Significance
Climate change is altering the structure and function of high-elevation ecosystems. Combining long-term observations with manipulative experiments is a powerful, yet rarely used way to test the sensitivity of such ecosystems to climatic change. Here, experimental evidence and meta-analysis confirm long-term observations that demonstrate climate warming and associated drying did not change net primary production, but did lead to a shift of allocation belowground. This observed shift was caused by a change in community composition. Although alpine grassland productivity appears to be resistant to warming, deeper root systems in response to warming could alter the amount of soil organic carbon stored in the subsoil, indicating that rooting depth should be taken into account when predicting soil organic carbon stocks under warming.
Keywords: alpine ecosystem, warming experiment, long-term monitoring, ecosystem functioning, Tibetan Plateau
Abstract
The structure and function of alpine grassland ecosystems, including their extensive soil carbon stocks, are largely shaped by temperature. The Tibetan Plateau in particular has experienced significant warming over the past 50 y, and this warming trend is projected to intensify in the future. Such climate change will likely alter plant species composition and net primary production (NPP). Here we combined 32 y of observations and monitoring with a manipulative experiment of temperature and precipitation to explore the effects of changing climate on plant community structure and ecosystem function. First, long-term climate warming from 1983 to 2014, which occurred without systematic changes in precipitation, led to higher grass abundance and lower sedge abundance, but did not affect aboveground NPP. Second, an experimental warming experiment conducted over 4 y had no effects on any aspect of NPP, whereas drought manipulation (reducing precipitation by 50%), shifted NPP allocation belowground without affecting total NPP. Third, both experimental warming and drought treatments, supported by a meta-analysis at nine sites across the plateau, increased grass abundance at the expense of biomass of sedges and forbs. This shift in functional group composition led to deeper root systems, which may have enabled plant communities to acquire more water and thus stabilize ecosystem primary production even with a changing climate. Overall, our study demonstrates that shifting plant species composition in response to climate change may have stabilized primary production in this high-elevation ecosystem, but it also caused a shift from aboveground to belowground productivity.
Climate change impacts the structure and function of terrestrial ecosystems, particularly in cold regions (1–6). The amount of net primary productivity (NPP) in an ecosystem influences carbon feedback between the terrestrial biosphere and the atmosphere. A growing number of experimental studies have explored how plant community composition and primary productivity respond to climate change (4, 7–9); however, fewer studies have done so in concert with companion long-term observations to explore how changes in community composition that are triggered by climate change may influence ecosystem function (but see refs. 9 and 10).
Alpine ecosystems are considered among the most sensitive ecosystems to climate change (8, 11), and 60% of the Tibetan Plateau is alpine (12). Over the past 50 y, annual temperature on the Tibetan Plateau has increased dramatically at a rate twice the global average (13, 14). Furthermore, data indicate that since 2000, the rate of warming may be accelerating even more than during the previous 40 y (15, 16). Alpine grasslands are susceptible to warming, are diverse, and harbor large stocks of carbon in the soil that if released, could lead to a positive feedback for climate warming (17–19).
The ubiquitous alpine grasslands on the Tibetan Plateau are important for the livelihood of native nomads and potentially susceptible to climate change, and several approaches have been taken to address the impact of climate change on this high-altitude, alpine ecosystem. Both process-based ecosystem models (11, 20) and long-term time-series datasets of the Normalized Difference Vegetation Index (NDVI) (21, 22) suggest increasing vegetation growth over the past several decades, although with pronounced geographical heterogeneity. However, these approaches, while informative at large spatial scales, do not provide detailed fine-scale information or insight into potential mechanisms. Long-term observations at the local plot scale have shown that NPP in alpine grasslands appeared to be insensitive to changes in either precipitation or air temperature from 1980 to 2000 (23). An observational approach such as the one taken in that study cannot explicitly address the underlying mechanism explaining why NPP was unresponsive to climate changes, however (10). Finally, a number of manipulative warming experiments on the Tibetan Plateau, using either open-top chambers or infrared heaters, have reported inconsistent results, some showing increases in NPP and grass biomass (24), while others finding decreased NPP or grass biomass (25). In addition, although experiments can be useful for exploring mechanisms and providing boundaries of possible change, they cannot capture the legacy effects induced by the background warming and do not necessarily reflect reality (26). Given these uncertainties, one important way forward is to combine long-term observational studies with experimental manipulations to examine the long-term patterns as well as the detailed mechanisms of community and ecosystem responses to climatic change.
In the present study, we combined 32 y of observational monitoring of alpine grassland ecosystems on the Tibetan Plateau (Fig. S1 A and B) with a manipulative experiment (Fig. S1C) to explore the effects of climate change on grassland productivity and community composition. In particular, we examined whether NPP and plant community composition were sensitive to climatic change in this high-elevation, cold ecosystem, and whether shifts in plant community composition amplified or stabilized changes in productivity. Finally, we explored the possible underlying mechanisms of the effects of warming on primary productivity.
Results
Long-Term Changes in Climate, Plant Productivity, and Species Composition.
