Abstract
Kinetic studies of protein dephosphorylation in photosynthetic thylakoid membranes revealed specifically accelerated dephosphorylation of photosystem II (PSII) core proteins at elevated temperatures. Raising the temperature from 22°C to 42°C resulted in a more than 10-fold increase in the dephosphorylation rates of the PSII reaction center proteins D1 and D2 and of the chlorophyll a binding protein CP43 in isolated spinach (Spinacia oleracea) thylakoids. In contrast the dephosphorylation rates of the light harvesting protein complex and the 9-kD protein of the PSII (PsbH) were accelerated only 2- to 3-fold. The use of a phospho-threonine antibody to measure in vivo phosphorylation levels in spinach leaves revealed a more than 20-fold acceleration in D1, D2, and CP43 dephosphorylation induced by abrupt elevation of temperature, but no increase in light harvesting protein complex dephosphorylation. This rapid dephosphorylation is catalyzed by a PSII-specific, intrinsic membrane protein phosphatase. Phosphatase assays, using intact thylakoids, solubilized membranes, and the isolated enzyme, revealed that the temperature-induced lateral migration of PSII to the stroma-exposed thylakoids only partially contributed to the rapid increase in the dephosphorylation rate. Significant activation of the phosphatase coincided with the temperature-induced release of TLP40 from the membrane into thylakoid lumen. TLP40 is a peptidyl-prolyl cis-trans isomerase, which acts as a regulatory subunit of the membrane phosphatase. Thus dissociation of TLP40 caused by an abrupt elevation in temperature and activation of the membrane protein phosphatase are suggested to trigger accelerated repair of photodamaged PSII and to operate as possible early signals initiating other heat shock responses in chloroplasts.
The most heat-sensitive function in green plants is photosynthesis (Berry and Björkman, 1980; Weis and Berry, 1988; Havaux and Tardy, 1996). High temperatures dramatically inhibit carbon dioxide fixation (Berry and Björkman, 1980; Feller et al., 1998). Moreover, the heat tolerance of leaves depends on the thermal sensitivity of the photochemical reactions in the thylakoid membranes of chloroplasts. The primary site of thermal damage to the photosynthetic function is believed to be associated with photosystem II (PSII; Berry and Björkman, 1980; Weis and Berry, 1988; Havaux and Tardy, 1996). Plants have evolved a number of molecular mechanisms to cope with the high temperature and to protect the photosynthetic system. In response to a few hours of a heat stress a nuclear encoded small heat shock protein is expressed and binds to the thylakoid membranes (Glaczinski and Kloppstech, 1988; Osteryoung and Vierling, 1994). This binding has been shown to protect thermolabile PSII and, consequently, whole-chain electron transport during the heat stress (Heckathorn et al., 1998). Several faster response mechanisms to elevation of the ambient temperature have also been proposed to occur in chloroplasts. These responses include the separation of PSII from light harvesting protein complex (LHCII; Sundby et al., 1986; Pastenes and Horton, 1996), temperature-induced conformational changes of PSII (Havaux, 1994), stabilization of thylakoid membranes and PSII due to violaxanthin to zeaxanthin conversion (Havaux and Tardy, 1996; Havaux et al., 1996; Tardy and Havaux, 1997) and, in some plants, isoprene synthesis (Sharkey, 1996; Singsaas et al., 1997). However, the mechanisms of immediate sensing of elevated temperatures and signals inducing the heat shock response in the photosynthetic machinery are still enigmatic.
Processes of protein phosphorylation comprise a universal molecular mechanism for adaptation of and regulation in living organisms. Specifically, phosphorylation of proteins was suggested to play a pivotal role in sensing elevated temperature by plants (Krishnan and Pueppke, 1987). In chloroplasts a unique redox-regulated protein phosphorylation has evolved (Bennett et al., 1980; Allen, 1992; Vener et al., 1998). This phosphorylation is induced by light that activates a redox-dependent membrane protein kinase (Vener et al., 1995, 1997; Gal et al., 1997; Snyders and Kohorn, 1999) and leads to phosphorylation of approximately twenty thylakoid membrane proteins. The major thylakoid phosphoproteins are those of the LHCII and polypeptides of PSII itself: the reaction center proteins D1, D2, CP43, and a 9-kD (PsbH) polypeptide. Phosphorylation of these proteins is reversible, and both integral and extrinsic membrane protein phosphatases appear to be involved in dephosphorylation of thylakoid phosphoproteins (Sun et al., 1989; Hast and Follmann, 1996; Hammer et al., 1997; Vener et al., 1999). Phosphorylation of PSII proteins has been shown to regulate the stability, degradation, and turnover of the reaction center proteins (Andersson and Aro, 1997; Barber et al., 1997; Kruse et al., 1997; Baena-Gonzalez et al., 1999). The D1 reaction center protein of PSII displays the highest turnover rate of all thylakoid proteins due to its light-induced damage. Repair of photosynthetic function requires proteolytic degradation of the damaged protein, followed by synthesis of a new D1 molecule and its integration into PSII (Aro et al., 1993). Under conditions of light stress the D1 protein becomes phosphorylated. However, the photodamaged D1 protein is subjected to proteolysis only after its dephosphorylation (Koivuniemi et al., 1995; Rintamäki et al., 1996). This is believed to be a key regulatory event in the coordination of degradation with integration and assembly of a new subunit during the protein turnover (Andersson and Aro, 1997). Thus, phosphorylation and dephosphorylation of PSII are critical regulatory factors and play a principal role in the control of PSII repair.
We have recently purified an intrinsic thylakoid membrane phosphatase and found that this enzyme is highly active in the dephosphorylation of PSII reaction center proteins (Vener et al., 1999). In the same study the phosphatase was found to be associated with and regulated by a cyclophilin-like protein, TLP40. The phosphatase activation in the presence of cyclosporin A was suggested to operate via release of TLP40 from the inner surface of the thylakoid membrane. In the present paper we report on a specific and rapid dephosphorylation of PSII core phosphoproteins, including the D1 protein, in response to abrupt elevation of temperature both in isolated thylakoids and in vivo. A heat-induced activation of protein dephosphorylation is shown to coincide with a release of TLP40 from the membrane into the thylakoid lumen. The protein phosphatase activation appears to represent an immediate, and probably the most profound, auxiliary enzymatic response to elevated temperatures in plant photosynthetic membranes.
