Significance
Borrelia burgdorferi is one of the few extracellular pathogens capable of establishing persistent infection. We show that a spirochete surface protein of unknown function, BBA57, is an unorthodox regulator of multiple virulent determinants. The protein orchestrates unique host immune evasion strategies crucial for early spirochete infection in mammals. It suppresses host complement-mediated killing and neutrophil-derived microbicidal responses, including induction of antimicrobial peptides, and promotes pathogen dissemination by regulating type I interferon. In the absence of BBA57, spirochetes can undergo nonheritable transcriptional reprograming events ultimately favoring pathogen persistence. These studies highlight the evolution of plasticity in immune evasion strategies of atypical pathogens like B. burgdorferi, which is remarkably adapted to persist in multiple hosts and on a long-term basis without host clearance.
Keywords: Borrelia burgdorferi, Lyme disease, immune evasion, microbial persistence, antimicrobial peptide
Abstract
Borrelia burgdorferi is one of the few extracellular pathogens capable of establishing persistent infection in mammals. The mechanisms that sustain long-term survival of this bacterium are largely unknown. Here we report a unique innate immune evasion strategy of B. burgdorferi, orchestrated by a surface protein annotated as BBA57, through its modulation of multiple spirochete virulent determinants. BBA57 function is critical for early infection but largely redundant for later stages of spirochetal persistence, either in mammals or in ticks. The protein influences host IFN responses as well as suppresses multiple host microbicidal activities involving serum complement, neutrophils, and antimicrobial peptides. We also discovered a remarkable plasticity in BBA57-mediated spirochete immune evasion strategy because its loss, although resulting in near clearance of pathogens at the inoculum site, triggers nonheritable adaptive changes that exclude detectable nucleotide alterations in the genome but incorporate transcriptional reprograming events. Understanding the malleability in spirochetal immune evasion mechanisms that ensures their host persistence is critical for the development of novel therapeutic and preventive approaches to combat long-term infections like Lyme borreliosis.
Although ∼1,400 species of diverse pathogens can infect humans, only a small number are capable of establishing persistent infection (1). One such example is the bacterial agent of Lyme disease, Borrelia burgdorferi, which can establish long-term infection in several mammalian and avian species, including those important for its zoonotic life cycle as well as accidental hosts such as humans. Remarkably, the spirochetes are capable of survival in a host even after antibiotic therapy (2–4). Despite the presence of elaborate host-derived immune surveillance mechanisms and potent microbicidal responses, these pathogens can evade host immunity utilizing different mechanisms, such as intragenic recombination via vlsE locus, inhibition of complement, evasion of phagocytosis, or by hijacking compounds in tick saliva (5–8). Those mechanisms are of high importance for survival of a pathogen like B. burgdorferi, which has an exclusively extracellular lifestyle compared with most other persistent pathogens that hide from host immune responses either by adopting an intracellular lifestyle or residing between extracellular and intracellular locations (9). Notably, B. burgdorferi does not resort to common adaptive events because it shows low overall recombination rate except for vlsE cassettes, as well as horizontal gene transfer events restricted to specific loci, and therefore is most likely unable to quickly acquire or alter genomic sequences that are important for evolution in many other persistent pathogens (10).
Lyme disease is now considered the most prevalent tick-borne illness in many parts of the globe, with at least 300,000 estimated new cases reported in the United States alone every year. The microbe thrives in an infection cycle involving diverse hosts, such as mammals, usually wild rodents, and an arthropod vector, Ixodes ticks (11). When infected ticks engorge on mammals, B. burgdorferi can be transmitted to the dermis and subsequently disseminate to various organs, including the heart, joints, and nervous system, where the spirochetes can evoke an inflammatory response leading to clinical complications. Although many aspects of spirochete infectivity are well studied, many questions remain, particularly regarding mechanisms that dictate the remarkable ability of the pathogen to adapt and persist in diverse hosts. This is partly due to the fact that the B. burgdorferi genome encodes for a vast majority of proteins of unknown functions, which are likely relevant to the unique biology of spirochetes and its infectious capacity (11). Among them, vlsE locus, which encodes an elaborate antigenic variation system in B. burgdorferi (6), has been well studied. The system assists in microbial evasion of host immunity through both random, segmental gene conversion as well as framework heterogeneity (12, 13). Despite possessing a highly unstable genome that encodes many pseudogenes and gene products of redundant functions, B. burgdorferi has evolved to subvert not only the innate but also the adaptive immune system of the host, ensuring their successful and long-term survival in an enzootic cycle (6, 7, 14–16). Gene manipulation studies have identified a series of spirochete proteins required for infection in mammalian hosts or tick vectors (17–20). In many cases, the mechanisms are based on interactions of spirochete proteins with host extracellular matrix proteins, for example, decorin binding protein A and B with decorin (21, 22); BBK32 (23), complement regulator-acquiring surface protein (CRASP)-1, RevA/RevB, and BB0347 with fibronectin (24); BBA33 and CRASP-1 with collagen (25, 26); BmpA with laminin (27); and Erp (28), Enolase (29–31), and OspC with plasminogen (14). Mutagenesis studies involving OspC further identified three key residues within its ligand binding domain that interacts with additional unidentified mammalian ligands (32). In addition to the ability of B. burgdorferi to inhibit the host complement system or regulatory molecules like factor H through specific binding via a series of CRASP proteins as well as OspC, further studies are required to fully understand how the Lyme disease pathogens evade the host innate immunity (8, 19, 25, 27, 33, 34).
Our recent studies identified a B. burgdorferi surface lipoprotein, BBA57, as a novel virulence determinant that supports pathogen survival during early mammalian infection while triggering inflammation at the late disseminated phase of infection (17). The protein is highly conserved among the B. burgdorferi sensu lato group with 60–70% identity between different species. As shown by database searches, other than possession of an actin-modulating domain (35), BBA57 lacks homology to proteins of known function; therefore, precisely how BBA57 supports spirochete infectivity remains undetermined. Here we show that BBA57 facilitates early murine infection by regulating a series of key microbial antigens that impair host complement and neutrophil-derived antimicrobial defense mechanisms, including the suppression of antimicrobial peptides, such as bactericidal/permeability-increasing protein (BPI). Our results underscore that BBA57 function is critical in supporting spirochete survival during early infection, facilitating its subsequence persistence in the host. Our results also suggest a unique plasticity of B. burgdorferi immune evasion strategies as loss of BBA57 triggers a nonheritable transcriptional/antigenic change(s) that allows BBA57-deficient mutants to compensate for gene loss during the later disseminated phase of infection. A better understanding of borrelial mediators of resistance to host innate responses and epigenetic adaptive mechanisms that dictate long-term host persistence will have far-reaching therapeutic and preventive implications for the control of infections like Lyme borreliosis.
