Abstract
Loss of adenosine deaminase activity leads to severe combined immunodeficiency (ADA-SCID); production and function of T, B, and natural killer (NK) cells are impaired. Gene therapy (GT) with an autologous CD34+-enriched cell fraction that contains CD34+ cells transduced with a retroviral vector encoding the human ADA cDNA sequence leads to immune reconstitution in most patients. Here, we report short- and medium-term safety analyses from 18 patients enrolled as part of single-arm, open-label studies or compassionate use programs. Survival was 100% with a median of 6.9 years follow-up (range, 2.3 to 13.4 years). Adverse events were mostly grade 1 or grade 2 and were reported by all 18 patients following GT. Thirty-nine serious adverse events (SAEs) were reported by 15 of 18 patients; no SAEs were considered related to GT. The most common adverse events reported post-GT include upper respiratory tract infection, gastroenteritis, rhinitis, bronchitis, oral candidiasis, cough, neutropenia, diarrhea, and pyrexia. Incidence rates for all of these events were highest during pre-treatment, treatment, and/or 3-month follow-up and then declined over medium-term follow-up. GT did not impact the incidence of neurologic/hearing impairments. No event indicative of leukemic transformation was reported.
Keywords: ADA-SCID, gene therapy, immune deficiency, opportunistic infection, immune reconstitution, safety, retroviral vector, adenosine deaminase
In 2016 the gene therapy, Strimvelis was approved for the treatment of patients with ADA-SCID for whom there is no suitable bone marrow donor. In this issue of Molecular Therapy, Aiuti et al. provide detailed safety data for treated patients. All patients are alive and without evidence of leukemic transformation.
Introduction
Severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID) is characterized by impaired function and differentiation of T, B, and natural killer (NK) cells.1, 2 Non-immunological abnormalities may also occur, including failure to thrive, hepatic disorders, renal disease, skeletal alterations, and neurological/cognitive/behavioral deficits.1, 3, 4, 5 ADA-SCID is rare: reported incidence rates range from 0.22 to 0.68 per 100,000 live births,6, 7, 8, 9 corresponding to fewer than 50 children per year in the United States and European Union combined.
From birth onward, patients with ADA-SCID are at risk of death due to life-threatening opportunistic infections; the disorder is fatal if untreated. Enzyme replacement therapy (ERT) in the form of polyethylene glycol-modified bovine adenosine deaminase (PEG-ADA) is beneficial for some patients with ADA-SCID and has been administered to over 150 patients.10, 11 However, immune reconstitution is variable and usually does not persist long term; approximately 10% of treated children develop neutralizing antibodies to PEG-ADA and require an increase in dosage, administration of corticosteroids, or cessation of therapy.12, 13, 14 For patients who received PEG-ADA between 1986 and 2008, the overall probability of 20-year survival on PEG-ADA was estimated to be 78%.1 A study of PEG-ADA treatment in 42 patients with ADA-SCID reported four deaths from infections.10
Patients with ADA-SCID may be effectively treated with hematopoietic stem cell transplant (HSCT). The best outcomes are achieved when a human leukocyte antigen (HLA)-matched sibling donor is used. However, this type of donor is available for less than 25% of patients with ADA-SCID.15 In contrast, the survival rate is low (less than 68%) for patients with ADA-SCID who receive HSCTs from HLA-matched unrelated donors.16 In a multicenter retrospective study of patients with ADA-SCID, the majority (63%) of deaths following all types of transplantations occurred within the first 100 days after HSCT. The reported causes of death were mainly related to pneumonitis/respiratory failure and sepsis, which caused more than 50% of all deaths. Other causes of death included graft versus host disease (GvHD) (15%) and fungal infection (incidence not reported). Only 2 of the 13 deaths that occurred after 100 days were attributed to HSCT-related complications.16
Gene therapy (GT) provides an opportunity to treat ADA-SCID while reducing the risk of side effects, such as GvHD and circumventing donor availability limitations. Treatment with an autologous cell fraction containing CD34+ cells carrying a human ADA cDNA sequence that has been inserted using a retroviral vector (RV) has previously been shown to result in immune system reconstitution for patients with ADA-SCID.17, 18, 19, 20, 21 While there are publications focusing on efficacy follow-up of ADA-SCID patients treated with HSCT or ERT, there is a paucity of published safety information. Similarly, high-level safety data for GT-treated ADA-SCID patients have been previously reported.19 Here are provided uniquely detailed analyses of short- and medium-term safety (up to 13 years) within a GT-treated ADA-SCID patient population, which were part of the dossier presented to European regulatory authorities for marketing authorization. These analyses specifically focus on areas of special interest, including infections, neurologic and hearing impairment, autoimmunity, hepatic findings (not previously reported), and malignancy.
Results
Unless otherwise noted, all results are from a May 2014 data cut as part of the dossier submitted to the European regulatory agency.
Study Population
Eighteen patients with ADA-SCID (11 male) were treated with GT (dose range, 0.9 to 18.2 × 106 CD34+ cells/kg; vector copy number range, 0.06 to 2.28 copies/cell).18, 19 The median age at GT was 1.7 years (range: 0.5 to 6.1 years); 15 patients had a previous history of ERT with PEG-ADA, and four had a previous history of unsuccessful haploidentical HSCT.
Efficacy
All 18 patients are alive as of June 2017 (A.A., unpublished data). Median follow-up at data cut (May 2014) was 6.9 years (range, 2.3 to 13.4 years). Engraftment of gene-modified cells led to immune reconstitution, demonstrated by immunoglobulin (IG) production, decreased intravenous (i.v.) IG use, antibody response after vaccination, normalization of T cell subsets (CD3+, CD4+, and CD8+ cells), evidence of thymopoiesis, and sustained T cell proliferative capacity. Three patients resumed long-term PEG-ADA treatment due to poor immune reconstitution after GT. One of these patients received a second GT treatment in the absence of further busulfan conditioning, but this treatment was also unsuccessful. That patient remains on ERT and is enrolled in long-term follow-up (LTFU). The other two patients received HLA-matched HSCTs from siblings (not originally available), followed by successful engraftment of donor stem cells and withdrawal from LTFU.
Adverse Events
A short summary of adverse events has been previously reported;19 additional details are below. In brief, adverse events (AEs) were mostly grade 1 or grade 2 in severity and were reported by all 18 patients. The incidence rates were greatest for the pre-treatment (start date after screening visit but prior to day −4), treatment (start date on day −4 up to and including start of GT [day 0], includes duration of busulfan conditioning), and 3-month follow-up (day 1, after infusion of investigational medicinal product, up to and including day 136) phases compared with the later phases (Table 1). The most frequently reported AEs were upper respiratory infection, gastroenteritis, diarrhea, and pyrexia. Two investigator-assessed treatment-related AEs were reported in a single patient: grade 1 hepatic steatosis and grade 2 abnormal white blood cell (decreased T cell receptor Vβ repertoire); both developed more than 8 years post-GT and were ongoing. Fifteen patients reported a total of 39 serious AEs (SAEs). Two patients experienced seven SAEs each. The most frequently reported SAEs were device-related infections, gastroenteritis, and pneumonia (Table 2). No SAEs were fatal or related to the GT medicinal product.
Table 1.