At the field site on the Tibetan Plateau, from 1980 to 2014, the mean annual air temperature increased by 1.2 °C (0.38 per decade) (Fig. S2A), annual precipitation did not vary systematically (P = 0.16; Fig. S2A), and the annual humidity index declined (P = 0.06; Fig. S2B). From 2002 to 2010, soil moisture at depths of 5 cm and 45 cm declined significantly (Fig. 1A). Overall, between 1980 and 2014, the site became both warmer and drier. Across the years, aboveground net primary productivity (ANPP) ranged from 298 to 484 g m−2 (average, 371 g m−2), but it did not vary systematically from year to year (P = 0.60; Fig. 1B). However, the relative contribution of plant functional groups (percentage of biomass) to total ANPP changed significantly; over time, the ANPP of grasses increased, that of sedges decreased, and that of forbs remained relatively stable (Fig. 1C).
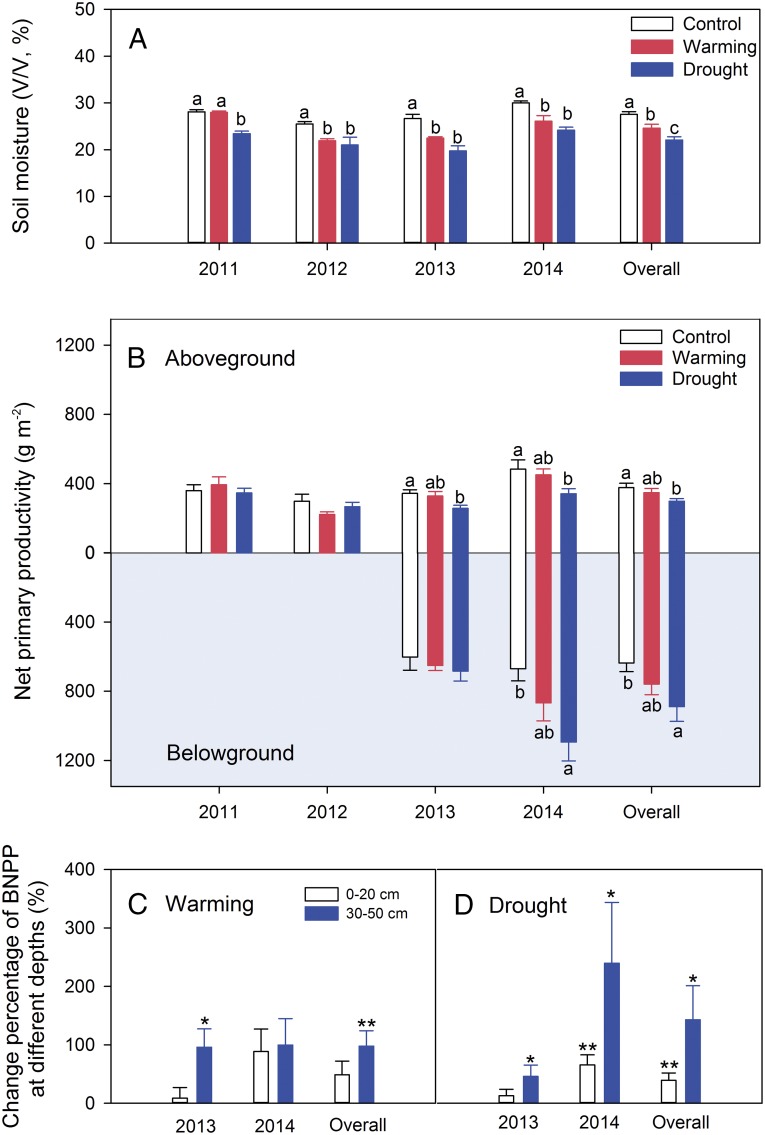
Fig. 1.
Long-term interannual variations in mean growing season soil moisture over 2002–2009 (A), and ANPP (B) and relative abundance of grasses, forbs, and sedges (C) over 1983–2014 in the Haibei research station where monitoring and the warming-drought experiment were conducted. Vertical bars represent SEM.
Experimental Warming and Drought Effects on Plant Productivity and Species Composition.
Over the 4-y experiment, both the warming and drought treatments significantly decreased growing season (May–September) soil moisture at 5 cm (Fig. 2A). However, warming did not significantly affect ANPP, belowground net primary productivity (BNPP), or total NPP (Fig. 2B). This was true across years, regardless of whether the individual year was wet and cold (2011, 2014) or dry and warm (2012, 2013) (Fig. S2C). In contrast, drought treatments decreased ANPP and increased BNPP (Fig. 2B), but without changing total NPP.
Fig. 2.
Warming and drought effects on soil moisture at 20 cm soil depth (A), ANPP and BNPP (B), and change percentage of BNPP [(warming or drought − control)/control × 100] at 0–20 cm and 30–50 cm soil depths (C and D) in the warming-drought experiment. Vertical bars represent SEM (n = 6). Letters above each bar (a, b, or c) indicate significant difference at P < 0.05 between treatments. Asterisks are significant at different levels between treatments and control: *P < 0.05, **P < 0.01.
