Significance
Recent clinical studies suggest that environmental insults, such as valproic acid (VPA) exposure, in utero can have adverse effects on brain function of the offspring in later life, although the underlying mechanisms of these impairments remain poorly understood. By focusing on the property of neural stem/progenitor cells (NS/PCs) residing in the adult hippocampus, we identified the mechanism of increased seizure sensitivity in prenatally VPA-exposed adult mice. Furthermore, we found that voluntary exercise can overcome the adverse effects through normalizing VPA-induced transcriptome alterations in NS/PCs. We believe that our study provides insights for further understanding and developing treatment strategies for neurological disorders induced by prenatal environmental insults.
Keywords: ectopic neurogenesis, neural stem cell, valproic acid, epilepsy, Cxcr4
Abstract
Epilepsy is a neurological disorder often associated with seizure that affects ∼0.7% of pregnant women. During pregnancy, most epileptic patients are prescribed antiepileptic drugs (AEDs) such as valproic acid (VPA) to control seizure activity. Here, we show that prenatal exposure to VPA in mice increases seizure susceptibility in adult offspring through mislocalization of newborn neurons in the hippocampus. We confirmed that neurons newly generated from neural stem/progenitor cells (NS/PCs) are integrated into the granular cell layer in the adult hippocampus; however, prenatal VPA treatment altered the expression in NS/PCs of genes associated with cell migration, including CXC motif chemokine receptor 4 (Cxcr4), consequently increasing the ectopic localization of newborn neurons in the hilus. We also found that voluntary exercise in a running wheel suppressed this ectopic neurogenesis and countered the enhanced seizure susceptibility caused by prenatal VPA exposure, probably by normalizing the VPA-disrupted expression of multiple genes including Cxcr4 in adult NS/PCs. Replenishing Cxcr4 expression alone in NS/PCs was sufficient to overcome the aberrant migration of newborn neurons and increased seizure susceptibility in VPA-exposed mice. Thus, prenatal exposure to an AED, VPA, has a long-term effect on the behavior of NS/PCs in offspring, but this effect can be counteracted by a simple physical activity. Our findings offer a step to developing strategies for managing detrimental effects in offspring exposed to VPA in utero.
Use of antiepileptic drugs (AEDs) is usually unavoidable for pregnant epileptic women to control seizure activity but is associated with a greater risk of major congenital malformation (1) and behavioral disorder in the offspring (2). Thus, the risk associated with the treatment is a major concern to women of childbearing age suffering from epilepsy. The AED valproic acid (VPA) is used worldwide and reportedly prescribed to more than 20% of pregnant women afflicted with epilepsy (1, 3). Accumulating epidemiological studies indicate that prenatal VPA exposure increases the risk of congenital malformations (4) and can have a long-lasting effect on brain function of children born to epileptic mothers (5, 6). Maternal use of VPA during pregnancy correlates with a significantly increased risk of autism spectrum disorder (7) and attention-deficit/hyperactivity disorder (8) in the offspring, both of which are known to often coincide with seizure disorders including epilepsy (9, 10). Further investigation is therefore warranted to reveal the basis of the correlation between maternal use of VPA and increased susceptibility to seizure in the offspring.
The adult mammalian brain retains neural stem/progenitor cells (NS/PCs) in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), and these NS/PCs continuously generate new neurons throughout life (11). Newborn neurons migrate, settle in the granular cell layer (GCL), and eventually become mature granular cells (GCs). Accurate migration of newborn neurons in the adult DG is critical for physiological hippocampal functions, and thus their mislocalization often leads to neuronal dysfunction through the formation of abnormal neuronal circuits (12). In this context, GCs ectopically located in the dentate hilus have been shown to be more excitable than those located normally in the GCL (13, 14); such ectopic neurons are frequently observed in animal models as well as in patients with temporal lobe epilepsy, which is the most common form of epilepsy in adults (15–17).
Previous reports have identified several genes (18–20), including CXC motif chemokine receptor 4 (Cxcr4) (21), as being responsible for the appropriate localization of newborn neurons in the adult hippocampal DG. Cxcr4 is a G protein-coupled receptor whose ligand is the chemoattractant Cxcl12 (22). In the adult hippocampal DG, Cxcr4 is expressed in NS/PCs and immature neurons, whereas Cxcl12 is expressed in GCs (23, 24).
In this study, we investigated the correlation between maternal use of VPA and increased seizure susceptibility in the offspring using a mouse model. Mechanistically, prenatal VPA exposure changed the property of adult hippocampal NS/PCs at the transcriptome level, for example by downregulating Cxcr4, and consequently increased the ectopic localization of newborn neurons in the hilus. Furthermore, we found that these adverse effects of embryonic VPA exposure were offset by voluntary exercise in a running wheel and that increased expression of Cxcr4 in hippocampal NS/PCs alone could recapitulate the effect of the exercise.
Results
Prenatal VPA Exposure Increases Seizure Susceptibility and Ectopic Hippocampal Neurogenesis in Adult Mice.