Summary of Adverse Events Reported in Three or More Patients
| Event (Rate per 100 Patient Years [No. of Patients]) | Pre-treatmenta (n = 17) | Treatment (n = 17) | 3-Month Follow-up (n = 17) | 3-Year Follow-up (n = 17) | 7-Year Follow-up (n = 13) | ≥8-Year Follow-up (n = 6b) | Total (n = 18) |
|---|---|---|---|---|---|---|---|
| Infections and Infestations | 445 (12) | 859 (2) | 844 (14) | 229 (17) | 127 (12) | 50 (5) | 203 (18) |
| Upper respiratory tract infection | 19 (1) | 0 | 64 (3) | 36 (8) | 17 (5) | 4 (1) | 24 (12) |
| Gastroenteritis | 39 (2) | 0 | 32 (2) | 22 (8) | 5 (2) | 0 | 13 (10) |
| Rhinitis | 39 (2) | 0 | 0 | 16 (8) | 12 (3) | 0 | 12 (9) |
| Bronchitis | 0 | 0 | 32 (1) | 16 (5) | 14 (3) | 0 | 12 (6) |
| Device-related infection | 0 | 0 | 64 (3) | 12 (4) | 0 | 0 | 8 (6) |
| Ear infection | 19 (1) | 0 | 16 (1) | 10 (3) | 9 (2) | 0 | 9 (6) |
| Oral candidiasis | 58 (3) | 0 | 111 (4) | 6 (2) | 2 (1) | 0 | 11 (6) |
| Nasopharyngitis | 19 (1) | 0 | 32 (2) | 10 (4) | 0 | 0 | 6 (5) |
| Pneumonia | 0 | 0 | 16 (1) | 2 (1) | 5 (2) | 4 (1) | 4 (5) |
| Sinusitis | 39 (2) | 0 | 0 | 4 (2) | 12 (5) | 0 | 7 (5) |
| Urinary tract infection | 0 | 0 | 32 (2) | 10 (3) | 5 (1) | 13 (2) | 9 (5) |
| Candida infection | 39 (2) | 0 | 16 (1) | 2 (1) | 0 | 0 | 3 (4) |
| Otitis media | 0 | 0 | 16 (1) | 4 (2) | 0 | 4 (1) | 3 (4) |
| Pharyngitis | 0 | 0 | 0 | 2 (1) | 5 (2) | 0 | 3 (4c) |
| Varicella | 0 | 0 | 0 | 6 (3) | 2 (1) | 0 | 3 (4) |
| Urinary tract infection (Escherichia coli) | 0 | 0 | 16 (1) | 10 (3) | 2 (1) | 0 | 5 (3) |
| Skin infection (fungal) | 0 | 0 | 32 (2) | 0 | 0 | 4 (1) | 2 (3) |
| Hemophilus infection | 0 | 0 | 0 | 6 (3) | 0 | 0 | 2 (3) |
| Influenza | 0 | 0 | 0 | 2 (1) | 5 (2) | 0 | 2 (3) |
| Respiratory tract infection | 19 (1) | 0 | 16 (1) | 8 (2) | 0 | 0 | 5 (3) |
| Sepsis (Staphylococcal) | 19 (1) | 0 | 16 (1) | 2 (1) | 0 | 0 | 2 (3) |
| Upper respiratory tract infection (bacterial) | 0 | 0 | 32 (2) | 8 (1) | 0 | 0 | 5 (3) |
| Urinary tract infection (Pseudomonal) | 19 (1) | 430 (1) | 48 (3) | 0 | 0 | 0 | 4 (3) |
| Investigations | 407 (12) | 859 (2) | 255 (10) | 53 (13) | 35 (10) | 17 (3) | 66 (17) |
| Antinuclear antibody positive test | 19 (1) | 0 | 0 | 0 | 9 (4) | 0 | 4 (5) |
| Increased blood IgE | 0 | 0 | 0 | 6 (3) | 7 (3) | 0 | 5 (5) |
| Increased hepatic enzyme | 0 | 0 | 80 (4) | 6 (2) | 0 | 0 | 6 (5) |
| Abnormal thorax CT | 58 (3) | 0 | 0 | 2 (1) | 0 | 0 | 3 (4) |
| Abnormal tympanometry | 19 (1) | 0 | 0 | 4 (2) | 2 (1) | 0 | 3 (4) |
| Increased blood alkaline phosphatase | 19 (1) | 430 (1) | 16 (1) | 2 (1) | 0 | 0 | 3 (3) |
| Abnormal protein electrophoresis | 0 | 0 | 16 (1) | 4 (2) | 0 | 0 | 2 (3) |
| Abnormal brain MRI | 58 (3) | 0 | 0 | 0 | 0 | 0 | 2 (3) |
| Abnormal pulmonary function test | 0 | 0 | 0 | 2 (1) | 5 (2) | 4 (1) | 3 (3) |
| Decreased weight | 0 | 0 | 0 | 4 (2) | 2 (1) | 0 | 2 (3) |
| Skin and subcutaneous tissue disorders | 194 (4) | 0 | 175 (7) | 30 (10) | 21 (5) | 8 (2) | 37 (16) |
| Atopic dermatitis | 19 (1) | 0 | 0 | 4 (2) | 5 (2) | 0 | 4 (5) |
| Skin lesion | 19 (1) | 0 | 16 (1) | 4 (2) | 0 | 0 | 3 (4) |
| Dermatitis | 0 | 0 | 32 (2) | 0 | 2 (1) | 0 | 2 (3) |
| Rash | 0 | 0 | 16 (1) | 4 (2) | 0 | 0 | 2 (3) |
| Blood and lymphatic system disorders | 97 (4) | 0 | 366 (11) | 26 (8) | 7 (2) | 0 | 34 (16) |
| Anemia | 19 (1) | 0 | 48 (3) | 6 (3) | 0 | 0 | 5 (7) |
| Neutropenia | 0 | 0 | 175 (5) | 4 (2) | 0 | 0 | 10 (6) |
| Eosinophilia | 19 (1) | 0 | 16 (1) | 4 (2) | 0 | 0 | 3 (4) |
| Respiratory, thoracic, and mediastinal disorders | 194 (7) | 0 | 32 (2) | 45 (12) | 24 (6) | 17 (3) | 38 (14) |
| Cough | 19 (1) | 0 | 0 | 14 (5) | 12 (3) | 4 (1) | 11 (8) |
| Interstitial lung disease | 39 (2) | 0 | 16 (1) | 0 | 0 | 0 | 2 (3) |
| Pneumonitis | 39 (2) | 0 | 16 (1) | 0 | 0 | 0 | 2 (3) |
| Productive cough | 19 (1) | 0 | 0 | 2 (1) | 2 (1) | 0 | 2 (3) |
| Gastrointestinal disorders | 97 (4) | 859 (2) | 159 (7) | 24 (7) | 21 (6) | 4 (1) | 30 (13) |
| Diarrhea | 58 (3) | 430 (1) | 64 (4) | 14 (6) | 12 (3) | 0 | 16 (10) |
| Vomiting | 39 (2) | 430 (1) | 32 (1) | 2 (1) | 2 (1) | 0 | 5 (6) |
| Enteritis | 0 | 0 | 16 (1) | 4 (1) | 2 (1) | 4 (1) | 4 (3) |
| General disorders and administration site conditions | 155 (6) | 1289 (3) | 16 (1) | 18 (9) | 12 (5) | 0 | 20 (12) |
| Pyrexia | 97 (4) | 430 (1) | 16 (1) | 12 (6) | 9 (4) | 0 | 13 (8) |
| Nervous system disorders | 78 (3) | 0 | 0 | 18 (7) | 12 (3) | 4 (1) | 15 (12) |
| Cognitive disorder | 0 | 0 | 0 | 6 (3) | 5 (2) | 0 | 4 (5) |
| Psychomotor hyperactivity | 19 (1) | 0 | 0 | 4 (2) | 0 | 0 | 2 (3) |
| Congenital, familial, and genetic disorders | 194 (8) | 0 | 0 | 22 (6) | 5 (2) | 0 | 18 (11) |
| Cryptorchism | 58 (3) | 0 | 0 | 6 (3) | 5 (2) | 0 | 6 (6) |
| Phimosis | 39 (2) | 0 | 0 | 10 (5) | 0 | 0 | 5 (6) |
| Hepatobiliary disorders | 39 (2) | 0 | 96 (4) | 12 (3) | 7 (2) | 8 (2) | 15 (10) |
| Hepatic steatosis | 0 | 0 | 32 (1) | 0 | 2 (1) | 8 (2) | 4 (4) |
| Hepatomegaly | 39 (2) | 0 | 0 | 2 (1) | 0 | 0 | 2 (3) |
| Musculoskeletal and connective tissue disorders | 78 (4) | 0 | 0 | 6 (2) | 12 (2) | 0 | 9 (7) |
| Foot deformity | 19 (1) | 0 | 0 | 0 | 5 (2) | 0 | 2 (3) |
| Muscle atrophy | 19 (1) | 0 | 0 | 0 | 5 (2) | 0 | 2 (3) |
| Endocrine disorders | 58 (3) | 0 | 0 | 4 (2) | 2 (1) | 4 (1) | 5 (6) |
| Hypothyroidism | 39 (2) | 0 | 0 | 4 (2) | 0 | 0 | 3 (4) |
| Vascular disorders | 39 (2) | 0 | 64 (3) | 2 (1) | 2 (1) | 0 | 6 (6) |
| Hypertension | 19 (1) | 0 | 64 (3) | 0 | 2 (1) | 0 | 5 (5) |
| Neoplasmsd | 39 (1) | 0 | 16 (1) | 6 (1) | 2 (1) | 13 (3) | 8 (5) |
| Skin papilloma | 0 | 0 | 0 | 0 | 2 (1) | 13(3) | 3 (3) |
AE, adverse event; CT, computed tomography; GT, gene therapy; IgE, immunoglobulin E, LTFU, long-term follow-up; SAE, serious adverse event. In any given phase, only those AEs that were new or worsened after the start of a particular phase were reported.
Pre-treatment AEs include any AEs with a start date from the screening visit but prior to day −4 from the date of GT. Pre-treatment events were originally recorded as concomitant diseases and were retrospectively reclassified as pre-treatment AEs. The events reported during the pre-treatment phase may have been ongoing at the time of study enrollment and may reflect concomitant disease history. However, events that may have been serious but did not meet criteria cannot be determined. The numbers of pre-treatment SAEs are therefore likely underestimated.
One patient started follow-up at year 13 but had AEs reported at year 8 and year 12, which are included.
An AE of pharyngitis occurred in the LTFU, but the dates were not known so it appears in the Total column only.
The neoplasms system organ class includes benign, malignant, and unspecified, including cysts and polyps.
Table 2.
Serious Adverse Events Following Gene Therapya
| Event | Study Phase | Duration, Days | Outcome | Maximum Toxicity Grade |
|---|---|---|---|---|
| Neutropeniab | 3-month follow-up | 29 | resolved | grade 4 |
| Pneumonia | 3-month follow-up | 46 | resolved | grade 4 |
| Autoimmune thrombocytopenia | 3-month follow-up | 12 | resolved | grade 3 |
| Device-related infection | 3-month follow-up | 12 | resolved | grade 3 |
| Device-related infection | 3-month follow-up | 16 | resolved | grade 3 |
| Epstein-Barr virus infection | 3-month follow-up | 9 | resolved | grade 3 |
| Hypertension | 3-month follow-up | 75 | resolved | grade 3 |
| Pancytopenia | 3-month follow-up | 22 | resolved | grade 3 |
| Sepsis (Staphylococcal) | 3-year follow-up | 9 | resolved | grade 4 |
| Aplastic anemia | 3-year follow-up | 120 | resolved | grade 3 |
| Autoimmune hepatitis | 3-year follow-up | 14 | resolved | grade 3 |
| Autoimmune thrombocytopenia | 3-year follow-up | 16 | resolved | grade 3 |
| Device-related infection | 3-year follow-up | 4 | resolved | grade 3 |
| Device-related infection | 3-year follow-up | 14 | resolved | grade 3 |
| Device-related infection | 3-year follow-up | 11 | resolved | grade 3 |
| Device-related infection | 3-year follow-up | 10 | resolved | grade 3 |
| Device-related infection | 3-year follow-up | 17 | resolved | grade 3 |
| Device-related infection | 3-year follow-up | 9 | resolved | grade 3 |
| Diarrhea | 3-year follow-up | 9 | resolved | grade 3 |
| Fever | 3-year follow-up | 4 | resolved | grade 3 |
| Fibrolipoma72 | 3-year follow-up | 4 | resolved | grade 3 |
| Gastroenteritis | 3-year follow-up | 6 | resolved | grade 3 |
| Gastroenteritis | 3-year follow-up | 7 | resolved | grade 3 |
| Guillain-Barré syndrome | 3-year follow-up | 4 | resolved | grade 3 |
| Meningitis | 3-year follow-up | 17 | resolved | grade 3 |
| Neutropenia | 3-month follow-up | 66 | resolved | grade 3 |
| Respiratory tract infection | 3-year follow-up | 3 | resolved | grade 3 |
| Upper respiratory tract infection (bacterial) | 3-year follow-up | 12 | resolved | grade 3 |
| Urinary tract infection | 3-year follow-up | 10 | resolved | grade 3 |
| Varicella | 3-year follow-up | 6 | resolved | grade 3 |
| Gastroenteritis | 3-year follow-up | 28 | resolved | grade 2 |
| Autoimmune aplastic anemia | 7-year follow-up | 10 | resolved | grade 3 |
| Pneumonia | 7-year follow-up | 10 | resolved | grade 3 |
| Pyoderma | 7-year follow-up | 20 | resolved | grade 3 |
| Varicella | 7-year follow-up | 8 | resolved | grade 3 |
| Fever | 7-year follow-up | 2 | resolved | grade 2 |
| Thermal burn | 7-year follow-up | 110 | resolved with sequelae | grade 2 |
| Pneumonia | ≥8-year follow-up | 8 | resolved | grade 3 |
| Urinary tract infection | ≥8-year follow-up | 4 | resolved | grade 2 |
Adapted from Cicalese et al.19
Identified as an event potentially related to autoimmunity (verbatim term: prolonged neutropenia [anti-neutrophils antibodies positive]).
