Abstract
The use of mesenchymal stromal cell (MSC) therapy for the treatment of type 2 diabetes (T2D) and T2D complications is promising; however, the investigation of MSC function in the setting of T2D has not been thoroughly explored. In our current study, we investigated the phenotype and function of MSCs in a simulated in vitro T2D environment. We show that palmitate, but not glucose, exposure impairs MSC metabolic activity with moderate increases in apoptosis, while drastically affecting proliferation and morphology. In co-culture with peripheral blood mononuclear cells (PBMCs), we found that MSCs not only lose their normal suppressive ability in high levels of palmitate, but actively support and enhance inflammation, resulting in elevated PBMC proliferation and pro-inflammatory cytokine release. The pro-inflammatory effect of MSCs in palmitate was partially reversed via palmitate removal and fully reversed through pre-licensing MSCs with interferon-gamma and tumor necrosis factor alpha. Thus, palmitate, a specific metabolic factor enriched within the T2D environment, is a potent modulator of MSC immunosuppressive function, which may in part explain the depressed potency observed in MSCs isolated from T2D patients. Importantly, we have also identified a robust and durable pre-licensing regimen that protects MSC immunosuppressive function in the setting of T2D.
Keywords: mesenchymal stromal cells, type 2 diabetes, palmitate, mesenchymal stem cells, immunomodulatory, obesity, cell therapy
Boland et al. demonstrate that palmitate, a saturated free fatty acid elevated in type 2 diabetic patients, is highly detrimental to the immunosuppressive ability of mesenchymal stromal cells. However, by pre-licensing MSCs with IFN-γ and TNF-α before palmitate exposure, MSC potency was restored.
Introduction
Clinical use of mesenchymal stromal cell (MSC) therapy has gained momentum in recent years because of notable successes in clinical trials for the treatment of graft-versus-host disease,1 critical limb ischemia,2 stroke,3 and type 1 diabetes.4 In contrast with standard pharmacological agents, MSC therapy offers the dynamism of a living system that can home to sites of injury or inflammation, sense factors within a specific environment, and respond with a tailored and multifactorial output.5 Many of the recent successes of MSC therapy have involved the immunomodulatory and pro-regenerative aspects of MSC function. MSCs secrete numerous factors that alter the fate of immune cells, while producing other trophic factors that support the function of non-immune cells.6 Because of this dynamic response, MSC therapy is particularly suited for pathological conditions characterized by a combination of inflammatory infiltration and tissue damage. Although clinical trials over the years have resulted in both successes and setbacks, the trajectory of MSC therapy continues to progress toward clinical adoption for multiple clinical indications.7 For MSC therapy to succeed upon broad clinical adoption, a number of unanswered questions must first be addressed, including the effect of the disease-specific transplant environment on MSC function. It is imperative to anticipate the diverse and complex needs of the general population and the potential differences in the underlying tissue environments within which MSCs will eventually need to function. For example, within the United States alone, nearly 30 million individuals have type 2 diabetes (T2D) and 86 million more are categorized as pre-diabetic.8 The meteoric rise in the prevalence of diabetes has led to both an associated rise in diabetic complications, while also making T2D a common comorbidity that may alter the course and efficacy of treatments for other conditions.9 Thus, for MSC therapy to be applied to the population at large, we must understand how MSC function is altered in the setting of T2D.
T2D is a complex systemic endocrinopathy characterized by metabolic derangement and chronic sub-clinical levels of inflammation, as well as the classical development of hyperglycemia secondary to loss of insulin sensitivity.10, 11, 12 The pathological serum and tissue environments of T2D have been shown to be detrimental to the function of a number of cells types within the mesenchymal and hematopoietic lineages, including endothelial progenitor cells,13 bone marrow mesenchymal progenitor cells,13, 14 and cardiac mesenchymal cells15 in mouse models of T2D, as well as within T2D patients. Recent work from Kizilay Mancini et al.16 and Serena et al.17 have shown that adipose-tissue-derived MSCs (ad-MSCs) isolated from patients with T2D show diminished immunosuppressive ability at baseline. However, the observation that MSCs isolated from T2D patients are immunosuppressively impaired has remained substantially correlative, with no specific effector molecules and mechanisms identified as causative of this decline in potency. In addition to abnormal ad-MSCs, several immune cell subtypes, including macrophages, B cells, and CD4+ T effector cell subsets, have also been shown to exhibit abnormal behavior in a T2D environment.18, 19, 20 Ip et al.21 identified that peripheral blood mononuclear cells (PBMCs) isolated from obese T2D patients show population distributions that are highly distinct from obese non-T2D patients, with obese T2D patients showing an enrichment in both the number and baseline cytokine secretion from pro-inflammatory Th17 and Th1 T cell subsets. In sum, these studies have identified the T2D environment as a distinct and potentially hostile environment for MSC immunosuppressive activity warranting our investigation of the direct effects of palmitate on MSC function.
Elevated serum levels of palmitate, a 16-carbon saturated fatty acid, have been implicated as a risk factor for the development of T2D.22 Both serum levels and the relative ratio of palmitate to other fatty acids in the adipose tissue of T2D patients have been reported to be elevated.13, 23 Physiologically, palmitate can signal through toll-like receptor 4 (TLR4)24 and peroxisome proliferator-activated receptor gamma (PPAR γ),13 integrate into membrane phospholipids,20, 25, 26 serve as an energy substrate,27 and become stored within lipid droplets.28 Consequences of elevated palmitate exposure tend to be cell type specific, although similar signaling pathways have been implicated in the pathophysiology affecting multiple cell types. Palmitate has been demonstrated to induce higher levels of pro-inflammatory cytokine production from PBMCs isolated from T2D patients versus non-T2D controls,19 while in other studies palmitate has significantly increased rates of apoptosis (i.e., lipoapoptosis) in cardiomyocytes.29 After 18 hr of palmitate exposure, skeletal muscle increases the release of monocyte chemoattractant factors, causing an increase in pro-inflammatory cytokines within the local environment of the muscle.30 In pancreatic β cells, a highly secretory cell type, palmitate exposure stimulates the unfolded-protein response (UPR), leading to endoplasmic reticulum (ER) stress and induction of a 4-fold increase in apoptosis within 48 hr.25 Given that MSCs rely on secreted factors to control inflammation, Lu et al.31 investigated whether palmitate treatment initiates a similar pathway of ER stress-induced apoptosis within MSCs. Both ER stress and increased apoptosis were evident in MSCs following palmitate exposure; however, whether this elevated ER stress was associated with a functional decline in the production of secreted or intracellular MSC immunomodulatory molecules has never been reported.
Currently, MSC therapy is being explored in active clinical trials for the treatment of several diabetic complications, including critical limb ischemia, diabetic foot ulcers, diabetic neuropathy, and peripheral artery disease.5 In addition to atherosclerosis32 and age16 being factors that depress MSC donor potency, several recent reports have demonstrated that ad-MSCs isolated from T2D patients are inferior at blocking T cell proliferation16, 17 and promoting wound healing33 than age- and sex-matched control MSCs. Though T2D patient MSCs appear to be functionally impaired, little is known about which factors within the diabetic environment contribute to this effect, if the impairment is permanent, or if allogenic MSCs harvested from healthy donors and then transplanted into a diabetic environment might similarly become impaired. To begin to address these questions, we studied the effects of two metabolic factors that are highly enriched in the serum of T2D patients, glucose and palmitate,27, 34 to determine how these specific factors affect the phenotype and immunomodulatory potency of human MSCs in order to gain an understanding of how MSC function might be altered upon transplantation into diabetic patients.
Results
Palmitate, but Not Glucose, Impairs MSC Metabolic Function and Increases Cell Death
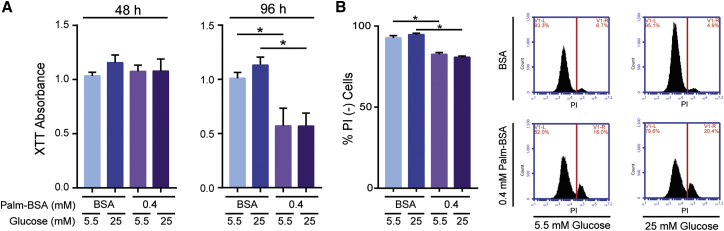
Elevated levels of extracellular glucose and palmitate induce cellular stress pathways in a diverse range of cell types leading to cellular growth arrest and apoptosis.31, 35 Additionally, both glucose and palmitate have been heavily implicated in the multi-organ disease progression characteristic of T2D and obesity.36, 37 To investigate the effect of glucose and palmitate on the metabolic and proliferative ability of MSCs in a simulated T2D environment, we cultured MSCs in high (25 mM) or low (5.5 mM) levels of glucose with or without a high, but physiologically relevant,13 dose of palmitate (0.4 mM Palm-BSA) or fatty acid–free BSA controls. After 48 and 96 hr of palmitate/glucose exposure, MSC metabolic activity was assessed via XTT. While no difference was observed between all groups after 48 hr, after 96 hr, elevated palmitate, but not glucose, led to a significant decline in XTT signal (Figure 1A).
Figure 1.