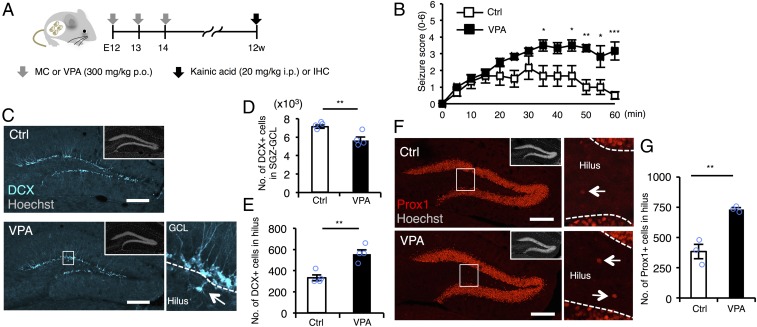
To explore the correlation between maternal use of VPA and increased susceptibility to seizure in the offspring using a mouse model, we first administered kainic acid (KA), a chemoconvulsant that activates glutamate receptors, to 12-wk-old (12w), prenatally VPA-exposed mice (VPA mice) (Fig. 1A). VPA mice developed more severe seizures than controls (Fig. 1B), indicating that prenatal VPA exposure increases seizure susceptibility in adulthood. Since pharmacological and genetic deletion of GABAergic interneurons in the hippocampus is reportedly associated with increased seizure susceptibility and epilepsy in mice (25, 26), we assessed the numbers of parvalbumin (PV)-, somatostatin (SST)-, and calretinin (CR)-positive interneurons in the hippocampus of control and VPA mice and found that they were comparable between the two groups (Fig. S1). We then focused on adult neurogenesis from NS/PCs in the SGZ of the hippocampal DG, because, as mentioned above, abnormal neuronal migration and subsequent generation of ectopic GCs (EGCs) are known to associate with epileptogenesis, in mice as well as humans. In the DG of VPA mice, the number of DCX-positive immature neurons located within the SGZ/GCL was lower than that in control mice (Fig. 1 C and D), indicating decreased adult hippocampal neurogenesis in VPA mice, in agreement with our previous study (27). Intriguingly, we discovered that the numbers of both DCX-positive immature and GC marker Prox1-positive neurons located ectopically in the hilus were increased in VPA mice (Fig. 1 C and E–G). These data imply that abnormal neuronal migration in the DG of VPA mice is associated with higher susceptibility to seizure.
Fig. 1.
Prenatal exposure to VPA increases seizure susceptibility and ectopic hippocampal neurogenesis in the adult. (A) Experimental scheme for investigating seizure susceptibility and adult neurogenesis. Control [administered with methylcellulose (MC)] and VPA mice were randomly assigned to the groups for scoring of seizure severity and immunohistochemistry (IHC). (B) Seizure response to KA treatment over time in control and VPA mice (n = 6 animals each). Two-way repeated measures ANOVA was used for statistical analysis (treatment: F1,130 = 59.08, P < 0.0001; time: F12,130 = 7.063, P < 0.0001; treatment × time interaction: F12,130 = 2.341, P = 0.0095, post hoc Bonferroni’s multiple comparison test). (C) Representative images of DCX-positive (cyan) immature neurons in the DG. The area outlined by a white rectangle in the lower main panel is enlarged to the right. The arrow indicates a DCX-labeled cell in the hilus, and dashed white lines mark the boundaries between GCL and hilus. (Insets) H33258 nuclear staining of each field. (Scale bar, 200 µm.) (D and E) Quantification of the number of DCX-positive cells in the SGZ/GCL (D) and hilus (E) (n = 4 animals each). (F) Representative images of Prox1-positive (red) GCs in the DG. The area outlined by a white rectangle in the lower main panel is enlarged to the right. The arrow indicates a Prox1-positive cell in the hilus, and the dashed white line marks the boundary between GCL and hilus. (Insets) H33258 nuclear staining (gray) of each field. (Scale bar, 200 µm.) (G) Quantification of the number of Prox1-positive cells in the hilus (n = 3 animals each). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Developmental Stage-Dependent Gene Expression Differences in NS/PCs Between Control and VPA Mice.