Laboratory and Clinical Values
Following an initial, transient decrease between baseline and day 14 (consistent with low-dose busulfan treatment), hematology parameters including hemoglobin, hematocrit, and platelet count increased toward pre-treatment values over 3 months to 1 year post-GT.19 Only white blood cell (WBC) counts increased above pre-treatment values, which is to be expected as lymphocyte counts rise as immune reconstitution progresses post-GT. There was no consistent pattern of concern for clinical chemistry or vital sign values (including heart rate and blood pressure).
Infections
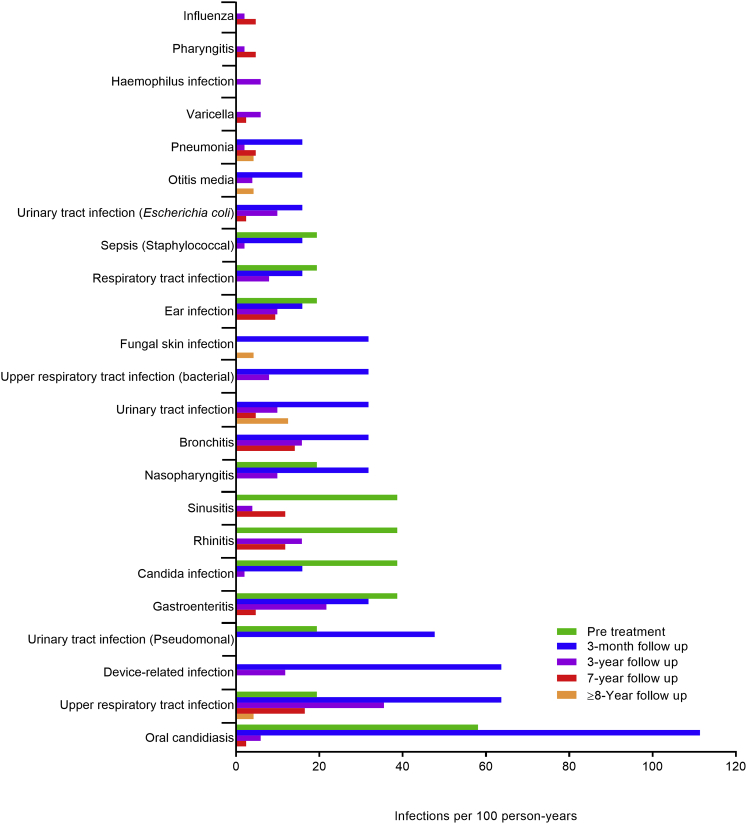
Infection AEs were reported for all 18 patients (Table 1), including those with unsuccessful GT. The three most frequently reported infections were normal, expected childhood infections:22, 23 upper respiratory tract infection, gastroenteritis, and rhinitis.19 The incidence rates for the most frequent infections peaked before or during the 3-month follow-up phase and then decreased (Figure 1). Twenty-four of the 39 SAEs reported post-treatment were infections; all resolved (Table 2). Six patients reported 10 AEs of central venous catheter (CVC) infections, of which eight were grade 3 SAEs that developed within 3 years of GT; all resolved. Most urinary tract infections (UTIs) occurred during 3-month follow-up and early in 3-year follow-up (Table 1). Three patients reported three or more UTIs each; two patients had UTI SAEs. Six of the 11 male patients reported UTIs; all six had conditions that predispose for UTIs. UTI events were not related to urinary catheter placement.
Figure 1.
Incidence of Infections in Three or More Patients by Study Phase
Incidence rate calculations include patients who resumed PEG-ADA treatment due to a lack of GT efficacy. In any given phase, only those AEs that were new or worsened after the start of a particular phase were reported.
There were 30 opportunistic infections reported following GT, 18 of them in the 3-month follow-up phase (Table 3). Most opportunistic infections were grade 1; all resolved. Post-GT, four patients reported Varicella zoster virus (VZV) infections, five reported oral candidiasis (three patients had pre-treatment AEs), three reported Clostridium difficile infections, and two reported Aspergillus infections.
Table 3.
Opportunistic Infections
| Event | Study Phase | Duration, Days | Outcome | Maximum Toxicity Grade |
|---|---|---|---|---|
| Candida infection | pre-treatment | 14 | resolved | grade1 |
| Candida infection | pre-treatment | NR | resolved | grade 1 |
| Oral candidiasis | pre-treatment | 25 | resolved | grade 1 |
| Oral candidiasis | pre-treatment | 4 | resolved | grade 1 |
| Oral candidiasis | pre-treatment | 51 | resolved | grade 1 |
| Skin Candida | pre-treatment | 13 | resolved | grade 1 |
| Epstein-Barr virus infection | 3-month follow-up | 9 | resolved | grade 3 (SAE) |
| Candida infection | 3-month follow-up | 13 | resolved | grade 2 |
| Clostridium difficile infection | 3-month follow-up | 45 | resolved | grade 2 |
| Epstein-Barr virus infection | 3-month follow-up | 26 | resolved | grade 2 |
| Epstein-Barr virus infection | 3-month follow-up | 6 | resolved | grade 2 |
| Positive Candida test | 3-month follow-up | 23 | resolved | grade 2 |
| Pulmonary mycosis | 3-month follow-up | 10 | resolved | grade 2 |
| Clostridium difficile colitis | 3-month follow-up | 5 | resolved | grade 1 |
| Clostridium difficile colitis | 3-month follow-up | 6 | resolved | grade 1 |
| Clostridium difficile infection | 3-month follow-up | 217 | resolved | grade 1 |
| Cytomegalovirus | 3-month follow-up | 22 | resolved | grade 1 |
| Oral candidiasis | 3-month follow-up | 7 | resolved | grade 1 |
| Oral candidiasis | 3-month follow-up | 13 | resolved | grade 1 |
| Oral candidiasis | 3-month follow-up | 12 | resolved | grade 1 |
| Oral candidiasis | 3-month follow-up | 120 | resolved | grade 1 |
| Oral candidiasis | 3-month follow-up | 6 | resolved | grade 1 |
| Oral candidiasis | 3-month follow-up | NR | resolved | grade 1 |
| Positive Clostridium test | 3-month follow-up | 15 | resolved | grade 1 |
| Varicella | 3-year follow-up | 6 | resolved | grade 3 (SAE) |
| Oral candidiasis | 3-year follow-up | NR | resolved | grade 2 |
| Oral candidiasis | 3-year follow-up | 10 | resolved | grade 2 |
| Varicella | 3-year follow-up | 23 | resolved | grade 2 |
| Varicella | 3-year follow-up | 19 | resolved | grade 2 |
| Aspergillus infection | 3-year follow-up | 16 | resolved | grade 1 |
| Candida infection | 3-year follow-up | 335 | resolved | grade 1 |
| Oral candidiasis | 3-year follow-up | 13 | resolved | grade 1 |
| Positive Aspergillus test | 3-year follow-up | 7 | resolved | grade 1 |
| Varicella | 7-year follow-up | 8 | resolved | grade 3 (SAE) |
| Gastroenteritis (cryptosporidial) | 7-year follow-up | NR | resolved | grade 1 |
| Oral candidiasis | 7-year follow-up | NR | resolved | grade 1 |
AE, adverse event; NR, not reported; SAE, serious adverse event. CMV and EBV reactivation occurred each in one patient early after treatment; primary Varicella infection occurred in four patients without complications, followed by specific antibody responses. In any given phase, only those AEs that were new or worsened after the start of a particular phase were reported.
Neurologic and Hearing Impairments
All patients had neurological assessments throughout follow-up via specialist exams, instrumental tests, and physical exams. Fourteen patients reported neurologic or hearing AEs at screening.19 Thirteen patients reported neurologic or hearing AEs post GT. Cognitive disorder and deafness were the most commonly reported events (Table 4). The majority of events were grade 1 or 2, none were serious, and none were considered by the investigator to be related to treatment. No convulsion AEs were reported.
Table 4.