MSCs’ Metabolic Activity Decreases and Cell Death Increases after Palmitate, but Not Glucose Exposure
(A) The metabolic activity of MSCs was determined using XTT after 48 or 96 hr of BSA or 0.4 mM Palm-BSA exposure in high (25 mM) or low (5.5 mM) glucose media (mean ± SEM, two-way ANOVA at 48 hr with Tukey correction for multiple comparisons: *p < 0.05; palmitate effect, not significant (n.s.), p = 0.8094; glucose effect, n.s., p = 0.4255; at 96 hr: palmitate effect, p = 0.0019; glucose effect, n.s., p = 0.612; n = 3 independent experiments). (B) Viability of MSCs after 96 hr of BSA or 0.4 mM Palm-BSA exposure in high or low glucose was determined using PI staining followed by flow cytometry analysis (mean ± SEM, two-way ANOVA with Tukey correction for multiple comparisons: palmitate effect, p < 0.0001; glucose effect, n.s., p = 0.9160; n = 3 independent experiments). PI+ population gating was determined using a staurosporine-treated positive control. Representative flow plot histograms of PI− (left of red line) versus PI+ (right of red line) MSC populations. Experiments were performed in MSC donor 00081.
Because XTT cannot distinguish between changes in cellular metabolism, proliferation, or viability, we quantified cell death after 96 hr of palmitate exposure by staining cells with propidium iodide (PI). After 96 hr of palmitate exposure, the fraction of PI-positive MSCs increased from 6% in fatty acid–free BSA controls to 18% in the 0.4 mM Palm-BSA condition (Figure 1B). Although a significant increase in death occurred in the 0.4 mM palmitate condition, it was surprising that >80% of the MSC population remained viable. Increasing glucose levels alone or in combination with elevated palmitate again resulted in no discernable differences in cell viability. Because PI staining alone cannot distinguish between necrotic and apoptotic cell death, we did additional staining with both Annexin V and PI. After 96 hr in 0.4 mM palmitate, there was a significant increase in both AnnexinV+PI− cells (early apoptotic) and AnnexinV+PI+ cells (late apoptotic), identifying apoptosis as one mechanism by which palmitate causes cell death in MSCs (Figure S1). Because the decline in MSC metabolic activity measured by XTT was much more pronounced than the accompanying elevation in cell death, our data implicated that palmitate was having a more complex effect on the phenotype of MSCs than could be explained through cell viability alone. Given that increased levels of glucose did not facilitate differences in MSC phenotype, all subsequent experiments were performed in high glucose levels to simplify experiments while more faithfully replicating serum conditions of a T2D patient.
Elevated Palmitate Levels Dramatically Alter MSC Morphology and Proliferation
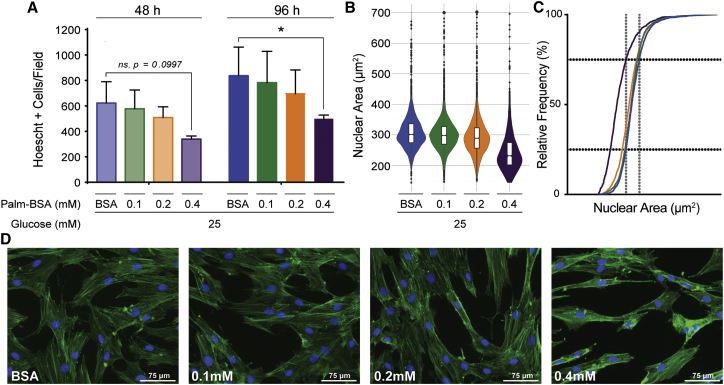
To further clarify the effect of palmitate on MSCs, we assessed cell proliferation by directly counting Hoechst-stained nuclei after 48 and 96 hr of palmitate exposure. In agreement with the XTT data, exposure to elevated palmitate levels decreased the proliferation of MSCs, with a statistically significant drop in cell number observed in the 0.4 mM Palm-BSA condition after 96 hr (Figure 2A). Interestingly, even at the highest dose of palmitate, MSCs did not fully halt proliferation, which confirmed the PI exclusion data that palmitate’s effects on MSCs were not solely cytotoxic and a large remnant of the population adapted, remaining viable and proliferative.
Figure 2.
Palmitate Inhibits MSC Proliferation and Dramatically Alters MSC Morphology
(A) Proliferation was assessed by direct cell counting of Hoechst 33342-stained MSCs via fluorescence microscopy after 48 or 96 hr of BSA and/or Palm-BSA exposure in high-glucose (25 mM) media. 10 images were taken at 5× magnification per treatment condition for each independent experiment (mean ± SEM, two-way ANOVA with Tukey correction for multiple comparisons, *p < 0.5, n = 3 independent experiments). (B) Nuclear area was determined on a per-cell basis using ImageJ software after staining MSCs with Hoechst 33342 and ActinGreen 488 ReadyProbe. Quantification was performed on 10 images per treatment condition for three independent experiments. Data are represented via violin plot with notched boxplots showing the 25th, 50th, and 75th percentiles. (C) Cumulative frequency distribution of cell’s nuclear area curve. Horizontal black dotted lines represent 25% and 75% relative frequency, respectively. Vertical gray dotted lines represent the 25th and 75th percentile of nuclear area in BSA controls. (D) Representative images of Hoechst 33342- and ActinGreen 488 ReadyProbe-stained MSCs taken at 20× magnification. Images were chosen to display the same approximate number of cells in each image (nuclei counts range from 21 to 25 cells per image). All experiments were performed with MSC donor 00081 at P3–P5. Blue represents BSA, green represents 0.1 mM Palm-BSA, orange represents 0.2 mM Palm-BSA, purple represents 0.4 mM Palm-BSA (color designation is maintained throughout all figures).
During microscopic evaluation of proliferation, we noticed that in addition to reduced cell numbers, MSCs cultured in elevated levels of palmitate took on an altered morphology. Thus, we further stained and analyzed MSCs with image processing to quantify changes in cytoplasmic and nuclear size. Fluorescence microscopy was used to acquire images followed by analysis via ImageJ to determine both nuclear and cytoplasmic area on an individual cell basis. From BSA to 0.2 mM Palm-BSA, the measure of nuclear area showed a normal, bell-shaped distribution within the cell population, with the median and mean nuclear area values nearly equivalent (Figure 2B). Within these same conditions, a small number of cells had nuclei that deviated more than 2 SDs from the mean, which likely represent cells actively undergoing S phase. However, in the 0.4 mM Palm-BSA condition, the measure of nuclear area showed a positively skewed distribution, with most cells having a much smaller nuclear area that differed in size from all other conditions (Figure 2B). Transformation of the data to a cumulative frequency distribution curve shows a clear divergence in the nuclear sizes between low- and high-dose palmitate exposure with 75% of the 0.4 mM Palm-BSA population having nuclei with sizes below the 25th percentile of BSA-treated controls (Figure 2C). Additional quantification of this image set using a published microscopy pipeline for cell-cycle analysis on a per-cell basis38 showed that at 0.4 mM Palm-BSA there was both a leftward shift of the mean integrated nuclear staining intensity, as well as nuclear area (Figure S2A). There was no evident mitotic peak across any of the palmitate conditions, which is likely a byproduct of the low mitotic rate of MSCs; however, quantification showed no significant difference in the relative frequency of cells deviating more than 2 SD from the mean of the BSA control. Ultimately, these data support a profile of palmitate-induced MSC nuclear condensation, without significant changes in progression through the cell cycle. In addition to having a dramatic effect on MSC nuclear size, high levels of palmitate caused a shrinking of the cytoplasm as evidenced by decreased overall F-actin staining (Figure 2D). The smaller overall size of the MSCs became more evident when analyzing forward scatter (FSC) during flow cytometric analysis. Mean FSC value of the 0.4 mM Palm-BSA condition was significantly smaller, denoting a smaller overall cell size, when compared with the BSA control (Figure S2B). Thus, palmitate profoundly altered the morphology of both MSC nuclei and cytoskeleton.
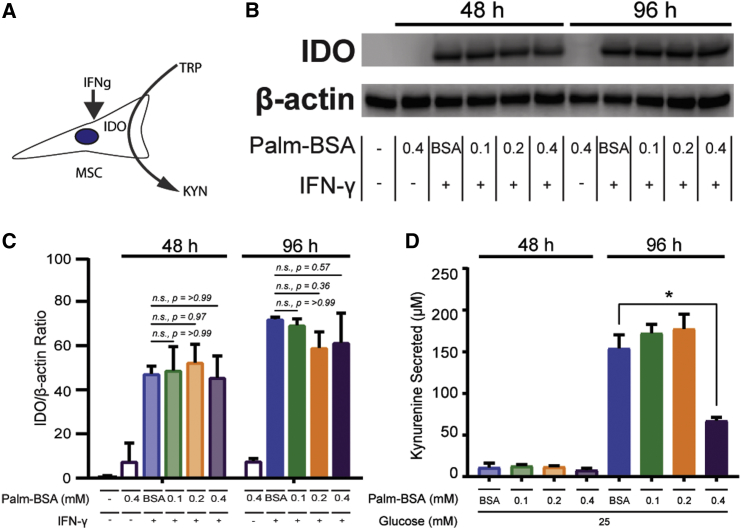
Palmitate Alone Does Not Compromise IDO Protein Levels, but It Does Impact Function
While previous studies have also reported a modest cytotoxic effect of palmitate on MSCs, we sought to determine whether MSC’s adaptation to the T2D environment compromised MSC’s immunomodulatory profile. The immunomodulatory potency of MSCs is heavily linked to production and secretion of proteins that act to inhibit the proliferation and activation of immune cells.39 A potent branch of MSC’s immunomodulatory profile is conversion of tryptophan to kynurenine by the intracellular enzyme indoleamine 2,3-dioxygenase (IDO).40 During basal conditions, IDO is not expressed in MSCs; however, upon exposure to inflammatory cues, specifically interferon gamma (IFN-γ), MSCs produce high amounts of IDO (Figure 3A). To determine whether palmitate interferes with IDO regulation, we exposed MSCs to palmitate with and without IFN-γ activation for 48 or 96 hr. As expected, palmitate treatment alone was not enough to elicit an increase in IDO levels, with nearly undetectable levels of IDO at either 48 or 96 hr (Figures 3B and 3C). Surprisingly, simultaneous treatment of MSCs with both IFN-γ and palmitate showed a normal increase in IDO production across all palmitate conditions and time points (Figure 3B). Simultaneous exposure to palmitate and IFN-γ did not, therefore, interfere with MSC’s ability to initiate and execute production of IDO. Furthermore, IDO enzymatic activity remained fully functional in the presence of palmitate up to 0.2 mM Palm-BSA, as evidenced by similar rates of kynurenine production as compared with controls at both 48 and 96 hr. However, the 0.4 mM Palm-BSA showed a significant decline in enzymatic activity at 96 hr despite equivalent protein levels across palmitate conditions (Figure 3D).