Results
BBA57 Is Nonessential for Survival Within the Tick but Required for Mammalian Transmission.
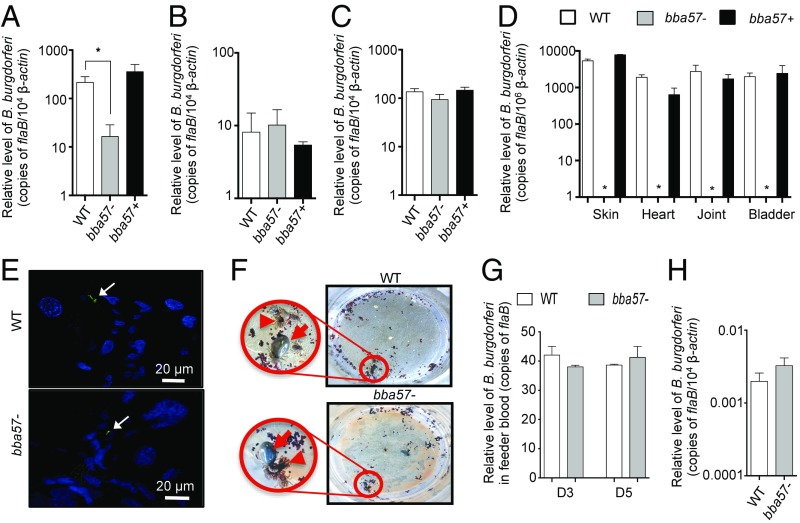
Since bba57 is highly expressed in the vector, we sought to assess its role in supporting B. burgdorferi life cycle in the tick, especially during pathogen entry, persistence, and transmission. To accomplish this, larval ticks were allowed to parasitize mice that had been infected by needle inoculation with the wild-type, bba57 mutant, or complemented isolate for 21 d, when the level of bacteria in mouse skin is similar between each group (17). The spirochete burden was then assessed by RT-qPCR analysis in ticks (36). Compared with wild-type and the complemented strain, the level of bba57 mutants was significantly lower in fully engorged larval ticks (Fig. 1A). However, analysis of newly molted unfed nymphs (Fig. 1B), following an intermolt period of 4 wk, showed similar burdens of bba57 mutant spirochetes, compared with wild-type and complemented strain. Collectively, these data suggest that BBA57 supports B. burgdorferi acquisition by ticks during their early survival in the vector but without a significant influence on long-term persistence. We then compared the fitness of the bba57 mutants during transmission from infected ticks to naive mice. Separate groups of molted infected nymphs were allowed to engorge on naive C3H/HeN mice. The RT-qPCR analysis showed comparable levels of mutants and wild-type isolates in engorged ticks, suggesting that BBA57 is not essential for spirochete persistence in fed ticks (Fig. 1C). However, 14 d after tick engorgement, bba57 mutant B. burgdorferi remained undetectable either by RT-qPCR (Fig. 1D) or by culture (SI Appendix, Table S1) in multiple tissues of mice, indicating that BBA57 is essential for the establishment of mammalian infection when transmitted via tick feeding.
Fig. 1.
BBA57 is required for mammalian infection but redundant for tick persistence. (A–C) Acquisition and persistence of bba57 mutants in ticks. Larvae were allowed to parasitize mice infected with wild-type B. burgdorferi (WT, white bars), bba57− mutant (bba57−, gray bars), or bba57 complemented isolates (bba57+, black bars). The burden in fed larvae (A), in freshly molted and unfed nymphs (B), and in infected nymphs fed on naive mice (C) was assessed by RT-qPCR. (D) B. burgdorferi burden in mice after transmission via infected nymphs. Mice were parasitized with nymphs infected with WT (white bars), bba57− (gray bars), or bba57+ (black bars). Infection was assessed by RT-qPCR analysis of pathogen burden in skin, joint, heart, and bladder samples obtained 2 wk after infection. Asterisk, not detectable. (E) B. burgdorferi migration to tick salivary glands. Salivary glands were isolated from wild-type or bba57− mutant–infected ticks, as shown in C, which parasitized naive mice for 3 d. Samples were fixed and stained with FITC-labeled anti-B. burgdorferi goat IgG and DAPI. (F) Tick engorgement on an artificial membrane feeding system. Top view of the capsules showing partly (arrowhead) or fully (arrow) engorged nymphs infected with either wild type (WT, Top) or mutants (bba57−, Bottom). Fed ticks were collected after complete feeding. (G) Assessment of B. burgdorferi exodus from ticks, during dissemination from infected ticks to feeder chamber containing naive blood. After tick engorgement, blood samples were collected and processed to analyze the levels of WT (white bars) and bba57− mutant (gray bars) by RT-qPCR. (H) Assessment of B. burgdorferi entry in naive ticks, from feeder chamber containing spirochete-infected blood (103 cells per mL). Ticks were collected after engorgement and assessed for burden of WT (white bars) or bba57− (gray bars) mutants by RT-qPCR targeting B. burgdorferi flaB gene. Bars represent the mean ± SEM of at least triplicate experiments. Data in A–H are representative of at least three independent experiments. *P < 0.05.
The inability of bba57 mutants to establish infection in the murine host could be due to an impaired migration through tick tissues. However, the mutants were readily detected in the salivary glands (Fig. 1E). To more conclusively prove that BBA57 is not required for spirochete dissemination within the tick, we adopted an in vitro membrane feeding system that omits mammalian host contact and studied spirochete exit from the vector during a blood meal intake from a membrane feeder (Fig. 1F). Based on the published protocol for artificial feeding of ticks (37), we used bovine blood for the assay. When infected ticks were allowed to engorge on the membrane feeder, equal levels of wild-type and bba57 mutants were detectable in the underlying chamber, confirming a nonessential role of BBA57 during spirochete exit from ticks (Fig. 1G). We also compared the acquisition of wild-type and bba57 mutants by naive ticks from spirochete-containing blood, using the artificial feeding system (Fig. 1H). Unlike direct host-derived blood engorgement (Fig. 1A), no deficiency was observed for the bba57 mutant entry into ticks, which could be due to lack of active components present in the blood meal acquired from a live host, such as complement. Together, these data suggest that the observed acquisition defect exhibited by the bba57 mutant through natural mouse feeding is due to specific events only linked to the vertebrate host. All together, these data establish that BBA57 function is nonessential for B. burgdorferi persistence in the vector, but the protein is required for B. burgdorferi early infection in mammals.