Neurologic, CNS, and Hearing-Impairment AEs
| Eventa | Study Phase | Duration, Days | Outcome | Maximum Toxicity Grade |
|---|---|---|---|---|
| Abnormal brain MRI | pre-treatment | NR | NR | NR |
| Abnormal tympanometry | pre-treatment | NR | NR | NR |
| Alteration in periventricular white matter on brain MRI | pre-treatment | 1,121 | resolved | NR |
| Broad-based walking | pre-treatment | ongoing | not resolved | NR |
| Enlarged lateral ventricular on brain MRI | pre-treatment | NR | NR | NR |
| Low-amplitude EEG waves with poor organization | pre-treatment | ongoing | not resolved | NR |
| Psychomotor hyperactivity | pre-treatment | ∼2 years | resolved | NR |
| Sensory neural hearing loss (bilateral) | pre-treatment | ongoing | not resolved | NR |
| Hypotonia | pre-treatment | 255 | resolved | grade 3 |
| Severe mental retardation | pre-treatment | ongoing | not resolved | grade 3 |
| Cerebral hematoma | pre-treatment | 112 | resolved | grade 2 |
| Deafness (bilateral) | pre-treatment | ongoing | not resolved | grade 2 |
| Psychomotor retardation | pre-treatment | ongoing | not resolved | grade 2 |
| Psychomotor retardation | pre-treatment | ongoing | not resolved | grade 2 |
| Abnormal brain MRI | pre-treatment | 1,122 | resolved | grade 1 |
| Abnormal brain MRI | pre-treatment | ongoing | not resolved | grade 1 |
| Abnormal brain scan | pre-treatment | ongoing | not resolved | grade 1 |
| Abnormal VEP | pre-treatment | 381 | resolved | grade 1 |
| Arnold-Chiari malformation | pre-treatment | ongoing | not resolved | grade 1 |
| Attention deficit/hyperactivity disorder | pre-treatment | 1,125 | resolved | grade 1 |
| Communication disorder | pre-treatment | 1,125 | resolved | grade 1 |
| Demyelination | pre-treatment | 1,318 | resolved | grade 1 |
| Developmental delay | pre-treatment | ongoing | not resolved | grade 1 |
| Gliosis | pre-treatment | ongoing | not resolved | grade 1 |
| Hypoacusia | pre-treatment | 964 | resolved | grade 1 |
| Hypothalamo-pituitary disorder | pre-treatment | ongoing | not resolved | grade 1 |
| Hypothalamo-pituitary disorder | pre-treatment | ongoing | not resolved | grade 1 |
| Impaired psychomotor skills | pre-treatment | ongoing | not resolved | grade 1 |
| Mental retardation | pre-treatment | 3003 | resolved | grade 1 |
| Plagiocephaly | pre-treatment | NR | resolved | grade 1 |
| Psychomotor retardation | pre-treatment | ∼2.5 years | resolved | grade 1 |
| Sensorineural hypoacusia at BAER | pre-treatment | ongoing | not resolved | grade 1 |
| Sleep disorder | pre-treatment | 462 | resolved | grade 1 |
| Tympanic membrane disorder | pre-treatment | ongoing | not resolved | grade 1 |
| VEP prolonged latency | pre-treatment | 672 | resolved | grade 1 |
| Gait disturbance | treatment | 37 | resolved | grade 1 |
| Middle ear inflammation | 3-month follow-up | 5 | resolved | grade 1 |
| Guillain-Barré syndrome | 3-year follow-up | 4 | resolved | grade 3 |
| Autism | 3-year follow-up | ongoing | not resolved | grade 2 |
| Speech disorder | 3-year follow-up | ongoing | not resolved | grade 2 |
| Abnormal tympanometry | 3-year follow-up | ongoing | not resolved | grade 1 |
| Abnormal tympanometry | 3-year follow-up | 1,167 | resolved | grade 1 |
| Cognitive disorder | 3-year follow-up | ongoing | not resolved | grade 1 |
| Cognitive disorder | 3-year follow-up | ongoing | not resolved | grade 1 |
| Cognitive disorder | 3-year follow-up | ongoing | not resolved | grade 1 |
| Deafness | 3-year follow-up | 198 | resolved | grade 1 |
| Deafness | 3-year follow-up | ongoing | not resolved | grade 1 |
| Deafness (bilateral) | 3-year follow-up | ongoing | not resolved | grade 1 |
| Deafness (bilateral) | 3-year follow-up | 338 | resolved | grade 1 |
| Developmental delay | 3-year follow-up | 2,212 | resolved | grade 1 |
| Impaired psychomotor skills | 3-year follow-up | ∼1.8 years | resolved | grade 1 |
| Psychomotor hyperactivity | 3-year follow-up | ∼1.1 years | resolved | grade 1 |
| Psychomotor hyperactivity | 3-year follow-up | ongoing | not resolved | grade 1 |
| Tympanic membrane disorder | 3-year follow-up | ongoing | not resolved | grade 1 |
| Hypoacusia | 7-year follow-up | ongoing | not resolved | grade 2 |
| Abnormal tympanometry | 7-year follow-up | ongoing | not resolved | grade 1 |
| Cognitive disorder | 7-year follow-up | ongoing | not resolved | grade 1 |
| Areflexia | 7-year follow-up | 871 | resolved | grade 1 |
| Cognitive disorder | 7-year follow-up | ongoing | not resolved | grade 1 |
| Deafness | 7-year follow-up | ongoing | not resolved | grade 1 |
| Disturbance in attention | 7-year follow-up | ongoing | not resolved | grade 1 |
| Impaired hearing | ≥8-year follow-up | ongoing | not resolved | grade 3 |
| Moderate mental retardation | ≥8-year follow-up | ongoing | not resolved | grade 3 |
| Obsessive-compulsive disorder | ≥8-year follow-up | ongoing | not resolved | grade 2 |
AE, adverse event; BAER, brainstem auditory evoked response; EEG, electroencephalogram; NR, not recorded; VEP, visual evoked potential. In any given phase, only those AEs that were new or worsened after the start of a particular phase were reported.
Relevant medical conditions ongoing at screening, which are not reported as AEs, were identified via manual review and are included in this table for completeness.
Autoimmunity
Manual record review revealed pre-treatment AEs potentially related to autoimmunity (positive anti-nuclear antibody [ANA] test, autoimmune haemolytic anemia, and hypothyroidism) in five patients. Following GT, 12 subjects reported 26 events related to autoimmunity.19 Grade 1 ANA positivity was the most frequently reported autoimmune event (one event each in five patients) (Table 5). Seven autoimmunity SAEs were reported and all resolved: anti-neutrophil antibody-induced neutropenia; Guillain-Barré syndrome; two autoimmune thrombocytopenia events in a single patient; and autoimmune aplastic anemia, aplastic anemia, and autoimmune hepatitis in another patient. Other than a single autoimmune aplastic anemia event, all autoimmunity SAEs occurred within the first 3 years post-GT. No systemic allergic events (angioedema, anaphylactic reaction, or severe cutaneous reactions) that could be indicative of immunogenicity to the GT medicinal product were observed.
Table 5.
AEs Potentially Related to Autoimmunity
| Event | Study Phase | Duration, Days | Outcome | Maximum Toxicity Grade |
|---|---|---|---|---|
| Autoimmune hemolytic anemia | pre-treatment | NR | resolved | NA |
| Chronic autoimmune hemolytic anemia | pre-treatment | NA | not resolved | NA |
| Hypothyroidism | pre-treatment | NA | not resolved | grade 2 |
| Antinuclear antibody positive test | pre-treatment | 3,005 | resolved | grade 1 |
| Hypothyroidism | pre-treatment | 1,116 | resolved | grade 1 |
| Hypothyroidism | pre-treatment | NA | not resolved | grade 1 |
| Neutropenia | 3-month follow-up | 29 | resolved | grade 4 (SAE) |
| Autoimmune thrombocytopenia | 3-month follow-up | 12 | resolved | grade 3 (SAE) |
| Anti-thyroid antibody positive test | 3-month follow-up | 648 | resolved | grade 1 |
| Aplastic anemiaa | 3-year follow-up | 120 | resolved | grade 3 (SAE) |
| Autoimmune hepatitis | 3-year follow-up | 14 | resolved | grade 3 (SAE) |
| Autoimmune thrombocytopenia | 3-year follow-up | 16 | resolved | grade 3 (SAE) |
| Guillain-Barré syndrome | 3-year follow-up | 4 | resolved | grade 3 (SAE) |
| Autoimmune thrombocytopenia | 3-year follow-up | 46 | resolved | grade 3 |
| Autoimmune thrombocytopenia | 3-year follow-up | NA | not resolved | grade 3 |
| Autoimmune haemolytic anemia | 3-year follow-up | NR | resolved | grade 2 |
| Hypothyroidism | 3-year follow-up | NA | not resolved | grade 2 |
| Hypothyroidism | 3-year follow-up | NA | not resolved | grade 2 |
| Autoimmune hepatitis | 3-year follow-up | 211 | resolved | grade 1 |
| Autoimmune aplastic anemia | 7-year follow-up | 10 | resolved | grade 3 (SAE) |
| Autoimmune haemolytic anemia | 7-year follow-up | 145 | resolved | grade 2 |
| Anti-neutrophil cytoplasmic antibody | 7-year follow-up | NA | not resolved | grade 1 |
| Anti-nuclear antibody positive test | 7-year follow-up | 1,093 | resolved | grade 1 |
| Anti-nuclear antibody positive test | 7-year follow-up | 1,023 | resolved | grade 1 |
| Anti-nuclear antibody positive test | 7-year follow-up | NA | not resolved | grade 1 |
| Anti-nuclear antibody positive test | 7-year follow-up | NA | not resolved | grade 1 |
| Anti-smooth muscle antibody positive test | 7-year follow-up | 1,373 | resolved | grade 1 |
| Anti-smooth muscle antibody positive test | 7-year follow-up | 877 | resolved | grade 1 |
| Positive direct Coombs test | 7-year follow-up | 1,242 | resolved | grade 1 |
| Autoimmune thyroiditis | ≥8-year follow-up | 743 | resolved | grade 1 |
| Antinuclear antibody positive test | ≥8-year follow-up | NA | not resolved | grade 1 |
AE, adverse event; NA, not applicable; NR, not reported; SAE, serious adverse event. In any given phase, only those AEs that were new or worsened after the start of a particular phase were reported.
Event was identified in SAE narrative as possibly related to autoimmunity.
Hepatic Events
Prior to GT, clinically relevant AEs included grade 1 cholelithiasis and liver haemangioma (one patient), grade 1 hepatomegaly and abnormal liver ultrasound (one patient), and grade 1 hepatomegaly (two patients). Following or during busulfan conditioning and GT, 12 patients showed 34 hepatic laboratory abnormalities or hepato-biliary disorders; 16 events were altered liver function tests (liver transaminases or alkaline phosphatase). Grade 1 hepatic steatosis was reported in one patient during 3-month follow-up and in three patients during LTFU. Potential contributing factors to hepatic steatosis included cholelithiasis, dyslipidemia, hepatic fibrosis, busulfan exposure, increased hepatic enzymes, and reactivated Epstein-Barr infection. Most hepatic events were grade 1. Autoimmune hepatitis was the only hepatic SAE reported.