Figure 3.
IDO Protein Levels Are Unaffected by Palmitate Exposure, but Enzymatic Activity Decreases Significantly
(A) Graphical representation of relationship between IFN-γ and IDO. (B) Representative western blot of IDO protein levels after 48 and 96 hr of simultaneous exposure to BSA, Palm-BSA, and/or IFN-γ. β-Actin provided as loading control (n = 3 independent experiments). (C) Densitometric quantification of IDO to β-actin protein levels (±SD, two-way ANOVA with Sidak correction for multiple comparisons to BSA control, n = 3 independent experiments). (D) IDO activity was measured via quantification of kynurenine secretion into the supernatant of MSCs exposed to 48 and 96 hr of BSA and/or Palm-BSA in high-glucose media (mean ± SEM, two-way ANOVA with Dunnett correction for multiple comparisons relative to BSA control, *p < 0.5; n = 3 independent experiments). Experiments were performed with MSC donor 00081.
Chronically High Levels of Palmitate Convert MSCs from an Anti-inflammatory to a Pro-inflammatory Phenotype across Multiple MSC Donors
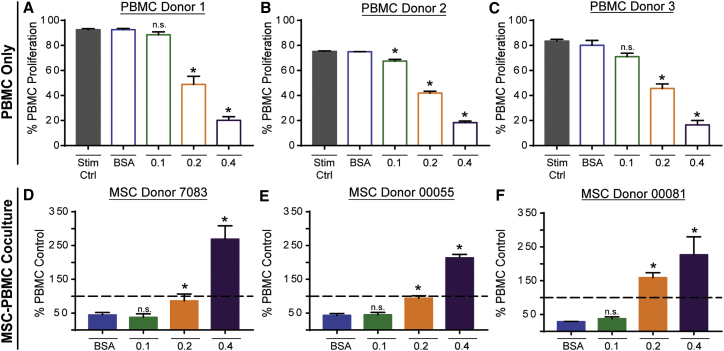
To determine whether palmitate exposure hinders MSCs’ ability to regulate the proliferation of human PBMCs, we conducted direct contact co-culture assays of MSCs and stimulated PBMCs with increasing doses of palmitate. In contrast with the IDO assay above, in which palmitate and IFN-γ exposure is simultaneous, MSC exposure to IFN-γ is delayed in co-cultures because of the time necessary for T cell activation. In order to account for any variance in donor susceptibility to palmitate’s effects on MSC immunosuppression, three allogeneic co-culture pairings of PBMCs (donors 1–3) and MSCs (7083, 00055, and 00081) were evaluated. In addition to co-cultures, PBMCs alone were exposed to the same increasing doses of palmitate as co-cultures and used to determine the relative suppressive potential of MSCs for each palmitate condition (dashed line on MSC-PBMC co-culture graphs represents the PBMC-only palmitate condition).
In response to palmitate exposure, PBMCs showed a dramatic dose-dependent decline in proliferation, which was consistent across three independent PBMC donors (Figures 4A–4C). At 0.2 and 0.4 mM Palm-BSA, PBMCs showed a 2- and 4-fold drop in proliferation, respectively, compared with BSA-treated cells given the same stimulatory cues (CD3/CD28 Dynabeads and recombinant human interleukin-2 [rh-IL-2]). Some variability was observed between the PBMC donors, with donor 1 achieving a maximum proliferation of 90% compared with 75% and 82% for donors 2 and 3, respectively. In addition, donor 2 was more sensitive to palmitate compared with donors 1 and 3, showing a significant drop of ∼10% in proliferation at 0.1 mM Palm-BSA. Notably, this decline in proliferation was independent of decreased cell viability, because no significant difference in PI+ cell numbers was observed across palmitate conditions (Figure S3).
Figure 4.
Exposure to Palmitate Severely Inhibits PBMC Proliferation and Causes MSCs to Convert from an Immunosuppressive to a Pro-inflammatory Phenotype
(A–C) CFSE-stained PBMCs for donor 1 (A), donor 2 (B), and donor 3 (C) were stimulated with CD3/CD28 Dynabeads and 100 U/mL rh-IL-2. Stim Ctrl is CFSE-stained PBMCs stimulated with CD3/CD28 Dynabeads and 100 U/mL rh-IL-2 without BSA or Palm-BSA. Gate for % proliferated was determined using an unstimulated CFSE-stained PBMC control (mean ± SEM, one-way ANOVA with Dunnett correction for multiple comparisons relative to BSA control, *p < 0.5; n = 3 independent experiments for each donor). (D–F) CFSE-stained PBMCs were co-cultured with MSCs at a 1:4 MSC/PBMC ratio (allogeneic co-culture pairings: D, PBMC donor 1 and MSC donor 7083; E, PBMC donor 2 and MSC donor 00055; F, PBMC donor 3 and MSC donor 00081). Proliferation of CFSE-stained PBMCs in co-cultures was normalized to proliferation in matched PBMC-only BSA and/or Palm-BSA-treated conditions (dashed line is proliferation in respective PBMC-only BSA or Palm-BSA condition) to demonstrate suppressive potential (mean ± SEM, one-way ANOVA with Dunnett correction for multiple comparisons relative to BSA control, *p < 0.5; n = 3 independent experiments for each allogeneic donor pairing).
In direct contact co-culture assays under low palmitate conditions (0.1 mM Palm-BSA), all MSC donors robustly suppressed PBMC proliferation to an equivalent level as compared with BSA co-culture controls (Figures 2D–2F). Exposure to 0.2 mM Palm-BSA led to an inability of any of the MSCs to suppress PBMC proliferation; in fact, with donor 00081, 0.2 mM Palm-BSA exposure led to an increase in PBMC proliferation at a rate 50% higher than when PBMCs were cultured alone in palmitate. At 0.4 mM Palm-BSA, all MSC donors caused a 200%–300% increase in the proliferation of PBMCs. An elevation in PBMC proliferation in the presence of MSCs and high palmitate represented not only a dramatic decline in the immunosuppressive ability of MSCs, but a reversal in MSC functionality, from anti-inflammatory to pro-inflammatory. To further dissect the context and mechanism of palmitate-induced conversion of MSC phenotype, we performed subsequent studies with donor 00081, which showed the greatest sensitivity to the effects of palmitate.
Direct contact co-culture assays allow for contact-dependent cytotoxic effects on MSCs via NK cells and CD8+ cytotoxic T cells present within the PBMC population.41 To determine whether the decline in MSC immunosuppressive function was secondary to contact-dependent cell death mechanisms, transwell co-culture experiments were conducted under the same conditions as described above. Decline in the immunosuppressive function of MSCs in high palmitate conditions remained in the transwell assays, ruling out contact-dependent cell death mechanisms as the dominant driver of the pro-inflammatory effect of palmitate on MSCs (Figure S4).
MSC Blockade of Pro-inflammatory Cytokine Production Is Abolished by Palmitate Exposure
The ability of MSCs to shift the cytokine environment is essential to halting the cascade of pro-inflammatory signaling that follows the initial activation of T cells.39 To assess the ability of MSCs to shift the cytokine environment from a pro-inflammatory to an anti-inflammatory profile, we analyzed supernatants from direct contact co-cultures with a multiplex cytokine bead array for cytokines characteristic of Th1, Th2, and Th17 profiles. In addition to a defect in the ability of MSCs to prevent proliferation of PBMCs, MSC’s ability to prevent the production of pro-inflammatory cytokines was compromised in the presence of elevated palmitate levels.
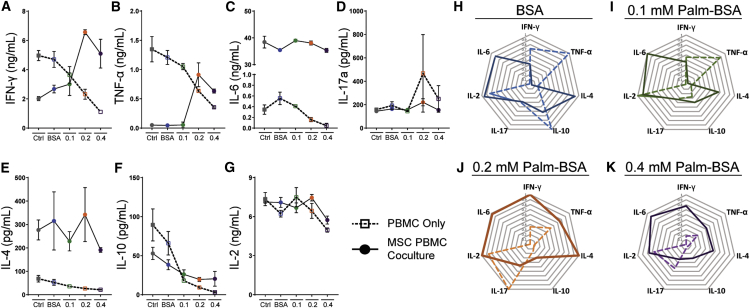
Production of cytokines in PBMC-only cultures showed a dose-dependent decline in all seven cytokines, confirming that palmitate causes not only a significant block on PBMC proliferation, but significantly compromises global PBMC cytokine secretion (Figures 5A–5G; Tables S1–S3). The cytokine profile from co-cultures at low palmitate levels shows that MSCs suppress PBMC production of Th1 cytokines, IFN-γ, and tumor necrosis factor alpha (TNF-α) to levels well below those produced by stimulated PBMCs exposed to the equivalent conditions (Figures 5A and 5B). However, in elevated palmitate conditions, MSCs become pro-inflammatory with IFN-γ and TNF-α levels peaking at 7 and 1 ng/mL, respectively, in the 0.2 mM Palm-BSA condition. Across all co-culture conditions, copious amounts of IL-6 were produced, 30–40 ng/mL, at levels much higher than that produced by either MSCs (Figure S5) or PBMCs alone (Figure 5C). No significant changes were seen in levels of IL-17A production across palmitate conditions (Figure 5D). IL-4, an anti-inflammatory cytokine known to be secreted by MSCs, was elevated across all palmitate conditions in co-cultures (Figure 5E), whereas IL-10, another anti-inflammatory cytokine, showed high levels in low palmitate conditions with a subsequent decrease in response to palmitate (Figure 5F). Elevated palmitate, therefore, compromised MSC’s ability to block the pro-inflammatory skewing of stimulated PBMCs. At low palmitate levels, MSCs continued to block pro-inflammatory skewing without a significant rise in measured anti-inflammatory cytokines when compared with stimulated controls (Figures 5H–5K).