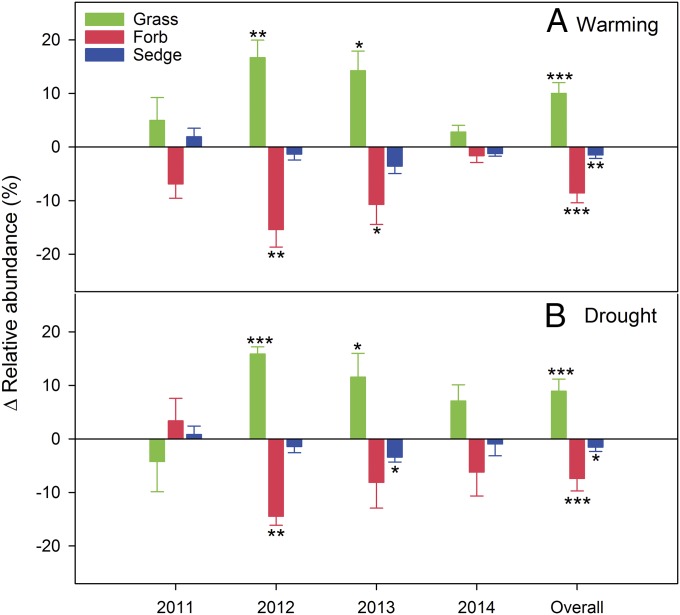
Across all 4 y, warming and drought both increased the relative abundance of grasses and decreased that of sedges and forbs (Fig. 3 and Table S1). These changes were evident in dry years (2012, 2013) but not in wet years.
Fig. 3.
Warming (A) and drought (B) effects on relative abundance (warming or drought − control) in different functional groups. Vertical bars represent SEM (n = 6). Asterisks are significant at different levels between treatments and control: *P < 0.05, **P < 0.01, ***P < 0.001.
To examine why different plant functional groups exhibited differing responses to the warming and drought treatments, we measured the root vertical distributions of nine species representing three functional groups (grass, forb, and sedge), which together accounted for 72% of total ANPP (Table S2). At our site, grasses generally had deeper roots (up to 85 cm soil depth) compared with sedges (up to 25 cm) and forbs (up to 30 cm) (Fig. S3). Such differences in vertical root distribution among functional groups (Fig. S3) and changes in functional group dominance with warming or drought treatments (Fig. 3) likely contributed to the shift in NPP allocation belowground, particularly to the subsoil (>30 cm). Warming did not change BNPP at 0–20 cm, but it increased BNPP at 30–50 cm (Fig. 2C and Fig. S4A). At the same time, drought significantly increased BNPP at both 0–20 cm and 30–50 cm (Fig. 2D and Fig. S4B).
Synthesis of Responses in Plant Productivity and Species Composition on the Tibetan Plateau.
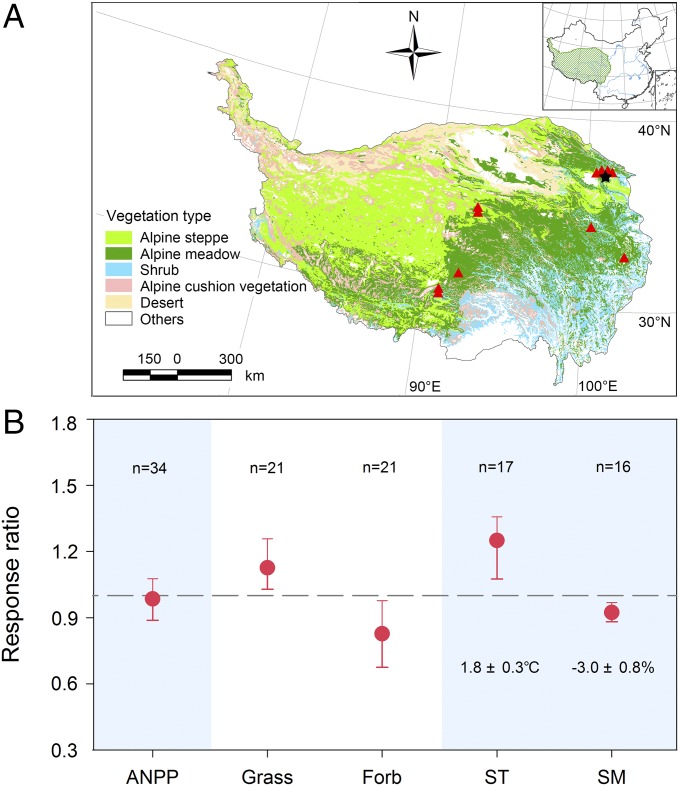
To examine whether the observed trend of no effect of warming on ANPP but an increase in the dominance of grasses and a decreased abundance of forbs were general patterns across Tibetan Plateau grasslands, we conducted a meta-analysis on the effect of experimental warming at nine sites distributed across the plateau (Fig. 4A and Table S3). Across all sites, experimental warming increased soil temperature by 1.8 °C and decreased soil moisture by 3.0%, but there was no strong or consistent effect of warming on ANPP; however, experimental warming at the nine sites led to shifts in community composition, with generally more grasses and fewer forbs (Fig. 4B). In the coldest sites, such as Fenghuoshan, Beiluhe, and Guoluo (mean annual temperature <−3.0 °C), warming tended to increase ANPP. In contrast, at those sites where the temperature was not as low, such as Damxung, Hongyuan, and Kakagou (mean annual temperature, −2.8 to 2.8 °C), warming tended to reduce ANPP (Table S4).