The bba57 Locus Facilitates Early Spirochete Infection in Skin Through Persistence Mechanisms That Are Adaptive and Nonheritable.
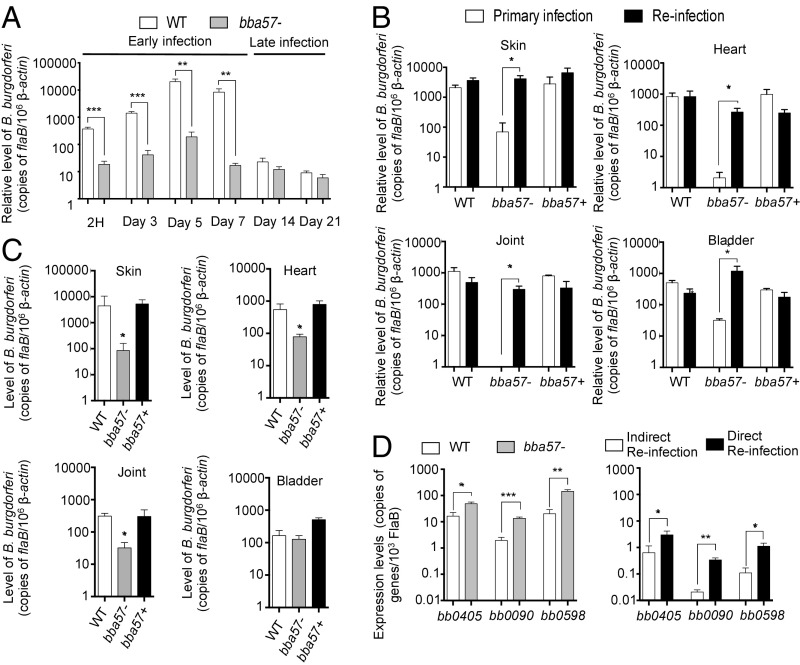
We have recently reported that B. burgdorferi surface protein BBA57 facilitates spirochete infection in the mammalian host (17). To test whether the protein is required for spirochete persistence at the dermal inoculum site, we injected an equal number of wild-type or bba57 mutants to mice and analyzed the pathogen burden at different time points. Analysis by RT-qPCR showed that wild-type B. burgdorferi persisted at high levels at the deposition site for a period of a week, followed by a decline 2 wk after infection and the maintenance of the bacterial levels up to 21 d (Fig. 2A). In contrast, spirochetes deficient for BBA57 suffered a dramatic reduction within the first 2 h after injection, which was sustained and remained significantly lower than their wild-type counterparts for a period of a week, although their levels were maintained and persisted at days 14 and 21 to a similar extent like wild-type spirochetes (Fig. 2A). These data suggested that BBA57 function is critical in the first several hours after the deposition of the bacteria in the skin and that its absence renders the spirochetes less able to establish an optimal initial infection.
Fig. 2.
BBA57 facilitates B. burgdorferi survival during early mammalian infection, whereas its absence uncovers bacteria adaptation strategy improving its persistence. (A) The B. burgdorferi burden at the dermal injection site. Mice were infected with wild-type B. burgdorferi (WT, white bars) or bba57− mutant (bba57−, gray bars). The skin injection site was collected at 2 h and days 3, 5, 7, 14, and 21 to analyze the bacteria burden by RT-qPCR. (B) B. burgdorferi burden in disseminated tissues after inoculation via a direct, host-to-host, reinfection strategy. Mice were infected with WT, bba57−, or bba57 complemented (bba57+) isolates. At 14 d postinfection, the pathogen burden in disseminated organs was assessed by RT-qPCR (primary infection, white bars) in disseminated organs. A group of mice was euthanized at 21 d of infection, and B. burgdorferi retrieved from the bladder were used to infect new sets of naive mice (reinfection, black bars). The spirochete burden was analyzed 14 d after infection in reinfected mice. (C) B. burgdorferi burden in disseminated tissues after indirect, culture-to-host reinfection strategy. Mice were infected with various spirochete isolates as shown in B with the exception of culturing isolated spirochetes in BSK media between primary and reinfection. (D) B. burgdorferi gene expression during persistence in the mammalian host. Out of 114 B. burgdorferi genes tested for expression, a set of limited genes (bb0405, bb0090, and bb0598) were differently expressed at 21 d between WT (white bars) and bba57− mutants (gray bars), as examined by RT-qPCR (Left) in the distant skin sites during primary infection. The same panel of genes expressed by bba57− mutant B. burgdorferi in the distant skin sites at 14 d between indirect reinfection (white bars) and directs reinfection (black bars) as analyzed by RT-qPCR (Right). Bars represent the mean ± SEM of at least triplicates. Data in A–D are representative of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Because bba57 mutants were able to rebound in disseminated organs to wild-type levels at later phases of infection (17), we investigated the adaptive changes in spirochetes that would allow their persistence in the host. To assess whether the bba57 mutant adaptation to the host was mediated through a genetic/epigenetic or nonheritable mechanism, we designed a reinfection experiment. Mice were infected with either the wild-type, bba57 mutants, or bba57 complemented isolates (primary infection). At 21 d of infection, when bba57 mutants were able to rebound to wild-type levels in disseminated organs (17), mice were euthanized, and infected bladder tissues were directly transferred to new sets of naive mice (reinfection). Mice from reinfection inoculum were euthanized at 14 d of infection, and dissemination to target organs was analyzed by RT-qPCR (Fig. 2B) and culture (SI Appendix, Table S1). Notably, although the levels of wild-type or complemented isolates did not significantly differ between primary and reinfection events, the bba57 mutant burden was remarkably increased during reinfection compared with the initial infection and was indistinguishable from those found when infecting with wild-type and bba57-complemented isolates. The host-specific adaptation shown in the absence of bba57 could not be genetically acquired because culturing B. burgdorferi between the primary infection and reinfection abolished the ability of the bba57 mutants to reach comparable levels than wild-type or complemented bacteria during reinfection in all organs tested except the bladder (Fig. 2C). Furthermore, whole genome next-generation Illumina sequencing of the bba57 mutants used for primary infection (original clones before host infection) as well as adapted clones (following 14 d of reinfection) confirmed the absence of any discernible genetic differences between two series of bba57 mutants, other than the expected changes at the vlsE1 region of plasmid lp28-1, as well as poor mapping and low read coverage due to the location at the end of that plasmid (SI Appendix, Fig. S1). However, despite being able to persist in reinfected hosts at similar levels to the wild-type or complemented isolate, bba57 mutants did not induce joint inflammation, because no obvious signs of ankle swelling were evident (SI Appendix, Fig. S2A). Moreover, bba57 mutants remained defective in their ability to survive in feeding ticks during their acquisition from reinfected hosts (SI Appendix, Fig. S2B) similar to the original, nonadapted mutants. We then investigated potential alterations in the B. burgdorferi transcriptome using skin samples collected during primary infection after 21 d (Fig. 2D, Left), once bba57 mutants are adapted, as well as after 14 d during reinfection (Fig. 2D, Right). Because transcriptome analysis by RNA sequencing is not possible due to extremely low spirochete burden in host tissues, we used a RT-qPCR-based assessment of infected host cDNAs targeting 114 B. burgdorferi genes (SI Appendix, Table S2). These genes were selected due to their known expression profile in vivo, potential extracellular exposure, and/or existence as outer membrane proteins with greater potentials for involvement in host–pathogen interaction (18). Analysis of RNA samples from adapted mutants showed a conserved set of differentially expressed genes. Notably, unlike cultured spirochetes grown in vitro (SI Appendix, Fig. S2C), expression of bb0405, bb0090, and bb0598 was found specifically induced in bba57 mutants in vivo (Fig. 2D). Overall, these results indicate that the absence of bba57 does not preclude, at least partially, the adaptation of the spirochete to the murine host but triggers adaptive and nonheritable changes in the transcription of a set of genes that promote persistence.