Malignancy
As of June 2017, no malignancies have been reported within this patient population (A.A., unpblished data). As of the May 2014 data cut, a benign fibrolipoma (one patient)24 and skin papillomas (warts) (three patients) were reported post-GT. Grade 1 abnormal protein electrophoresis was reported in three patients; all of these events resolved. One patient reported an AE of increased IG that resolved over 3 years. All patients have a polyclonal T cell Vβ repertoire with more than a single Vβ family represented. Bone marrow morphology evaluations and immunophenotype revealed developing lymphocyte, myeloid, and erythroid cells but no clinically significant alterations. Cytogenetic analyses (karyotypes) were normal in bone marrow and peripheral blood; no abnormal blast values were found in either peripheral blood or bone marrow. All tests for replication competent retrovirus in bone marrow and peripheral blood yielded negative results.
Previously, we reported retrovirus insertion site (RIS) analysis24, 25 that was complemented by further analysis on 14 patients at a single time point.19 To validate this data, nine patients were re-tested using whole-blood or peripheral-blood mononuclear cells collected at more recent time points. Bioinformatics analysis of this new sequencing data (comprising 29,777,849 reads) identified a total of 3,225 insertion sites. Comparison revealed that 547 (14.5%) of the RIS previously detected were also recovered during this new analysis. Collision rates between samples were high, and following bioinformatics processing the insertion site diversity ranged between 22 and 441 for the patients tested. Test-retest data showed that both the reproducibility and sensitivity of the assay were low. Due to the limitations of the testing method, the data were not suitable for drawing conclusions on integration abundance, repertoire, and ultimately on the safety profile of GT. A new test system has now been developed and will be the subject of future publications.
Discussion
Here, we provide comprehensive safety evaluation from a GT-treated cohort of patients with ADA-SCID that contributed to the data package for the approval of autologous GT (Strimvelis) for ADA-SCID in the EU in 2016. The listings provided here reveal unique insights into the clinical history of both pre- and post-treatment ADA-SCID. Importantly, AE grade, treatment phase, and duration are available for opportunistic infections; neurologic, CNS, and hearing events; and events related to autoimmunity. This first-of-its-kind dataset comprehensively describes the overall health of these patients, including areas of specific safety interest, in addition to their response to GT. In contrast, previous publications of alternative ADA-SCID treatments have focused on efficacy and death due to specific causes but did not provide detailed analyses of AEs and other safety-related outcomes, as they were not designed to collect events after treatment.26, 27, 28, 29
Children with ADA-SCID commonly suffer from a variety of opportunistic infections, including viral, fungal, and mycobacterial infections. Due to defects in B cells and IG production, they are also susceptible to infection with encapsulated bacteria such as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.30, 31 Persistent infections with opportunistic organisms, including Candida albicans, VZV, Aspergillus, and Cryptosporidium are not uncommon and may lead to serious complications or death.23, 32, 33 Infections of the respiratory tract (upper respiratory tract, rhinitis, bronchitis, nasopharyngitis, pneumonia, and sinusitis) and gastrointestinal tract (gastroenteritis/enteritis along with diarrhea) were the most frequently reported AEs in this study. UTIs were identified as events of special interest because the observed overall incidence (especially in males) was higher than expected.34 Importantly, all male patients with UTIs also had conditions that predispose for UTI,35 and the rate of UTIs in the 3-year follow-up period was comparable to those estimated for the general US pediatric population.36 No males reported UTIs during the 7-year and >8-year follow-up phases.
During the 3-year and 7-year follow-up phases, the rate of Candida infection was higher than that published for the general UK pediatric population,37 but the rate of Varicella infection was comparable to other socialized European children.38, 39 During the 3-year follow-up period, gastroenteritis occurred at a higher rate than is observed for the general pediatric population,40 but there were no reports of gastroenteritis during the 7-year and ≥8-year follow-up periods. Infection incidence rates were highest in the pre-treatment to 3-month hospitalization period and decreased with time, a finding expected in subjects undergoing immune reconstitution.18, 19 Generally, incidence rates dropped over time until they were similar to those reported for the general pediatric population.
AEs in the blood and lymphatic system organ class (including anemia, neutropenia, and thrombocytopenia) were most frequently reported during the 3-month follow-up phase. As expected, the majority of these events occurred within the first 35 days post-GT, indicating that they were likely a consequence of conditioning with busulfan; granulocytopenia, thrombocytopenia, and anemia are all adverse drug reactions reported for busulfan therapy.41 Hepatic events were identified as special interest due to the known association of busulfan with elevations in transaminases and hepatic veno-occlusive disease (VOD). The incidence of hepatic enzyme increases peaked immediately following busulfan treatment during 3-month follow-up and then declined. These events are consistent with those commonly associated with bone marrow transplantation and are likely reduced in number and severity due to the low-dose regimen applied here. No VOD events were reported.
CNS abnormalities are frequently reported manifestations of ADA-SCID, including in long-term survivors of bone marrow transplantation (BMT).5, 10, 42, 43, 44, 45 Reported neurological deficits include cognitive impairment (mental retardation, low IQ, reduced verbal skills, and learning disabilities), behavioral abnormalities, sensorineural hearing deficits, motor dysfunction, developmental delays, hypotonia, and abnormal brain MRIs. PEG-ADA treatment prior to SCT does not seem to correct these impairments,10, 42, 43, 44, 45 despite the previously reported improvement of neurologic manifestations in two patients following reduction of metabolite concentrations.46 In a cross-sectional study of 105 patients with congenital immunodeficiencies surviving after SCT (56 of whom had SCID or ADA-SCID), it was reported that the underlying genetic disease, presence of ADA-SCID, and consanguinity were each associated with a worse intelligence outcome.47 Moreover, patients who had a more severe clinical course (i.e., admission to the intensive care unit) had a worse neurocognitive outcome post-SCT. Lower socioeconomic status was also associated with poor outcomes, while the age at transplantation, length of stay in the hospital, and conditioning regimen were not considered significant risk factors. Unsurprisingly, most patients described here reported neurological findings at baseline despite prior receipt of ERT. These manifestations may have been related to the underlying ADA-SCID pathology, sequelae from previous infections (e.g., meningitis, otitis media), and/or prior antibiotic treatment (e.g., gentamycin). Here, we report additional neurologic and hearing AEs post GT; however, we are unable to differentiate between the underlying disease state and GT-related procedures/medications41, 48, 49, 50 (SCT, busulfan conditioning, and antibiotic prophylaxis/treatment) as potentially contributing factors. There was no indication that baseline neurological AEs improved following GT.
Patients with ADA-SCID may experience autoimmune AEs after SCT (and/or during ERT) that are thought to be related to immune dysregulation prior to achieving complete immune reconstitution.51, 52 The more common autoimmune manifestations include hemolytic anemia, hypothyroidism, and immune thrombocytopenia.1, 53, 54 Following treatment with GT, HSCT, and/or PEG-ADA, early-onset ADA-SCID patients may have a response (atopy) to common allergens such as food or inhalants.55 Reports of autoimmunity and allergy-related events have also been described in delayed-onset ADA-SCID.1, 56, 57 Several autoimmune events occurred pre-treatment. The majority of post-GT events occurred within 3-year follow-up, mainly consisted of positive autoantibody tests without associated clinical manifestations, and were consistent with ongoing immune reconstitution. Peripheral blood CD19+ B cells and CD3+ T cells, red blood cell deoxyadenosine nucleotide (dAXP) levels, and anti-CD3 monoclonal antibody (mAb) proliferative responses were similar between patients with and without autoimmune events (data not shown). Autoimmune events are commonly associated with HSCT, PEG-ADA treatment, and the underlying disease state.58 Importantly, there were no indications that any autoimmune events were specifically attributable to the GT medicinal product.
GT with integrating vectors introduces a risk of insertional mutagenesis through activation of proto-oncogenes, the formation of de novo gene expression products, and knockout of tumor suppressor gene function.59 As a result, leukemic transformation is a leading safety concern for GT. Hematologic malignancies and abnormalities following RV GT have been observed in patients treated for X-linked SCID,60 chronic granulomatous disease,61, 62, 63 and Wiskott-Aldrich Syndrome.64 Importantly, no cases of leukemia have been reported in the cohort described here, despite more than a decade of follow-up in several patients. Moreover, none of the other 22 patients with ADA-SCID who underwent alternative GT regimens over the past decade have developed leukemia.21, 65 Additional RIS analysis confirmed the existence of several thousand unique insertion sites. As is expected for a pseudo-randomly inserting vector, RIS were detected in areas of known risk.55 However, clones with these inserts are stable and are not causing clinical safety issues for patients. Insertion site diversity (the number of recovered sites) was comparable to similar gamma retroviral GT trials.66
All 18 patients who have been treated with RV GT for ADA-SCID are alive, clinically well, and without signs of leukemic transformation. The safety data presented here provide a first-of-its-kind assessment describing detailed short-term and medium-term follow-up, including infections, hepatic events, autoimmune manifestations, and neurologic/hearing impairments. Monitoring of this cohort is ongoing and will be incorporated into a registry for these and future patients, planned in agreement with pharmacovigilance guidelines for GT products. Such a registry is vital to establish the long-term safety of GT for the treatment of patients with ADA-SCID and to allow accurate comparisons of this new treatment with SCT, ERT, and the emerging class of lentiviral-based GT vectors.67 Long-term safety and efficacy data from the registry will be made publicly available as the cohort matures.
The safety data reported here are consistent with those available for the general ADA-SCID population29 and indicate that GT can safely restore immune function in these patients. Immune reconstitution leads to a decrease in the rate of infections following GT, in many cases to rates similar to those within the general pediatric population. The patients discussed here, for whom other treatment options were limited, safely underwent GT and in most cases corrected the metabolic defect and achieved immune reconstitution.
