Significance
Cigarette smoking is the leading cause of preventable disease and death in the United States. Severe withdrawal symptoms are the reason most people have a hard time quitting smoking. AMP-activated protein kinase (AMPK) is a master regulator of energy homeostasis and is activated in response to cellular stressors. We discovered that the AMPK pathway is activated following chronic nicotine use but is repressed following nicotine withdrawal. We reasoned that increasing pAMPK levels pharmacologically might reduce symptoms of nicotine withdrawal. After giving mice metformin, an AMPK activator, we were able to reduce nicotine withdrawal, and found that this was dependent on the presence of the AMPK protein in the hippocampus. This study suggests that AMPK activation could be a target for smoking cessation.
Keywords: nicotine, metformin, 5′ AMP-activated protein kinase, CREB
Abstract
Cigarette smoking is the leading cause of preventable disease and death in the United States, with more persons dying from nicotine addiction than any other preventable cause of death. Even though smoking cessation incurs multiple health benefits, the abstinence rate remains low with current medications. Here we show that the AMP-activated protein kinase (AMPK) pathway in the hippocampus is activated following chronic nicotine use, an effect that is rapidly reversed by nicotine withdrawal. Increasing pAMPK levels and, consequently, downstream AMPK signaling pharmacologically attenuate anxiety-like behavior following nicotine withdrawal. We show that metformin, a known AMPK activator in the periphery, reduces withdrawal symptoms through a mechanism dependent on the presence of the AMPKα subunits within the hippocampus. This study provides evidence of a direct effect of AMPK modulation on nicotine withdrawal symptoms and suggests central AMPK activation as a therapeutic target for smoking cessation.
Cigarette smoking constitutes a major health burden in the United States, with smoking accounting for more than 500,000 deaths annually nationwide (1). Despite the more than 30 reports issued by the Surgeon General and multiple public health campaigns addressing the negative impact of smoking on the health and well-being of Americans, the cessation rate remains modest (1, 2). Multiple obstacles prevent successful cessation, including irritability, anger, depressed mood, anxiety, and difficulty concentrating. There are currently three Food and Drug Administration-approved therapies used to target the symptoms of withdrawal: nicotine replacement therapy, a nicotinic acetylcholine receptor (nAChR) partial agonist (varenicline/Chantix), and an antidepressant (bupropion/Zyban) (3). However, although 70% of smokers express a desire to quit, current therapies achieve only a 15% success rate (1, 4).
In cigarette smokers, nicotine has been shown to reduce anxiety. Given the lack of studies on the effects of nicotine in nonsmokers, it is unclear whether nicotine reduces anxiety in the absence of withdrawal; however, numerous studies in rodent models have demonstrated dose-dependent anxiolytic effects of nicotine in drug-naïve subjects (5, 6). There is considerable evidence that withdrawal-associated anxiety contributes to poor adherence to smoking cessation treatments (7, 8) as well as relapse rates, with many smokers experiencing anxiety symptoms during acute abstinence periods (7, 9). A recent functional imaging study in smokers correlated affect with aberrant activation in the hippocampus during smoking cue presentation (10), and hippocampal volume has been correlated with successful quit attempts (11). Thus, the hippocampus plays an important role in the anxiolytic effects of nicotine and nicotine-withdrawal associated negative effects in both humans and animal models.
We have previously shown that the transcription factor CREB is required for nicotine reward (12), and through genomic analyses have identified the AMPK pathway as a CREB target following chronic nicotine exposure and withdrawal (13). We discovered that within the hippocampus, CREB binding is enriched in a number of pathways (Ingenuity Pathway Analysis; complete gene list in ref. 13). We found that in the category of “cell-to-cell signaling, carbohydrate metabolism, lipid metabolism,” CREB binds to the cis-regulatory elements in 12 of 24 members of the energy-sensory AMP-activated protein kinase (AMPK) pathway, as listed in the Reactome Database (Table 1). These include several subunits of AMPK itself (the genes encoding the β1, β2, γ1, and γ2 subunits) and the upstream regulator of AMPK, Liver Kinase B1 (LKB1; also known as Serine/Threonine Kinase 11).