BBA57 Modulates the Expression of Multiple B. burgdorferi Proteins.
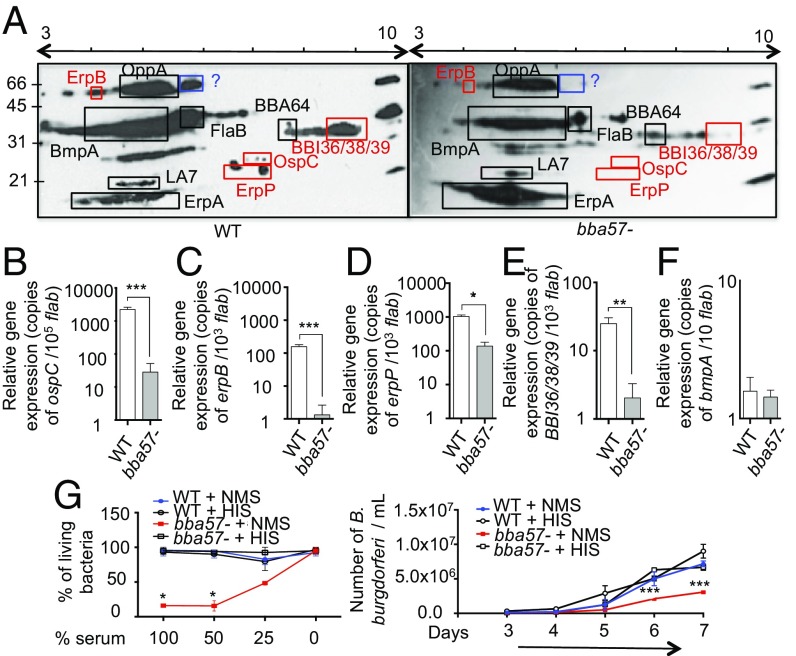
To assess the mechanism by which BBA57 supports early mammalian infection, we first explored whether its absence resulted in alterations in the transcriptome and proteome of B. burgdorferi. The low spirochete levels in infected tissues at early time points of infection prevented a direct and global analysis of transcripts and proteins. However, because hosts might have generated antibodies against these in vivo expressed genes, at least partly, we therefore assessed the profile of B. burgdorferi proteins using serological immunoblotting on 2D gels followed by expression analysis using gene-specific primers. The 2D immunoblot analysis using sera collected from 21-d–infected mice showed that the mutation in bba57 resulted in significantly different antibody responses compared with wild-type spirochetes (Fig. 3A), possibly due to different pattern of protein expression or immune accessibility. This differential antigen expression was specific to bba57 deletion and not associated with the loss of a membrane protein, because a similar 2D immunoblot pattern was recorded between the wild-type and a control isogenic mutant, bbh06 (38), lacking a surface-exposed B. burgdorferi outer membrane protein also produced during mammalian infection (SI Appendix, Fig. S3A). The protein identification, based on size and isoelectric point, was performed using published information (39). Among the major differences in antibody responses, OspC, BBI36/38/39, ErpP, and ErpB proteins were the most conspicuous (39), being absent in bba57 mutants. These differences in antibody response levels were also reflected by the early reduction in transcript levels for ospC, bbi36/38/39, erpP, and erpB genes in bba57 mutants at day 7 of infection (Fig. 3 B–E). As a control, we observed that a similar antibody response against BmpA (bb0383) in both wild-type and bba57 mutants correlated with no significant differences in expression levels for the bb0383 gene (Fig. 3F). The decrease in transcript levels is specific to the mammalian host environment because it is not observed either in ticks during feeding (SI Appendix, Fig. S3B) or in cultured cells grown in vitro after pH and temperature shift (SI Appendix, Fig. S3C), as shown with ospC expression. In addition to these proteins, other unknown antigens were also differentially recognized by murine infected sera from wild-type and bba57 mutant B. burgdorferi (Fig. 3A). Because we noticed the absence of ErpB and ErpP production by bba57 mutants, which are known to protect B. burgdorferi against complement activity (40, 41), we conducted an in vitro bactericidal assay by incubating bba57 mutants or wild-type B. burgdorferi with heat-inactivated or normal serum collected from murine hosts (Fig. 3G). The percentage of living bba57 mutants was significantly reduced in a dose-dependent fashion only after incubation with normal, complement active mouse serum, compared with wild type (Fig. 3G, Left). The growth of bba57 mutants was also decreased after incubation with serum with active complements (Fig. 3G, Right). This result suggests that the BBA57-mediated regulation of ErpP and ErpB proteins contributes to the protection of B. burgdorferi from host complement-mediated killing activity. All together, these results show that the absence of BBA57 results in differential gene expression changes and immune recognition during establishment of early murine infection.
Fig. 3.