Materials and Methods
Study Design
Two pilot studies,17, 18, 19 one pivotal study with a LTFU component,18, 19 and a compassionate use program19 (CUP; established in accordance with the Italian Ministerial Decree of 8 May 2003) enrolled patients with ADA-SCID. LTFU is ongoing and permitted enrollment of patients from the pilot studies and CUP to participate in long-term assessments beyond the initial follow-up period. All studies were non-randomized, single-arm, and open label. The pivotal study and LTFU are registered at https://www.clinicaltrials.gov as #NCT00598481.
One patient was enrolled and treated at Hadassah Hebrew University Hospital in Jerusalem (Israel). The second pilot study, pivotal study, LTFU, and CUP were administered at San Raffaele Scientific Institute in Milan (Italy) and approved by the Institutional Ethics Committee and the Italian National Regulatory Authorities. All studies complied with Declaration of Helsinki protocols.
Ad hoc data regarding survival and malignancies were provided in June 2017 (A.A., unpublished data); it is not from a validated database or a pre-specified analysis. Data cut for all other results was May 2014 (to match the data provided in the regulatory filings).
Patients
All patients’ parents provided written informed consent. All patients were under 18 years of age, diagnosed with ADA-SCID, and lacked an HLA-identical sibling donor. All patients had either previously received ≥6 months of PEG-ADA treatment with demonstrated suboptimal efficacy/intolerance, or PEG-ADA was not a long-term treatment option for them.
Pre-treatment
A CVC was placed during the pre-treatment phase. Back-up HSCs were harvested and cryopreserved in case of poor engraftment or technical issues with product manufacture. Patients receiving ERT ceased PEG-ADA administration 10 to 22 days before treatment. The chemotherapy agent busulfan was given as non-myeloablative conditioning to prepare the bone marrow compartment for HSC engraftment. Administration was divided into eight doses of 0.5 mg/kg, administered over study days −3 and −2. Equivalent oral doses (four doses of 0.6 mg/kg) were administered to one patient when i.v. dosing was not feasible. Busulfan dose was reduced if plasma levels exceeded 4000 ng/mL*h, as measured after the first and fifth doses. This regimen is approximately 20%–25% of the typical dosage used in a myeloablative conditioning regimen41, 68 and was selected with the intent to promote enhanced gene-modified HSC engraftment while minimizing toxicities associated with intensive chemotherapy.18
GT
Mononuclear cells were harvested from bone marrow under general anesthesia. An RV was used to transduce purified CD34+ cells with the human ADA cDNA sequence.17, 18 On the day of GT, patients were intravenously infused with the transduced autologous CD34+-enriched cell fraction (Strimvelis, GSK2696273).
Concomitant therapy usually included pentamidine as first-line Pneumocystis jirovecii prophylaxis from before GT until recovery of white blood cell (WBC) count, then switched to cotrimoxazole.69 i.v./oral antibacterial and antifungal prophylaxis was administered according to SCT guidelines.70 Acyclovir was used as a herpes simplex prophylaxis and was typically administered until complete immunological reconstitution was achieved. Pre-emptive therapy with ganciclovir or foscarnet was administered according to SCT guidelines for patients who were Cytomegalovirus positive by PCR. Pre-emptive therapy with rituximab was used in patients who were Epstein-Barr virus positive by PCR and experienced a substantial increase in cellular viral load.
Outcomes
The key efficacy endpoint assessed was survival. Efficacy endpoints are reported elsewhere.19
Safety assessments included vital signs, physical examination (normal/abnormal), electrocardiogram (normal/abnormal), urinalysis, bone marrow morphology, bone marrow immunophenotype, bone marrow karyotype, peripheral blood karyotype, and replication competent retrovirus testing. Hematologic assessments included red blood cells (RBCs), WBCs, neutrophils, lymphocytes, monocytes, eosinophils, basophils, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets, reticulocytes, and erythrocyte sedimentation rate (ESR). Clinical chemistry assessments included AST, alanine aminotransferase (ALT), C-reactive protein (CRP), lactate dehydrogenase (LDH), total bilirubin, indirect bilirubin, glucose, blood urea nitrogen (BUN), creatinine, creatinine phosphokinase (CPK), sodium, potassium, calcium, chlorine, magnesium, phosphorus, alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), total protein, albumin, iron, transferrin, ferritin, FT3, FT4, thyroid-stimulating hormone (TSH), XDP, prothrombin time (PT), activated partial prothrombin time (aPTT), and fibrinogen. Specialist examinations included ophthalmology, neurology, psychology, cardiology, and ear/nose/throat. Instrumental tests included electrocardiograms, chest X-ray, abdominal ultrasound scan, chest CT, echocardiogram, brain/sinus MRI, brainstem auditory evoked potential, visual evoked potential, tympanometry/audiometry, electroencephalography, hand bone X-ray, and respiratory function tests (for patients >5 years of age). Assessments were performed at baseline and then at intervals during the first 8 years post-GT. Subsequently, patients continue to be evaluated clinically at either their local hospital or San Raffaele Hospital.
AEs
AE reporting was started from the date of GT onward. Per protocol, pre-treatment events were recorded as concomitant diseases. These events were then retrospectively reported as AEs to provide a baseline, conform to GSK standards, and accommodate regulatory requirements. All AEs were assigned to 1 of 6 phases: pre-treatment (start date after screening visit but prior to day −4); treatment (start date on day −4 up to and including start of GT [day 0], includes the timing for busulfan conditioning); 3-month follow-up window (day 1, after infusion of investigational medicinal product, up to and including day 136); 3-year follow-up window (day 137 to day 1,278); 7-year follow-up window (day 1,279 to day 2,739), and ≥8-year follow-up window (≥day 2,740). However, except for those events that required hospitalization or for which severity was specified in the available clinical records, pre-treatment events that may have been serious cannot be specifically determined. The numbers of pre-treatment SAEs are, therefore, likely underestimated. The events reported during the pre-treatment phase may have been ongoing at the time of study enrollment and may reflect concomitant disease history. During the treatment phase, only unexpected or severe conditions were reported as AEs. In any given phase, only those AEs that were new or worsened after the start of a particular phase were reported. Any AE with an unknown outcome,or with a missing resolution date on the clinical report form was recorded as unresolved in the database. A manual review was conducted to identify any conditions ongoing at screening and potentially related to central CNS, autoimmunity, hepatic disorders, and opportunistic infections. AE toxicity was classified using standard Common Terminology Criteria for Adverse Events (CTCAE) (version 4) criteria.71
Based on the expected events in patients with ADA-SCID, the risks of GT, regulatory guidance, and the reporting of treatment-related AEs, the following events were identified as AEs of special interest: infections; neurologic and hearing AEs; immune reactions (including AEs possibly related to autoimmunity); oncogenesis events; and hepatic laboratory abnormalities and events. These denote events that the study sponsors and/or regulatory officials felt necessitated specific tracking due to their anticipated frequency or potential impact. Where available, Standardized MedDRA queries have been used to facilitate identification of cases of interest from the AE database that contain terms related to signs, symptoms, diagnoses, syndromes, physical findings, or laboratory test data that may be associated with a medical condition of interest. Custom searches were developed in some instances where a standardized query was not available or was inadequate.
A manual search of available AE listings (by preferred term and verbatim term) and of listings for prior medical conditions was performed. SAE narratives and AEs known to be associated with autoimmunity were reviewed to identify additional events. Laboratory testing for autoimmune antibodies (anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, anti-smooth muscle antibody, direct Coombs test, liver kidney microsomal antibody, and mitochondrial antibody) was conducted during LTFU but was not checked at each visit for every patient. Antibody positivity detection was recorded as an AE by the investigator, even when not associated with clinically relevant events.
Safety Analysis
Not all patients had data available for all time periods. All patients, with the exception of one patient, had data available from pre-treatment through 3-year follow-up. Two patients withdrew from follow-up after receiving successful matched-sibling SCTs. All other patients are enrolled in LTFU and are contacted each year by the clinic center but have varying durations of follow-up. Safety data were summarized only; statistical analysis was not performed.
RIS Analysis
Each patient sample was run in triplicate (three biological replicates), and each biological replicate was digested with three restriction enzymes. After bioinformatic analysis, the data for the three enzymes were combined to create one dataset for each biological replicate. The variability of the assay in terms of RIS recovery concordance and the standard deviation of the abundance estimates between the three biological replicates from each patient were analyzed.
Author Contributions
Conceptualization, A.A., M.G.R.; Validation, A.A., J.A., K.R., M.G.R.; Formal Analysis, E.D.B., K.R., R.R.R.; Investigation, A.A., J.A., L.C., M.P.C., F.F., M.G.R.; Writing – Review and Editing, A.A., J.A., L.C., M.P.C., E.D.B, F.F., K.R., R.R.R., M.G.R.; Supervision, A.A., J.A., R.R.R., M.G.R.; Project Administration, A.A., J.A., R.R.R., M.G.R.; Funding Acquisition, A.A.
Conflicts of Interest
J.A., E.D.B., R.R.R., and K.R. are employees of, and own shares in, GlaxoSmithKline. A.A. is the PI of the ADA-SCID clinical trial sponsored by GSK. The remaining authors (L.C., M.P.C., F.F., and M.G.R.) declare no competing financial interests.
Acknowledgments
The authors would like to thank all the medical and nurse personnel of the Paediatric Immunohematology and Hematology and Bone Marrow Transplant Unit of San Raffaele Hospital (Milan), the personnel of the SR-Tiget Clinical Trial Office, local referring physicians who helped with patient management, and all patients who participated in this study and their families. The authors thank Luca Biasco and Francesca Dionisio (both of SR-Tiget) for performing the RIS integration studies, and Michela Gabaldo (SR-Tiget) for her continuous support in the alliance between Telethon/San Raffaele and GSK. The authors wish to thank Sam Garthside, Younan Chen, and Andrea Campanile of GSK for statistical, programming, operational, and data management support for the clinical development program. Writing assistance was provided by Molly Nixon of Synchrogenix (funded by GSK). Project management support was provided by Barbara Kravitz of GSK. In addition, the authors acknowledge Miriam Casiraghi, Giuliana Tomaselli, and Samih El Hossary (all of SR-Tiget) for their support to patients. Financial support for these studies was provided to A.A. by the Fondazione Telethon, European Union Project FP7 CELL PID, and GlaxoSmithKline.
