | 100 | 134.583 ± 17.926** | 43.750 ± 8.159** |
| TFR low-dose group | 50 | 104.750 ± 12.263* | 36.667 ± 5.805* |
Note: Compared with the model group.
P < 0.05.
P < 0.01.
3.2. Effect of memory on the perimenopausal period in mice
From the Table 2, compared with the blank group, the latent period and the number of shocks were significantly increased in the model group (P < 0.01). Reflecting the decline in memory of the perimenopausal model of mice (Sindhu et al., 2017). Compared with the model group, the GNA capsule group, SIS capsule group, high and mid dose group of TFR could significantly improve the incubation period of mice in avoid dark experiments and reduce the number of shocks (P < 0.01), improve mice memory. Low dose group of TFR could reduce the number of shocks (P < 0.05).
Table 2.
Effects of TFR on the incubation period and the number of shocks in the mice perimenopausal model ( ± s, n = 12).
| Group | Dose (mg/kg) | Icubation period (S) | Number of shocks (frequency) |
|---|---|---|---|
| Blank group | – | 191.000 ± 52.401** | 1.917 ± 1.730** |
| Model group | – | 79.000 ± 22.066 | 6.500 ± 1.314 |
| GNA capsule group | 675 | 163.833 ± 38.879** | 3.000 ± 1.537** |
| SIS capsule group | 250 | 166.667 ± 24.403** | 3.333 ± 0.888** |
| TFR high-dose group | 200 | 160.167 ± 26.219** | 2.000 ± 0.953** |
| TFR mid -dose group | 100 | 151.833 ± 32.755** | 2.667 ± 0.985** |
| TFR low -dose group | 50 | 94.917 ± 7.891 | 4.167 ± 1.642* |
Note: Compared with the model group.
P < 0.05.
P < 0.01.
3.3. Effect of organ index on the perimenopausal period in mice
From the Table 3, compared with the blank group, the uterine index of the model group was significantly decreased (P < 0.01), indicating that ovariectomy led to perimenopausal model mice uterine atrophy, removal of ovaries and thus estrogen deficiency lead to perimenopausal mice immune organ atrophy. Compared with the model group, the GNA capsule group, SIS capsule group, high and mid dose group of TFR could significantly improve thymus, spleen, uterine index (P < 0.01).
Table 3.
Effects of TFR on the thymus, spleen, uterine index in the mice perimenopausal model ( ± s, n = 12).
| Group | Dose (mg/kg) | Thymus index (mg/g) | Spleen index (mg/g) | Uterine index (mg/g) |
|---|---|---|---|---|
| Blank group | – | 3.116 ± 0.613** | 6.115 ± 0.904** | 3.116 ± 0.613** |
| Model group | – | 1.685 ± 0.276 | 3.868 ± 0.966 | 1.699 ± 0.525 |
| GNA capsule group | 675 | 2.996 ± 0.092** | 5.851 ± 0.641** | 2.971 ± 0.341** |
| SIS capsule group | 250 | 3.067 ± 0.623** | 5.793 ± 1.114** | 3.073 ± 0.870** |
| TFR high-dose group | 200 | 2.949 ± 0.559** | 5.935 ± 0.959** | 2.946 ± 0.426** |
| TFR mid -dose group | 100 | 2.893 ± 0.111** | 5.791 ± 0.932** | 2.903 ± 0.416** |
| TFR low -dose group | 50 | 1.964 ± 0.547 | 4.563 ± 1.082 | 2.006 ± 0.644 |
Note: Compared with the model group.
* P < 0.05.
P < 0.01.
3.4. Effect of serum sex hormone level on the perimenopausal period in mice
From the Table 4, compared with the blank group, the levels of E2 and T were significantly decreased (P < 0.01), and the levels of LH and FSH were significantly increased in the model group mice serum (P < 0.01). Indicating that the removal of ovarian lead to perimenopausal model mice serum levels of sex hormones disorder, perimenopausal mouse model replication success. Compared with the model group, the GNA capsule group, SIS capsule group, high and mid dose group of TFR could significantly increase the levels of E2 and T, decreased the levels of FSH and LH in the mice serum of the perimenopausal model (P < 0.01). Low dose group of TFR could increase the levels of T and decreased the levels of FSH (P < 0.05).