BBA57 modulates the expression of several B. burgdorferi gene products involved in spirochete evasion of host innate immunity. (A) Differential antibody responses in mice infected with wild-type B. burgdorferi (WT) or bba57− mutant (bba57−). Serum samples from mice infected for 21 d with WT (Left) or bba57− (Right) B. burgdorferi was collected and used as a probe for immunoblotting against lysates prepared from wild-type B. burgdorferi. Protein spots present in both immunoblot samples were indicated by black squares, whereas red and blue squares denote identified and unidentified proteins, respectively, and ones up-regulated or only present in WT-infected mice. Protein identification was based on comparison with published information about electrophoretic migration of B. burgdorferi proteins and the characterization of their isoelectric points (39). (B–F) Reduced transcript levels of representative B. burgdorferi genes produced by the mutants at the injection site. Mice were injected with WT (white bars) or bba57− (gray bars) isolates. After 7 d, the injection site was retrieved and processed for expression analysis of ospC (B), erpB (C), erpP (D), bbi36/38/39 (E), and bmpA (F) expression by RT-qPCR. (G) BBA57 protects B. burgdorferi against serum complement-mediated killing activity; B. burgdorferi were exposed to different concentration of normal mouse serum (NMS) or heat inactivated mouse serum (HIS) for 18 h. The percentage of living spirochetes was determined based on bacteria motility using dark-field microscopy (Left). Right denotes regrowth assay. B. burgdorferi were grown in BSK medium after exposure with NMS or HIS for 48 h. The spirochetes were counted every day for a week using dark-field microscopy. Bars represent the mean ± SEM of at least triplicates. Data in A–G are representative three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
BBA57 Is Required for Early Innate Immune Evasion, Including Protection from Neutrophil Microbicidal Responses.
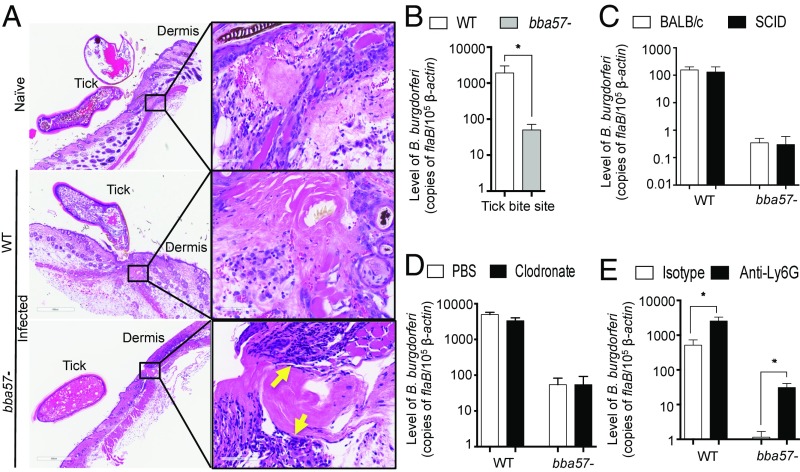
To further investigate the contribution of BBA57 to the establishment of B. burgdorferi infection, we assessed its effects on the local immune response. Histological analysis of murine tick bite sites suggested a slight increase in immune cell infiltration in response to ticks infested with the bba57 mutant compared with those infested with wild-type B. burgdorferi or naive ticks (Fig. 4A). Infiltrating cell populations were similar across groups and were composed predominantly of neutrophils, macrophages, lymphocytes, and eosinophils. Similar cell populations were observed when spirochetes were needle injected, although the immune responses were markedly reduced in comparison with tick bite sites, and eosinophils were not as prevalent (SI Appendix, Fig. S4). This immune response correlated with a decreased bacterial burden in animals infested with bba57 mutant-infected tick (Fig. 4B).
Fig. 4.
BBA57 protects B. burgdorferi at the dermal inoculum site against neutrophil-derived bactericidal responses. (A) Histology of murine skin at tick bite sites 5 d after placement of naive nymphal ticks or infested nymphs shows increased cellular infiltrates at the tick-bite site in those animals exposed to bba57− B. burgdorferi (yellow arrows). (B) B. burgdorferi burden at the tick bite site. The WT (white bars) or bba57− mutant B. burgdorferi (gray bars)-infected ticks were placed on naive mice. Five days later, the bite sites were collected and processed for bacterial burden analysis by RT-qPCR. (C) The levels of B. burgdorferi at the injection site in immunodeficient mice. Groups of BALB/c (white bars) or SCID mice (black bars) were injected with pathogens, and their burdens were detected at the injection site after 5 d by RT-qPCR. (D) Burden of B. burgdorferi at the injection site following macrophages depletion. Mice were treated with liposomes containing either PBS (white bars) or clodronate (black bars). Following cell depletion, mice were injected pathogens, and their burdens were detected after 5 d by RT-qPCR, as shown in C. (E) B. burgdorferi burden at the injection site after neutrophil depletion. Mice were treated with control (white bars) or anti-Ly6G (clone 1A8) antibodies (black bars) to deplete neutrophils. Following cell depletion, mice were injected pathogens, and their burdens were detected after 5 d by RT-qPCR, as shown in C. Bars represent the mean ± SEM of at least triplicates. Data in A–E are representative of at least six mice and two independent experiments. *P < 0.05.
Because bba57 mutants are cleared from the dermal inoculum site during tick-borne transmission, we next assessed the involvement of different immune cells in spirochete clearance. As expected, syringe injection of BALB/c and SCID mice with bba57 mutants resulted in an identical phenotype, indicating that BBA57-mediated immune evasion is targeting innate immune cells (Fig. 4C). We next identified macrophages and neutrophils in the murine dermis, based on immunoreactivity using specific antibodies against F4/80 and Ly6G, respectively. We first focused on macrophages because these cells are highly phagocytic for B. burgdorferi (42). However, the clodronate liposome-mediated depletion of macrophages, as visualized by confocal microscopic staining (SI Appendix, Fig. S5A), did not lead to any significant increase in the mutant burden at the injection site (Fig. 4D). Next, we assessed the involvement of neutrophils because these cells are also phagocytic and produce a variety of bactericidal effector molecules (43). The depletion of neutrophils with anti-Ly6G monoclonal antibodies (SI Appendix, Fig. S5B) resulted in a dramatic increase in the levels of bba57 mutants compared with a more modest increase of wild-type B. burgdorferi after needle inoculation (Fig. 4E). In addition, confocal microscopy revealed that during early infection, bba57 mutants are less protected against neutrophil insults than wild-type spirochetes (SI Appendix, Fig. S6A). Together, these data indicate that neutrophils are major contributors to the local control of B. burgdorferi at the site of deposition and that BBA57 exerts a protective role against such neutrophil-mediated spirochete killing. We also found that the mechanism by which neutrophils suppress the levels of bba57 mutants is independent of Toll (Myd88-dependent) pathways because bba57 mutants showed impaired persistence in the myd88-deficient mouse tissues as in wild-type animals (SI Appendix, Fig. S6B). Taken together, these results suggest that BBA57 protects B. burgdorferi from dermal neutrophil-mediated killing at the infection site.