Fig. 4.
Locations of the nine sites included in the meta-analysis (A) and the response ratios from the meta-analysis (B). The star indicates the location of the experimental warming-by-precipitation site used in this study. B shows the response ratios of experimental warming on ANPP, relative abundance of grass and forb functional groups, soil temperature (ST), and soil moisture (SM). The error bars represent 95% CI. If the data did not overlap with 1, a significant warming-induced response was indicated. The numbers of studies used in analysis are shown above the bars.
Discussion
Long-term changes in climate may lead to overall changes in NPP, a pattern observed in several monitoring studies (9, 27, 28). A meta-analysis of 20 warming experiments indicated that warming increased ANPP by ∼19% (29) and BNPP by 52% (30). In contrast, even though the Tibetan Plateau has been warming and drying for longer than 32 y, NPP at our alpine grassland site did not significantly change over time or with experimental warming. Surprisingly, this pattern was consistent when we synthesized results from nine other warming experiments conducted across the Tibetan Plateau (Fig. 4B). Given the rapid climate change in a climate-sensitive region that is often referred to as the Third Pole, our results are particularly important for assessing ecosystem sensitivity and predicting how cold-adapted ecosystems may respond to global change.
While some previous studies have found that NPP varies systematically with spatial or temporal variations in climate (31, 32), we posit a mechanism to explain why the response of ANPP on the Tibetan Plateau may be different from that reported previously. The response of productivity to climate change depends on the stabilizing effect of compensatory interactions among major functional groups (33, 34). In both long-term monitoring and experimental manipulation, climate warming and associated drying led to increases in deep-rooted grasses and forbs and unchanged or reduced production of shallow-rooted sedges. In this alpine ecosystem, water content is higher in the subsoil than in the surface soil during drought periods; thus, an increase in the abundance of deep-rooted grasses could enable the aggregate community to access more water. An increase in soil moisture availability could buffer the community during dry periods and lead to a stabilization of ANPP over time—even under drier conditions. This proposed mechanism is supported by the results of a study from temperate grasslands in Inner Mongolia, where the community-level stability in primary production over time arose from compensatory interactions among major functional groups (33).
Aside from the foregoing mechanism, we cannot rule out the possibility of other nonmutually exclusive mechanisms. For instance, it could be the case that NPP is stable under climate warming because of phenological changes that occur over the growing season; that is, an increase in NPP in the beginning of the growing season is canceled out by lower NPP at the end of the growing season (35, 36). Another possible mechanism in our system is that plasticity in key functional plant traits facilitates persistence in the face of climate change (37–39).
In agreement with our long-term observations and experimental manipulation, a meta-analysis of data from nine experimental sites in the Tibetan alpine grassland showed similar trends in production and functional group composition; however, the mechanisms responsible for these trends might differ among sites (Table S4). At the coldest sites, warming tended to stimulate ANPP because temperature rather than precipitation was the primary limiting factor for plant growth. At those sites subject to seasonal drought stress, warming tended to reduce ANPP because the indirect warming effect on soil moisture overrode the direct warming effect on plants. The response observed on the Tibetan Plateau may hold true in grassland ecosystems worldwide. The influence of warming on plant production generally depends on the balance between the positive effect of warming on plant growth and the negative effect of warming on soil moisture. In cold or humid grassland ecosystems, warming tends to increase production; for instance, warming stimulates ecosystem production by increasing shrub production in tundra (40) and by increasing C4 grass production in tallgrass prairie (41). In contrast, however, in ecosystems that face seasonal water shortages, warming tends to decrease production; for example, warming decreases ecosystem production by reducing grass productivity in semiarid grassland (42) and reduces subalpine meadow production by reducing shallow-rooted forbs (9). Our study site is a mesic alpine grassland subject to seasonal droughts where the temperature is not severely limited, so a neutral response of primary production to climate warming is not surprising.
It should be noted that the productivity responses to a 4-y drought experiment and 32 y of increasingly warmer and drier climates might differ when extended to long-term successional scales. Importantly, the changes documented in our study do not include shifts in functional group composition—for example, from grasses to shrubs—that may constrain the responses of ANPP to altered precipitation (43). Over the long term and with succession, the plant community should converge on a potential water use efficiency under drought conditions (44); thus, with reduced precipitation, ANPP should be reduced as well. This may explain why short-term productivity may be less responsive to warming than responses, coupled with succession, over longer periods.