Table 4.
Effects of TFR on the serum sex hormone level in the mice perimenopausal model ( ± s, n = 12).
| Group | Dose (mg/kg) | E2 (pmol/L) | T (pg/mL) | FSH (U/mL) | LH (U/mL) |
|---|---|---|---|---|---|
| Blank group | – | 30.623 ± 4.055** | 369.548 ± 43.958** | 11.739 ± 5.326** | 1.013 ± 0.300** |
| Model group | – | 20.595 ± 3.567 | 207.167 ± 49.358 | 23.558 ± 4.553 | 2.184 ± 0.361 |
| GNA capsule group | 675 | 28.208 ± 1.965** | 328.833 ± 33.177** | 13.773 ± 4.900** | 1.272 ± 0.332** |
| SIS capsule group | 250 | 28.975 ± 2.692** | 334.310 ± 41.010** | 14.587 ± 3.661** | 1.177 ± 0.220** |
| TFR high-dose group | 200 | 28.017 ± 2.249** | 309.615 ± 73.141* | 13.159 ± 4.847** | 1.204 ± 0.111** |
| TFR mid-dose group | 100 | 27.208 ± 1.205** | 355.000 ± 43.311** | 14.601 ± 4.256** | 1.279 ± 0.117** |
| TFR low-dose group | 50 | 21.097 ± 2.038 | 273.910 ± 32.231* | 17.261 ± 3.676* | 1.666 ± 0.507 |
Note: Compared with the model group.
P < 0.05.
P < 0.01.
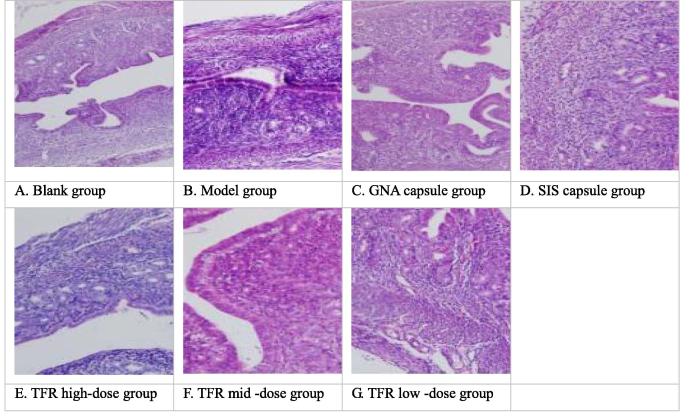
3.5. Effect of uterine path morphology on the perimenopausal period in mice
From the Table 5, compared with the blank group, in model group, there were significant pathological changes in uterus (P < 0.01), endometrial epithelial cells, glands were obvious atrophy (Fig. 1B). Compared with the model group, the GNA capsule group, SIS capsule group, mid dose group of TFR could significantly improve the pathological changes of the uterus in mice (P < 0.01), endometrial epithelial cells, glands small part of atrophy (Fig. 1C, D, F). High dose group of TFR could improve the pathological changes of the uterus in mice (P < 0.05), endometrial epithelial cells, glands partial atrophy (Fig. 1E).
Table 5.
Effects of TFR on uterine path morphology in the mice perimenopausal model ( ± s, n = 12).
| Group | Dose (mg/kg) | − | + | ++ | +++ | P |
|---|---|---|---|---|---|---|
| Blank group | – | 11 | 1 | 0 | 0 | |
| Model group | – | 0 | 2 | 4 | 6 | △△ |
| GNA capsule group | 675 | 3 | 5 | 3 | 1 | ** |
| SIS capsule group | 250 | 3 | 4 | 5 | 0 | ** |
| TFR high-dose group | 200 | 3 | 4 | 3 | 1 | * |
| TFR mid -dose group | 100 | 4 | 6 | 2 | 0 | ** |
| TFR low -dose group | 50 | 0 | 3 | 6 | 3 |
Note: Compared with the model group.