BBA57 Confers Spirochetes Resistance Against Innate Immunity by Regulating the Production of Antimicrobial Peptides.
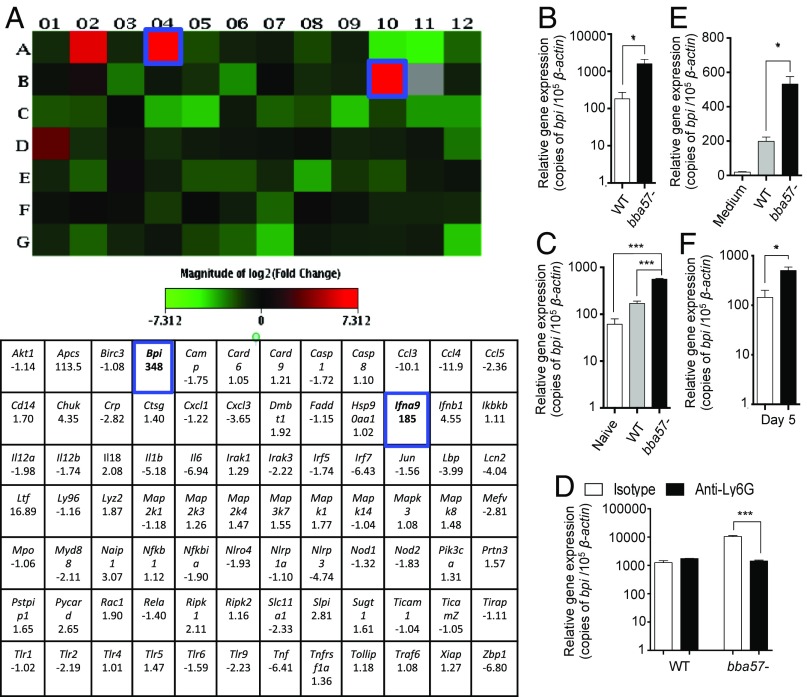
We next assessed the mechanisms by which BBA57 protects B. burgdorferi from neutrophil-mediated killing. To identify host innate immune mediators that are altered during infection with bba57 mutants, we used a PCR expression array screen. Mice were infected with wild-type or bba57 mutant spirochetes (105 cells per mouse), and after 5 d, the expression of innate immune genes in the skin was analyzed using commercially available PCR expression arrays. The array profiled the expression of 84 key genes involved in various aspects of murine innate immune response to bacteria. As expected, the lower pathogen burdens at the time of analysis correlated with decreased levels of expression of several proinflammatory factors, including the cytokines Il1b, Il6, or Tnf or the chemokines Ccl3, Cxcl3, or Ccl5. Furthermore, we also observed lower transcriptional levels of the pathogen recognition receptors or their downstream signaling intermediaries, including Tlr2, Tlr9, Nlrp3, Myd88, Irak3, or Irf7 (Fig. 5A). These results suggested that the lower bacterial burdens in mice infected with bba57 mutants induced a limited activation of infiltrating innate immune cells compared with bacteria containing the gene.
Fig. 5.
BBA57 alters the host antimicrobial response. (A) Assessment of host antibacterial inflammatory response at the dermal injection site. Equal numbers of wild-type B. burgdorferi (WT) or bba57− mutant (bba57−) (105 cells per animal) were injected into the skin. Five days following inoculum, the injection sites were retrieved and processed for a PCR array for simultaneous analysis of expression of 84 host antibacterial response genes. The table summarizes the fold change of gene expression of bba57− mutant compared with WT. Genes up-regulated during mutant infection are represented in red. Blue squares show the most dramatically induced genes, bactericidal/permeability-increasing protein (BPI), and an IFN gene (Ifna9) explored in this study. (B) Up-regulation of BPI during syringe-inoculated infection with bba57 mutants. Equal numbers of WT (white bars) or bba57− (black bars) isolates (105 cells per animal) were injected into mouse skin. Injection sites were retrieved following 5 d of inoculum and analyzed for BPI expression by RT-qPCR. (C) Induction of BPI during tick-borne infection with bba57 mutants. Naive nymphal ticks or nymphs infected with WT (gray bars) or bba57− mutants (black bars) were allowed to parasitize naive mice. After 5 d of feeding, the bite site was retrieved and BPI expression was analyzed by RT-qPCR. (D) Reduction of bba57 mutant-induced BPI expression following depletion of neutrophils. Mice were treated for cell depletion as shown in Fig. 3E. The expression of BPI was measured at the injection site by RT-qPCR after 5 d of infection. (E) BPI induction by neutrophils following exposure with spirochetes in culture. Primary neutrophils were isolated from mice and then immediately incubated in vitro with only medium (white bar), WT B. burgdorferi (gray bar), or bba57− mutants (black bar). Following spirochete exposure, neutrophils were collected, and BPI expression was measured by RT-qPCR. (F) Up-regulation of BPI during infection with an isogenic mutant lacking a key BBA57-modulated gene, ospC. Equal numbers (105 cells) of the WT B. burgdorferi (white bar) or ospC mutants (black bar) were injected into mouse skin. Injection sites at the dermis were retrieved after 5 d of inculum and processed for BPI expression by RT-qPCR. Bars represent the mean ± SEM of at least triplicates. Data in A–F are representative of at least eight mice and two independent experiments. *P < 0.05; ***P < 0.001.