Our results have important implications for ecosystem carbon dynamics in response to climate change. At this site, subsoils store approximately 50% of total soil organic carbon in the top 1 m. A shift in species composition to deeply rooted species might affect the stability of soil organic carbon in the subsoil. One possibility is that deeper-rooting species may stimulate microbial activity and soil organic matter decomposition, rendering the carbon stored in the subsoil vulnerable to climate change (45–47) (Fig. S5). Alternatively, increased deep roots may increase carbon storage in the subsoil through inputs of root material, microbial processing, and matrix stabilization (48, 49). Given that soil carbon stocks are determined by the balance between carbon input and carbon stabilization and microbial activity, predicting long-term ecosystem dynamics in this increasingly variable climate is complex.
In conclusion, we found that across a major high-elevation biome, ANPP is resistant to climate warming, in contrast to our a priori prediction. We show observationally, experimentally, and with a regional meta-analysis that while ANPP did not change with warming and drying over decades, the composition of the plant community shifted toward more deeply rooted species. Given the important contributions of roots to soil carbon formation and soil organic carbon stability, these findings could be important when predicting carbon feedbacks to the atmosphere.
Methods
Study Site.
The study site is located at Haibei National Field Research Station in the Alpine Grassland Ecosystem (37°36′ N, 101°19′ E, 3,215 m a.s.l.) in the northeastern part of the Tibetan Plateau, in Qinghai Province. The climate is characterized by a long, cold winter and a short, cool summer. The mean annual air temperature and precipitation during 1980–2014were −1.2 °C and 489.0 mm, respectively. Approximately 80% of precipitation was concentrated in the growing season from May to September. This mesic alpine grassland is dominated by Stipa aliena, Elymus nutans, and Helictotrichon tibeticum. The soil is classified as mollisols according to the US Department of Agriculture Soil Taxonomy. Average soil bulk density, organic carbon concentration, and pH are 0.8 g cm−3, 63.1 g kg−1, and 7.8 at 0–10 cm soil depth, respectively.
Long-Term Monitoring Observations.
Plant aboveground production and functional group composition have been monitored since 1980. The climatic data were obtained from the meteorological station that was installed in 1980. Aboveground biomass was harvested every month from May to September. Five or more quadrats of 50 cm × 50 cm were randomly harvested within an area of 250 m × 230 m before 2005. Starting in 2005, a systematic sampling method was used. An area of 150 m × 150 m was equally divided into 25 blocks and marked permanently; five of these blocks on a diagonal were selected. Each selected block was further separated into 25 6 m × 6 m cells. Five 0.25 m × 0.25 m quadrats were harvested from one of the 25 cells in each set of five diagonal blocks.
After harvesting, all plant samples were oven-dried at 65 °C to a constant weight. In 1983–1985, 1989, 1998–2000, and 2006–2014, the living plants were further sorted into forb, grass, and sedge functional groups. The measured peak biomass served as a proxy for ANPP.
Warming Experiment Design and Measurements.
A warming and precipitation manipulation experiment was established in July 2011, approximately 50 m from the long-term observational area. The manipulative experiment was a full factorial design, including two warming levels (control and warming) and three precipitation levels (drought, ambient, and wet). Each treatment had six replicates and 36 2.2 m × 1.8 m plots that were divided at random into six blocks. Each warming treatment plot was warmed by two medium-wave heaters (220 V, 1,200 W, 1.0 m long, 0.22 m wide), and each control plot was outfitted with two dummy heaters. The precipitation treatment was controlled by four transparent Panlite sheet channels (PC-1151; Teijin Chemicals) installed at a 15° angle above each plot. The drought treatment was controlled by the nonslotted channels, and 50% of the intercepted rainwater was collected and stored. The wet treatment was provided by slotted channels that sprinkled the collected water from the drought plots immediately after the rain, resulting in a 50% increase compared with the control. The control was also installed with four dummy slotted channels. To avoid surface runoff, metal plates were inserted to a soil depth of 15 cm with 5 cm remaining above the surface around each plot. During the 4-y experiment (2011–2014), 2011 and 2014 were wet years, while 2012 and 2013 were dry years. Air temperature, annual or growing season (May to September), exhibited no significant variations during the experimental period.
ANPP and BNPP were measured in each plot. Peak aboveground biomass as a proxy for ANPP was harvested; separated into grasses, sedges, and forbs; and oven-dried at 65 °C to a constant weight in three 0.25 m × 0.25 m quadrats of each plot. BNPP was estimated using an ingrowth-core method. The preexisting root biomass was removed by soil cores (5 cm diameter, 50 cm long) in September 2012. Then sieved soils from the same soil depths outside the plots with polyester mesh bags (mesh size 1 mm) were refilled back to the holes. The soil cores collected from the holes were further divided into five soil layers: 0–5, 5–10, 10–20, 20–30 and 30–50 cm. Following soil sampling at the end of growing season, roots were washed carefully in sieves (0.5 mm) to remove the black debris and attached soil, dried, and weighed.