RESULTS
Heat-Stimulation of Thylakoid Protein Dephosphorylation in Vitro
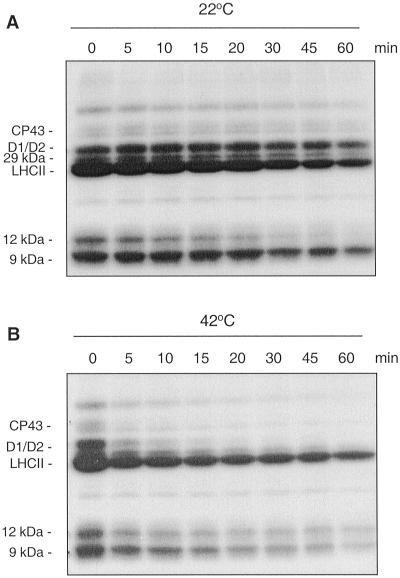
To elucidate a possible influence of elevated temperature on thylakoid protein phosphorylation/dephosphorylation the membrane proteins of isolated spinach (Spinacia oleracea) thylakoids were first phosphorylated by [γ-32P]ATP, employing endogenous light-activated protein kinase. Then, a dephosphorylation kinetics for the 32P-labeled membrane proteins was assayed at 22°C (Fig. 1A) and 42°C (Fig. 1B). Comparison of the dephosphorylation rates at 22°C and 42°C revealed accelerated dephosphorylation of all thylakoid phosphoproteins at 42°C (Fig. 1). However, the extent of activated dephosphorylation differed significantly for various proteins. The most striking observation was an extremely fast dephosphorylation of the D1, D2, and CP43 proteins of PSII in particular. These PSII core phosphoproteins lost their major amount of phosphate already during the first 10 min of heat treatment (Fig. 1B). As revealed by western analysis, the content of D1 protein in the thylakoids did not change upon heating during this short period, excluding degradation of D1 or loss due to aggregation of the protein at the top of the gel during electrophoresis (data not shown). Thus elevated temperature elicits an extremely high rate of dephosphorylation for several PSII proteins. The reversible phosphorylation of the PSII proteins has been studied extensively before (Elich et al., 1993; Silverstein et al., 1993; Carlberg and Andersson, 1996; Rintamäki et al., 1996; Fulgosi et al., 1998), but under neither of the other conditions studied has D1 and D2 dephosphorylation been found to be so rapid.
Figure 1.
Dephosphorylation of thylakoid proteins in vitro at 22°C and 42°C. Spinach thylakoid membranes were isolated and phosphorylated in the presence of [γ-32P]ATP under a photon flux density (PFD) 300 μmol photons m−2 s−1. The autoradiograms show dephosphorylation of thylakoid phosphoproteins by endogenous phosphatases in darkness either at 22°C (A) or 42°C (B). Positions of the major thylakoid phosphoproteins are indicated.
As a next step we performed a detailed study on the dephosphorylation kinetics of the major thylakoid phosphoproteins at 22°C, 27°C, 35°C, and 42°C. Table I shows the data on the dephosphorylation of CP43, D1 and D2, the 9-kD protein, LHCII, and the 29-kD protein. The identity of 29-kD phosphoprotein in spinach thylakoids is not clear; however, its phosphorylation/dephosphorylation behavior is similar to LHCII. Elevation of temperature from 22°C to 35°C increased the dephosphorylation rate of PSII reaction center proteins D1 and D2 about 6-fold. Only a 3-fold increase was observed in the dephosphorylation rates of the LHCII and 29-kD phosphoproteins, as well as of CP43. Further temperature increase to 42°C did not enhance dephosphorylation of LHCII and the 9-kD protein. On the other hand the dephosphorylation of the D1, D2 proteins and CP43 was further accelerated resulting in more than a 10-fold total increase upon raising the temperature from 22°C to 42°C (Table I). Therefore, short heat exposure of isolated thylakoids leads to specific increase in the dephosphorylation rates of the PSII reaction center phosphoproteins.
Table I.
Temperature dependence of the dephosphorylation rates for the major phosphoproteins in isolated thylakoids
| Temperature | t1/2
|
|||
|---|---|---|---|---|
| 22°C | 27°C | 35°C | 42°C | |
| min | ||||
| Phosphoprotein | ||||
| CP43 | 22 ± 8 | 33 ± 5 | 9 ± 4 | 2 ± 0.6 |
| D1/D2 | 49 ± 12 | 49 ± 7 | 8 ± 4 | 4 ± 2 |
| 9-kD | 52 ± 26 | 55 ± 10 | 9 ± 6 | 19 ± 10 |
| LHCII | 35 ± 10 | 37 ± 7 | 12 ± 10 | 18 ± 7 |
| 29-kD | 43 ± 9 | 35 ± 8 | 14 ± 4 | 13 ± 3 |
The data are presented as the half-times (minutes). The half-times were calculated from the first-order rate fitting of the dephosphorylation versus time curves obtained from four experiments at each temperature.
Heat-Stimulated Thylakoid Protein Dephosphorylation in Vivo
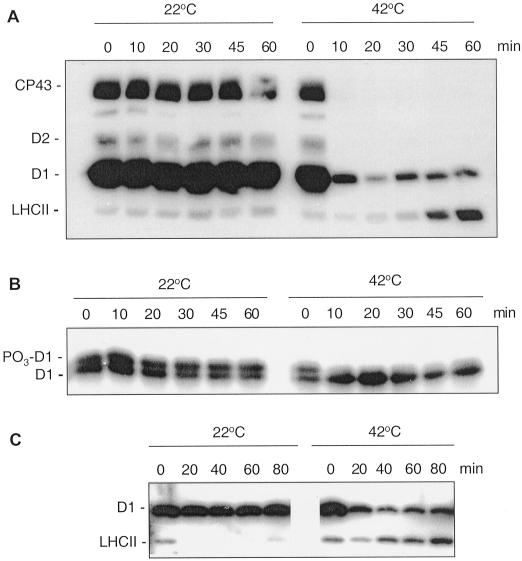
To investigate the physiological relevance of the temperature-induced specific dephosphorylation of PSII we performed studies in vivo. Illumination of spinach leaf discs under relatively high light intensity induced phosphorylation of D1, D2, and CP43, whereas LHCII proteins existed mostly in unphosphorylated form as reported earlier (Rintamäki et al., 1997). Only slow dephosphorylation of the D1, D2, and CP43 proteins occurred in the leaf discs during a 60 min incubation in darkness at 22°C as judged by analysis with antibody against phospho-Thr (P-Thr; Fig. 2A). However, dephosphorylation of these proteins after incubation of the leaf discs at 42°C was strikingly faster and reached completion within 10 min or even less (Fig. 2A). Quantification of the immunoblot data from the in vivo studies (four experiments at each temperature) revealed that heating from 22°C to 42°C decreased the dephosphorylation half-times from 41 ± 5 min to 3 ± 0.4 min for the CP43 protein, from 230 ± 30 min to 12 ± 1 min for the D1 protein, and from 58 ± 9 min to 3 ± 0.4 min for the D2 protein. The fast dephosphorylation of the phospho-D1 protein induced by elevated temperature in vivo was confirmed using high-resolution gels, which allow electrophoretic separation of the phosphorylated and non-phosphorylated forms of this reaction center protein (Elich et al., 1992; Koivuniemi et al., 1995). As detected by the specific D1 antibody, the electrophoretic migration of phospho- D1 is retarded compared with the non-phosphorylated form of the protein. Figure 2B shows that both forms of the D1 protein were present in the leaves and their ratio did not change during incubation in darkness at 22°C. However, after a short exposure of leaf discs to 42°C the amount of the phospho-D1 was drastically reduced (Fig. 2B) in agreement with the observation made using the P-Thr antibody for detection.