Table 1.
Genes of the AMPK pathway bound by CREB in the mouse hippocampus
| Gene symbol | Gene name |
| Acacb | Acetyl-CoA carboxylase beta |
| Acsl1 | Acyl-CoA synthetase |
| Cab39L | Calcium-binding protein 39 |
| Ppm1a | Protein phosphatase 1A |
| Prkab1 | AMPK, beta 1 subunit |
| Prkab2 | AMPK, beta 2 subunit |
| Prkag1 | AMPK, gamma 1 subunit |
| Prkag2 | AMPK, gamma 2 subunit |
| Rheb | Ras homolog enriched in brain |
| Rptor | Raptor, regulator of MTO |
| Stk11 | Serine/threonine kinase 11 |
| Tsc1 | Tuberous sclerosis 1 |
Although the role of AMPK in peripheral tissues is well established, few studies have focused on its effect within the central nervous system, and none have examined its relation to nicotine dependence, outside of the hypothalamus (14). Of importance, recent studies illustrate that AMPK signaling is functionally linked to behaviors, including cognition and negative affect (15–17), that contribute to relapse during nicotine withdrawal. Therefore, we wanted to determine whether controlling AMPK activity during nicotine withdrawal could ameliorate the affective symptoms of nicotine withdrawal.
Results
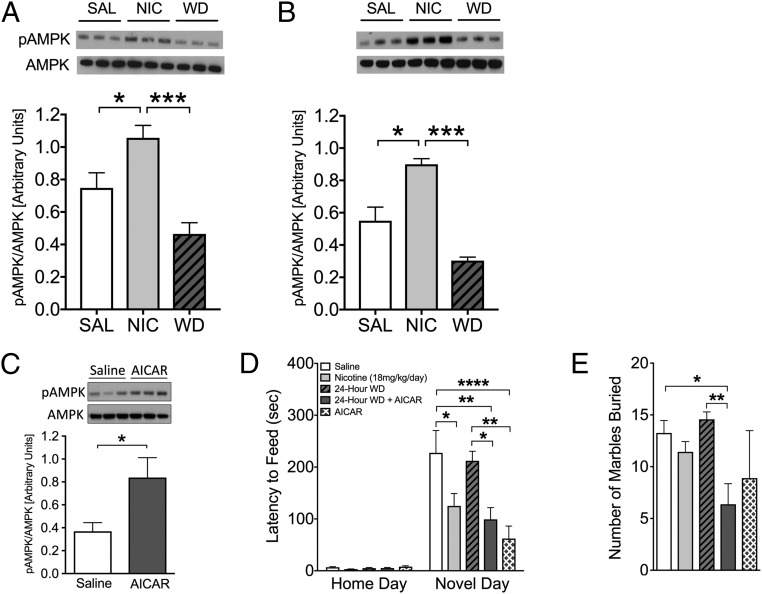
First, we evaluated the effects of chronic nicotine and 24-h withdrawal on AMPK pathway activation in the brain. Nicotine treatment caused a significant activation of this pathway in the hippocampus, as shown by the elevated pAMPK/AMPK ratio and the increased phosphorylation of the AMPK target acetyl-CoA carboxylase (ACC) (Fig. 1 A and B). Of interest, this is the sole brain region examined that shows this pattern of pAMPK activity (Fig. S1). Next, we determined whether pharmacologic activation of the AMPK pathway could ameliorate nicotine withdrawal symptoms. To this end, we treated mice with 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an allosteric activator of AMPK (18). AICAR caused a significant activation of the AMPK pathway in the hippocampus, indicating that it crosses the blood-brain barrier (Fig. 1C). Nicotine withdrawal causes anxiety-like behaviors in mice, which can be evaluated using two behavioral tests: novelty-induced hypophagia (NIH) and marble-burying (MB). A reduction in anxiety-like behavior was demonstrated by a reduced latency to feed in the NIH test and a reduced number of marbles buried in the MB test. In both assays, AICAR treatment completely abolished the effects of nicotine withdrawal (Fig. 1 D and E).
Fig. 1.