Figure 5.
MSC Blockade of Pro-inflammatory Cytokine Production Is Completely Abrogated by Palmitate Exposure
(A–G) A multiplex Th1/Th2/Th17 cytokine bead array (CBA) ELISA was performed on supernatants isolated from PBMC-only (open square with dashed line) and MSC PBMC co-cultures (closed circle with solid line) for (A) IFN-γ, (B) TNF-α, (C) IL-6, (D) IL-17a, (E) IL-4, (F) IL-10, and (G) IL-2. Mean ± SEM, two-way ANOVA with Tukey correction for multiple comparisons was performed; significance tables of all comparisons are provided in Tables S1–S3; n = 3 independent experiments. Ctrl (for PBMC only), CFSE-stained PBMCs stimulated with CD3/CD28 Dynabeads and 100 U/mL rh-IL-2 without BSA or Palm-BSA; Ctrl (for MSC PBMC co-culture), 1:4 ratio of MSC to CFSE-stained PBMCs stimulated with CD3/CD28 Dynabeads and 100 U/mL rh-IL-2 without BSA or Palm-BSA. (H–K) Radial plot demonstrating differences in overall cytokine milieu between PBMC-only and MSC PBMC co-cultures in BSA (H), 0.1 (I), 0.2 (J), and 0.4 (K) Palm-BSA condition. Vertices represent magnitude of individual cytokines that were normalized to the maximum value, with highest value (1.0) at the ring farthest from the center and 0 for each cytokine at the center. All data points are from the allogeneic pairing of PBMC donor 3 and MSC donor 00081.
Immunosuppressive Potency of MSCs Returns following Removal of Palmitate
Serum free fatty acid (FFA) levels fluctuate depending on the fed versus fasted state of the person, with obese and diabetic patients exhibiting an elevated baseline concentration of saturated FFA.13, 22, 23, 27, 34, 42 The previous co-culture assays simulated chronically elevated saturated FFA levels; however, exposure to these levels will vary over time in a patient. To determine whether palmitate exposure permanently damages MSC’s immunosuppressive potency, we designed and performed a recovery experiment. MSCs were exposed to 96 hr of increasing doses of palmitate. Following palmitate exposure, MSCs were thoroughly washed to remove residual palmitate, counted via a hemocytometer, and then plated into co-cultures with stimulated PBMCs at a ratio of 1 MSC to 4 PBMCs (Figure 6A). MSCs exposed to 0–0.2 mM Palm-BSA regained immunosuppressive potency, whereas those in 0.4mM Palm-BSA did not (Figure 6B). As we accounted for any changes in cell number after palmitate exposure by washing, counting, and re-plating at precise ratios, the impaired immunosuppressive potency seen with 0.4 mM Palm-BSA exposure cannot be attributed to a modest increase in cell death (Figures 1B and S1) and instead suggests a change in cell phenotype itself.
Figure 6.
MSCs Can Regain Immunosuppressive Potency after Palmitate Exposure but Show a Dose-Dependent Delay in Recovery that Can Be Partially Modulated by TLR4 Inhibition
(A) Timeline of recovery experiment involving exposure of MSCs to Palm-BSA prior to MSC PBMC Co-culture. (B) Division index (average number of cell divisions from a cell in the original CFSE-stained PBMC population, includes parent peak in calculation) was calculated using FlowJo software in 1:4 MSC:PBMC co-cultures after MSC exposure to BSA and/or Palm-BSA for 96 hr (mean ± SEM, one-way ANOVA with Dunnett correction for multiple comparisons to BSA control, *p < 0.5; n = 4 independent experiments). PBMC ctrl, PBMCs stimulated with CD3/CD28 Dynabeads and 100 U/mL rh-IL-2 without BSA and/or Palm-BSA; MSC+PBMC Ctrl, 1:4 ratio of MSC:PBMCs stimulated with CD3/CD28 Dynabeads and 100 U/mL rh-IL-2 without BSA and/or Palm-BSA. (C) Representative peak-fit analysis of flow cytometry histograms showing the number of PBMC generations proliferated in MSC PBMC co-culture after MSC exposure to 96-hr BSA and/or Palm-BSA. All representative histograms were from the same experiment to demonstrate suppression relative to stimulated PBMC Ctrl. (D) Percent proliferation of PBMCs from MSC:PBMC coculture (1:4) with MSCs pre-exposed to BSA and/or Palm-BSA in the presence or absence of the TLR4 inhibitor, C34 (10 μg/mL). Data show mean ± SEM, two-way ANOVA with Sidak correction for multiple comparisons to non-TLR4 inhibitor-treated condition; n = 4 independent experiments. (E) Representative flow cytometry histograms showing MFI of CFSE-stained PBMCs co-cultured with MSCs pre-exposed to 0.4 mM Palm-BSA ± TLR4 inhibition. (F) Quantification of MFI from CFSE-stained PBMCs in MSC:PBMC co-cultures (1:4) with MSCs pre-exposed to 0.4 mM Palm-BSA ± TLR4 inhibition (mean ± SEM, two-way ANOVA with Sidak correction for multiple comparisons to non-TLR4 inhibitor-treated condition, *p < 0.5, n = 4 independent experiments).
Interestingly, peak fit analysis of PBMC proliferation revealed that the immunosuppressive potency of MSCs was recovered in a dose-dependent manner. MSCs allowed more generations of PBMCs to proliferate before regaining immunosuppressive potency as the palmitate dose increased. On average, only 1 daughter generation was allowed to proliferate in the 0.1 mM Palm-BSA condition compared with 1.5 and 4 daughter generations in 0.2 and 0.4 mM Palm-BSA, respectively (Figure 6C). These data indicate that extended exposure to elevated levels of palmitate causes a time-dependent delay in recovery of immunosuppressive function.
Because palmitate has been shown to act as a TLR4 ligand,24 we next wanted to determine whether blocking TLR4 signaling could rescue MSC immunosuppressive potency from palmitate-induced damage. To do this, we followed the same experimental design as was previously shown for the recovery experiments (Figure 6A), with or without the addition of the TLR4 inhibitor, C34, during the initial palmitate exposure. After the 96-hr exposure to BSA and/or Palm-BSA in the presence or absence of TLR4 inhibition, we washed and re-plated the MSCs with stimulated PBMCs in regular media. Analyzing our initial metric of absolute PBMC proliferation, we once again saw a loss of immunosuppressive potency in the 0.4 mM Palm-BSA condition that was not significantly rescued by TLR4 inhibition (Figure 6D). Interestingly, when we analyzed the mean fluorescence intensity (MFI) of CFSE-stained PBMCs in the 0.4 mM Palm-BSA condition, there was a consistent rightward shift in the MFI distribution, denoting less overall PBMC stimulation and therefore earlier MSC recovery of immunosuppression (Figure 6E). Quantification of the MFI across palmitate conditions showed a small, but significant change in the MFI of PBMCs suppressed by TLR4-inhibited 0.4 mM Palm-BSA-exposed MSCs (Figure 6F). TLR4 inhibition, therefore, does appear to play a part in the damage induced in MSCs by palmitate; however, given the broad range of roles that palmitate can have, from ligand to energy substrate to membrane component, it is unlikely that blocking one single signaling pathway will rescue MSCs from palmitate’s diverse effects.