References
- 1.Gaspar H.B., Aiuti A., Porta F., Candotti F., Hershfield M.S., Notarangelo L.D. How I treat ADA deficiency. Blood. 2009;114:3524–3532. doi: 10.1182/blood-2009-06-189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapel H., Geha R., Rosen F., IUIS PID (Primary Immunodeficiencies) Classification committee Primary immunodeficiency diseases: an update. Clin. Exp. Immunol. 2003;132:9–15. doi: 10.1046/j.1365-2249.2003.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer A.V., Aiuti A. New insights into the pathogenesis of adenosine deaminase-severe combined immunodeficiency and progress in gene therapy. Curr. Opin. Allergy Clin. Immunol. 2009;9:496–502. doi: 10.1097/ACI.0b013e3283327da5. [DOI] [PubMed] [Google Scholar]
- 4.Nikolajeva O., Worth A., Hague R., Martinez-Alier N., Smart J., Adams S., Davies E.G., Gaspar H.B. Adenosine deaminase deficient severe combined immunodeficiency presenting as atypical haemolytic uraemic syndrome. J. Clin. Immunol. 2015;35:366–372. doi: 10.1007/s10875-015-0158-0. [DOI] [PubMed] [Google Scholar]
- 5.Sauer A.V., Hernandez R.J., Fumagalli F., Bianchi V., Poliani P.L., Dallatomasina C., Riboni E., Politi L.S., Tabucchi A., Carlucci F. Alterations in the brain adenosine metabolism cause behavioral and neurological impairment in ADA-deficient mice and patients. Sci. Rep. 2017;7:40136. doi: 10.1038/srep40136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryser O., Morell A., Hitzig W.H. Primary immunodeficiencies in Switzerland: first report of the national registry in adults and children. J. Clin. Immunol. 1988;8:479–485. doi: 10.1007/BF00916954. [DOI] [PubMed] [Google Scholar]
- 7.Yee A., De Ravin S.S., Elliott E., Ziegler J.B., Contributors to the Australian Paediatric Surveillance Unit Severe combined immunodeficiency: a national surveillance study. Pediatr. Allergy Immunol. 2008;19:298–302. doi: 10.1111/j.1399-3038.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 8.Verbsky J.W., Baker M.W., Grossman W.J., Hintermeyer M., Dasu T., Bonacci B., Reddy S., Margolis D., Casper J., Gries M. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008-2011) J. Clin. Immunol. 2012;32:82–88. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 9.Vogel B.H., Bonagura V., Weinberg G.A., Ballow M., Isabelle J., DiAntonio L., Parker A., Young A., Cunningham-Rundles C., Fong C.T. Newborn screening for SCID in New York State: experience from the first two years. J. Clin. Immunol. 2014;34:289–303. doi: 10.1007/s10875-014-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth C., Hershfield M., Notarangelo L., Buckley R., Hoenig M., Mahlaoui N., Cavazzana-Calvo M., Aiuti A., Gaspar H.B. Management options for adenosine deaminase deficiency; proceedings of the EBMT satellite workshop (Hamburg, March 2006) Clin. Immunol. 2007;123:139–147. doi: 10.1016/j.clim.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar H.B. Bone marrow transplantation and alternatives for adenosine deaminase deficiency. Immunol. Allergy Clin. North Am. 2010;30:221–236. doi: 10.1016/j.iac.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Chaffee S., Mary A., Stiehm E.R., Girault D., Fischer A., Hershfield M.S. IgG antibody response to polyethylene glycol-modified adenosine deaminase in patients with adenosine deaminase deficiency. J. Clin. Invest. 1992;89:1643–1651. doi: 10.1172/JCI115761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun J.D., Lee N., Kobayashi R.H., Chaffee S., Hershfield M.S., Stiehm E.R. Suppression of an antibody to adenosine-deaminase (ADA) in an ADA-deficient patient receiving polyethylene glycol modified adenosine deaminase. Ann. Allergy. 1993;70:462–466. [PubMed] [Google Scholar]
- 14.Lainka E., Hershfield M.S., Santisteban I., Bali P., Seibt A., Neubert J., Friedrich W., Niehues T. polyethylene glycol-conjugated adenosine deaminase (ADA) therapy provides temporary immune reconstitution to a child with delayed-onset ADA deficiency. Clin. Diagn. Lab. Immunol. 2005;12:861–866. doi: 10.1128/CDLI.12.7.861-866.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschhorn R., Grunebaum E., Roifman C., Candotti F. Immunodeficiency due to defects of purine metabolism. In: Ochs H.D., Smith C.I.E., Puck J.M., editors. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. Third Edition. Oxford University Press; 2014. pp. 188–230. [Google Scholar]
- 16.Hassan A., Booth C., Brightwell A., Allwood Z., Veys P., Rao K., Hönig M., Friedrich W., Gennery A., Slatter M., Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation and European Society for Immunodeficiency Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120:3615–3624. doi: 10.1182/blood-2011-12-396879. quiz 3626. [DOI] [PubMed] [Google Scholar]
- 17.Aiuti A., Slavin S., Aker M., Ficara F., Deola S., Mortellaro A., Morecki S., Andolfi G., Tabucchi A., Carlucci F. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 18.Aiuti A., Cattaneo F., Galimberti S., Benninghoff U., Cassani B., Callegaro L., Scaramuzza S., Andolfi G., Mirolo M., Brigida I. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 19.Cicalese M.P., Ferrua F., Castagnaro L., Pajno R., Barzaghi F., Giannelli S., Dionisio F., Brigida I., Bonopane M., Casiraghi M. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood. 2016;128:45–54. doi: 10.1182/blood-2016-01-688226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Zhang F., Adams S., Bjorkegren E., Bayford J., Brown L., Davies E.G. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 2011;3:97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- 21.Candotti F., Shaw K.L., Muul L., Carbonaro D., Sokolic R., Choi C., Schurman S.H., Garabedian E., Kesserwan C., Jagadeesh G.J. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120:3635–3646. doi: 10.1182/blood-2012-02-400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay A.D., Heron J., Ness A., ALSPAC study team The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam. Pract. 2005;22:367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 23.Hershfield M. Adenosine Deaminase Deficiency. In: Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., Stephens K., editors. GeneReviews® [Internet], updated 2014. University of Washington; 2006. http://www.ncbi.nlm.nih.gov/books/NBK1483/?report=classic [Google Scholar]
- 24.Aiuti A., Cassani B., Andolfi G., Mirolo M., Biasco L., Recchia A., Urbinati F., Valacca C., Scaramuzza S., Aker M. Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J. Clin. Invest. 2007;117:2233–2240. doi: 10.1172/JCI31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biasco L., Ambrosi A., Pellin D., Bartholomae C., Brigida I., Roncarolo M.G., Di Serio C., von Kalle C., Schmidt M., Aiuti A. Integration profile of retroviral vector in gene therapy treated patients is cell-specific according to gene expression and chromatin conformation of target cell. EMBO Mol. Med. 2011;3:89–101. doi: 10.1002/emmm.201000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booth C., Gaspar H.B. Pegademase bovine (PEG-ADA) for the treatment of infants and children with severe combined immunodeficiency (SCID) Biologics. 2009;3:349–358. [PMC free article] [PubMed] [Google Scholar]
- 27.Cancrini C., Ferrua F., Scarselli A., Brigida I., Romiti M.L., Barera G., Finocchi A., Roncarolo M.G., Caniglia M., Aiuti A. Role of reduced intensity conditioning in T-cell and B-cell immune reconstitution after HLA-identical bone marrow transplantation in ADA-SCID. Haematologica. 2010;95:1778–1782. doi: 10.3324/haematol.2010.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanegane H., Taneichi H., Nomura K., Wada T., Yachie A., Imai K., Ariga T., Santisteban I., Hershfield M.S., Miyawaki T. Successful bone marrow transplantation with reduced intensity conditioning in a patient with delayed-onset adenosine deaminase deficiency. Pediatr. Transplant. 2013;17:E29–E32. doi: 10.1111/j.1399-3046.2012.01762.x. [DOI] [PubMed] [Google Scholar]
- 29.Baffelli R., Notarangelo L.D., Imberti L., Hershfield M.S., Serana F., Santisteban I., Bolda F., Porta F., Lanfranchi A. Diagnosis, treatment and long-term follow-up of patients with ADA deficiency: a single-center experience. J. Clin. Immunol. 2015;35:624–637. doi: 10.1007/s10875-015-0191-z. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham-Rundles C. Autoimmunity in primary immune deficiency: taking lessons from our patients. Clin. Exp. Immunol. 2011;164(Suppl 2):6–11. doi: 10.1111/j.1365-2249.2011.04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uzzaman A., Fuleihan R.L. Chapter 27: approach to primary immunodeficiency. Allergy Asthma Proc. 2012;33(Suppl 1):S91–S95. doi: 10.2500/aap.2012.33.3560. [DOI] [PubMed] [Google Scholar]
- 32.Stephan J.L., Vlekova V., Le Deist F., Blanche S., Donadieu J., De Saint-Basile G., Durandy A., Griscelli C., Fischer A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J. Pediatr. 1993;123:564–572. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 33.Buckley R.H., Schiff R.I., Schiff S.E., Markert M.L., Williams L.W., Harville T.O., Roberts J.L., Puck J.M. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J. Pediatr. 1997;130:378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 34.Zorc J.J., Kiddoo D.A., Shaw K.N. Diagnosis and management of pediatric urinary tract infections. Clin. Microbiol. Rev. 2005;18:417–422. doi: 10.1128/CMR.18.2.417-422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiraoka M., Tsukahara H., Ohshima Y., Mayumi M. Meatus tightly covered by the prepuce is associated with urinary infection. Pediatr. Int. 2002;44:658–662. doi: 10.1046/j.1442-200x.2002.01633.x. [DOI] [PubMed] [Google Scholar]
- 36.Sood A., Penna F.J., Eleswarapu S., Pucheril D., Weaver J., Abd-El-Barr A.E., Wagner J.C., Lakshmanan Y., Menon M., Trinh Q.D. Incidence, admission rates, and economic burden of pediatric emergency department visits for urinary tract infection: data from the nationwide emergency department sample, 2006 to 2011. J. Pediatr. Urol. 2015;11:246e1–246e8. doi: 10.1016/j.jpurol.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Oeser C., Lamagni T., Heath P.T., Sharland M., Ladhani S. The epidemiology of neonatal and pediatric candidemia in England and Wales, 2000-2009. Pediatr. Infect. Dis. J. 2013;32:23–26. doi: 10.1097/INF.0b013e318275612e. [DOI] [PubMed] [Google Scholar]
- 38.Dubos F., Grandbastien B., Hue V., Martinot A., Hospital Network for Evaluating Management of Common Childhood Diseases Epidemiology of hospital admissions for paediatric varicella infections: a one-year prospective survey in the pre-vaccine era. Epidemiol. Infect. 2007;135:131–138. doi: 10.1017/S0950268806006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pozza F., Piovesan C., Russo F., Bella A., Pezzotti P., Emberti Gialloreti L. Impact of universal vaccination on the epidemiology of varicella in Veneto, Italy. Vaccine. 2011;29:9480–9487. doi: 10.1016/j.vaccine.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Leino T., Ollgren J., Salo H., Tiihonen P., Kilpi T. First year experience of rotavirus immunisation programme in Finland. Vaccine. 2012;31:176–182. doi: 10.1016/j.vaccine.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 41.Busilvex (2014). Summary of Product Characteristics. Pierre Fabre Médicament: France.