The AMPK activator AICAR eliminates anxiety-like behaviors following nicotine withdrawal. (A and B) Nicotine causes activation of the AMPK pathway in the hippocampus, as indicated by representative Western blot analyses of AMPK and pAMPK (n = 9) and ACC and pACC tissue (n = 3). *P < 0.05; ***P < 0.001. (C–E) Chronic AICAR administration increases pAMPK level in the hippocampus and reduces anxiety-like behavior at 24 h after cessation of nicotine. (C) Systemic AICAR treatment results in significant activation of the AMPK pathway in the hippocampus, indicating that the drug crosses the blood-brain barrier (n = 4; P < 0.05). (D and E) Anxiety-like behavior precipitated by nicotine withdrawal is reduced by AICAR. (D) NIH was tested at 24 h after nicotine withdrawal. AICAR before nicotine withdrawal prevented the increase in latency to feed observed in saline-treated mice undergoing 24-h withdrawal. Bars represent mean latency ± SEM (n = 7–13). *P < 0.05; **P < 0.01. (E) The MB test was performed at 48 h after nicotine withdrawal. Data represent the mean ± SEM number of marbles buried over 15 min (n = 7–8). **P < 0.01.
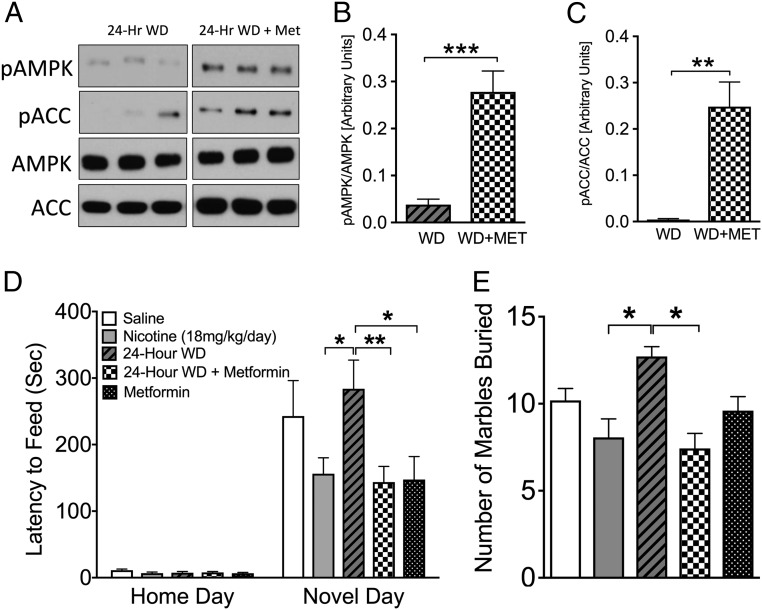
Next, we used the Food and Drug Administration-approved diabetes drug metformin, which targets AMPK activation in the liver (19, 20). One week of systemic metformin pretreatment resulted in activation of the AMPK pathway following nicotine withdrawal compared with saline-pretreated animals (Fig. 2 A–C). Strikingly, systemic metformin also completely prevented anxiety-like behaviors caused by nicotine withdrawal (Fig. 2 D and E) at a dose that did not impact body weight, food consumption, or glucose levels under both fed and fasted conditions (Fig. S2). Of note, both AICAR (Fig. 1D) and metformin (Fig. 2D) reduced the latency to feed in the NIH test in naïve mice, suggesting that activation of AMPK may provide a novel target for general anxiolytic activity as well as anxiety induced from nicotine withdrawal.
Fig. 2.
Systemically administered metformin increases hippocampal pAMPK and reduces anxiety-like behavior during nicotine withdrawal. (A–C) Western blot analysis of hippocampal AMPK, pAMPK, ACC, and pACC levels at 24 h after nicotine withdrawal in the presence and absence of i.p. metformin (n = 6–7). **P < 0.01; ***P < 0.001. (D) Mice chronically treated with nicotine exhibited a reduced latency to approach a palatable food in a novel environment compared with mice at 24 h after nicotine withdrawal. Metformin reverses the increase in anxiety-like behavior caused by nicotine withdrawal. Bars represent mean latency ± SEM (n = 13–15). *P < 0.05; **P < 0.01. (E) Metformin treatment reduced anxiety-like behavior as measured by the number of marbles buried. Data represent the mean ± SEM number of marbles buried over 15 min (n = 7–16). *P < 0.05.