“−” endometrial epithelial cells, glands, muscle, serous are normal; “+” endometrial epithelial cells, glands small part of atrophy, muscle serous membrane are normal; “++” endometrial epithelial cells, glands partial atrophy, muscle slightly shrinking, serous are normal; “+++” endometrial epithelial cells, glands were obvious atrophy, serous are normal.
P < 0.05.
P < 0.01.
Fig. 1.
Effect of uterine path morphology on the perimenopausal period in mice (HE × 200).
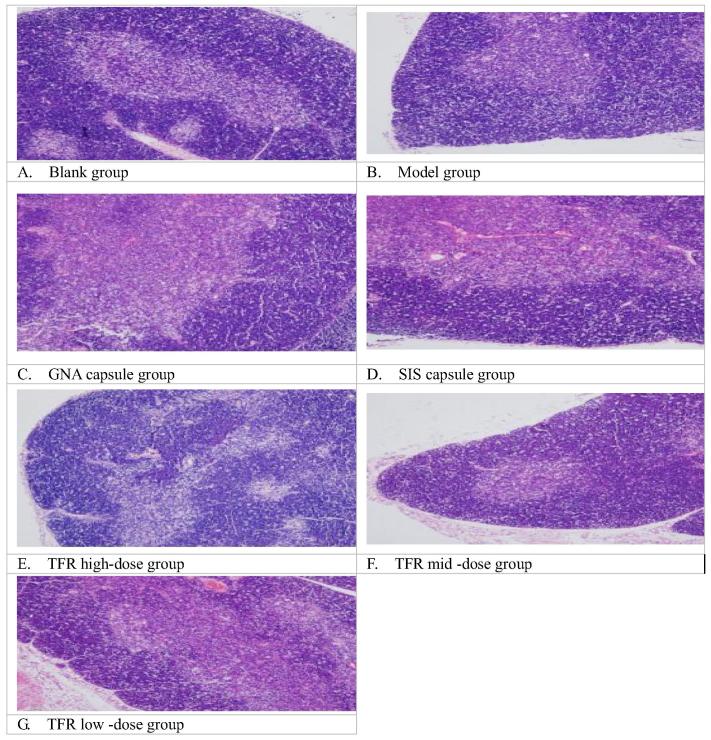
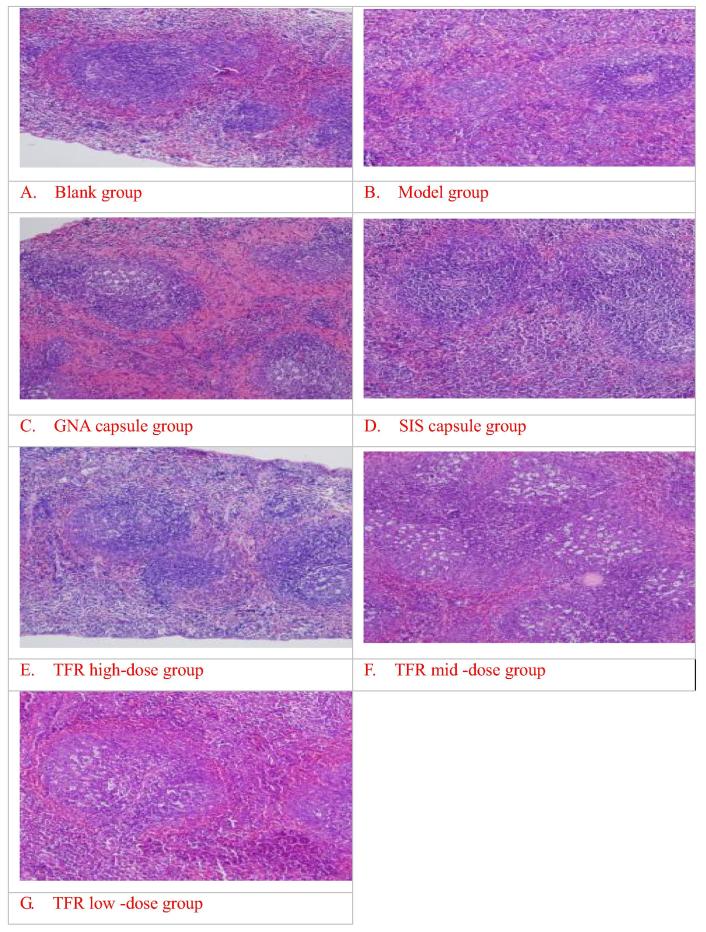
3.6. Effect of thymus, spleen path morphology on the perimenopausal period in mice
From the Table 6, compared with the blank group, in the model group, the thickness of thymic cortex and the volume of splenic nodules were significantly decreased (P < 0.01); the thymic lobule cortex, the medulla clear boundaries, the cortical atrophy thinning, lymphocytes decreased significantly (Fig. 2B); the red pulp of spleen white pulp clear boundaries, splenic nodule was obviously reduced, lymphocyte sparse decreased obviously (Fig. 3B); indicating that after the perimenopausal model the mice of thymus, spleen volume atrophy (Zaheer et al., 2017). Compared with the model group, the GNA capsule group, SIS capsule group, high dose group of TFR could significantly thickened the volume of splenic nodules and the thickness of thymic cortex (P < 0.01), in this three groups, the thymic lobule cortex, medulla, clear boundary, the cortical thickening, denser lymphocyte (Fig. 2C, D, E); the spleen red and white pulp, clear boundary, splenic corpuscle increased significantly, lymphocyte dense (Fig. 3C, D, E); the mid dose group of TFR could thicken the thickness of thymic cortex (P < 0.05). The cortical, medullary thymic lobule, clear boundary, cortical atrophy thinning dense, lymphocyte (Fig. 2F).