Pre-licensing MSCs with IFN-γ and TNF-α prior to Palmitate Exposure Fully Restores MSC’s Immunosuppressive Potency via a JAK1/JAK2-Dependent Pathway
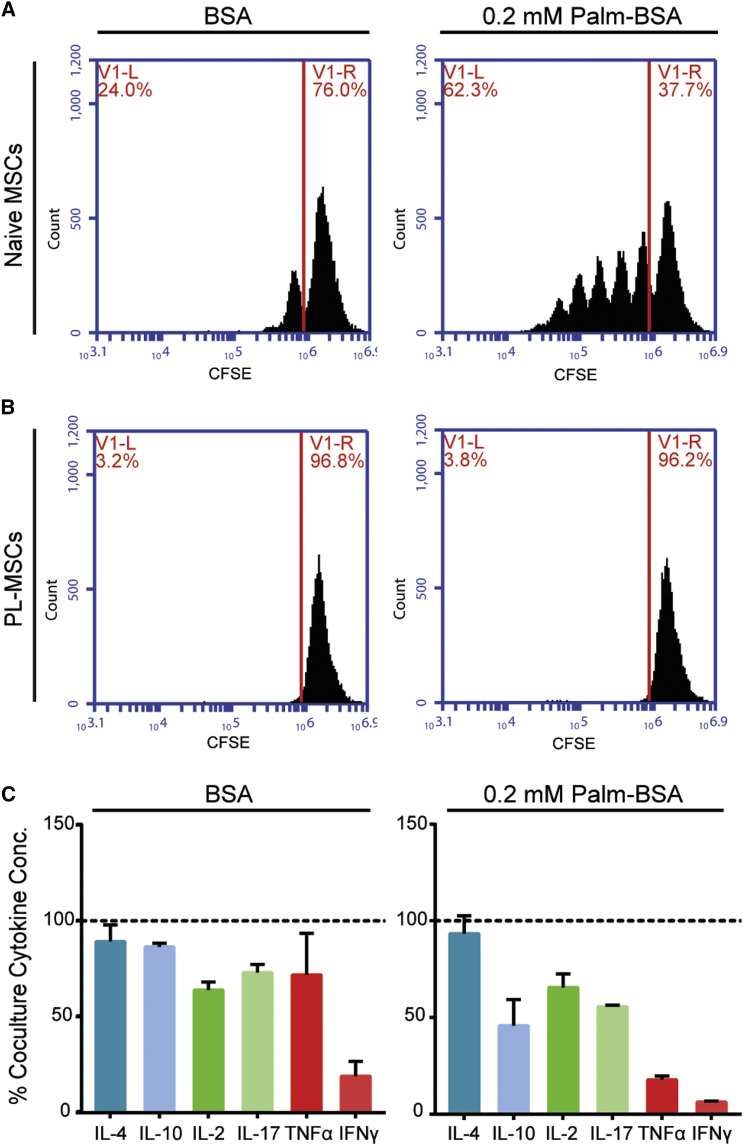
Two cytokines, IFN-γ and TNF-α, were found to be significantly increased in co-culture supernatants after exposure to high levels of palmitate for 6 days. Interestingly, both IFN-γ and TNF-α have been extensively investigated as pre-licensing agents to enhance MSC immunosuppressive potency.43, 44, 45, 46, 47 Pre-licensing MSCs involves exposing MSCs to stimulatory cytokines prior to transplanting the MSCs into an inflammatory environment. To investigate the role of the sequence of exposure to palmitate and inflammatory cytokines on the ability of MSCs to remain functional, MSCs were pre-licensed with 10 ng/mL IFN-γ and 1 ng/mL TNF-α for 24 hr prior to co-culture with stimulated PBMCs. Cytokine levels were chosen to mimic the end-point concentrations observed in previous co-culture assays. Pre-licensed MSCs (PL-MSC) were able to fully suppress PBMC proliferation in the presence of palmitate at both 1:4 (Figures 7A and 7B) and 1:8 (Figure S6) MSC/PBMC ratios. The degree of enhancement in potency was enough to make PL-MSCs in high palmitate conditions more potent than naive MSCs in palmitate-free co-cultures. Analysis of supernatants from co-cultures with and without PL-MSCs showed PL-MSCs also greatly decreased the production of IFN-γ and TNF-α to levels only 18% and 6% of the co-culture controls, respectively (Figure 7C). Interestingly, although all cytokines were suppressed to some degree, the pro-inflammatory cytokines IFN-γ and TNF-α displayed the largest change in concentration.
Figure 7.
IFN-γ and TNF-α Pre-licensing Completely Blocks the Pro-inflammatory Skewing of MSCs in the Presence of Palmitate
(A and B) Representative flow cytometry histograms showing proliferated PBMCs (proliferated population left of the red line) in MSC PBMC co-cultures with naive (A) and pre-licensed (B) MSCs in BSA and/or Palm-BSA conditions. (C) Cytokine production in PL-MSC PBMC co-cultures was assessed using a seven-cytokine bead array (IL-6 levels were higher than the top ELISA standard and were therefore excluded from this analysis). Cytokine levels were normalized to the naive MSC PBMC co-culture cytokine levels (dashed line) in order to demonstrate suppression of pro-inflammatory cytokine release in the presence of PL-MSCs. All experiments were conducted with MSC donor 00081 with PBMC donor 3 (mean ± SEM, n = 3 independent experiments).
To explore the mechanism by which pre-licensing blocks palmitate-induced pro-inflammatory conversion of MSCs, we explored immediate and delayed aspects of the IFN-γ signal transduction cascade after palmitate exposure in naive and PL-MSCs. PL-MSCs and naive MSCs exposed to palmitate for 96 hr were equally able to detect IFN-γ exposure as evidenced by equivalent levels of STAT-1 phosphorylation after 2–10 min of IFN-γ exposure (Figure S7). Thus, the ability of MSCs to sense IFN-γ was not impacted by palmitate exposure.
To determine whether the rescue of MSC immunosuppressive behavior is dependent on early mediators of the IFN-γ signal transduction cascade, we pre-licensed MSCs with IFN-γ and TNF-α in the presence or absence of either ruxolitinib (500 nM), a JAK1/JAK2 inhibitor which is downstream of the interferon gamma receptor (IFNGR), or tofacitinib (500 nM), a JAK3 inhibitor independent of the IFNGR, prior to being added into co-cultures with PBMCs. Ruxolitinib, but not tofacitinib, blocked nearly half of the rescue effect of pre-licensing (Figure 8A, 0.2 mM Palm-BSA: non-PL, mean = 118.5%, PL, mean = 16.9%, Rux-PL, mean = 59.7%, Tof-PL, mean = 23.3%), resulting in elevated levels of PBMC proliferation compared with IFN-γ and TNF-α pre-licensing alone. The slight decline in the potency of PL-MSCs in the presence of tofacitinib is likely due to its off-target inhibitory activity at JAK1/JAK2. These data confirm that the mechanism through which pre-licensing is facilitating a blockade of the pro-inflammatory skewing of MSCs in palmitate is dependent, at least in part, on an intact IFN-γ/JAK1/2 signaling cascade.
Figure 8.
IFN-γ and TNF-α Pre-licensing Requires Intact IFNGR Signaling and Fixes MSCs into an Anti-inflammatory Phenotype, Even after Extended Exposure to Palmitate
(A) MSC:PBMC co-cultures were performed with naive PL-MSCs, ruxolitinib-treated (500 nM, JAK1/2 inhibitor) PL-MSCs, and tofacitinib-treated (500 nM, JAK3 inhibitor) PL-MSCs. JAK inhibitors were added to pre-licensing media 2 hr before adding in pre-licensing cytokines (10 ng/mL IFN-γ and 1 ng/mL TNF-α), and MSCs were subsequently cultured for 24 hr (mean ± SEM, one-way ANOVA with Dunnett correction for multiple comparisons to naive co-culture, *p < 0.5; n = 5 independent experiments of MSC donor 00081 with PBMC donors 1–5.) (B) Gene expression analysis of naive and PL-MSCs after 96 hr of BSA and/or Palm-BSA exposure followed by replacement with 24 hr of IFN-γ (10 ng/mL) media (data from one representative experiment, mean ± SD, one-way ANOVA with Dunnett correction for multiple comparisons with naive MSCs treated with BSA followed by 24 hr of IFN-γ stimulation, *p < 0.5; n = 3 independent experiments with MSC donor 00081).
Finally, we looked further downstream at transcriptional changes secondary to IFN-γ and TNF-α pre-licensing to identify alterations in MSC transcriptional response to IFN-γ stimulation. PL-MSCs and naive MSCs were exposed to 96-hr palmitate, followed by stimulation with 10 ng/mL IFN-γ for 24 hr, and then analyzed via qRT-PCR. In addition to the functional deficit MSCs exhibit in co-cultures in the presence of palmitate, naive MSCs exposed to palmitate display a transcriptional shift displaying increases in pro-inflammatory mediators like prostaglandin-endoperoxidase synthase 2 (PTGS2; also known as cyclooxygenase 2 [COX-2]) and IL-6 (Figure 8B), while PL-MSCs maintain a fixed transcriptional signature in response to palmitate exposure, with no difference between BSA or 0.2 mM Palm-BSA-treated PL-MSCs. Unexpectedly, the effects of the 24-hr pre-licensing regimen on MSC’s transcriptional phenotype was maintained even after removal from the pre-licensing stimuli for 120 hr.
Discussion
MSCs isolated from patient populations with metabolic disorders and chronic inflammatory conditions have frequently been shown to exhibit an abnormal phenotype characterized by initiation of senescence, elevated levels of apoptosis, and diminished immunosuppressive potency.14, 15, 32, 33, 48, 49, 50 Herein, we have shown that despite an initial increase in apoptosis, after 96 hr of exposure to doses of palmitate that are physiological for T2D patients, the vast majority of the MSCs (80%) remain viable and adapt, with demonstrable phenotypic and morphological changes (Figure 1B). Palmitate exposure led to a dose-dependent decline in the metabolic activity of the cell, attributable at least in part to a decrease in the proliferative rate of the MSCs (Figure 1A). MSC morphology also gradually shifted culminating at 0.4 mM Palm-BSA exposure, resulting in condensed nuclei, with 75% of cells having nuclei smaller than the 25th percentile of control MSCs (Figures 2B–2D). Surprisingly, we found that palmitate alone does not decrease the production of IDO (Figures 3B and 3C), a major enzyme often used to benchmark MSC potency,51 but can negatively impact enzymatic function at high palmitate levels. IDO’s ability to convert tryptophan to kynurenine was significantly reduced by 0.4 mM Palm-BSA exposure; however, even this highest dose did not fully quench IDO activity. Additionally, analysis of MSC-secreted cytokines via a multiplex ELISA bead array showed a dose-dependent increase in both IL-6 and IL-4 in the supernatants of MSCs exposed only to palmitate for 96 hr (Figure S5).