To investigate root vertical distribution pattern of pant species in the community, nine species from three plant functional groups—grasses (Stipa aliena, Elymus nutans, and Helictotrichon tibeticum), forbs (Gentiana straminea, Tibetia himalaica, Saussurea pulchra, and Medicago ruthenica), and sedges (Kobresia humilis and Carex przewalskii) —were chosen outside the permanent plots. By digging soil monoliths and washing the attached debris and soil, three intact root systems for each species were obtained. The roots were cut into 5-cm segments, and dry weights were recorded.
Starting in August 2011, soil temperature and moisture were measured hourly at 5-, 10-, and 20-cm depths hourly using three sets of sensors (EM 50; Decagon Devices) for three blocks.
Data Analyses for Monitoring and Experiment.
For the long-term observations, the humidity index was obtained by calculating the ratio of precipitation to potential evapotranspiration (PET) (50), while the PET was calculated based on air temperature and relative humidity with the following function (51):
where Tair is the monthly air temperature and RH is the monthly relative humidity. Linear regressions were used to analyze the interannual trends in ANPP, relative abundance of different functional groups, air temperature, precipitation, humidity index, and soil moisture.
For the field manipulative experiment, paired t tests were used to determine the differences in the relative abundance of functional groups and in the percentage change of BNPP at different depths between control and warming/drought. One-way analysis of variance and Turkey’s honest significant difference test were used to test differences in soil moisture, ANPP, BNPP, and NPP among control, warming and drought plots. All statistical analyses were conducted using R 2.15.1 software (R Core Team). Differences were considered significant at P < 0.05 unless stated otherwise.
Meta-Analysis for the Warming Experiments over the Tibetan Plateau.
Literature searches were conducted through the search engines of Web of Science and China National Knowledge Infrastructure. Only warming experiments conducted in alpine steppe or alpine meadow ecosystems in the Tibetan Plateau were included. For each selected study, a variety of data was collected from control and warming treatments, including ANPP, vegetation coverage, biomass of grasses and forbs, soil temperature, and moisture. Site information for all field stations, including longitude, latitude, altitude, mean annual temperature and annual precipitation, was collected as well. For experiments with data reported for multiple years, only data in the most recent year were used, to guarantee independence of the observations.
The responses of ANPP, relative abundance of grasses and forbs, soil temperature, and soil moisture to warming manipulations were assessed by meta-analysis. The effect size of each variable was calculated using the following equation:
where lnRR is the natural logarithm of response ratio and XT and XC are the means of the treatment and control groups, respectively (52). Metawin (Sinauer Associates) was used to calculate mean response ratios and 95% bootstrap confidence intervals (CIs). If the 95% bootstrap CI did not overlap 1, then the effect caused by warming was considered significant.
Supplementary Material
Acknowledgments
We thank Jingyun Fang, Guirui Yu, and Huifeng Hu for organizing this special issue, and Dr. T. Chapin III, the subject editor, and anonymous reviewers for their insightful comments and suggestions on an earlier version of this manuscript. This work was supported by Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDA05050000; National Basic Research Program of China Grant 2014CB954000; National Natural Science Foundation of China Grants 31630009, 31570394, and 31321061; and a Semper Ardens grant from the Carlsberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700299114/-/DCSupplemental.
References
- 1.Chapin FS, III, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of arctic tundra to experimental and observed changes in climate. Ecology. 1995;76:694–711. [Google Scholar]
- 2.Luo Y. Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol Syst. 2007;38:683–712. [Google Scholar]
- 3.Elmendorf SC, et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol Lett. 2012;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 4.Van der Wal R, Stien A. High-arctic plants like it hot: A long-term investigation of between-year variability in plant biomass. Ecology. 2014;95:3414–3427. [Google Scholar]
- 5.Rudgers JA, et al. Responses of high-altitude graminoids and soil fungi to 20 years of experimental warming. Ecology. 2014;95:1918–1928. doi: 10.1890/13-1454.1. [DOI] [PubMed] [Google Scholar]
- 6.Crowther TW, et al. Quantifying global soil carbon losses in response to warming. Nature. 2016;540:104–108. doi: 10.1038/nature20150. [DOI] [PubMed] [Google Scholar]