- 42.Rogers M.H., Lwin R., Fairbanks L., Gerritsen B., Gaspar H.B. Cognitive and behavioral abnormalities in adenosine deaminase deficient severe combined immunodeficiency. J. Pediatr. 2001;139:44–50. doi: 10.1067/mpd.2001.115023. [DOI] [PubMed] [Google Scholar]
- 43.Albuquerque W., Gaspar H.B. Bilateral sensorineural deafness in adenosine deaminase-deficient severe combined immunodeficiency. J. Pediatr. 2004;144:278–280. doi: 10.1016/j.jpeds.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 44.Hönig M., Albert M.H., Schulz A., Sparber-Sauer M., Schütz C., Belohradsky B., Güngör T., Rojewski M.T., Bode H., Pannicke U. Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2007;109:3595–3602. doi: 10.1182/blood-2006-07-034678. [DOI] [PubMed] [Google Scholar]
- 45.Nofech-Mozes Y., Blaser S.I., Kobayashi J., Grunebaum E., Roifman C.M. Neurologic abnormalities in patients with adenosine deaminase deficiency. Pediatr. Neurol. 2007;37:218–221. doi: 10.1016/j.pediatrneurol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Hirschhorn R., Paageorgiou P.S., Kesarwala H.H., Taft L.T. Amerioration of neurologic abnormalities after “enzyme replacement” in adenosine deaminase deficiency. N. Engl. J. Med. 1980;303:377–380. doi: 10.1056/NEJM198008143030706. [DOI] [PubMed] [Google Scholar]
- 47.Titman P., Pink E., Skucek E., O’Hanlon K., Cole T.J., Gaspar J., Xu-Bayford J., Jones A., Thrasher A.J., Davies E.G. Cognitive and behavioral abnormalities in children after hematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood. 2008;112:3907–3913. doi: 10.1182/blood-2008-04-151332. [DOI] [PubMed] [Google Scholar]
- 48.Gurney J.G., Ness K.K., Rosenthal J., Forman S.J., Bhatia S., Baker K.S. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: results from the Bone Marrow Transplant Survivor study. Cancer. 2006;106:1402–1408. doi: 10.1002/cncr.21752. [DOI] [PubMed] [Google Scholar]
- 49.Weber C., Schaper J., Tibussek D., Adams O., Mackenzie C.R., Dilloo D., Meisel R., Göbel U., Laws H.J. Diagnostic and therapeutic implications of neurological complications following paediatric haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:253–259. doi: 10.1038/sj.bmt.1705905. [DOI] [PubMed] [Google Scholar]
- 50.Esposito S., Canevini M.P., Principi N. Complications associated with antibiotic administration: neurological adverse events and interference with antiepileptic drugs. Int. J. Antimicrob. Agents. 2017;50:1–8. doi: 10.1016/j.ijantimicag.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 51.Etzioni A. Immune deficiency and autoimmunity. Autoimmun. Rev. 2003;2:364–369. doi: 10.1016/s1568-9972(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 52.Daikeler T., Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract. Res. Clin. Haematol. 2007;20:349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Neven B., Leroy S., Decaluwe H., Le Deist F., Picard C., Moshous D., Mahlaoui N., Debré M., Casanova J.L., Dal Cortivo L. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113:4114–4124. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 54.Sauer A.V., Brigida I., Carriglio N., Hernandez R.J., Scaramuzza S., Clavenna D., Sanvito F., Poliani P.L., Gagliani N., Carlucci F. Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood. 2012;119:1428–1439. doi: 10.1182/blood-2011-07-366781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence M.G., Barber J.S., Sokolic R.A., Garabedian E.K., Desai A.N., O’Brien M., Jones N., Bali P., Hershfield M.S., Stone K.D. Elevated IgE and atopy in patients treated for early-onset ADA-SCID. J. Allergy Clin. Immunol. 2013;132:1444–1446. doi: 10.1016/j.jaci.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santisteban I., Arredondo-Vega F.X., Kelly S., Mary A., Fischer A., Hummell D.S., Lawton A., Sorensen R.U., Stiehm E.R., Uribe L. Novel splicing, missense, and deletion mutations in seven adenosine deaminase-deficient patients with late/delayed onset of combined immunodeficiency disease. Contribution of genotype to phenotype. J. Clin. Invest. 1993;92:2291–2302. doi: 10.1172/JCI116833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shovlin C.L., Simmonds H.A., Fairbanks L.D., Deacock S.J., Hughes J.M., Lechler R.I., Webster A.D., Sun X.M., Webb J.C., Soutar A.K. Adult onset immunodeficiency caused by inherited adenosine deaminase deficiency. J. Immunol. 1994;153:2331–2339. [PubMed] [Google Scholar]
- 58.Sauer A.V., Brigida I., Carriglio N., Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front. Immunol. 2012;3:265. doi: 10.3389/fimmu.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight S., Collins M., Takeuchi Y. Insertional mutagenesis by retroviral vectors: current concepts and methods of analysis. Curr. Gene Ther. 2013;13:211–227. doi: 10.2174/1566523211313030006. [DOI] [PubMed] [Google Scholar]
- 60.Cavazzana-Calvo M., Fischer A., Hacein-Bey-Abina S., Aiuti A. Gene therapy for primary immunodeficiencies: Part 1. Curr. Opin. Immunol. 2012;24:580–584. doi: 10.1016/j.coi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Grez M., Reichenbach J., Schwäble J., Seger R., Dinauer M.C., Thrasher A.J. Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol. Ther. 2011;19:28–35. doi: 10.1038/mt.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aiuti A., Bacchetta R., Seger R., Villa A., Cavazzana-Calvo M. Gene therapy for primary immunodeficiencies: Part 2. Curr. Opin. Immunol. 2012;24:585–591. doi: 10.1016/j.coi.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Siler U., Paruzynski A., Holtgreve-Grez H., Kuzmenko E., Koehl U., Renner E.D., Alhan C., de Loosdrecht A.A., Schwäble J., Pfluger T. Successful combination of sequential gene therapy and rescue allo-HSCT in two children with X-CGD—importance of timing. Curr. Gene Ther. 2015;15:416–427. doi: 10.2174/1566523215666150515145255. [DOI] [PubMed] [Google Scholar]
- 64.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D. Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee S., Thrasher A.J. Gene therapy for PIDs: progress, pitfalls and prospects. Gene. 2013;525:174–181. doi: 10.1016/j.gene.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deichmann A., Brugman M.H., Bartholomae C.C., Schwarzwaelder K., Verstegen M.M., Howe S.J., Arens A., Ott M.G., Hoelzer D., Seger R. Insertion sites in engrafted cells cluster within a limited repertoire of genomic areas after gammaretroviral vector gene therapy. Mol. Ther. 2011;19:2031–2039. doi: 10.1038/mt.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaspar B., Buckland K., Rivat C., Booth C., Gilmour K., Cornetta K. Immunological and metabolic correction after lentiviral vector mediated haematopoietic stem cell gene therapy for ADA deficiency [abstract] J. Clin. Immunol. 2014;34 S167. Abstract ESID-0018. [Google Scholar]
- 68.Bartelink I.H., Bredius R.G., Belitser S.V., Suttorp M.M., Bierings M., Knibbe C.A., Egeler M., Lankester A.C., Egberts A.C., Zwaveling J., Boelens J.J. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol. Blood Marrow Transplant. 2009;15:231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan J.E., Benson C., Holmes K.K., Brooks J.T., Pau A., Masur H., Centers for Disease Control and Prevention (CDC) National Institutes of Health. HIV Medicine Association of the Infectious Diseases Society of America Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm. Rep. 2009;58:1–207. quiz CE1–CE4. [PubMed] [Google Scholar]
- 70.European Society for Blood and Marrow Transplantation (EBMT), European Society for Immunodeficiencies (ESID) (2017). Guidelines for haematopoietic stem cell transplantation for primary immunodeficiencies. https://esid.org/layout/set/print/Working-Parties/Inborn-Errors-Working-Party-IEWP/Resources/UPDATED!-EBMT-ESID-GUIDELINES-FOR-HAEMATOPOIETIC-STEM-CELL-TRANSPLANTATION-FOR-PI.
- 71.National Cancer Institute (2009). Common Terminology Criteria for Adverse Events (CTCAE) v 4.0. National Institutes of Health.
- 72.Grunebaum E., Chung C.T., Dadi H., Kim P., Brigida I., Ferrua F., Cicalese M.P., Aiuti A., Roifman C.M. Purine metabolism, immune reconstitution, and abdominal adipose tumor after gene therapy for adenosine deaminase deficiency. J. Allergy Clin. Immunol. 2011;127:1417–1419.e3. doi: 10.1016/j.jaci.2011.04.014. [DOI] [PubMed] [Google Scholar]