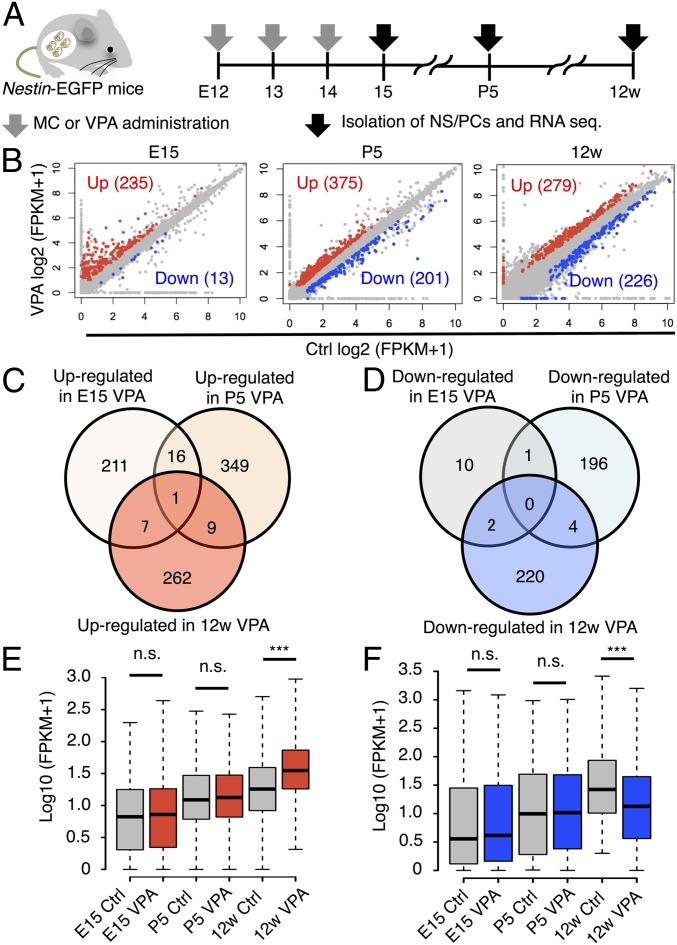
To gain a deeper insight into how prenatal VPA exposure induces aberrant and/or ectopic neurogenesis in the adult DG, we performed RNA sequencing using EGFP-positive NS/PCs isolated from Nestin-EGFP mice with or without prenatal VPA exposure. NS/PCs were isolated from forebrain (E15) to investigate the acute effect of VPA and from the DG [postnatal day (P) 5 and 12w] to explore the long-term effects of VPA on the developing (28) and mature DG (Fig. 2A). Hierarchical clustering identified three distinct clusters, each composed of NS/PCs derived from a single developmental stage (Fig. S2A), suggesting that prenatal VPA exposure did not affect gene expression in NS/PCs as strongly as it intermingles the clustering between distinct developmental stages. Therefore, we next sought to identify differentially expressed genes (DEGs), whose expression levels were significantly changed by VPA exposure within each developmental stage (q-value < 0.05, fold change ≥ 1.5). In NS/PCs derived from E15 mice, most of the DEGs were up-regulated genes (235 out of all 248 DEGs), most likely because VPA is a histone deacetylase inhibitor (29) that positively regulates gene expression by promoting histone acetylation (Fig. 2B). Furthermore, gene set enrichment analysis (GSEA) using the E15 NS/PC transcriptome revealed a significant increase in the expression of neuron differentiation- and nervous system development-related genes in VPA mice compared with control, suggesting that VPA promotes the differentiation of NS/PCs into neurons (Fig. S2B), in accordance with previous reports (27, 30).
Fig. 2.
Transcriptome analysis of NS/PCs from control and VPA mice at distinct developmental stages. (A) Schematic representation of the isolation of NS/PCs from Ctrl and VPA mice at E15, P5, and 12w. RNAs were extracted from these cells and subjected to sequence analysis. (B) Scatter plots of genes expressed in NS/PCs from Ctrl and VPA mice. Up- (red) and down-regulated (blue) DEGs are highlighted. (C and D) Venn diagrams of up- (C) and down-regulated (D) DEGs at each developmental stage. (E and F) Box plot of up- (E) and down-regulated (F) DEG expression in NS/PCs of 12w hippocampal DG. In contrast to 12w, expression levels of the DEGs are comparable between Ctrl and VPA mice at E15 and P5. ***P ≤ 0.001. n.s., not significant.
We then asked whether these gene expression changes in E15 NS/PCs induced by VPA exposure are sustained in hippocampal NS/PCs at P5 and 12w. Among up- and down-regulated DEGs at each developmental stage, only one DEG persisted throughout these stages (Fig. 2 C and D). These data indicate that prenatal VPA exposure initially triggers differential gene expression in NS/PCs, but that the identity of the DEGs changes as development progresses. In support of this, when we examined the DEGs identified in NS/PCs of 12w mice at the earlier developmental stages (E15 and P5), we observed no differences in their expression between control and VPA mice (Fig. 2 E and F).
Prenatal VPA Exposure Leads to Perturbation in the Expression of Cell Migration-Associated Genes in Adult NS/PCs.