To directly examine the impact of elevated palmitate on MSC’s immunosuppressive potency, we simulated a T2D environment and performed co-cultures with human PBMCs from multiple donors. We discovered that MSCs undergo a remarkable conversion in immunomodulatory phenotype, transitioning from their potent immunosuppressive state to drivers of inflammation (Figures 4D–4F). This phenotypic conversion from anti-inflammatory to pro-inflammatory was revealed in elevated physiologically relevant palmitate levels across multiple allogeneic donor pairings, consistently leading to enhanced proliferation of PBMCs and highly elevated pro-inflammatory cytokine levels (Figures 5A–5K). The pro-inflammatory conversion of MSCs upon exposure to the T2D environment has potential implication for the treatment of inflammatory complications in T2D patients. Thus, there is a need to further understand the impact of the diabetic microenvironment on MSCs upon transplantation and to design strategies to control MSC potency in the presence of a diabetic environment. To date, efficacy and safety studies have been performed in T2D patients with both autologous and allogeneic MSC sources, and have shown moderate effectiveness for the treatment of T2D itself, leading to decreases in blood glucose levels and lower basal insulin concentrations.52, 53 However, clinical trial data concerning use of MSCs expressly for treating inflammation in the setting of T2D is not yet available. Given the defective immunosuppressive phenotype of T2D-derived MSCs described by several groups,16, 17 autologous sources of MSCs may be inferior to an allogeneic alternative. Ultimately, the potency of allogeneic MSC therapy needs to be tailored and controlled to effectively modulate inflammation in the setting of T2D, where transplanted MSCs will be affected by both local and systemic factors.
Having discovered a dramatic re-polarization of MSCs from immunosuppressive to inflammatory in simulated T2D conditions, we sought to determine whether MSCs could be directed to remain immunosuppressive. We have previously shown pre-licensing MSCs with IFN-γ is able to augment MSC potency in normal co-cultures,51 and that the effect is durable enough to last through cryopreservation.54 To our surprise, pre-licensing MSCs with IFN-γ and TNF-α before palmitate exposure was extremely potent and durable. Not only did pre-licensing completely block the pro-inflammatory skewing of MSCs, it enhanced MSC baseline suppressive ability (Figures 7A–7C). While naive MSCs respond to palmitate by upregulating pro-inflammatory genes, PL-MSCs appear to be protected from the effects of palmitate even after 96 hr in an environment that we have shown affects both their proliferation and metabolic activity (Figure 8B). Recently, IFN-γ pre-licensing has been shown to remodel the promoter region of the IDO1 gene to facilitate a more permissive structure that enables earlier synthesis of IDO upon a secondary exposure to IFN-γ.55 Remodeling of specific promoter regions of immunomodulatory genes may account for why our PL-MSCs remained fixed within a distinct transcriptional phenotype even 5 days after removal from the original pre-licensing environment. This finding is particularly noteworthy in relation to translational applications of MSCs because the persistence of a fixed phenotype post-transplantation is still a major hurdle to expanding MSC therapy to the population at large.
While in the current study we utilized a pre-licensing regimen consisting of exposure to IFN-γ and TNF-α, multiple pre-licensing regimens have been recently developed, with each polarizing MSCs into a unique transcriptional and functional state.43, 56, 57, 58 The transcriptional response of MSCs to pre-licensing cytokine combinations is highly diverse and depends on the specific combinations of pre-licensing cytokines used. IFN-γ and TNF-α exposure initiates transcriptional upregulation of pathways related to antigen presentation, as well as acute-phase response, death receptor, nuclear factor κB (NF-κB), and IL-17a signaling in MSCs.46 Additionally, lipidomic screening of MSCs pre-licensed with IFN-γ and TNF-α showed a decrease in total cellular palmitate compared with non-pre-licensed controls.59 Interestingly, IFN-γ pre-licensing alone has been shown in a separate report to downregulate genes associated with lipid and sterol biosynthesis, which may be one pathway by which our PL-MSCs are protected from the effects of palmitate.58 Future work in our laboratory will focus on unraveling the specific mechanisms by which MSCs are impaired by the T2D environment and identifying the optimal pre-licensing regimen necessary to produce MSCs that will maintain therapeutic potency post-transplantation into a T2D patient.
In conclusion, our present study identifies a specific metabolic factor within the T2D environment that explicitly reverses MSC’s immunomodulatory potency in an in vitro model using primary human cells. Additionally, our study has identified a potent pre-licensing strategy for MSCs, using IFN-γ and TNF-α, that fully blocks the pro-inflammatory effects of palmitate. The effects of pre-licensing are durable, remaining fixed over an extended period, making it a potentially translatable strategy. Further tailoring of this T2D-specific pre-licensing strategy has the potential not only to enhance the potency of allogeneic MSCs within an in vivo T2D environment, but may also rescue the depressed immunosuppressive phenotype of MSCs isolated from T2D patients, making autologous MSC therapy an option for T2D patients.
Materials and Methods
Human MSC Culture
Pre-characterized human MSCs (donor 7083) were acquired from Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from the Office of the Director of the NIH, Grant P40OD011050, resource ID SCR_005522. Two additional pre-characterized sets of human donor MSCs (00081 and 00055) were acquired from RoosterBio. All MSCs were acquired from healthy donors after informed consent with characterization conforming to MSC minimal criteria of the International Society for Cellular Therapy: all donors were >95% positive for CD73a, CD90, and CD105, but <2% positive for CD11, CD14, CD19, CD34, CD45, CD79a, and HLA-II with tri-lineage differentiation ability. All cells used for experiments were maintained for at least two passages out of cryostorage in MEM-alpha (Invitrogen) supplemented with 15% fetal bovine serum (Premium Select, Atlanta Biologicals), 1% (v/v) penicillin/streptomycin (Life Technologies), and 1% (v/v) L-glutamine (Life Technologies) before being used for experiments at P3–P5, representing ∼6–11 population doublings.
Palmitate-BSA Conjugation
Palmitate-BSA conjugation was adapted from a protocol as previously described.60, 61 In brief, sodium palmitate (Sigma-Aldrich) was solubilized in 0.1 N NaOH in a 70°C water bath to a final concentration of 100 mM. A 20% w/v fatty acid-free BSA (Sigma-Aldrich) solution was prepared in an adjacent 50°C water bath. Once fully solubilized, 4 mL of sodium palmitate solution was added to 36 mL of BSA at 50°C for a final concentration of 10 mM Palm-BSA. Both the Palm-BSA-conjugated solution and 20% w/v BSA were then filter sterilized using a 0.44-μm pore filter. Filtered aliquots were stored at −20°C followed by warming to 37°C prior to experimental plating. To accommodate for pH changes from BSA addition, all treated conditions received the same volume of 20% w/v BSA and/or Palm-BSA in the following ratios, respectively: BSA only (4:0), 0.1 mM (1:3), 0.2 mM (2:2), and 0.4 mM (0:4).
Metabolic Function and Viability Assays
To characterize the effect of palmitate on MSC metabolic function, we used an XTT Cell Viability Assay Kit (Biotium). 15,000 MSCs were plated in 96-well plates in 25 mM glucose followed by a 48-hr exposure to 0.1, 0.2, or 0.4 mM Palm-BSA and/or 20% w/v FFA-BSA in 100 μL of total well volume. After 48 hr, 50 μL of activator/XTT Buffer solution was added to each well followed by incubation at 37°C for 1 hr. After 1 hr, absorbance at 475 and 660 nm were read via microplate reader. Wells without Palm-BSA or BSA, one-third of the initial cell number, or no MSCs were used as internal controls for each experiment. To determine cell viability, we incubated MSCs with BSA or 0.4 mM Palm-BSA for 96 hr, media were collected, and attached cells were lifted from the cell culture substrate via Accutase (Innovative Cell Technologies) and added to the collected media. Cells were pelleted and then stained with 1 μL of PI (Life Technologies) to a final concentration of 1.5 μM, and the cells were allowed to incubate in suspension on ice for 10 min. Cells were subsequently washed twice with PBS and analyzed via flow cytometry using an Accuri C6 flow cytometer (BD Biosciences).
Cell Proliferation and Morphology
Cell proliferation was determined via direct count of nuclei stained with Hoechst 33342+ (Life Technologies) after 48 and 96 hr of palmitate exposure. Tile scans were performed to count all nuclei within each well, and ImageJ (NIH) was used to quantify nuclei. For morphological evaluation, MSCs were fixed for 5 min with 10% formalin followed by permeabilization for 5 min with 0.1% Triton X-100 in PBS. Cells were subsequently stained with Hoechst 33342+ for 10 min. After washing, two drops of ActinGreen 488 ReadyProbe (Molecular Probes) was added to the well, and the protocol was followed according to manufacturer’s instructions. Imaging was performed at 20× magnification on a fluorescence microscope (Leica DMI6000). Quantification of nuclear and cytoplasmic area was performed using a custom-written Macro for ImageJ (NIH), as well as a published microscopy pipeline for Cell Profiler.38
IDO Protein and Activity Assays
To assess the effect of palmitate exposure on IDO protein levels, we conducted western blot analysis as previously prescribed.54 In brief, 50,000 MSCs were plated in six-well plates. After 1 hr, 10 ng/mL IFN-γ, 120 μL of 20% w/v BSA and/or 10 mM Palm-BSA, and 250 μM L-tryptophan (for subsequent IDO activity assays) were added to each well. Conditioned media and cell lysates were harvested at 48 and 96 hr by washing wells with ice-cold PBS three times followed by addition of 40 μL of ice-cold radioimmunoprecipitation assay (RIPA) buffer (10 μL/mL PMSF solution, 10 μL/mL sodium orthovanadate, and 10 μL/mL protease inhibitor cocktail). Lysate was then clarified by centrifugation at 8,000 relative centrifugal force (rcf) at 4°C for 10 min. Total protein concentration was determined by microBCA (Thermo Scientific). 10–20 μg of protein was added to a 4%–12% Bis-Tris gradient gel followed by electrophoresis and transfer. Following transfer, the membrane was blocked with 5% nonfat dry milk in TBS with 0.01% Tween. Primary antibodies, rabbit anti-IDO (1:1,000, 12006S; Cell Signaling) and mouse anti-β-actin (1:20,000, 1406030; Ambion, Thermo Scientific), were then used to probe for protein bands. Horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000 goat anti-rabbit IgG HRP and 1:10,000 goat anti-mouse IgG HRP; Fisher Scientific) and WesternBright ECL HRP Substrate (Advansta) were used to visualize protein bands followed by densitometric quantification using the LI-COR C-Digit Scanner. Conditioned media from the wells were collected to determine the effect of palmitate on IDO activity levels by assessing kynurenine production as previously described.54 In brief, protein in the supernatant was removed by addition of 100 μL of trichloroacetic acid (30%, w/v) and incubated at 52°C for 30 min to facilitate conversion of N-formylkynurenine to kynurenine. Samples were then centrifuged at 2,500 rcf for 10 min to pellet proteins, and 100 μL of the resultant supernatant for each sample was added to a 96-well plate. 100 μL of Ehrlich’s reagent (0.8% p-dimethylaminobenzaldehyde in glacial acetic acid) was added to each well to induce a colorimetric change. Samples were then incubated at room temperature for 10 min followed by absorbance reading on a microplate reader at 490 nm.