Table 6.
Effects of TFR on the thymus, spleen path morphology in the mice perimenopausal model ( ± s, n = 12).
| Group | Dose (mg/kg) | Thymic cortex thickness (μm) | Splenic nodule (μm) |
|---|---|---|---|
| Blank group | – | 337.94 ± 26.16** | 427.88 ± 58.96** |
| Model group | – | 215.51 ± 28.11 | 261.52 ± 33.22 |
| GNA capsule group | 675 | 364.53 ± 36.82** | 381.28 ± 59.62** |
| SIS capsule group | 250 | 321.42 ± 66.84** | 340.71 ± 52.41** |
| TFR high-dose group | 200 | 285.96 ± 42.13** | 324.13 ± 35.51** |
| TFR mid-dose group | 100 | 271.46 ± 37.77* | 285.24 ± 25.81 |
| TFR low-dose group | 50 | 279.91 ± 58.15 | 257.07 ± 27.90 |
Note: Compared with the model group.
P < 0.05.
P < 0.01.
Fig. 2.
Effect of thymus path morphology on the perimenopausal period in mice (HE × 200).
Fig. 3.
Effect of spleen path morphology on the perimenopausal period in mice (HE × 200).
4. Discussion
Ovaries castration produce menopausal model in mice, model group mice appeared the number of activities and standing significantly reduced; the curiosity of the fresh environment is reduced; the latency of entering the darkroom is significantly shorter, the number of electric shocks increased significantly, the memory of the mice decline; the levels of E2 and T are significantly decreased, and the levels of LH and FSH are significantly increased, sex hormone levels in mice serum disorder; uterine lesions, thymus, spleen volume atrophy; indicating that the perimenopausal mice model is successfully replicated. Given the high, mid and low dose TFR after a period, the phenomenon has been improved and relieved, the results showed that TFR had a good therapeutic effect on the perimenopausal model of mice.