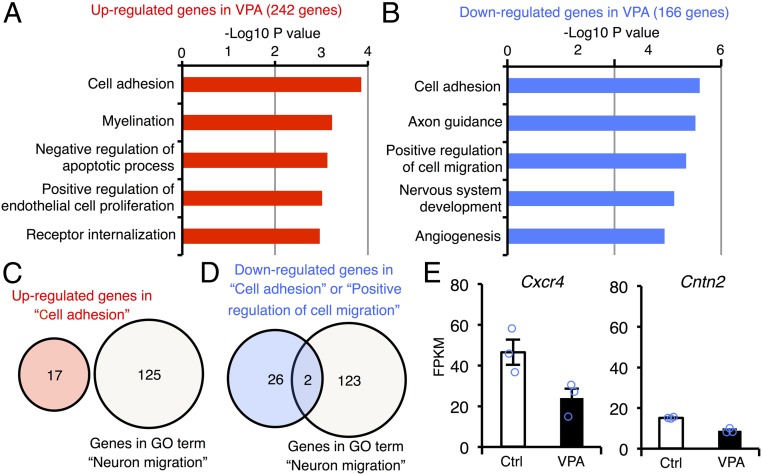
Having observed that aberrant neurogenesis occurs in 12w VPA mice, we hypothesized that genes responsible for this impairment would be among the DEGs in 12w NS/PCs. To identify such genes, we subjected DEGs in 12w NS/PCs to Gene Ontology (GO) analysis of biological processes and found that both up-regulated and down-regulated genes are significantly associated with two cell migration-related terms, “cell adhesion” and “positive regulation of cell migration” (Fig. 3 A and B). Among the genes categorized in these GO terms, we next asked whether they overlapped with genes in the GO term “neuron migration,” since we are focusing on neuronal mislocalization (Fig. 3 C and D), and identified two down-regulated genes that did so, contactin 2 (Cntn2) and Cxcr4 (Fig. 3 D and E). Cntn2 encodes one of the members of the contactin family, which are reported to modulate the migration of cortical interneurons in embryonic brain (31), although no role has hitherto been shown for Cntn2 in adult neurogenesis. In contrast, the conditional deletion of Cxcr4 in NS/PCs has been shown to induce ectopic positioning of newborn neurons in the adult hippocampus (21). Therefore, we decided to further focus on Cxcr4 as a candidate contributor to the aberrant neuronal migration and enhanced seizure sensitivity in VPA mice in the following experiments.
Fig. 3.
Prenatal VPA exposure alters the expression level of cell migration-related genes in NS/PCs of adult DG. (A and B) Functional annotation of up- (A) and down-regulated (B) genes in NS/PCs of adult VPA mice relative to control mice. The top five GO terms in each gene group are displayed. (C) Venn diagram of up-regulated genes categorized in the GO term “cell adhesion” and the gene list associated with “neuron migration.” There was no overlap between genes in each category. (D) Identification of two candidate genes for the ectopic neuronal migration in VPA mice at 12w. Down-regulated genes categorized in the GO terms “cell adhesion” or “positive regulation of cell migration” overlapped with two genes in the GO term “neuron migration.” (E) Expression levels of the two genes identified in D.
Voluntary Exercise Ameliorates Enhanced Seizure Susceptibility and Abnormal Neuronal Migration in VPA Mice by Normalizing Perturbed Gene Expression in NS/PCs.
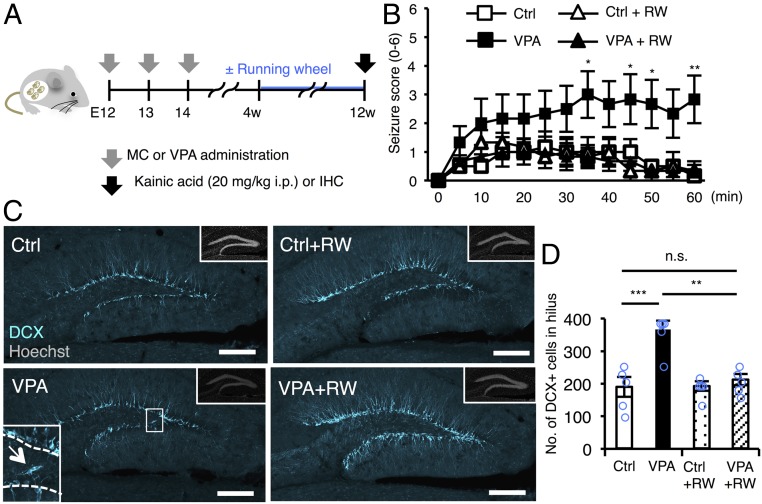
Voluntary exercise in a running wheel, a widely accepted neurogenic stimulus (32, 33), decreases seizure susceptibility in rats (34–36), although the mechanism underlying this effect is unknown. Therefore, we next evaluated the effect of this physical activity on seizure susceptibility and neuronal migration in the hippocampus in VPA mice (Fig. 4A). We found that 8 wk of voluntary running alleviated the time-dependent increase of seizure score in VPA mice to the level in control mice (Fig. 4B), coincident with decreased abnormal neuronal migration (Fig. 4 C and D).
Fig. 4.
Voluntary exercise alleviates increased seizure susceptibility and abnormal neuronal migration in VPA mice. (A) Experimental scheme for investigating the effect of voluntary running on seizure susceptibility and neuronal migration in the DG. Control (administered with MC) and VPA mice were randomly assigned to the groups for scoring of seizure severity and IHC. (B) Seizure response to KA treatment over time in control and VPA mice with or without voluntary running as indicated (n = 6 animals each). Two-way repeated measures ANOVA was used for statistical analysis (treatment: F3,240 = 3.052, P = 0.0522; time: F12,240 = 9.837, P < 0.0001; treatment × time interaction: F36,240 = 2.428, P < 0.0001, post hoc Bonferroni’s multiple comparison test for VPA vs. VPA+RW, *P ≤ 0.05, **P ≤ 0.01). (C) Representative images of DCX-positive immature neurons (cyan) in the DG. The area outlined by the white rectangle in the lower left is magnified in the inset. The arrow indicates a DCX-positive cell in the hilus, and the dashed white line marks the boundary between hilus and GCL. (Insets) H33258 nuclear staining (gray) of each field. (Scale bar, 200 µm.) (D) Quantification of the number of DCX-positive cells in the hilus (n = 5 animals each). One-way ANOVA was used for statistical analysis (F3,16 = 13.31, P < 0.0001, post hoc Tukey’s multiple comparison test, **P ≤ 0.01, ***P ≤ 0.001. n.s., not significant).