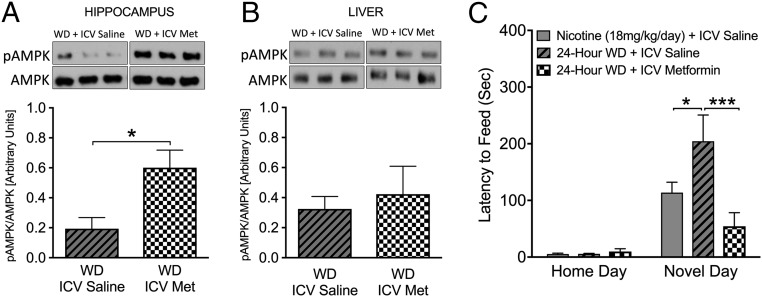
Metformin is known to have multiple peripheral targets, including liver and skeletal muscle, and activation of the AMPK pathway in these organs might indirectly contribute to behaviors observed following withdrawal from nicotine. To determine whether metformin acts centrally to improve nicotine withdrawal symptoms or whether peripheral effects contribute, we implanted mice with osmotic minipumps connected to intracerebroventricular (i.c.v.) guide cannulas to centrally deliver either metformin (50 μg/d) or saline for 1 wk before withdrawal. This dose of i.c.v. metformin was chosen because it does not alter peripheral glucose metabolism (21) (Fig. S2). As expected, 7 d of i.c.v. metformin caused AMPK activation in the hippocampus, but not in the liver (Fig. 3 A and B). Importantly, centrally administered metformin completely prevented nicotine withdrawal-induced anxiety behaviors, as shown by a reduced latency to consume novel food (Fig. 3C). These data strongly support the notion that central, not peripheral, actions of metformin ameliorate nicotine withdrawal symptoms.
Fig. 3.
Intracerebroventricular (i.c.v.) metformin activates hippocampal AMPK levels and reduces nicotine withdrawal symptoms. (A and B) Western blot analysis demonstrates increased hippocampal (A) but not hepatic (B) pAMPK levels following 1 wk of i.c.v. administration of metformin compared with mice given i.c.v. saline following nicotine withdrawal (n = 4). *P < 0.05. (C) Metformin given i.c.v. prevents anxiety-like withdrawal symptoms precipitated by nicotine withdrawal as determined by the NIH test, as demonstrated by a reduced latency to feed at 24 h after withdrawal compared with mice administered saline. Bars represent mean latency ± SEM (n = 7–8), *P < 0.05; ***P < 0.001.
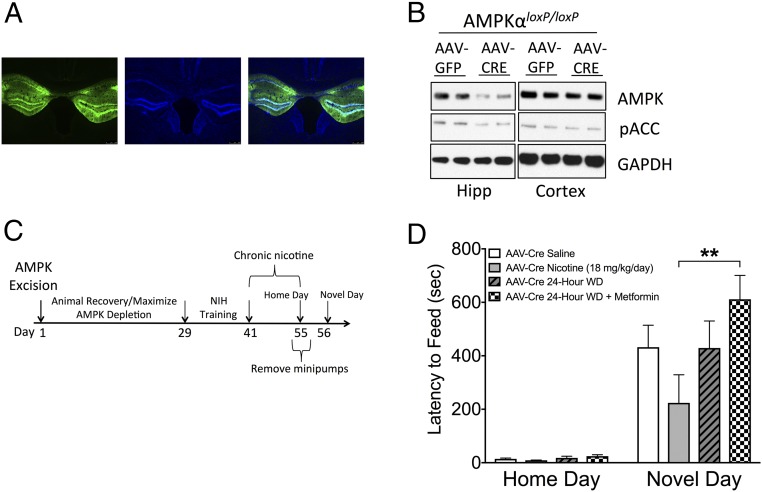
Metformin has been shown to activate a variety of cellular targets in addition to AMPK (19, 20, 22–24). To confirm that AMPK is the relevant target of metformin-mediated reduction of anxiety-like behaviors following nicotine withdrawal, we used Ampkα1loxP/loxP;Ampkα2loxP/loxP mice harboring conditional alleles for both α-subunits of AMPK (AMPKαloxP/loxP hereinafter). An adeno-associated virus (AAV) encoding Cre recombinase (AAV-Cre) was microinjected directly into the dorsal and ventral hippocampi of AMPKαloxP/loxP mice, generating site-specific deletion of AMPKα (Fig. 4A). To assess the anatomic and temporal resolution of our injection coordinates, AAV-GFP (green fluorescence protein) was injected into the hippocampi of AMPKαloxP/loxP mice, and its presence in the expected location was confirmed 8 wk later through GFP immunohistochemistry (Fig. 4A). Western blot analysis confirmed that total levels of AMPKα were decreased in the hippocampi at 8 wk after AAV-Cre injection in AMPKαloxP/loxP mice, but remained unchanged in other brain regions, such as the cortex (Fig. 4B). Furthermore, levels of phospho-acetyl CoA carboxylase (pACC) were also reduced in the hippocampi, demonstrating a functional reduction in the pathway (Fig. 4B).