MSC-PBMC Direct Contact Co-culture
PBMCs from three de-identified, consenting, blood donors were retrieved from leukapheresis reduction cones provided by the DeGowin Blood Center at the University of Iowa Hospitals and Clinics. For co-culture plating, MSCs were harvested and 62,500 MSCs were added to each well of a 24-well plate and allowed to attach for 2 hr. During the time for MSC attachment, PBMCs were labeled using a CFSE Cell Proliferation Kit (Invitrogen) at a final dye concentration of 1 μM. Prior to being added to each well, PBMCs were incubated for 15 min with an equal concentration of Human T Activator CD3/CD28 Dynabeads (Invitrogen). After this time, 250,000 PBMCs and 250,000 Dynabeads were added to each well to achieve a 1:4 ratio of MSCs to PBMCs. All conditions, except the co-culture and PBMC-only controls, were cultured in high glucose (25 mM) to mimic the hyperglycemic condition of T2DM. Total culture volume of each well was standardized to 750 μL of RPMI supplemented with 10% FBS, 1% (v/v) L-glutamine, and 1% (v/v) penicillin/streptomycin. 30 μL of 20% w/v BSA and/or 10 mM Palm-BSA was added to each co-culture once MSCs and PBMCs were plated, as well as recombinant human IL-2 (100 U/mL) to ensure T cell activation. Stimulated and unstimulated PBMCs without addition of glucose or palmitate were used as controls in both the co-culture and PBMC-only plates. MSC-PBMC co-cultures were maintained for 6 days, after which time the media from the wells (containing the suspended PBMCs) were collected and centrifuged to pellet the PBMCs. Supernatants from all wells were collected and analyzed in subsequent bead-based cytokine arrays. PBMCs from cultures with and without MSCs were re-suspended in RPMI 1640 followed by analysis on an Accuri C6 flow cytometer to assess PBMC proliferation in the presence and absence of MSCs and/or palmitate.
Th1/Th2/Th17 Cytokine Bead Array
Endpoint analysis of cytokines produced from co-culture and PBMC experiments was conducted using a seven-cytokine bead-based ELISA array (BD Biosciences) to determine the levels of IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α, and IFN-γ produced in the presence and absence of MSCs at increasing concentrations of palmitate. In brief, 25 μL of co-culture supernatants or standards was diluted 1:1 with assay diluent, followed by a 1:1 mixing of diluted supernatant with all seven capture bead populations re-suspended in assay diluent. PE-conjugated detection antibodies were then added and the solution was incubated for 3 hr in the dark at room temperature. After 3 hr, the samples were washed with 1 mL of wash buffer and centrifuged at 200 × g for 5 min, followed by aspiration of the supernatant and resuspension of the beads with 300 μL of wash buffer. Samples were analyzed on an Accuri C6 flow cytometer. Linear standard curves for each cytokine were established using Prism 7 and used to interpolate the concentration of each cytokine in each sample.
Gene Expression
RNA was isolated from MSCs using 1 mL of Trizol reagent for every 1 million cells followed by phase separation using chloroform. RNA was precipitated using isopropanol followed by washing with ethanol. RNA was used for downstream experiments only if the 260 nm/280 nm ratio fell between 1.8 and 2.0 following NanoDrop analysis. 2 μg of RNA was reverse transcribed to cDNA using a high-capacity cDNA reverse transcription kit with RNase inhibitors (Applied Biosystems). Quantitative gene expression was performed on an Applied Biosystems 7900HT using PowerUp SYBR Green (Applied Biosystems) at the Iowa Institute of Human Genetics Genomics Division. The following PrimeTime primers (Integrated DNA Technologies) were used: GAPDH, Hs.PT.39a.22214836; PTGS2, Hs.PT.58.77266; IL6, Hs.PT.58.40226675; IDO, Hs.PT.58.924731. Fold change in gene expression was calculated using the comparative cycle threshold (Ct) method and expressed relative to the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical Analysis
Quantitative data were graphed using GraphPad Prism 7. Statistical analysis was performed with GraphPad Prism using one-way and two-way ANOVA depending on the assay. Statistical details are listed in the caption of each figure. For all post hoc analyses, p < 0.05 was considered statistically significant.
Author Contributions
Conceptualization, L.B., V.A.L., and J.A.A.; Methodology, L.B., V.A.L., and J.A.A.; Formal Analysis, L.B., A.J. Brown, and J.A.A.; Investigation, L.B., A.J. Burand, A.J. Brown, D.B., and J.A.A.; Writing – Original Draft, L.B. and J.A.A.; Writing – Review & Editing, L.B., V.A.L., and J.A.A.; Visualization, L.B. and J.A.A.; Funding Acquisition, V.A.L. and J.A.A.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was funded in part by grants from the Diabetes Action Research and Education Foundation, BD Biosciences Research Grant Program, and the National Blood Foundation awarded to J.A.A. Additional support was provided by the Fraternal Order of Eagles Diabetes Research Center to V.A.L. and J.A.A. Special thanks to Prof. Jon Houtman and Dr. Michael Zhang for providing rh-IL-2 and PBMCs.
Footnotes
Supplemental Information includes seven figures and three tables and can be found with this article online at https://doi.org/10.1016/j.ymthe.2017.12.013.
Supplemental Information
References
- 1.Miyamura K. Insurance approval of mesenchymal stem cell for acute GVHD in Japan: need of follow up for some remaining concerns. Int. J. Hematol. 2016;103:155–164. doi: 10.1007/s12185-015-1930-x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta P.K., Chullikana A., Parakh R., Desai S., Das A., Gottipamula S., Krishnamurthy S., Anthony N., Pherwani A., Majumdar A.S. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J. Transl. Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg G.K., Kondziolka D., Wechsler L.R., Lunsford L.D., Coburn M.L., Billigen J.B., Kim A.S., Johnson J.N., Bates D., King B. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson P.-O., Schwarcz E., Korsgren O., Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 5.Ankrum J., Karp J.M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol. Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Gao F., Chiu S.M., Motan D.A.L., Zhang Z., Chen L., Ji H.-L., Tse H.F., Fu Q.L., Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan J., Tie G., Xu T.Y., Cecchini K., Messina L.M. Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Rev. 2013;9:360–372. doi: 10.1007/s12015-013-9433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolajczyk B.S., Jagannathan-Bogdan M., Shin H., Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–250. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat. Rev. Endocrinol. 2011;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 12.Skyler J.S., Bakris G.L., Bonifacio E., Darsow T., Eckel R.H., Groop L., Groop P.H., Handelsman Y., Insel R.A., Mathieu C. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–255. doi: 10.2337/db16-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trombetta A., Togliatto G., Rosso A., Dentelli P., Olgasi C., Cotogni P., Brizzi M.F. Increase of palmitic acid concentration impairs endothelial progenitor cell and bone marrow-derived progenitor cell bioavailability: role of the STAT5/PPARγ transcriptional complex. Diabetes. 2013;62:1245–1257. doi: 10.2337/db12-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Januszyk M., Sorkin M., Glotzbach J.P., Vial I.N., Maan Z.N., Rennert R.C., Duscher D., Thangarajah H., Longaker M.T., Butte A.J., Gurtner G.C. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes. 2014;63:3047–3056. doi: 10.2337/db13-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vecellio M., Spallotta F., Nanni S., Colussi C., Cencioni C., Derlet A., Bassetti B., Tilenni M., Carena M.C., Farsetti A. The histone acetylase activator pentadecylidenemalonate 1b rescues proliferation and differentiation in the human cardiac mesenchymal cells of type 2 diabetic patients. Diabetes. 2014;63:2132–2147. doi: 10.2337/db13-0731. [DOI] [PubMed] [Google Scholar]
- 16.Kizilay Mancini O., Shum-Tim D., Stochaj U., Correa J.A., Colmegna I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res. Ther. 2015;6:140. doi: 10.1186/s13287-015-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serena C., Keiran N., Ceperuelo-Mallafre V., Ejarque M., Fradera R., Roche K., Nuñez-Roa C., Vendrell J., Fernández-Veledo S. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34:2559–2573. doi: 10.1002/stem.2429. [DOI] [PubMed] [Google Scholar]
- 18.DeFuria J., Belkina A.C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J.D., Nersesova Y.R., Markham D., Strissel K.J., Watkins A.A., Zhu M. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe C.M.O., Abreu L.F.M., Gomes P.S., Gonzaga R.M., Veloso C.A., Nogueira-Machado J.A. The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid. Med. Cell. Longev. 2014;2014:479587. doi: 10.1155/2014/479587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei X., Song H., Yin L., Rizzo M.G., Sidhu R., Covey D.F., Ory D.S., Semenkovich C.F. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip B., Cilfone N.A., Belkina A.C., DeFuria J., Jagannathan-Bogdan M., Zhu M., Kuchibhatla R., McDonnell M.E., Xiao Q., Kepler T.B. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity (Silver Spring) 2016;24:102–112. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma W., Wu J.H., Wang Q., Lemaitre R.N., Mukamal K.J., Djoussé L., King I.B., Song X., Biggs M.L., Delaney J.A. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am. J. Clin. Nutr. 2015;101:153–163. doi: 10.3945/ajcn.114.092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clore J.N., Allred J., White D., Li J., Stillman J. The role of plasma fatty acid composition in endogenous glucose production in patients with type 2 diabetes mellitus. Metabolism. 2002;51:1471–1477. doi: 10.1053/meta.2002.35202. [DOI] [PubMed] [Google Scholar]