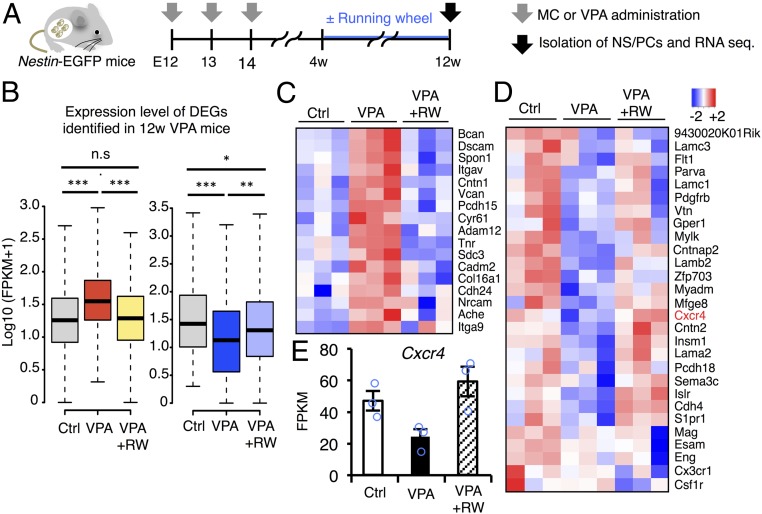
In light of these findings, we next attempted to examine the effects of voluntary running on the transcriptome profile of NS/PCs in VPA mice (Fig. 5A). To our surprise, the running mostly amended both positively and negatively distorted gene expression in the adult hippocampal NS/PCs of VPA mice (Fig. 5B). Of note, the altered expression of genes categorized as cell migration-related genes, including Cxcr4, in VPA mice was largely normalized by the physical activity (Fig. 5 C–E).
Fig. 5.
Voluntary running normalizes transcriptomic alteration of adult NS/PCs in VPA mice. (A) Schematic representation of the isolation of NS/PCs from Ctrl and VPA mice at 12w. RNAs were extracted from these cells and subjected to sequence analysis. (B) Box plots of up- (Left) and down-regulated (Right) DEG expression in NS/PCs of 12w hippocampal DG. Changes of gene expression, in both directions, after VPA exposure were largely normalized by running (RW); *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, n.s indicates not significant. (C) Heat map showing the expression level of up-regulated DEGs in adult VPA mice categorized in the GO term “cell adhesion” (Fig. 2 B and D) in control, VPA, and VPA+RW mice. (D) Heat map indicating the expression level of down-regulated genes in the adult VPA mice categorized in GO term “cell adhesion” and “positive regulation of cell migration” (Fig. 2 C and E) in control, VPA, and VPA+RW mice. (E) Expression level of Cxcr4 in NS/PCs of 12w control, VPA, and VPA+RW mice.
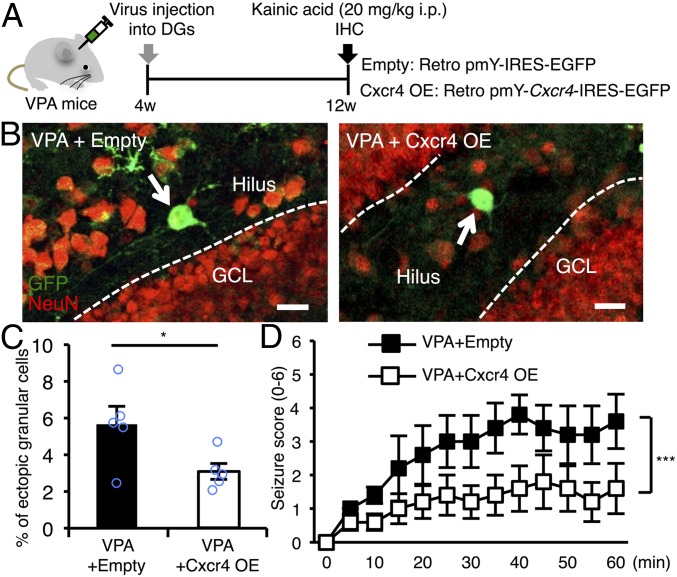
Having observed that running suppressed abnormal neuronal migration and decreased seizure susceptibility and, even more interestingly, normalized the reduced expression of Cxcr4 in VPA mice, we wanted to examine whether expression of Cxcr4 alone in NS/PCs is capable of overcoming these prenatal VPA exposure-induced impairments. To do so, we used a retrovirus expressing Cxcr4 together with GFP to selectively transduce proliferating NS/PCs in the DG. Before performing in vivo experiments, we first confirmed that infection by the Cxcr4-expressing virus can transduce NS/PCs to express Cxcr4 protein in vitro and found that it did so (Fig. S3 A and B). Since control and VPA mice at 4w displayed no difference yet in hippocampal neurogenesis or seizure susceptibility (Fig. S4), we infected hippocampal NS/PCs of VPA mice with control and Cxcr4-expressing retroviruses at this stage to examine the effect of Cxcr4 expression 8 wk later (Fig. 6A). In the DG of these mice, newly generated mature neurons from NS/PCs were labeled with GFP and NeuN (a mature neuron marker) at 12w, 8 wk after the viral injection (Fig. 6A and Fig. S3C). We found that restoration of Cxcr4 expression reduced the mislocalization of GFP- and NeuN-positive newly generated neurons (Fig. 6 B and C). Although there was no significant interaction with time course, Cxcr4 overexpression significantly decreased the seizure score induced by KA injection (Fig. 6D). These results indicate that replenishment of the reduced Cxcr4 expression in NS/PCs of VPA mice is effective enough to prevent the aberrant neuronal migration in the DG and to counteract the aggravated seizure susceptibility caused by prenatal VPA exposure.