A handful of innate immune genes displayed a dramatic up-regulation, most prominently, the antimicrobial peptides (AMP) bactericidal/permeability-increasing protein (Bpi), Lactotransferrin (Ltf), or Secretory leukocyte proteinase inhibitor (Slpi) (Fig. 5A). Because BPI is known to kill B. burgdorferi and is predominantly produced by neutrophils (44), we focused our analysis on this AMP. Subsequently, a series of independent gene expression analysis using syringe and tick-borne infections confirmed a significant BPI induction in bba57 mutant-infected mice following 5 d after injection (Fig. 5B) and during the last day of tick feeding (Fig. 5C). In contrast, BPI expression was decreased during engorgement with naive and wild-type infected ticks. BPI up-regulation by bba57 mutants was minimal in neutrophil-depleted mice (Fig. 5D), indicating that this cell type is the primary producer of the AMP in response to the presence of the spirochete. Moreover, the stimulation of murine neutrophils confirmed the ability of bba57 mutants to induce more Bpi transcripts than wild-type spirochetes (Fig. 5E). Similarly to bba57 mutants (Fig. 5F), ospC mutants also induced higher expression levels of BPI than wild-type spirochetes at the injection site when analyzed 5 d after infection. These results suggest that the absence of OspC protein observed in bba57 mutants (Fig. 3) could contribute to the up-regulation of BPI.
We then analyzed the antimicrobial activity of BPI against wild-type or bba57 mutant B. burgdorferi in vitro. As shown by confocal immunofluorescence microscopy (SI Appendix, Fig. S7A), the number of dead spirochetes increased with the addition of higher BPI concentration but did not significantly differ between wild-type and bba57 mutants (SI Appendix, Fig. S7B). At 100 µg/mL, BPI was able to kill 30% of B. burgdorferi. Moreover, B. burgdorferi exposed to the highest concentration of BPI was unable to regrow when inoculated in a fresh media (SI Appendix, Fig. S7C). Taken together, these data indicate that BBA57 influences the expression of innate immune-related molecules, including BPI, rather than directly blocking the activity of these antimicrobial peptides. These results also show that BBA57 supports spirochete persistence during early infection by modulating the host expression of neutrophilic antimicrobial peptides like BPI. However, because we noticed that the burden of bba57 mutants remained lower than wild type in neutrophil-depleted mice (Fig. 4E), it suggests that other cell types also contribute to the secretion of BPI or that other nonneutrophilic factors also impair the persistence of mutants.
BBA57 Influence the Host IFN Associated Responses.
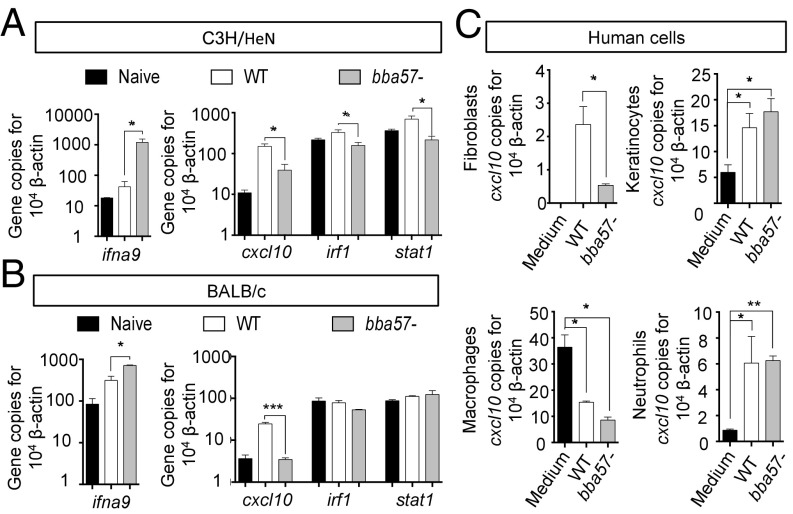
Apart from the near clearance at the inoculum site, bba57 mutants also displayed a defect in their ability to disseminate to distant organs. Because the results of the host response PCR array (Fig. 5A) also indicated modulation of a host type I IFN gene (ifna9) during infection with bba57 mutants, we further tested its expression, together with a group of genes known as IFN-stimulated genes (ISGs), particularly those whose up-regulations have been previously linked to spirochete dissemination events (45, 46). The gene expression analysis in C3H/HeN and BALB/c mice showed the induction of ifna9 by bba57 mutants but not wild-type B. burgdorferi (Fig. 6 A and B, Left), associated with an up-regulation of selected ISGs (cxcl10, irf1, and stat1) by wild-type but not bba57 mutant spirochetes (Fig. 6 A and B, Right). This result suggests a negative regulation of ISGs by IFNa9, albeit the expression of the ISGs analyzed could also be independently regulated from the induction of IFNa9 by the mutants. Either way, the chemokine CXCL10 is specifically associated with skin disorders during Lyme borreliosis including erythema migrans (47). We therefore assessed the modulation of this chemokine by BBA57 using primary human cells. The fibroblasts, keratinocytes, neutrophils, and macrophages isolated from healthy human donors were exposed to wild-type or bba57 mutants in culture. The results showed that CXCL10 was significantly more induced by wild-type B. burgdorferi than by the bba57 mutant in fibroblasts but not in the other cell types tested (Fig. 6C), including human macrophages where B. burgdorferi negatively regulates CXCL10 expression independent of BBA57. Taken together, these results further suggested a specific immune modulatory role of BBA57, including host IFN associated responses that influence the dissemination of B. burgdorferi during murine and potentially human Lyme borreliosis.
Fig. 6.