Figure 2.
Dephosphorylation of thylakoid proteins in vivo at 22°C and 42°C. Spinach leaf discs were illuminated 60 min at 22°C and then transferred to darkness and incubated at 22°C or 42°C. Dephosphorylation was terminated at the indicated time points by freezing the leaf discs in liquid nitrogen. Thylakoid membranes were isolated and the extent of protein phosphorylation was determined using a P-Thr antibody (A and C) or a D1-specific antibody (B). In the latter case the upper band of the D1 doublet represents the phosphorylated form of the protein, indicated by PO3-D1. Before conducting the dephosphorylation experiments different light intensities were used for induction of higher in vivo phosphorylation levels of either PSII core proteins or LHCII. The leaf discs were illuminated under a PFD 1,000 μmol photons m−2 s−1 for more effective phosphorylation of PSII proteins (A, B) or under a PFD 80 μmol photons m−2 s−1 for induction of LHCII phosphorylation (C).
The relative amount of phospho-LHCII formed during the incubation of leaf discs at low light did not decrease upon transfer of leaves to darkness at elevated temperature. On the contrary, incubation of leaf discs at 42°C in darkness increased phosphorylation of LHCII proteins (Fig. 2C). These data suggest prevalence of the LHCII kinase activity over that of the respective protein phosphatase at elevated temperature (Fig. 2, A and C), which could be due to temperature-induced structural changes in LHCII. Indeed the LHCII phosphorylation has recently been shown to be regulated at the substrate level by reversible light-induced conformational changes exposing the LHCII phosphorylation site (Zer et al., 1999).
Photosystem II Is Dephosphorylated by a Membrane-Bound Phosphatase
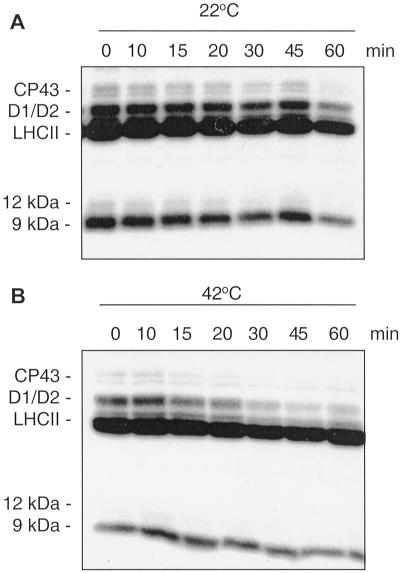
To elucidate whether the heat-stimulated dephosphorylation of the PSII proteins is catalyzed by membrane-bound or extrinsic protein phosphatases the extrinsic proteins were removed from the phosphorylated thylakoids by high-salt washings. As shown in Figure 3, the 12-kD phosphoprotein was removed from the membranes after this treatment, as well (compare with Fig. 1). Comparison of the protein dephosphorylation rates at two temperatures revealed that the membranes preserved the heat-induced phosphatase activity toward the PSII core phosphoproteins after the removal of the extrinsic proteins (Fig. 3). The dephosphorylation of the PSII core proteins in the “stripped” membranes at 42°C proceeded in a fast and specific way (Fig. 3B). On the contrary, dephosphorylation of LHCII and the 9-kD phosphoprotein was heavily retarded in the stripped membranes. These results are consistent with our previous observation that purified thylakoid membrane phosphatase has a higher specificity toward PSII core proteins rather than toward LHCII (Vener et al., 1999). Moreover, all previously described extrinsic thylakoid phosphatases have been shown to dephosphorylate mostly LHCII, but not PSII proteins (Sun et al., 1993; Hast and Follmann, 1996; Hammer et al., 1997). Although the involvement of extrinsic phosphatases in the PSII dephosphorylation could not completely be ruled out, a slower dephosphorylation of PSII proteins in the stripped membranes than in the non-washed thylakoids (compare Figs. 1 and 3) is more likely a result of partial inactivation of the membrane phosphatase upon the high-salt-treatment. Furthermore, stimulation of CP43, D1, and D2 dephosphorylation by the high temperature in the membranes depleted of extrinsic proteins provided another strong indication that this process is catalyzed by a membrane bound phosphatase.
Figure 3.
Dephosphorylation of thylakoid proteins in NaBr-washed thylakoids. Radioactively labeled thylakoid membranes were washed with 2 m NaBr to remove extrinsic protein phosphatases. Subsequently the thylakoids were incubated in darkness either at 22°C (A) or 42°C (B) to follow protein dephosphorylation as presented on autoradiograms.
Lateral Migration of PSII in Relation to Protein Dephosphorylation
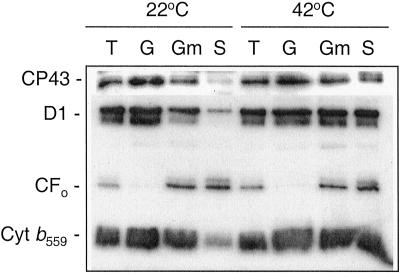
Thylakoid membranes are highly appressed and PSII is concentrated in the stacked grana regions (Aro et al., 1993), which may make it poorly accessible to either a soluble phosphatase or the catalytic domain of a membrane enzyme. Therefore the reasons for accelerated dephosphorylation of PSII upon heating could be a partial temperature-induced dissociation of LHCII from PSII, partial unstacking of thylakoids, and lateral migration of PSII to the stroma-exposed thylakoid regions (Gounaris et al., 1984; Sundby et al., 1986; Pastenes and Horton, 1996). To verify the extent of heat-induced lateral migration of PSII under the chosen experimental conditions, thylakoid membranes were fractionated into grana- and stroma-exposed thylakoids after 5 min of incubation either at 22°C or 42°C. First, the high temperature significantly decreased the amount of the appressed thylakoid fraction indicating unstacking of the grana regions, in accordance with previous studies (Gounaris et al., 1984; Pastenes and Horton, 1996). Second, the elevated temperature induced a migration of PSII from the residual grana regions to the stroma-exposed membranes, in accordance with Sundby et al. (1986). At 22°C the majority of PSII complexes was found in the grana regions as compared with the stroma membranes (Fig. 4). However, 5 min incubation at 42°C caused a lateral migration of PSII from grana as judged from an equal distribution of the D1 protein, CP43, and cytochrome b559 between stacked and unstacked thylakoid fractions. Taking into account the higher phosphatase activity in stromal thylakoids (Carlberg and Andersson, 1996) and enrichment of TLP40, which associates with the membrane protein phosphatase in the stroma-exposed membrane regions (Vener et al., 1999), the heat-induced lateral migration of PSII, as well as the partial destacking of thylakoids could contribute to the accelerated dephosphorylation of the PSII core proteins.