Chinese medicine treatment of perimenopausal syndrome with kidney deficiency pathogenesis theory (Liu, 2012, Yang and Zhou, 2012), women into the perimenopausal period, kidney qi deficiency, red any two losses, lack of refined gas, menstrual disorders, as well as menopause, and accompanied by a series of symptoms, such as fanre, insomnia, more dreams. Kidney deficiency is the root cause of perimenopausal syndrome (Zhang and Miao, 2011, Gohar et al., 2017). Recent studies have shown that the drug for kidney yang have estrogen-like effects, on perimenopausal syndrome have a better therapeutic effect (Wei and Miao, 2013), and no side effects and contraindications. Western medicine treatment with hormone replacement method, has certain limitations. Raspberry is Rosaceae Rubus plants, medicine and food, and now has been widely used in medicine, food, health products, cosmetics and other industries (Hummer, 2010, Yan et al., 2013). Rubus plants contain rich flavonoids (Li, 2009, Jiang et al., 2013), with anti-tumor, thrombosis, hypolipidemic, hypoglycemic, promote blood circulation, eliminate free radicals (Bao et al., 2011, Wei et al., 2012), anti-aging effected (Chang et al., 2013). Chinese medicine raspberry tonifying kidney, solid fine, shrink urine, commonly used in the treatment of kidney deficiency enuresis, urinary frequency, impotence premature ejaculation, spermatorrhea and so on (He et al., 2013). Modern pharmacological studies have shown that raspberry has the effect of eliminating fatigue, improving immunity, improving learning and memory, and regulating gonadal axis, etc. (Cheng et al., 2012).
In this study, mice were removed by ovariectomy, to study the effect of TFR on perimenopausal models in mice, the results showed that TFR had better effect on the autonomic activity, memory and hormone level of perimenopausal mice. The ovariectomized animal model is relatively mature and has been widely used, however, the simulation of human perimenopausal syndrome is still inadequate, 90% of menopausal women ovarian is complete, the direct removal of the ovaries so that the body's estrogen levels suddenly decline, and also affected other hormone levels, such as luteinizing hormone, androgen and follicle-stimulating hormone (Rocca et al., 2011, Gao et al., 2017, Jamal et al., 2017). Should further improve the method of model replication, simulation more in line with human perimenopausal animal model. Perimenopausal syndrome caused by metabolic disorders in the body, the symptoms are diverse, in addition to the above test indicators, but also the establishment of other corresponding indicators, more scientific and more comprehensive reflection of TFR on perimenopausal models of mice. TFR play a role in the treatment mechanism is still not clear, the need for further research. Comprehensive and thorough study of TFR on perimenopausal treatment, to develop a safe, reliable, significant treatment of perimenopausal drugs.
Acknowledgments
The research work is supported by Henan province science and technology research cooperation special (142107000102); team of outstanding scientific and technological innovation in Henan province (TCJ2014-391); Henan Province basic and cutting-edge technology research plan (132300410019).
Footnotes
Peer review under responsibility of King Saud University.