Fig. 6.
Replenishment of Cxcr4 expression in NS/PCs of the DG alleviates the increased seizure susceptibility and abnormal neuronal migration in VPA mice. (A) Experimental scheme for investigating the effect of Cxcr4 expression in NS/PCs on seizure susceptibility and neuronal migration in VPA mice. (B) Representative images of GFP (green) and NeuN (red) dual-positive (GFP+NeuN+) newborn neurons located in the hilus (arrows). Dashed white lines indicate the boundary between hilus and GCL. (Scale bar, 20 µm.) (C) Quantification of percentages of the number of ectopically located GFP+NeuN+ cells among total GFP+NeuN+ cells in the DG (n = 5 animals each). (D) Seizure response to KA treatment over time in VPA mice that received control and Cxcr4-expressing retrovirus injection (n = 5 animals each). Two-way repeated measures ANOVA was used for statistical analysis (Cxcr4: F1,104 = 33.14, P < 0.0001; time: F12,104 = 3.344, P = 0.0004; Cxcr4 × time interaction: F12,104 = 0.5549, P = 0.8731). *P ≤ 0.05, ***P ≤ 0.001.
Discussion
Accumulating evidence indicates that environmental insults including exposure to medical drugs in utero have long-lasting effects on brain function of the offspring (37, 38). In the present study, we have shown that prenatal VPA exposure impairs neuronal migration in the adult DG through the decreased expression of Cxcr4 in NS/PCs and consequently increases seizure susceptibility, whereas voluntary running overcomes these adverse effects (Fig. S5). Consistent with our findings, a previous study has proposed that the deletion of a chromosome region including the Cxcr4 locus is strongly linked to a seizure disorder (39). However, further clinical research is needed to determine whether Cxcr4 deficiency and/or mutations are indeed bona fide risk factors for epilepsy.
Since VPA induces changes in the expression of many genes as an epigenetic drug (40), we initially assumed that the altered expression of numerous genes in NS/PCs induced by prenatal VPA exposure would be sustained until adulthood. However, that was not the case: Only one DEG at the embryonic stage (E15) persisted until the adult stage, even though there existed hundreds of DEGs in NS/PCs between control and VPA mice at each developmental stage (E15, P5, and 12w). These findings imply that transient transcriptome changes in NS/PCs in response to VPA exposure at the embryonic stage led directly or indirectly to the alteration of gene expression at later stages, eventually causing neurological defects in adulthood. Although the mechanisms underlying the alterations in the adult NS/PC transcriptome, including reduced Cxcr4 expression in VPA mice, have yet to be elucidated, future studies such as analyzing the epigenetic profile of NS/PCs in mice treated with VPA during development should help to reveal them.
In addition to its effects on NS/PCs as shown in this study, VPA also influences the behavior of other cell types in the CNS. Previous reports have revealed that VPA suppresses inhibitory synaptic formation by repressing the expression of a vesicular GABA transporter and glutamate decarboxylases in neurons (41, 42). GABA, the principal inhibitory neurotransmitter, provides a counterbalance to neuronal excitation. If the balance tips toward excitation, it induces seizure. VPA is also thought to affect the synaptic excitatory/inhibitory balance indirectly by changing gene expression in astrocytes (43). Based on these findings, we cannot completely exclude the possibility that prenatal VPA exposure increases seizure susceptibility by altering the behavior of cells other than NS/PCs. Nevertheless, our observation that replenishing Cxcr4 expression alone in NS/PCs was sufficient to overcome the aberrant migration of newborn neurons and the increased seizure sensitivity in VPA mice clearly demonstrates that VPA-increased seizure susceptibility is attributable to the dysfunction of NS/PCs.