Figure 4.
Lateral migration of the PSII induced by high temperature. Isolated thylakoid membranes (T) incubated for 5 min at 22°C or 42°C were subfractionated into grana (G), grana margins (Gm), and stroma-exposed thylakoids (S) using digitonin and differential centrifugation. Marker PSII proteins, CP43, the D1 protein, and cytochrome b559 (Cyt b559), were detected using specific corresponding antibodies. An antibody against ATP synthase subunit CFo was used as a control for proteins with permanent location in stroma-exposed thylakoid membranes and grana margins.
To obtain further information about the role of substrate accessibility on the activation of PSII core protein dephosphorylation by the membrane protein phosphatase, the influence of the membrane structure was eliminated. Thylakoid membranes were solubilized with N-dodecyl-β-d-maltoside (DM), which is the mild detergent with respect to the activity of the membrane protein phosphatase (Vener et al., 1999). Analysis of protein dephosphorylation in the radioactively labeled DM-solubilized thylakoids revealed that the dephosphorylation rates of D1/D2 and CP43 increased 1.2- to 1.5-fold upon solubilization (data not shown). Comparison of the PSII core protein dephosphorylation at 22°C and 42°C in the solubilized thylakoids did not reveal a significant increase of the reaction rates upon heating. Taken together these results indicate that lateral migration of PSII could contribute to, but cannot totally explain, one order of magnitude of acceleration of the dephosphorylation upon heat treatment of thylakoids. Thus additional regulatory factors for activation of the PSII core protein phosphatase must exist.
Temperature Dependence of the Activity of Isolated Phosphatase
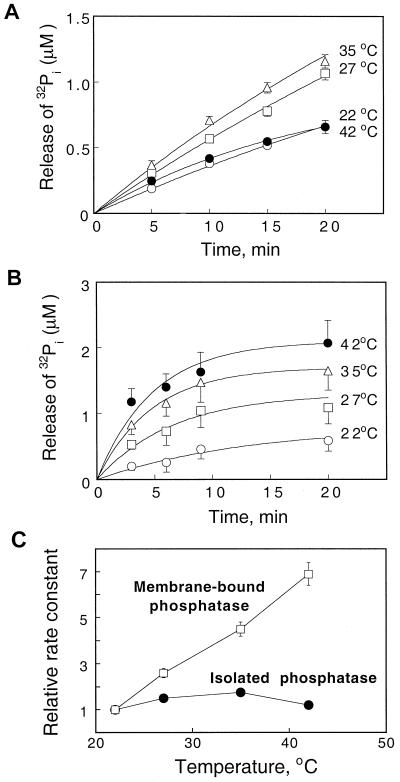
To determine whether the thylakoid membrane protein phosphatase itself is highly activated by elevated temperatures, the activity of the isolated enzyme was assayed at different temperatures using truncated thylakoid phosphopeptides as substrates. These 32P-labeled peptides were cleaved from membranes with trypsin and hence comprised a mixture of phosphorylated peptides. Only one-third of the total label was readily released from the phosphopeptides upon incubation with the isolated phosphatase or with the high-salt-washed thylakoid membranes. Analysis of the peptide mixture by HPLC and electrospray mass spectrometry revealed that this fraction represented the phosphopeptides of PSII proteins: D1 (Ac-pTAILER and Ac-pTAILERR), D2 (Ac-pTIAVGK), and CP43 (Ac-pTLFNGTLTLAGR; A.V. Vener, unpublished data). A common trait of these phosphopeptides is the presence of N-terminal acetylated and phosphorylated Thr residues, which could be important for the specificity of the membrane phosphatase. In contrast, trypsin released from the LHCII proteins only short and nonacetylated phosphopeptides: pTAGK and pTVK. As shown in Figure 5A, an increase of the temperature from 22°C to 27°C and then to 35°C led only to a limited stimulation of phosphopeptide hydrolysis by the isolated phosphatase: i.e. 1.5- and 1.75-fold, respectively. Further increase of the temperature to 42°C led to irreversible deactivation of the isolated enzyme. Thus the dephosphorylation capability of the isolated protein phosphatase itself is not significantly stimulated in response to elevated temperatures.
Figure 5.
Temperature dependence of the phosphatase activity of the isolated membrane enzyme and the enzyme bound to thylakoid membranes. Phosphatase assays were performed with 32P-labeled phosphopeptides as a substrate. The 32P-labeled phosphopeptides were obtained from radioactively labeled thylakoid membranes by trypsin treatment. The phosphatase activity of isolated phosphatase (A) and of intact thylakoids (B) was measured at 22°C, 27°C, 35°C, or 42°C. The initial phosphopeptide concentrations were 10 μm, based on the 32P content. C, Comparison of the increase in dephosphorylation rate constants at different temperatures for the isolated phosphatase and the membrane-bound enzyme.