References
- Bao Y.M., Kang D.W., Zhang L. Determination of ramulus RuBi flavonoids in the Mongolian medicine and study of its antioxidant effect in vitro. J. Inner Mongolia Univ. for Nationalities (Nat. Sci.) 2011;26(1):14–16. [Google Scholar]
- Chang J., Wang X.C., Li Y.P. Recent advances in the study of structure activity relationship of natural flavonoids. Nat. Prod. Res. Dev. 2013;25(7):1006–1010. [Google Scholar]
- Cheng D., Li J., Zhou B. Study on chemical constituents and pharmacological effect of raspberry. J. Chin. Med. Mater. 2012;35(11):1873–1876. [Google Scholar]
- Fan G., Su L.N., Guo M.J. Progress of experimental model on perimenopausal syndrome. Liaoning J. Tradit. Chin. Med. 2014;41(1):184–185. [Google Scholar]
- Gao W., Wang Y., Wang W., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar S., Abbas G., Sajid S., Sarfraz M., Ali S., Ashraf M., Aslam R., Yaseen K. Prevalence and antimicrobial resistance of Listeria monocytogenes isolated from raw milk and dairy products. Matrix Sci. Medica. 2017;1(1):10–14. [Google Scholar]
- He J.M., Sun N., Wu W.D. HPLC determine the content of ellagic acid, flavonoids and raspberry-F5 in raspberries. China J. Chin. Mater. Med. 2013;38(24):4351–4356. [PubMed] [Google Scholar]
- Hummer K. Rubus pharmacology: antiquity to the present. Hortscience. 2010;45(11):1587–1591. [Google Scholar]
- Jamal M., Shareef M., Sajid S. Lincomycin and tetracycline resistance in poultry. Rev. Matrix Sci. Pharma. 2017;1(1):33–38. [Google Scholar]
- Jiang H.M., Wang X.Q., Shi C.H. Refluxing extraction optimization of total flavonoids from the leaves of rubus coreanus. Guizhou Agric. Sci. 2013;41(5):144–146. [Google Scholar]
- Li D. Study on extraction, purification and hypoglycemic effect of total flavonoids from raspberry leaves. Agric. Univ. Hunan. 2009:2–6. [Google Scholar]
- Li, J.C., Yu, T., Zhang, S., 2016. Research Progress in the treatment of female Perimeno Pausal syndrome. Chinese Community Doctors, 32(23), 9–11.
- Liu Y. Chinese medicine treatment of climacteric syndrome. Chin. J. Ethnomed. Ethnopharmacy. 2012;4:81–82. [Google Scholar]
- Rashid M., Saleem M.I., Deeba F., Khan M.S., Mahfooz S.A., Butt A.A., Abbas M.W. Effect of season on occurrence of caprine mastitis in beetal in faisalabad premises. Matrix Sci. Medica. 2017;1(1):19–21. [Google Scholar]
- Rocca W.A., Grossardt B.R., Shuster L.T. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu Z.U.D., Shafiq Z., Naseer M.U., Khan M.N., Saleemi M.K., Aslam B., Abbas R.Z., Khan M.K. Prevalence of ectoparasitic fauna and efficacy of two commercial acaricides against argus persicus in layer poultry. Matrix Sci. Pharma. 2017;1(1):39–40. [Google Scholar]
- Wei Z.B., Sun J.M., Li P.F. Study on the antioxidant constituents from the roots of raspberry leaves. China J. Chin. Materia Med. 2012;37(23):3591–3594. [PubMed] [Google Scholar]
- Wei Z.Z., Miao M.S. Characteristic analysis of reinforcing kidney traditional chinese medicine in the treatment of perimenopausal syndrome. China J. Chin. Med. 2013;11:1688–1691. [Google Scholar]
- Yan C.X., Ding X.Q., Xia Y. Rubus chingii Hu industry development strategy. Guangdong Agric. Sci. 2013;20:234–236. [Google Scholar]
- Yang Y.Q., Zhou L.Y. Nourishing kidney and blood method in the treatment of climacteric syndrome: clinical observation of 48 cases. Jilin J. Tradit. Chin. Med. 2012;6:604–605. [Google Scholar]
- Zaheer Z., Rahman S.U., Zaheer I., Abbas G., Younas T. Methicillin-resistant staphylococcus aureus in poultry- an emerging concern related to future epidemic. Matrix Sci. Medica. 2017;1(1):15–18. [Google Scholar]
- Zhang Y., Miao M.S. Study on treatment of climacteric syndrome with traditional Chinese medicine of tonifying kidney and strengthening Yang. China J. Chin. Med. 2011;26(9):1084–1087. [Google Scholar]