- 24.Huang S., Rutkowsky J.M., Snodgrass R.G., Ono-Moore K.D., Schneider D.A., Newman J.W., Adams S.H., Hwang D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boslem E., Weir J.M., MacIntosh G., Sue N., Cantley J., Meikle P.J., Biden T.J. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J. Biol. Chem. 2013;288:26569–26582. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borradaile N.M., Han X., Harp J.D., Gale S.E., Ory D.S., Schaffer J.E. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Boon H., Blaak E.E., Saris W.H.M., Keizer H.A., Wagenmakers A.J.M., van Loon L.J.C. Substrate source utilisation in long-term diagnosed type 2 diabetes patients at rest, and during exercise and subsequent recovery. Diabetologia. 2007;50:103–112. doi: 10.1007/s00125-006-0482-2. [DOI] [PubMed] [Google Scholar]
- 28.Ali A.H., Koutsari C., Mundi M., Stegall M.D., Heimbach J.K., Taler S.J., Nygren J., Thorell A., Bogachus L.D., Turcotte L.P. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes. 2011;60:2300–2307. doi: 10.2337/db11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Fu H., Chang F., Wang J., Zhang S., Caudle Y., Zhao J., Yin D. Sodium orthovanadate suppresses palmitate-induced cardiomyocyte apoptosis by regulation of the JAK2/STAT3 signaling pathway. Apoptosis. 2016;21:546–557. doi: 10.1007/s10495-016-1231-8. [DOI] [PubMed] [Google Scholar]
- 30.Pillon N.J., Li Y.E., Fink L.N., Brozinick J.T., Nikolayev A., Kuo M.-S., Bilan P.J., Klip A. Nucleotides released from palmitate-challenged muscle cells through pannexin-3 attract monocytes. Diabetes. 2014;63:3815–3826. doi: 10.2337/db14-0150. [DOI] [PubMed] [Google Scholar]
- 31.Lu J., Wang Q., Huang L., Dong H., Lin L., Lin N., Zheng F., Tan J. Palmitate causes endoplasmic reticulum stress and apoptosis in human mesenchymal stem cells: prevention by AMPK activator. Endocrinology. 2012;153:5275–5284. doi: 10.1210/en.2012-1418. [DOI] [PubMed] [Google Scholar]
- 32.Kizilay Mancini Ö., Lora M., Shum-Tim D., Nadeau S., Rodier F., Colmegna I. A proinflammatory secretome mediates the impaired immunopotency of human mesenchymal stromal cells in elderly patients with atherosclerosis. Stem Cells Transl. Med. 2017;6:1132–1140. doi: 10.1002/sctm.16-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinh N.T., Yamashita T., Ohneda K., Kimura K., Salazar G.T., Sato F., Ohneda O. Increased expression of EGR-1 in diabetic human adipose tissue-derived mesenchymal stem cells reduces their wound healing capacity. Stem Cells Dev. 2016;25:760–773. doi: 10.1089/scd.2015.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sertoglu E., Kurt I., Tapan S., Uyanik M., Serdar M.A., Kayadibi H., El-Fawaeir S. Comparison of plasma and erythrocyte membrane fatty acid compositions in patients with end-stage renal disease and type 2 diabetes mellitus. Chem. Phys. Lipids. 2014;178:11–17. doi: 10.1016/j.chemphyslip.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Dasu M.R., Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab. 2011;300:E145–E154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ertunc M.E., Hotamisligil G.S. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016;57:2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan L.-J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J. Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roukos V., Pegoraro G., Voss T.C., Misteli T. Cell cycle staging of individual cells by fluorescence microscopy. Nat. Protoc. 2015;10:334–348. doi: 10.1038/nprot.2015.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nauta A.J., Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 40.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinnadurai R., Copland I.B., Garcia M.A., Petersen C.T., Lewis C.N., Waller E.K., Kirk A.D., Galipeau J. Cryopreserved mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNγ licensing. Stem Cells. 2016;34:2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reaven G.M., Hollenbeck C., Jeng C.Y., Wu M.S., Chen Y.D. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 43.Klinker M.W., Marklein R.A., Lo Surdo J.L., Wei C.H., Bauer S.R. Morphological features of IFN-γ-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. USA. 2017;114:E2598–E2607. doi: 10.1073/pnas.1617933114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia. 2011;25:1408–1414. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 45.Ryan J.M., Barry F., Murphy J.M., Mahon B.P. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin P., Zhao Y., Liu H., Chen J., Ren J., Jin J., Bedognetti D., Liu S., Wang E., Marincola F., Stroncek D. Interferon-γ and tumor necrosis factor-α polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci. Rep. 2016;6:26345. doi: 10.1038/srep26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H., Wang W., Wang G., Hou Y., Xu F., Liu R., Wang F., Xue J., Hu T., Luan X. Interferon-γ and tumor necrosis factor-α promote the ability of human placenta-derived mesenchymal stromal cells to express programmed death ligand-2 and induce the differentiation of CD4(+)interleukin-10(+) and CD8(+)interleukin-10(+)Treg subsets. Cytotherapy. 2015;17:1560–1571. doi: 10.1016/j.jcyt.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Cramer C., Freisinger E., Jones R.K., Slakey D.P., Dupin C.L., Newsome E.R., Alt E.U., Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19:1875–1884. doi: 10.1089/scd.2010.0009. [DOI] [PubMed] [Google Scholar]
- 49.Acosta L., Hmadcha A., Escacena N., Pérez-Camacho I., de la Cuesta A., Ruiz-Salmeron R., Gauthier B.R., Soria B. Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes. 2013;62:4266–4269. doi: 10.2337/db13-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo G., Meng Y., Tan W., Xia Y., Cheng C., Chen X., Gu Z. Induction of apoptosis coupled to endoplasmic reticulum stress through regulation of CHOP and JNK in bone marrow mesenchymal stem cells from patients with systemic lupus erythematosus. J. Immunol. Res. 2015;2015:183738. doi: 10.1155/2015/183738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ankrum J.A., Dastidar R.G., Ong J.F., Levy O., Karp J.M. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci. Rep. 2014;4:4645. doi: 10.1038/srep04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhansali A., Asokumar P., Walia R., Bhansali S., Gupta V., Jain A., Sachdeva N., Sharma R.R., Marwaha N., Khandelwal N. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23:1075–1085. doi: 10.3727/096368913X665576. [DOI] [PubMed] [Google Scholar]
- 53.Skyler J.S., Fonseca V.A., Segal K.R., Rosenstock J., MSB-DM003 Investigators Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care. 2015;38:1742–1749. doi: 10.2337/dc14-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gramlich O.W., Burand A.J., Brown A.J., Deutsch R.J., Kuehn M.H., Ankrum J.A. Cryopreserved mesenchymal stromal cells maintain potency in a retinal ischemia/reperfusion injury model: toward an off-the-shelf therapy. Sci. Rep. 2016;6:26463. doi: 10.1038/srep26463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rovira Gonzalez Y.I., Lynch P.J., Thompson E.E., Stultz B.G., Hursh D.A. In vitro cytokine licensing induces persistent permissive chromatin at the Indoleamine 2,3-dioxygenase promoter. Cytotherapy. 2016;18:1114–1128. doi: 10.1016/j.jcyt.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song Y., Dou H., Li X., Zhao X., Li Y., Liu D., Ji J., Liu F., Ding L., Ni Y., Hou Y. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 57.Pricola K.L., Kuhn N.Z., Haleem-Smith H., Song Y., Tuan R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell. Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sivanathan K.N., Rojas-Canales D., Grey S.T., Gronthos S., Coates P.T. Transcriptome profiling of IL-17A preactivated mesenchymal stem cells: a comparative study to unmodified and IFN-γ modified mesenchymal stem cells. Stem Cells Int. 2017;2017:1025820. doi: 10.1155/2017/1025820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campos A.M., Maciel E., Moreira A.S.P., Sousa B., Melo T., Domingues P., Curado L., Antunes B., Domingues M.R., Santos F. Lipidomics of mesenchymal stromal cells: understanding the adaptation of phospholipid profile in response to pro-inflammatory cytokines. J. Cell. Physiol. 2016;231:1024–1032. doi: 10.1002/jcp.25191. [DOI] [PubMed] [Google Scholar]
- 60.Spector A.A. Structure and lipid binding properties of serum albumin. Methods Enzymol. 1986;128:320–339. doi: 10.1016/0076-6879(86)28077-5. [DOI] [PubMed] [Google Scholar]
- 61.Cousin S.P., Hügl S.R., Wrede C.E., Kajio H., Myers M.G., Jr., Rhodes C.J. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.