Fig. 4.
Anxiolytic effects of metformin during nicotine withdrawal are dependent on hippocampal AMPKα. (A) Representative GFP expression in the hippocampus at 10× magnification. From left to right: GFP, DAPI, merge. (B) Western blot analysis of AMPKα and pACC in the hippocampus and cortex of AAV-Cre–treated AMPKαloxP/loxP mice indicating the specificity of gene ablation. (C) Overview of the experimental paradigm for the NIH test. Here 2-mo-old AMPKαloxP/loxP mice were injected stereotactically with AAV-Cre. Four weeks later, the mice were trained for the NIH test and treated with chronic nicotine, and NIH behavior was examined at 24 h after withdrawal from nicotine. (D) Chronic metformin treatment does not reduce anxiety in AMPKαloxP/loxP mice injected with AAV-Cre, as evidenced by an increased latency to consume a palatable food in a novel environment at 24 h after withdrawal compared with nicotine-treated AMPKαloxP/loxP mice injected with AAV-Cre. Bars represent mean latency ± SEM (n = 6–7). **P < 0.01.
To assess anxiety-like behaviors, mice were trained in the NIH paradigm at 4 wk after intrahippocampal injection of AAV-Cre and tested at 8 wk after viral injection to ensure full AMPK ablation (Fig. 4C). Mice were treated with nicotine and metformin for 1 wk, followed by 24 h of nicotine withdrawal before behavioral testing. As shown in Fig. 4D, the AMPK-deficient mice treated with metformin showed a dramatic and significant increase in the latency to feed on the novel food compared with the nicotine-treated mice, indicating that the positive effects of metformin on nicotine withdrawal-induced anxiety are mediated, at least in large part, by the presence of AMPK in the hippocampus.
Discussion
Multiple preclinical and clinical studies have explored the influence of targeting nicotinic acetylcholine receptors (nAChRs) for smoking cessation therapies. However, the limited success and adverse side effects of these aids call for investigation beyond targeting nAChRs to abrogate nicotine withdrawal symptoms, such as negative affect. Here we have used pharmacologic, behavioral, and genetic approaches to identify central AMPK activation as a potential novel therapeutic approach for nicotine cessation therapy.
Withdrawal from nicotine produces physiological, affective, and cognitive effects (25, 26). The negative affect experienced during withdrawal is directly associated with early smoking relapse, with successful quitters exhibiting a greater positive affect compared with those who relapse (27). Smokers also report that one of their top concerns when considering the consequences of abstinence is the loss of nicotine as a tool to cope with negative affect (28).