- 7.Sistla SA, et al. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature. 2013;497:615–618. doi: 10.1038/nature12129. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, et al. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob Chang Biol. 2013;19:2940–2955. doi: 10.1111/gcb.12277. [DOI] [PubMed] [Google Scholar]
- 9.Harte J, Saleska SR, Levy C. Convergent ecosystem responses to 23-year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long-term climate-soil carbon feedback. Glob Chang Biol. 2015;21:2349–2356. doi: 10.1111/gcb.12831. [DOI] [PubMed] [Google Scholar]
- 10.Elmendorf SC, et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc Natl Acad Sci USA. 2015;112:448–452, and erratum (2015) 112:E4156. doi: 10.1073/pnas.1410088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piao S, et al. Impacts of climate and CO2 changes on the vegetation growth and carbon balance of Qinghai-Tibetan grasslands over the past five decades. Global Planet Change. 2012;98:73–80. [Google Scholar]
- 12.Zhang X, Sun S, Yong S, Zhou Z, Wang R. Vegetation Map of the People’s Republic of China (1: 1000000) Geological Publishing House; Beijing: 2007. [Google Scholar]
- 13.Dong M, Jiang Y, Zheng C, Zhang D. Trends in the thermal growing season throughout the Tibetan Plateau during 1960-2009. Agric For Meteorol. 2012;166–167:201–206. [Google Scholar]
- 14.Hansen J, Ruedy R, Sato M, Lo K. Global surface temperature change. Rev Geophys. 2010;48:1–52. [Google Scholar]
- 15.Yao T, Pu J, Lu A, Wang Y, Yu W. Recent glacial retreat and its impact on hydrological processes on the Tibetan Plateau, China, and surrounding regions. Arct Antarct Alp Res. 2007;39:642–650. [Google Scholar]
- 16.Christensen JH, et al. The physical science basis. In: Stocker TF, et al., editors. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2013. pp. 1217–1308. [Google Scholar]
- 17.Wu H, Guo Z, Peng C. Land use induced changes of organic carbon storage in soils of China. Glob Chang Biol. 2003;9:305–315. [Google Scholar]
- 18.Yang Y, et al. Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob Chang Biol. 2008;14:1592–1599. [Google Scholar]
- 19.Tang X, et al. Carbon pools across China’s terrestrial natural ecosystems: New estimations based on an intensive field inventory. Proc Natl Acad Sci USA. 2018;115:4021–4026. doi: 10.1073/pnas.1700291115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang Q, et al. Carbon dynamics of terrestrial ecosystems on the Tibetan Plateau during the 20th century: An analysis with a process-based biogeochemical model. Glob Ecol Biogeogr. 2010;19:649–662. [Google Scholar]
- 21.Zhang G, Zhang Y, Dong J, Xiao X. Green-up dates in the Tibetan Plateau have continuously advanced from 1982 to 2011. Proc Natl Acad Sci USA. 2013;110:4309–4314. doi: 10.1073/pnas.1210423110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen M, et al. Evaporative cooling over the Tibetan Plateau induced by vegetation growth. Proc Natl Acad Sci USA. 2015;112:9299–9304. doi: 10.1073/pnas.1504418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, et al. Stability of alpine meadow ecosystem on the Qinghai-Tibetan Plateau. Chin Sci Bull. 2008;51:320–327. [Google Scholar]
- 24.Wang S, et al. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology. 2012;93:2365–2376. doi: 10.1890/11-1408.1. [DOI] [PubMed] [Google Scholar]
- 25.Klein JA, Harte J, Zhao XQ. Experimental warming, not grazing, decreases rangeland quality on the Tibetan Plateau. Ecol Appl. 2007;17:541–557. doi: 10.1890/05-0685. [DOI] [PubMed] [Google Scholar]
- 26.Wolkovich EM, et al. Warming experiments underpredict plant phenological responses to climate change. Nature. 2012;485:494–497. doi: 10.1038/nature11014. [DOI] [PubMed] [Google Scholar]
- 27.Hudson JM, Henry GH. Increased plant biomass in a high Arctic heath community from 1981 to 2008. Ecology. 2009;90:2657–2663. doi: 10.1890/09-0102.1. [DOI] [PubMed] [Google Scholar]
- 28.Epstein HE, et al. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ Res Lett. 2012;7:1–12. [Google Scholar]
- 29.Rustad L, et al. GCTE-NEWS A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126:543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob Chang Biol. 2011;17:927–942. [Google Scholar]
- 31.Zhao M, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science. 2010;329:940–943. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]
- 32.Chu C, et al. Does climate directly influence NPP globally? Glob Chang Biol. 2016;22:12–24. doi: 10.1111/gcb.13079. [DOI] [PubMed] [Google Scholar]
- 33.Bai Y, Han X, Wu J, Chen Z, Li L. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- 34.Connell SD, Ghedini G. Resisting regime-shifts: The stabilising effect of compensatory processes. Trends Ecol Evol. 2015;30:513–515. doi: 10.1016/j.tree.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Moore DJ, Burns SP, Monson RK. Longer growing seasons lead to less carbon sequestration by a subalpine forest. Glob Chang Biol. 2010;16:771–783. [Google Scholar]
- 36.Parmentier FJW, et al. Longer growing seasons do not increase net carbon uptake in the northeastern Siberian tundra. J Geophys Res. 2011;116:1–11. [Google Scholar]