Activation of the Membrane-Bound Phosphatase and Release of TLP40 from the Thylakoid Membrane
We have recently found that the peptidyl-prolyl cis-trans isomerase TLP40 can regulate the activity of the protein phosphatase within the thylakoid membrane (Vener et al., 1999). Although TLP40 is located in the thylakoid lumen and can reversibly associate with the inner membrane surface, the phosphatase active site is situated on the opposite side of the membrane facing the chloroplast stroma. It was therefore proposed that binding of TLP40 to the intrinsic phosphatase depresses the dephosphorylation activity, whereas activation of the phosphatase occurs upon release of TLP40 into the lumen (Vener et al., 1999). To check whether such a mechanism could be responsible for the heat shock-induced stimulation of the thylakoid protein dephosphorylation, a temperature-induced activation of the phosphatase was studied in intact thylakoids with externally added phosphopeptides (Fig. 5B). This approach allowed the elimination of the factor of endogenous substrate accessibility and enabled us to study the correlation between phosphatase activation and the amount of TLP40 bound to membranes. The results presented in Figure 5B show that elevated temperature indeed caused a significant increase of the phosphatase activity, as judged by accelerated release of the inorganic phosphate (32Pi) from the added phosphopeptides. The temperature rise from 22°C to 27°C, 35°C, and 42°C led to 2.6-, 4.3-, and 6.9-fold increases in the initial rates of 32Pi release. Clearly, high temperatures induced a prominent activation of the phosphatase only when the enzyme was in the membrane-bound, but not in the isolated form (Fig. 5C). This favored the possibility of the enzyme activation via the release of TLP40 from the inner membrane surface into the thylakoid lumen.
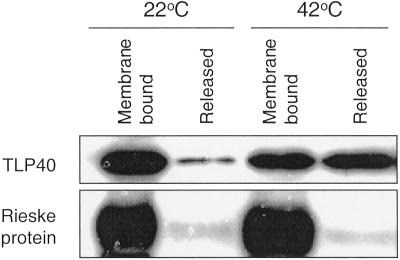
Consequently, the effect of increasing temperature on the distribution of TLP40 between the soluble and membrane-bound fractions was studied by western analysis before and after incubation of thylakoids at 42°C for 5 min. At 22°C most TLP40 was bound to the membrane, whereas only a small population of the protein was soluble in the lumen (Fig. 6). After 5 min of heating the distribution of TLP40 had changed: a much greater fraction of the protein was released from the membrane (Fig. 6). Therefore, a fast detachment of the TLP40 from the membrane upon the heat treatment of thylakoids correlates with an activation of the membrane protein phosphatase as described above.
Figure 6.
Release of TLP40 from thylakoid membranes as a result of a heat treatment. Isolated thylakoid membranes were incubated 5 min at 22°C or 42°C and then frozen in liquid nitrogen. Subsequently the membranes were disrupted with DM, and the membrane fraction and lumenal fraction, containing the released proteins, were separated by centrifugation. The proportions of membrane-bound and released TLP40 were determined with a specific antibody. As a control the content of the Rieske iron-sulfur protein was determined in the same fractions. The Rieske protein is located in thylakoid lumen and bound to the membrane via a single transmembrane anchoring span (Karnauchov et al., 1997).
DISCUSSION
In this communication we describe findings of rapid dephosphorylation of the PSII reaction center proteins D1 and D2, as well as of the chlorophyll a binding protein, CP43, in response to elevated temperature. In isolated thylakoid membranes the high temperature effect on the acceleration of the dephosphorylation was quite specific for the PSII reaction center proteins as compared with other phosphoproteins, including LHCII. Furthermore, in vivo experiments revealed that heat shock induced an even more rapid dephosphorylation of the PSII phosphoproteins, but there was no increase in the LHCII dephosphorylation. This specific dephosphorylation of the PSII reaction center proteins under heat stress conditions may play an important regulatory role, as dephosphorylation of D1 and D2 proteins does in the repair cycle of PSII in response to light stress (Aro et al., 1993; Andersson and Aro, 1997). Phosphorylation does not protect D1 protein against light-induced damage; however, it does prevent the proteolytic degradation of the damaged D1 (Andersson and Aro, 1997). Only after dephosphorylation can the D1 protein be proteolytically degraded (Koivuniemi et al., 1995; Rintamäki et al., 1996). Thus rapid dephosphorylation of the PSII reaction center proteins in response to abrupt elevation of temperature appears to be an immediate regulatory reaction to heat shock in plant photosynthetic membranes.
We have established that the heat-stimulated PSII dephosphorylation process is catalyzed by an intrinsic thylakoid protein phosphatase, since dephosphorylation was preserved after removal of extrinsic phosphatase activities by salt washings. Moreover, the protein phosphatase isolated from thylakoid membranes was found to exert a high activity and specificity in dephosphorylation of the PSII phosphoproteins, but not of phospho-LHCII (Vener et al., 1999). Therefore, the selectivity of the temperature-induced dephosphorylation of PSII compared with LHCII is likely a result of involvement of different phosphatases in dephosphorylation of these protein complexes. This conclusion is in agreement with several previous studies (Silverstein et al., 1993; Sun et al., 1993; Carlberg and Andersson, 1996; Hammer et al., 1997).
The mechanism behind the heat stimulation of the membrane protein phosphatase appears to be complex. The activity of the isolated phosphatase did not exhibit significant temperature dependence. The high temperature-induced partial destacking of thylakoid membranes and acceleration of lateral migration of membrane protein complexes could contribute to the activation of the PSII dephosphorylation. Under our experimental conditions PSII migrated toward stroma lamellae and became equally distributed between the grana and stroma domains of the thylakoid system within 5 min at 42°C, in agreement with previous studies (Gounaris et al., 1984; Sundby et al., 1986). Protein dephosphorylation rates have previously been shown to be higher in the stroma thylakoids than in grana domains (Carlberg and Andersson, 1996). Consequently, the lateral migration of phospho-PSII delivers the substrate closer to the enzyme. However, enhanced accessibility of phospho-PSII to phosphatase seems to have only a limited impact on the stimulation of dephosphorylation, as is evident from experiments with solubilized membranes. Hence, the very modest heat activation of the phosphatase itself and the lateral migration of the phosphorylated PSII cannot explain the 10- and 20-fold acceleration of dephosphorylation induced by heat shock in vitro and in vivo, respectively. Therefore, additional factor should account for the stimulation of the membrane protein phosphatase.
The use of phosphopeptides as thylakoid phosphatase substrates revealed a requirement of the membrane integrity for the fast heat-induced dephosphorylation to occur. When in membrane-associated form, the phosphatase was activated 7-fold at the high temperature. Recently we found that the thylakoid protein phosphatase is regulated by TLP40, a peptidyl-prolyl cis-trans isomerase located in the thylakoid lumen (Fulgosi et al., 1998). Binding of cyclosporin A, an immunosuppressive drug, to the active site of TLP40 led to a pronounced activation of thylakoid protein dephosphorylation by the membrane protein phosphatase. On the other hand, prolyl-containing peptidyl-prolyl cis-trans isomerase substrate peptides inhibited phosphatase activity (Vener et al., 1999). TLP40 was found to interact transiently with the inner face of the thylakoid membrane potentially serving as a regulatory subunit of the membrane phosphatase. It was proposed that binding of TLP40 to a lumen-exposed epitope of the transmembrane protein phosphatase suppressed the phosphatase activity, whereas release of TLP40 into the lumen activated the phosphatase (Vener et al., 1999). Dissociation of TLP40 from the membrane and concomitant activation of the PSII-specific phosphatase after a brief exposure of thylakoids to elevated temperatures is the additional confirmation of the proposed regulatory model provided by the present study. The present data connect specific dephosphorylation of the PSII phosphoproteins upon the heat shock conditions with the release of TLP40 from the thylakoid membrane.