Since GCs newly generated from NS/PCs are reported to become fully mature within 8 wk (44), we injected Cxcr4-expressing retroviruses into the DG of 4-wk-old VPA mice and analyzed them 8 wk later. We found that seizure susceptibility and ectopic neurogenesis were suppressed in these mice. Because retroviruses mainly infect proliferating NPCs rather than long-term dividing NSCs (44), we concluded that Cxcr4 replenishment in NPCs was responsible for the proper migration of their progeny to the GCL, thereby counteracting VPA-enhanced seizure sensitivity; however, we cannot currently explain why only one injection of Cxcr4-expressing retroviruses into 4-wk-old mice was sufficient to overcome the detrimental effect of prenatal VPA exposure. It is possible that some long-term dividing NSCs were also infected by the retroviruses, thus perhaps continuously suppressing mislocalization of newly generated neurons.
Ectopic neurogenesis in the hippocampal DG is observed in epileptic patients (17). In addition, physical exercise is known to have therapeutic effects on patients with epilepsy (45, 46). However, the functional link between these processes and the underlying mechanisms has yet to be established. We have shown here that voluntary exercise normalizes the expression of cell migration-associated genes in adult NS/PCs, leading to decreased seizure susceptibility in VPA mice through restoration of abnormal neuronal migration. Cognitive deficiency is also associated with epilepsy (47). In this regard, we have reported previously that voluntary exercise improves hippocampal cognitive function in VPA mice (27). In agreement with these findings, ablation of aberrant neurogenesis in the adult mouse hippocampus is known to suppress cognitive decline and chronic seizure frequency (15), suggesting that recurrent seizure as well as memory deficits can be prevented by precisely controlling seizure-induced aberrant neurogenesis.
Our results in this study indicate that prenatal environmental insults such as exposure to AEDs induce long-lasting impairments in NS/PC behavior and lead to deficiencies in brain activity in the offspring, but also that these adverse effects can be reversed by a simple physical activity. Our findings should pave the way for therapeutic strategies to treat offspring who have experienced an unfavorable intrauterine environment including exposure to medical drugs.
Methods
No statistical methods were used to predetermine sample sizes, but our sample sizes correspond to those reported in previous publications (48). Statistical analysis was performed using GraphPad Prism software (GraphPad Software). Data distribution was assumed to be normal but this was not formally tested. Statistical analysis was done using unpaired t tests and one-way ANOVA with post hoc analysis using Tukey’s multiple comparison test. Data from the 1-h trial of KA treatment were analyzed by two-way repeated-measures ANOVA, and post hoc analysis was done using Bonferroni’s multiple comparison test. Data are presented as mean ± SEM. Results were considered significant when P ≤ 0.05.
All aspects of animal care and treatment were carried out according to the guidelines of the Experimental Animal Care Committee of Kyushu University.
Additional information is provided in SI Methods.
Supplementary Material
Acknowledgments
We appreciate the technical assistance from the Research Support Center, Kyushu University Graduate School of Medical Sciences. We thank Y. Ohkawa for performing RNA sequencing and N. Hamazaki, H. Noguchi, S. Katada, and T. Imamura for discussions. This study is supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research) from the Japan Agency for Medical Research and Development, grants from the “FUKUOKA” OBGYN Researcher’s Charity Foundation Fund, The Clinical Research Promotion Foundation, and Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid for Scientific Research (KAKENHI) Grants 16H06527 and 17H01390 (to K.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE101239).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716479115/-/DCSupplemental.
References
- 1.Tomson T, et al. EURAP study group Dose-dependent risk of malformations with antiepileptic drugs: An analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 2.Dean JC, et al. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002;39:251–259. doi: 10.1136/jmg.39.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viinikainen K, Heinonen S, Eriksson K, Kälviäinen R. Community-based, prospective, controlled study of obstetric and neonatal outcome of 179 pregnancies in women with epilepsy. Epilepsia. 2006;47:186–192. doi: 10.1111/j.1528-1167.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 4.Jentink J, et al. EUROCAT Antiepileptic Study Working Group Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185–2193. doi: 10.1056/NEJMoa0907328. [DOI] [PubMed] [Google Scholar]
- 5.Meador KJ, et al. NEAD Study Group Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meador KJ, et al. NEAD Study Group Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): A prospective observational study. Lancet Neurol. 2013;12:244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen J, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MJ, et al. NEAD Study Group Fetal antiepileptic drug exposure: Adaptive and emotional/behavioral functioning at age 6 years. Epilepsy Behav. 2013;29:308–315. doi: 10.1016/j.yebeh.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuchman R, Cuccaro M, Alessandri M. Autism and epilepsy: Historical perspective. Brain Dev. 2010;32:709–718. doi: 10.1016/j.braindev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Davis SM, et al. Epilepsy in children with attention-deficit/hyperactivity disorder. Pediatr Neurol. 2010;42:325–330. doi: 10.1016/j.pediatrneurol.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012;53:109–115. doi: 10.1111/j.1528-1167.2012.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan RZ, Timofeeva O, Nadler JV. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol. 2010;104:3293–3304. doi: 10.1152/jn.00663.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102:670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho KO, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33:8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 18.Jessberger S, et al. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korn MJ, Mandle QJ, Parent JM. Conditional disabled-1 deletion in mice alters hippocampal neurogenesis and reduces seizure threshold. Front Neurosci. 2016;10:63. doi: 10.3389/fnins.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultheiß C, et al. CXCR4 prevents dispersion of granule neuron precursors in the adult dentate gyrus. Hippocampus. 2013;23:1345–1358. doi: 10.1002/hipo.22180. [DOI] [PubMed] [Google Scholar]