To mechanistically examine the anxiogenic effect of nicotine withdrawal in preclinical models, we used behavioral paradigms that reliably demonstrate the anxiety impairments observed in humans following withdrawal. The NIH and MB tests are two validated tests for evaluating anxiety-like behavior in rodents. Chronic nicotine use reduces anxiety-like behavior, as measured by a decreased latency to eat a palatable food in a novel environment in the NIH test. In contrast, latency is significantly increased following nicotine withdrawal (13, 29, 30). Similarly, chronic nicotine use decreases anxiety-like behavior as measured by a reduction in the number of marbles buried in the MB test, while withdrawal increases the number of marbles buried in this test (6, 29).
Based on our findings demonstrating the preclinical efficacy of metformin in alleviating anxiety-like behavior following nicotine withdrawal, we propose that AMPK activation in the brain via metformin can be repurposed as a novel pharmacotherapy for nicotine cessation. The proven preclinical efficacy of metformin in alleviating withdrawal symptoms along with its well-established safety profile for diabetes treatment should encourage investigators to translate these findings into future clinical trials for higher continuous abstinence rates in smokers, with the added benefit of normalizing glycemic control.
Materials and Methods
Study Design.
The aim of this study was to examine the impact of AMPK activation on mediating anxiety-like behavior and cognition in preclinical models of nicotine withdrawal. To this end, mouse studies were conducted in which mice received treatment with an osmotic minipump containing saline or nicotine (18 mg/kg/d) for 2 wk. Spontaneous withdrawal from nicotine occurred following physical removal of the minipump. All behavioral responses were measured at 24–48 h after nicotine withdrawal, because the first 2 d of withdrawal are associated with the highest rate of relapse in humans (31) and with peak affective withdrawal symptoms in mice (32). AMPK activators (AICAR and metformin) were chronically administered before nicotine cessation, and behavioral outcomes were evaluated using two behavioral paradigms, the NIH and MB tests. Experimenters blind to treatment group assignment assessed two behavioral endpoints: latency to consume (NIH) and number of marbles buried (MB). Western blot analysis was used to quantify protein levels of AMPK within hippocampal tissue following AICAR or metformin administration. Finally, we used an AAV vector to deliver Cre recombinase into AMPKαloxP/loxP mice harboring a conditional allele for AMPK to determine whether AMPK is necessary for mediating the anxiolytic effects of metformin during withdrawal from nicotine.
Animals.
Male 129SvEv; C57BL/6J F1 hybrid mice age 6–8 wk were obtained from The Jackson Laboratory and used in the behavioral paradigm tests, i.c.v. drug experiments, and molecular studies. All AAV-Cre experiments were conducted in previously derived and described AMPKαloxP/loxP mice (AMPKα1loxP/loxP mice previously crossed with AMPKα2loxP/loxP mice) (23, 33) from the Viollet laboratory (Institut Cochin, INSERM). All mice were maintained on a standard light cycle (lights on between 0600 and 1800 h) and had free access to food and water. All experimental procedures were approved by the University of Pennsylvania’s Animal Care and Use Committee and were conducted in compliance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (34).
Nicotine Delivery, Dose, and Withdrawal.
Mice were anesthetized with an isoflurane/oxygen mixture (1–3%), and osmotic minipumps (model 1002; Alzet) were placed s.c. using aseptic surgery techniques. (−)-Nicotine hydrogen tartrate salt (MP Biomedicals) was dissolved in sterile 0.85% saline and administered via the osmotic minipumps at a dose of 18 mg/kg/d (dose expressed as freebase weight) for 2 wk. Control (saline-treated) mice received osmotic minipumps filled with 0.85% saline. Following treatment, the minipumps were surgically removed from one-half of the mice receiving saline and the mice treated chronically with nicotine to initiate spontaneous withdrawal.
In Vivo Drug Treatments.
Metformin (Spectrum) was dissolved in saline and administered at 250 mg/kg by i.p. injection. AICAR (Toronto Research Chemicals) was dissolved in saline and administered at 500 mg/kg by i.p. injection. Vehicle was 0.85% saline administered by i.p. injection. All injections were started at 7 d before withdrawal from nicotine and were given once daily between 0900 and 1000 hours.
Intracerebroventricular Administration of Metformin.