Photosynthetic functions and, primarily, the function of PSII are the most heat-sensitive processes in plant cells. The heat tolerance limit of leaves coincides with the thermal stability of photochemical reactions in the thylakoid membrane (Berry and Björkman, 1980; Weis and Berry, 1988; Havaux and Tardy, 1996). Acclimation of plants to elevated temperatures via reprogramming cellular activities and synthesis of heat shock proteins proceeds in a time scale of hours (Schöffl et al., 1998). The heat shock proteins bind to thylakoids at elevated temperatures (Glaczinski and Kloppstech, 1988; Osteryoung and Vierling, 1994). The transition of the non-binding to the binding status of the heat shock proteins is comparatively sharp and occurs between 36°C and 40°C (Glaczinski and Kloppstech, 1988). In contrast, TLP40 protein is present already before the heat shock conditions and rapidly discharges from the membrane in response to abrupt temperature increase. The TLP40 release from the membrane is paralleled by a profound activation of the PSII protein phosphatase.
The known immediate events occurring upon abrupt exposure of leaves to high temperature are destacking of thylakoids (Gounaris et al., 1984), release of extrinsic components of the oxygen evolving complex from PSII, and increase in permeability and fluidity of the photosynthetic membrane (Havaux et al., 1996; Tardy and Havaux, 1996). As emergency mechanisms of thylakoid membrane stabilization under heat shock, rigidifying carotenoids are provided by the xanthophyll cycle (Tardy and Havaux, 1997) and isoprene is synthesized in various plants (Sharkey and Singsaas, 1995; Sharkey, 1996; Singsaas et al., 1997). However, the signaling events induced by heat stress that lead to higher thermotolerance of cellular activities are not yet fully understood. Our finding of an immediate activation of the thylakoid protein phosphatase in response to a steep rise of ambient temperature favors the involvement of reversible protein phosphorylation in the signaling chain. The protein phosphorylation/dephosphorylation cascade has already been suggested to connect light perception in the thylakoid membrane of green alga and expression of a nuclear cab gene (Escoubas et al., 1995). Specific inhibitors of eukaryotic Ser/Thr protein phosphatases, in particular, okadaic acid, microcystin, and tautomycin, blocked this signaling. It is remarkable that the protein phosphatase, purified from the thylakoid membrane and described in the present study, is also inhibited by the same inhibitors (Vener et al., 1999). Thus we propose that selective activation of the thylakoid membrane phosphatase in response to heat shock may be involved not only in dephosphorylation of PSII core phosphoproteins and regulation of PSII turnover, but could also be a more general cellular signal leading to plant acclimative responses to heat stress and heat shock.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Spinach (Spinacia oleracea) plants were grown hydroponically under a PFD of 400 μmol photons m−2 s−1 with a 10-h light/14-h dark rhythm at 25°C. Fully expanded leaves were used in all experiments.
Isolation and Subfractionation of Thylakoid Membranes
For in vitro phosphorylation/dephosphorylation assays thylakoid membranes were isolated according to Andersson et al. (1976) and resuspended in reaction buffer consisting of 50 mm Tricine (N-[tris(hydroxymethyl)methyl]Gly), pH 7.8, 100 mm sorbitol, and 5 mm MgCl2. For measurements of protein phosphorylation levels in vivo the thylakoid membranes were isolated from spinach leaf discs and resuspended in a small volume of storage buffer consisting of 10 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-NaOH, pH 7.5, 100 mm Suc, 5 mm NaCl, 10 mm NaF, and 10 mm MgCl2. Thylakoid subfractionation into grana and stroma-exposed membrane regions was performed using digitonin and differential centrifugation according to Leto et al. (1985).
Phosphorylation and Dephosphorylation of Thylakoid Proteins in Vitro
Thylakoids (0.3 mg chlorophyll mL−1) were phosphorylated in the presence of 0.1 mm [γ-32P]ATP (0.02 mCi/mg chlorophyll) under a PFD of 300 μmol photons m−2 s−1 at room temperature for 30 min. Excess of radioactivity was removed by washing the membranes with 50 mm Tricine, pH 7.8, 100 mm sorbitol, and 5 mm MgCl2. The thylakoids were finally resuspended in the same buffer and incubated in darkness at 22°C, 27°C, 35°C, and 42°C for different periods to follow protein dephosphorylation. Dephosphorylation was terminated by addition of electrophoresis sample buffer. To study dephosphorylation of thylakoid proteins by the intrinsic membrane phosphatase, the 32P-labeled thylakoids were depleted from extrinsic proteins by washing with 2 m NaBr (Hurt and Hauska, 1981). In some experiments 32P-labeled thylakoid membranes solubilized with 10 mm DM in the presence of 2 mm dithiothreitol were used for protein dephosphorylation studies at 22°C or 42°C.
Phosphorylation and Dephosphorylation of Thylakoid Proteins in Vivo
Spinach leaf discs (3 cm in diameter), floating on distilled water, were illuminated under a PFD of 1,000 μmol photons m−2 s−1 at 22°C for 60 min to phosphorylate PSII proteins (Rintamäki et al., 1997). To induce maximal LHCII phosphorylation, leaf discs were illuminated at low light (a PFD 80 μmol photons m−2 s−1) for 60 min. A metal-halide lamp (HQI-T 250 watts/daylight) served as a light source. After light treatment the leaf discs were transferred to darkness and incubated further at 22°C or 42°C for up to 60 min. Samples for thylakoid isolation were taken during the time course of incubation, frozen in liquid nitrogen, and stored at −80°C.