- 22.Bagri A, et al. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- 23.Berger O, Li G, Han SM, Paredes M, Pleasure SJ. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. doi: 10.1159/000096210. [DOI] [PubMed] [Google Scholar]
- 24.Kolodziej A, et al. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobos I, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 26.Zipancic I, Calcagnotto ME, Piquer-Gil M, Mello LE, Alvarez-Dolado M. Transplant of GABAergic precursors restores hippocampal inhibitory function in a mouse model of seizure susceptibility. Cell Transplant. 2010;19:549–564. doi: 10.3727/096368910X491383. [DOI] [PubMed] [Google Scholar]
- 27.Juliandi B, et al. Reduced adult hippocampal neurogenesis and cognitive impairments following prenatal treatment of the antiepileptic drug valproic acid. Stem Cell Reports. 2015;5:996–1009. doi: 10.1016/j.stemcr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi H, et al. DNA methyltransferase 1 is indispensable for development of the hippocampal dentate gyrus. J Neurosci. 2016;36:6050–6068. doi: 10.1523/JNEUROSCI.0512-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denaxa M, Chan CH, Schachner M, Parnavelas JG, Karagogeos D. The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development. 2001;128:4635–4644. doi: 10.1242/dev.128.22.4635. [DOI] [PubMed] [Google Scholar]
- 32.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 34.Arida RM, Scorza FA, dos Santos NF, Peres CA, Cavalheiro EA. Effect of physical exercise on seizure occurrence in a model of temporal lobe epilepsy in rats. Epilepsy Res. 1999;37:45–52. doi: 10.1016/s0920-1211(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 35.Setkowicz Z, Mazur A. Physical training decreases susceptibility to subsequent pilocarpine-induced seizures in the rat. Epilepsy Res. 2006;71:142–148. doi: 10.1016/j.eplepsyres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Holmes PV, Reiss JI, Murray PS, Dishman RK, Spradley JM. Chronic exercise dampens hippocampal glutamate overflow induced by kainic acid in rats. Behav Brain Res. 2015;284:19–23. doi: 10.1016/j.bbr.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Heindel JJ, Skalla LA, Joubert BR, Dilworth CH, Gray KA. Review of developmental origins of health and disease publications in environmental epidemiology. Reprod Toxicol. 2017;68:34–48. doi: 10.1016/j.reprotox.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Yin P, et al. Maternal immune activation increases seizure susceptibility in juvenile rat offspring. Epilepsy Behav. 2015;47:93–97. doi: 10.1016/j.yebeh.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Michaud JL, et al. The genetic landscape of infantile spasms. Hum Mol Genet. 2014;23:4846–4858. doi: 10.1093/hmg/ddu199. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, et al. PI3K/AKT/mTOR signaling mediates valproic acid-induced neuronal differentiation of neural stem cells through epigenetic modifications. Stem Cell Reports. 2017;8:1256–1269. doi: 10.1016/j.stemcr.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuchi M, et al. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res. 2009;65:35–43. doi: 10.1016/j.neures.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Kumamaru E, Egashira Y, Takenaka R, Takamori S. Valproic acid selectively suppresses the formation of inhibitory synapses in cultured cortical neurons. Neurosci Lett. 2014;569:142–147. doi: 10.1016/j.neulet.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 43.Wang CC, et al. Valproic acid mediates the synaptic excitatory/inhibitory balance through astrocytes–A preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:111–120. doi: 10.1016/j.pnpbp.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arida RM, de Almeida AC, Cavalheiro EA, Scorza FA. Experimental and clinical findings from physical exercise as complementary therapy for epilepsy. Epilepsy Behav. 2013;26:273–278. doi: 10.1016/j.yebeh.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Pimentel J, Tojal R, Morgado J. Epilepsy and physical exercise. Seizure. 2015;25:87–94. doi: 10.1016/j.seizure.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Lhatoo SD, Sander JW. The epidemiology of epilepsy and learning disability. Epilepsia. 2001;42:6–9, discussion 19–20. doi: 10.1046/j.1528-1157.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda T, et al. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun. 2015;6:6514. doi: 10.1038/ncomms7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
- 50.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura T, et al. Retrovirus-mediated gene transfer and expression cloning: Powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- 52.Fischer J, et al. Prospective isolation of adult neural stem cells from the mouse subependymal zone. Nat Protoc. 2011;6:1981–1989. doi: 10.1038/nprot.2011.412. [DOI] [PubMed] [Google Scholar]
- 53.Walker TL, Kempermann G. 2014. One mouse, two cultures: Isolation and culture of adult neural stem cells from the two neurogenic zones of individual mice. J Vis Exp, e51225.
- 54.Patel RK, Jain M. NGS QC toolkit: A toolkit for quality control of next generation sequencing data. PLoS One. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.