Osmotic minipumps (model 1002; Alzet) for i.c.v. delivery were filled with either 0.85% saline or metformin (50 μg/d), connected to silicone tubing (CT24SR; PlasticsOne), push-fit into guide cannula (3300/SP; PlasticsOne), and allowed to equilibrate to 37 °C overnight. One week after mice were implanted with nicotine minipumps, mice were again anesthetized with vaporized isoflurane. The minipump was inserted contralateral to the nicotine minipump, and the guide cannula was placed above the right lateral ventricle (anteroposterior, −0.8 mm; mediolateral, +1.5 mm) and fixed with dental cement. The nicotine minipumps were removed at 6 d after the surgery, and behavioral testing was performed at 24 h after withdrawal.
Western Blot Analysis.
Protein analysis was performed as described previously (35, 36). In brief, tissue was lysed in 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 15% glycerol, Phosphatase Inhibitor Mixtures 2 and 3 (Sigma-Aldrich), and Protease Inhibitor Mixtures 1 and 2 (Roche). Equal amounts of protein were run on NuPage 4–12% Bis-Tris gels (Invitrogen). Following SDS/PAGE and blocking, membranes were incubated overnight at 4 °C with one of the following antibodies: AMPK, phospho-AMPKα, ACC, phospho-ACC, or GAPDH (Cell Signaling Technology). Anti-rabbit Ig horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) and SuperSignal West Pico chemiluminescence reagent (Thermo Fisher Scientific) were used for detection. Protein quantification was performed using ImageJ software according to the provided protocol. Densities were calculated for each sample and analyzed across conditions.
Viruses and Microinjections.
The packaging, purification, and determination of vector titers for the AAVs AAV9.CMV.PI.eGFp.WPRE.bGH and AAV9.CMV.PI.CRE.rB were performed at the University of Pennsylvania Vector Core. Injection titers and stereotaxic coordinates were determined as described previously (37). AAV-Cre or AAV-GFP virus was stereotaxically microinjected into the dorsal and ventral hippocampi (anteroposterior −2.1, lateral ± 1.4, dorsoventral −2.0 and anteroposterior −2.9, lateral ± 3.0, dorsoventral −3.8, respectively) at a final titer of 6.534 × 1010 gc/μL over 20 min using a pressure-injection system (KD Scientific) at 0.1 μL/min, 5 min per coordinate. Behavioral testing began at 8 wk after surgery to allow time for recovery and viral expression.
Marble Burying.
The MB test was conducted as described previously (6). After 1 h of acclimation in the room in which MB testing was performed, the mice were placed in a small cage (26 × 20 × 14 cm) with 5-cm deep bedding, on top of which were placed 20 equally distributed marbles. The mice were left undisturbed for 15 min. After 15 min, an investigator blinded to the treatment group returned the mouse to its home cage and counted the number of marbles that were buried in bedding (3/4 or more coverage).
Novelty-Induced Hypophagia.
The NIH test was conducted as described previously (13) with mice housed in groups of two. The mice were placed in a testing room with dividers separating their home cages and allowed to acclimate for 1 h before presentation of a palatable food (peanut butter chips; Nestle). Training was repeated over 10 d with latency to consume the food measured on days 4–10. Following training, mice were randomly assigned to a treatment group, and the osmotic minipumps were implanted. Following treatment, latency to consume peanut butter chips over a 15-min period was measured in the home cage as a baseline, and then measured again 24 h later in a novel environment. The novel environment consisted of a standard cage with no bedding placed within a white box with bright light (approximately 2,150 Lux) and a novel smell (1:10 Pine Sol).
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software), and all data are presented as mean ± SEM. Comparisons between two groups (e.g., Western blot quantification) were done using Student’s t test. Comparisons among multiple groups at one time point (e.g., MB test) were done using one-way ANOVA. Finally, comparisons between multiple groups at different time points (e.g., NIH test) were performed using repeated-measures two-way ANOVA to determine significant differences, with time (home day, novel day) as the within, repeated-measures independent factor and drug treatment as the dependent variable. The threshold for statistical significance was set at P < 0.05, and Bonferroni’s multiple-comparison test was used for all post hoc analyses.