Detection of Thylakoid Proteins and Phosphoproteins
Thylakoids from in vivo experiments were solubilized in the presence of 6 m urea, and polypeptides were separated by SDS-PAGE (Laemmli, 1970) using 15% (w/v) acrylamide gels with 6 m urea. One microgram of chlorophyll was loaded into each well. Proteins from in vitro experiments or after thylakoid subfractionation were separated on gels without urea with 2 μg of chlorophyll loaded per well. The patterns of the 32P-labeled phosphoproteins were revealed by autoradiography using x-ray films after the gels were stained and dried. For immunoblotting, the polypeptides were transferred to a polyvinylidene difluoride membrane and proteins detected with antibodies raised against the D1 protein, CP43, TLP40, the ATP synthase subunit CFo, cytochrome b559, and the Rieske iron-sulfur protein. The D1 specific antibody was raised against a synthetic peptide corresponding to amino acids 234 to 242 of the D-E loop in Synechocystis 6803 (Research Genetics, Huntsville, AL). The TLP40 antibody was raised against the protein overexpressed in Escherichia coli (Fulgosi et al., 1998). The phosphorylation level of proteins after in vivo experiments was detected with an antibody raised against P-Thr (Zymed Laboratories, San Francisco). The relative content of specific proteins on immunoblots was determined using enhanced chemiluminescence (Amersham, Buckinghamshire, UK) fluorography or chemiluminescent kit from New England BioLabs (Beverly, MA). The quantities of particular protein or phosphoprotein were determined by scanning the x-ray films with a laser densitometer, using the software package Image Quant from Molecular Dynamics (Sunnyvale, CA).
Assay of Protein Phosphatase Activity with Phosphopeptide Substrates
32P-labeled phosphopeptides were prepared from thylakoids phosphorylated with [γ-32P]ATP in the light, and cleaved from membranes with trypsin, as described in Vener et al. (1999). To assay protein phosphatase activity, the phosphopeptides were resuspended in a buffer containing 50 mm Tricine, pH 7.8, 100 mm sorbitol, and 5 mm MgCl2. Then 5 μL of the phosphopeptides (20 μm, 6,000–9,000 cpm) and 5 μL of thylakoids (1.2 mg chlorophyll mL−1) or a fraction containing the purified phosphatase were mixed and incubated for 3 to 20 min at 22°C, 27°C, 35°C, and 42°C. The amount of 32P-label remaining in the phosphopeptides and the released labeled 32Pi were determined by counting in the liquid scintillation system LS 6000TA (Beckman, Fullerton, CA) following the acid-molybdate extraction of 32Pi as described before (Vener et al., 1999).
Purification of the Thylakoid Protein Phosphatase
The membrane protein phosphatase was purified from spinach thylakoid membranes approximately 9,000- to 13,000-fold as described in Vener et al. (1999). In short, thylakoids (1 mg chlorophyll mL−1) washed with 2 m NaBr were solubilized using Triton X-100 (1% [v/v], final concentration) and subsequently subjected to three consecutive anion-exchange chromatographic steps on Sepharose Q and Resource Q (Pharmacia, Piscataway, NJ). At each step detergents were changed from 0.5% (w/v) Triton X-100 to 2 mm DM and 8 mm CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid). After the last step the phosphatase containing fractions were concentrated on Centricone (Amicon, Beverly, MA) and further purified by size-exclusion FPLC on Superose 12. The phosphatase activity was determined using the phosphopeptide assay.
Chlorophyll and Protein Determination
Chlorophyll concentrations and chlorophyll a/b ratios were determined according to Arnon (1949). Protein determination was performed according to Bradford (1976).
Footnotes
Support for this work was provided by the Swedish Natural Science Research Council, by The Academy of Finland, by the Swedish Council for Forestry and Agricultural Research, by Nordiskt Kontaktorgan för Jordbruksforskning, by The Nordic Energy Research Program, by The German Research Foundation (SFB 184), and by the Human Frontier Science Program.
LITERATURE CITED
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Andersson B, Åkerlund H-E, Albertsson P-Å. Separation of subchloroplast membrane particles by counter-current distribution. Biochim Biophys Acta. 1976;423:122–132. doi: 10.1016/0005-2728(76)90106-7. [DOI] [PubMed] [Google Scholar]
- Andersson B, Aro E-M. Proteolytic activities and proteases of plant chloroplasts. Physiol Plant. 1997;100:780–793. [Google Scholar]
- Arnon DJ. Copper enzymes in isolated chloroplasts. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Barbato R, Aro E-M. Role of phosphorylation in repair cycle and oligomeric structure of photosystem two. Planta. 1999;208:196–204. [Google Scholar]
- Barber J, Nield J, Morris EP, Zheleva D, Hankamer B. The structure, function and dynamics of photosystem two. Physiol Plant. 1997;100:817–827. [Google Scholar]
- Bennett J, Steinback KE, Arntzen CJ. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci USA. 1980;77:5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Andersson B. Phosphatase activities in spinach thylakoid membranes: effectors, regulation and location. Photosynth Res. 1996;47:145–156. doi: 10.1007/BF00016177. [DOI] [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem. 1992;267:3523–3529. [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Dephosphorylation of photosystem II core proteins is light-regulated in vivo. EMBO J. 1993;12:4857–4862. doi: 10.1002/j.1460-2075.1993.tb06175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas JM, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 1998;116:539–546. doi: 10.1104/pp.116.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgosi H, Vener AV, Altschmied L, Herrmann RG, Andersson B. A novel multi-functional chloroplast protein: identification of a 40 kDa immunophilin-like protein located in the thylakoid lumen. EMBO J. 1998;17:1577–1587. doi: 10.1093/emboj/17.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Zer H, Ohad I. Redox-controlled thylakoid protein phosphorylation: news and views. Physiol Plant. 1997;100:869–885. [Google Scholar]
- Glaczinski H, Kloppstech K. Temperature-dependent binding to the thylakoid membranes of nuclear-coded chloroplast heat-shock proteins. Eur J Biochem. 1988;173:579–583. doi: 10.1111/j.1432-1033.1988.tb14038.x. [DOI] [PubMed] [Google Scholar]
- Gounaris K, Brain ARR, Quinn PJ, Williams WP. Structural reorganisation of chloroplast thylakoid membranes in response to heat-stress. Biochim Biophys Acta. 1984;766:198–208. [Google Scholar]