- 37.Geng Y, Wang L, Jin D, Liu H, He J-S. Alpine climate alters the relationships between leaf and root morphological traits but not chemical traits. Oecologia. 2014;175:445–455. doi: 10.1007/s00442-014-2919-5. [DOI] [PubMed] [Google Scholar]
- 38.Reich PB, Wright IJ, Lusk CH. Predicting leaf physiology from simple plant and climate attributes: A global GLOPNET analysis. Ecol Appl. 2007;17:1982–1988. doi: 10.1890/06-1803.1. [DOI] [PubMed] [Google Scholar]
- 39.Grime JP, et al. Long-term resistance to simulated climate change in an infertile grassland. Proc Natl Acad Sci USA. 2008;105:10028–10032. doi: 10.1073/pnas.0711567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmendorf SC, et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Chang. 2012;2:453–457. [Google Scholar]
- 41.Xu X, et al. Interannual variability in responses of belowground net primary productivity (NPP) and NPP partitioning to long-term warming and clipping in a tallgrass prairie. Glob Chang Biol. 2012;18:1648–1656. [Google Scholar]
- 42.Yang HJ, et al. Community structure and composition in response to climate change in a temperate steppe. Glob Chang Biol. 2011;17:452–465. [Google Scholar]
- 43.Lauenroth WK, Sala OE. Long-term forage production of North American shortgrass steppe. Ecol Appl. 1992;2:397–403. doi: 10.2307/1941874. [DOI] [PubMed] [Google Scholar]
- 44.Huxman TE, et al. Convergence across biomes to a common rain-use efficiency. Nature. 2004;429:651–654. doi: 10.1038/nature02561. [DOI] [PubMed] [Google Scholar]
- 45.Fontaine S, et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature. 2007;450:277–280. doi: 10.1038/nature06275. [DOI] [PubMed] [Google Scholar]
- 46.Mobley ML, et al. Surficial gains and subsoil losses of soil carbon and nitrogen during secondary forest development. Glob Chang Biol. 2015;21:986–996. doi: 10.1111/gcb.12715. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt MW, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 48.Lorenz K, Lal R. The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv Agron. 2005;88:35–66. [Google Scholar]
- 49.Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob Chang Biol. 2013;19:988–995. doi: 10.1111/gcb.12113. [DOI] [PubMed] [Google Scholar]
- 50.Gao Y, Li X, Leung LR, Chen D, Xu J. Aridity changes in the Tibetan Plateau in a warming climate. Environ Res Lett. 2015;10:034013. [Google Scholar]
- 51.Tabari H, Grismer M, Trajkovic S. Comparative analysis of 31 reference evapotranspiration methods under humid conditions. Irrig Sci. 2013;31:107–117. [Google Scholar]
- 52.Hedges L, Gurevitch J, Curtis P. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- 53.Ashe X. 2013. Effects of warming and precipitation regime on plant phenology and productivity in an alpine meadow, northwestern Sichuan, China. Master’s dissertation (Chengdu University of Technology, Chengdu, China)
- 54.Chen J, et al. Differential responses of ecosystem respiration components to experimental warming in a meadow grassland on the Tibetan Plateau. Agric For Meteorol. 2016;220:21–29. [Google Scholar]
- 55.Li YN, et al. Effects of a 5-years mimic temperature increase to the structure and productivity of Kobresia humilis meadow. Acta Ecol Sin. 2004;12:236–239. [Google Scholar]
- 56.Ganzhu Z. 2013. Ecosystem carbon exchange under warming and precipitation enhancement in Kobresia pygmaea meadow in northern Tibet. Master’s dissertation (Chinese Academy of Agricultural Sciences, Beijing, China)
- 57.Chen J, et al. The influence of short-term experimental warming on alpine steppe of bird island. J Arid Land. 2014;28:127–133. [Google Scholar]
- 58.Li N, et al. Short-term effects of temperature enhancement on community structure and biomass of alpine meadow in the Qinghai-Tibet Plateau. Acta Ecol Sin. 2011;31:895–905. [Google Scholar]
- 59.Fu G, et al. Clipping alters the response of biomass production to experimental warming: A case study in an alpine meadow on the Tibetan Plateau, China. J Mt Sci. 2015;12:935–942. [Google Scholar]
- 60.Zong N, et al. Responses of ecosystem CO2 fluxes to short-term experimental warming and nitrogen enrichment in an alpine meadow, Northern Tibet Plateau. Sci World J. 2013;2013:1–11. doi: 10.1155/2013/415318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao JZ. 2012. The responses of plant community to simulating warming in the Kobresia humilis meadow. PhD dissertation (Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining, China)
- 62.Shi FS, Ning WU. Effect of temperature enhancement on community strcture and biomass of subalpine meadow in Northwestern Sichuan. Acta Ecol Sin. 2008;28:5286–5293. [Google Scholar]
- 63.Zhang Y, Welker JM. Tibetan alpine tundra responses to simulated changes in climate: Aboveground biomass and community responses. Arct Alp Res. 1996;28:203–209. [Google Scholar]
- 64.Xu M, et al. Effects of warming and clipping on plant and soil properties of an alpine meadow in the Qinghai-Tibetan Plateau, China. J Arid Land. 2015;7:189–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.