Supplementary Material
Acknowledgments
We thank Dr. Benoit Viollet for providing the AMPKα1loxP/loxP;AMPKα2loxP/loxP mice, and Gavin Huang for assisting with the experiments. This work was supported by National Institutes of Health Grants T32-GM008076 (to B.G.L.), R01 DA041180 (to J.A.B.), DK084336 (to S.F.K.), and National Center for Advancing Translational Sciences Award TL1TR000138.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707047115/-/DCSupplemental.
References
- 1.Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking–50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120:1914–1916. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiore MC, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. US Department of Health and Human Services, US Public Health Service; Rockville, MD: 2008. [Google Scholar]
- 3.US FDA Administration 2012 FDA 101: Smoking Cessation Products. Available at https://www.speakcdn.com/assets/1441/fda_101.pdf. Accessed March 27, 2018.
- 4.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 5.File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. Eur J Pharmacol. 2000;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- 6.Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 8.Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine Tob Res. 2010;12:574–581. doi: 10.1093/ntr/ntq047. [DOI] [PubMed] [Google Scholar]
- 9.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 10.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 11.Froeliger B, et al. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology (Berl) 2010;210:577–583. doi: 10.1007/s00213-010-1862-3. [DOI] [PubMed] [Google Scholar]
- 12.Walters CL, Cleck JN, Kuo Y-C, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Turner JR, et al. Evidence from mouse and man for a role of neuregulin 3 in nicotine dependence. Mol Psychiatry. 2014;19:801–810. doi: 10.1038/mp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez de Morentin PB, et al. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes. 2012;61:807–817. doi: 10.2337/db11-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagon Y, et al. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem. 2005;280:42142–42148. doi: 10.1074/jbc.M507607200. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Zhai X, Li H, Ji L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J Affect Disord. 2014;156:171–177. doi: 10.1016/j.jad.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S, et al. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res. 2014;1576:81–90. doi: 10.1016/j.brainres.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 19.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 20.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portela LV, et al. Intracerebroventricular metformin decreases body weight but has pro-oxidant effects and decreases survival. Neurochem Res. 2015;40:514–523. doi: 10.1007/s11064-014-1496-7. [DOI] [PubMed] [Google Scholar]
- 22.Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RA, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- 26.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 27.al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Hendricks PS, Wood SB, Hall SM. Smokers’ expectancies for abstinence: preliminary results from focus groups. Psychol Addict Behav. 2009;23:380–385. doi: 10.1037/a0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BG, Anastasia A, Hempstead BL, Lee FS, Blendy JA. Effects of the BDNF Val66Met polymorphism on anxiety-like behavior following nicotine withdrawal in mice. Nicotine Tob Res. 2015;17:1428–1435. doi: 10.1093/ntr/ntv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yohn NL, Turner JR, Blendy JA. Activation of α4β2*/α6β2* nicotinic receptors alleviates anxiety during nicotine withdrawal without upregulating nicotinic receptors. J Pharmacol Exp Ther. 2014;349:348–354. doi: 10.1124/jpet.113.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes JR, et al. Smoking cessation among self-quitters. Health Psychol. 1992;11:331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- 32.Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- 33.Viollet B. AMPK: Lessons from transgenic and knockout animals. Front Biosci. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Research Council 2011. Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
- 35.Bang S, et al. AMP-activated protein kinase is physiologically regulated by inositol polyphosphate multikinase. Proc Natl Acad Sci USA. 2012;109:616–620. doi: 10.1073/pnas.1119751109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Bang S, Park S, Shi H, Kim SF. Acyl-CoA-binding domain containing 3 modulates NAD+ metabolism through activating poly(ADP-ribose) polymerase 1. Biochem J. 2015;469:189–198. doi: 10.1042/BJ20141487. [DOI] [PubMed] [Google Scholar]
- 37.Gundersen BB, et al. Increased hippocampal neurogenesis and accelerated response to antidepressants in mice with specific deletion of CREB in the hippocampus: role of cAMP response-element modulator τ. J Neurosci. 2013;33:13673–13685. doi: 10.1523/JNEUROSCI.1669-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.