- Hammer MF, Markwell J, Sarath G. Purification of a protein phosphatase from chloroplast stroma capable of dephosphorylating the light-harvesting complex-II. Plant Physiol. 1997;113:227–233. doi: 10.1104/pp.113.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hast T, Follmann H. Identification of two thylakoid-associated phosphatases with protein phosphatase activity in chloroplasts of the soybean (Glycine max) Photochem Photobiol. 1996;36:313–319. [Google Scholar]
- Havaux M. Temperature-dependent modulation of the photoinhibition-sensitivity of photosystem II in Solanum tuberosum leaves. Plant Cell Physiol. 1994;35:757–766. [Google Scholar]
- Havaux M, Tardy F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: possible involvement of xanthophyll-cycle pigments. Planta. 1996;198:324–333. [Google Scholar]
- Havaux M, Tardy F, Ravenel J, Chanu D, Parot P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of xanthophyll content. Plant Cell Environ. 1996;19:1359–1368. [Google Scholar]
- Heckathorn SA, Downs CA, Sharkey TD, Coleman JS. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E, Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981;117:591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Karnauchov I, Herrmann RG, Klosgen RB. Transmembrane topology of the Rieske Fe/S protein of the cytochrome b6/f complex from spinach chloroplasts. FEBS Lett. 1997;408:206–210. doi: 10.1016/s0014-5793(97)00427-4. [DOI] [PubMed] [Google Scholar]
- Koivuniemi A, Aro E-M, Andersson B. Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry. 1995;34:16022–16029. doi: 10.1021/bi00049a016. [DOI] [PubMed] [Google Scholar]
- Krishnan HB, Pueppke SG. Heat shock triggers rapid protein phosphorylation in soybean seedlings. Biochem Biophys Res Commun. 1987;148:762–767. doi: 10.1016/0006-291x(87)90941-7. [DOI] [PubMed] [Google Scholar]
- Kruse O, Zheleva D, Barber J. Stabilization of photosystem two dimers by phosphorylation: implication for the regulation of the turnover of D1 protein. FEBS Lett. 1997;408:276–280. doi: 10.1016/s0014-5793(97)00439-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto KJ, Bell E, McIntosh L. Nuclear mutants lead to an accelerated turnover of chloroplast encoded 48 kDa and 34.5 kDa proteins in thylakoids lacking photosystem II. EMBO J. 1985;4:1645–1653. doi: 10.1002/j.1460-2075.1985.tb03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Vierling E. Dynamics of small heat shock protein distribution within the chloroplasts of higher plants. J Biol Chem. 1994;269:28676–28682. [PubMed] [Google Scholar]
- Pastenes C, Horton P. Effect of high temperature on photosynthesis in beans. Plant Physiol. 1996;112:1245–1251. doi: 10.1104/pp.112.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Kettunen R, Aro E-M. Differential D1 dephosphorylation in functional and photodamaged photosystem II centers: dephosphorylation is a prerequisite for degradation of damaged D1. J Biol Chem. 1996;271:14870–14875. doi: 10.1074/jbc.271.25.14870. [DOI] [PubMed] [Google Scholar]
- Rintamäki E, Salonen M, Suoranta UM, Carlberg I, Andersson B, Aro E-M. Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo: application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J Biol Chem. 1997;272:30476–30482. doi: 10.1074/jbc.272.48.30476. [DOI] [PubMed] [Google Scholar]
- Schöffl F, Prandl R, Reindl A. Regulation of the heat-shock response. Plant Physiol. 1998;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. Isoprene synthesis by plants and animals. Endeavor. 1996;20:74–78. doi: 10.1016/0160-9327(96)10014-4. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL. Why plants emit isoprene. Nature. 1995;374:769. [Google Scholar]
- Silverstein T, Cheng L, Allen JF. Chloroplast thylakoid protein phosphatase reactions are redox-independent and kinetically heterogeneous. FEBS Lett. 1993;334:101–105. doi: 10.1016/0014-5793(93)81690-2. [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyders S, Kohorn BD. TAKs, thylakoid membrane protein kinases associated with energy transduction. J Biol Chem. 1999;274:9137–9140. doi: 10.1074/jbc.274.14.9137. [DOI] [PubMed] [Google Scholar]
- Sun G, Bailey D, Jones MW, Markwell J. Chloroplast thylakoid protein phosphatase is a membrane surface-associated activity. Plant Physiol. 1989;89:238–243. doi: 10.1104/pp.89.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Sarath G, Markwell J. Phosphopeptides as substrates for thylakoid protein phosphatase activity. Arch Biochem Biophys. 1993;304:490–495. doi: 10.1006/abbi.1993.1380. [DOI] [PubMed] [Google Scholar]
- Sundby C, Melis A, Mäenpää P, Andersson B. Temperature-dependent changes in antenna size of photosystem II: reversible conversion of photosystem IIα to photosystem IIβ. Biochim Biophys Acta. 1986;851:475–483. [Google Scholar]
- Tardy F, Havaux M. Photosynthesis, chlorophyll fluorescence, light-harvesting system and photoinhibition resistance of a zeaxanthin-accumulating mutant of Arabidopsis thaliana. J Photochem Photobiol B. 1996;34:87–94. doi: 10.1016/1011-1344(95)07272-1. [DOI] [PubMed] [Google Scholar]
- Tardy F, Havaux M. Thylakoid membrane fluidity and thermostability during the operation of the xanthophyll cycle in higher-plant chloroplasts. Biochim Biophys Acta. 1997;1330:179–193. doi: 10.1016/s0005-2736(97)00168-5. [DOI] [PubMed] [Google Scholar]
- Vener AV, Ohad I, Andersson B. Protein phosphorylation and redox sensing in chloroplast thylakoids. Curr Opin Plant Biol. 1998;1:217–223. doi: 10.1016/s1369-5266(98)80107-6. [DOI] [PubMed] [Google Scholar]
- Vener AV, Rokka A, Fulgosi H, Andersson B, Herrmann RG. A cyclophilin-regulated PP2A-like protein phosphatase in thylakoid membranes of plant chloroplasts. Biochemistry. 1999;38:14955–14965. doi: 10.1021/bi990971v. [DOI] [PubMed] [Google Scholar]
- Vener AV, Van Kan PJ, Gal A, Andersson B, Ohad I. Activation/deactivation cycle of redox-controlled thylakoid protein phosphorylation: role of plastoquinol bound to the reduced cytochrome bf complex. J Biol Chem. 1995;270:25225–25232. doi: 10.1074/jbc.270.42.25225. [DOI] [PubMed] [Google Scholar]
- Vener AV, Van Kan PJM, Rich PR, Ohad I, Andersson B. Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc Natl Acad Sci USA. 1997;94:1585–1590. doi: 10.1073/pnas.94.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis E, Berry JA. Plants and high temperature stress. Symp Soc Exp Biol. 1988;42:329–346. [PubMed] [Google Scholar]
- Zer H, Vink M, Keren N, Dilly-Hartwig HG, Paulsen H, Herrmann RG, Andersson B, Ohad I. Regulation of thylakoid protein phosphorylation at the substrate level: reversible light-induced conformational changes expose the phosphorylation site of the light-harvesting complex II. Proc Natl Acad Sci USA. 1999;96:8277–8282. doi: 10.1073/pnas.96.14.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]