BBA57 regulates IFN-stimulated genes. (A) Expression of type I IFN genes in C3H mice is influenced by BBA57. Groups of C3H mice were inoculated with equal levels (105 per animal) of WT B. burgdorferi (white bars) or bba57− mutants (gray bars). Five days after infection, skin injection sites were collected and processed for RT-qPCR to analyze expression of ifna9 (Left) and several other IFN-stimulated genes (Right). (B) Expression of type I IFN genes in BALB/c mice is influenced by BBA57. The animals were infected and analyzed as detailed in A. (C) Modulation of expression of cxcl10 chemokine by BBA57 in primary dermal and myeloid human cells. Human fibroblasts, keratinocytes, macrophages, and neutrophils were incubated with medium only (black bars), WT B. burgdorferi (white bars), or bba57− mutant (gray bars) at a MOI of 50. After 24 h of incubation with fibroblasts (Top Left), keratinocytes (Top Right), or macrophages (Bottom Left) or after 12 h of incubation with neutrophils (Bottom Right), cells were collected and processed for cxcl10 expression using RT-qPCR. Bars represent the mean ± SEM of at least triplicates. Data in A–C are representative of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The persistent infection of pathogens in mammals must rely on their adaptive ability to evade sophisticated host immune responses. This is especially relevant for the agent of Lyme borreliosis, a tick-borne pathogen, which lacks the ability for transovarial transmission through Ixodes eggs and, therefore, must establish long-term infections in mammalian reservoir hosts to be acquired by vector ticks during a blood meal engorgement (11). Here we show that B. burgdorferi surface lipoprotein BBA57, originally discovered as a novel virulence determinant (17), assists in spirochete infection by facilitating microbial evasion of host innate immunity. Loss of BBA57 impairs a series of previously unrecognized and multifaceted spirochete defense strategies against host complement, neutrophil factors, and IFN responses associated with the dysregulation of several bacterial surface proteins. BBA57 could be directly involved in the recognition of unidentified host factors and transduce signals to regulate the expression of other surface protein (48). As a surface protein itself, it is hard to envision how BBA57 can function as a transcriptional modulator, influencing expression of multiple B. burgdorferi antigens. Therefore, the role of BBA57 in host–pathogen interactions or pathogenesis could be indirect as a consequence of the deletion of the gene. Although BBA57 protects the spirochete against innate immune-derived cell envelope stress, its absence is destabilizing, forcing the mutant to mount a compensatory response that is only partially protective early on and not required during later infection. Nevertheless, these studies uncover plasticity or adaptability in major immune evasion strategies of spirochetes, like one mediated by BBA57, because its loss triggers a nonheritable host-specific adaptation that features a transcriptional reprograming cascade involving a subset of genes associated with metabolism, biosynthesis, and virulence (20, 49). Ultimately, this strategy ensures the persistence of the mutants in the mammalian host (Fig. 7). Given the apparent lack of known mechanisms of persistence in B. burgdorferi, such as toxin/antitoxin modules that are critical for host persistence of other extracellular bacteria like Staphylococcus aureus and Pseudomonas aeruginosa (50), our study highlights the evolution of a unique adaptive mechanism in spirochetes. Notably, many key spirochete genes associated with immune evasion or host adaptation encode unique surface proteins (such as BBA57, OspC, and BB0405) that lack orthologs in any other species and are thus specifically associated with the special biology and persistence of the Lyme disease pathogen (11, 20, 51).
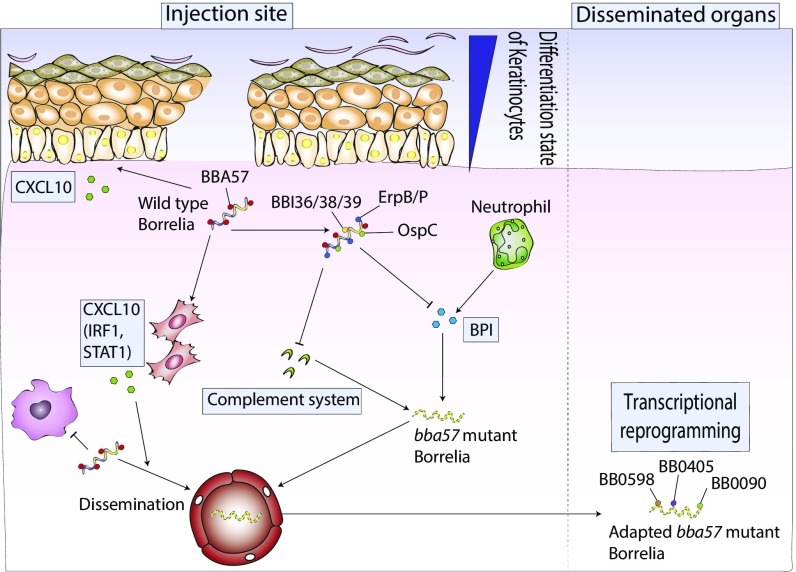
Fig. 7.
A proposed model showing BBA57 modulation of host innate immune response to promote spirochete persistence. During early host infection process, B. burgdorferi dramatically up-regulated its surface lipoprotein BBA57 that subsequently governs the expression of other surface lipoprotein gene products such as OspC, BBI36/38/39, ErpB, and ErpP. These events facilitate suppression of antimicrobial peptide expression including BPI, largely expressed by neutrophils. The BPI suppression is most likely mediated by OspC. The BBA57 function also assists the pathogen to protect from the host complement system, possibly through the capture of factor H via Erp proteins (at least by Erp B and ErpP). Therefore, bba57− mutants with impaired expression of OspC and Erp proteins are more susceptible to BPI and complement killing. Besides, expression of surface proteins like BBA57 also favors induction of host IFN-stimulated genes such as cxcl10. This chemokine might promote the dissemination of the bacteria to disseminated organs. After evasion of early innate immunity response, residual spirochetes disseminate and colonize distant organs (skin, heart, joint, or bladder), where they eventually establish persistent infection. bba57 mutants, despite their near clearance at the dermal inoculum site, are also able to accomplish persistent infection following a nonheritable adaptive process that likely involves up-regulation of specific microbial genes, notably, bb0598, bb0405, and bb0090.
During early infection, mammals respond to invading pathogens by triggering robust innate immune responses, some of which are effective within minutes following microbial entry (52). Neutrophils are one of the fast responders and earliest immune cells recruited at the site of pathogen inoculation with the capacity to generate a variety of microbicidal effector mechanisms (53). In the context of arthropod-borne infections, such as Leishmania major transmitted by sand flies, neutrophils can limit infection through necrosis induced by Toll-like receptors (TLRs) (54). In addition, other antibacterial responses like phagocytosis, oxidative burst, and AMP secretion have also been shown to participate in microbial clearance, including B. burgdorferi during early infection (15, 42, 55–57). Here we show that Lyme disease spirochetes evolved countermeasures mediated through BBA57 to evade some of these neutrophil-derived insults, possibly via the regulation of multiple spirochete surface proteins. Notably, a domain known to be involved in pathogen–host interactions (35) has recently been annotated in the BBA57 sequence, as shown in the NCBI database for conserved domains. The newly recognized domain in BBA57, known as “Actin assembly-inducing protein or ActA” domain, has been originally identified in Listeria monocytogenes (35). It mediates the assembly of the host cell actin filaments at the bacterial surface, resulting in actin polymerization and propulsion of the bacterium through the cell. Given that B. burgdorferi adopts an exclusively extracellular lifestyle, the spirochete could potentially use this actin polymerization domain in BBA57 to modulate host immune defenses, for example, escaping neutrophil extracellular traps (56). Although B. burgdorferi are susceptible to microbicidal responses generated by neutrophils, including killing by AMPs (55), spirochetes generally either have decreased susceptibility or are resistant against some AMPs, such as LL37 or cathelicidin (58). Nevertheless, previous studies on antimicrobial susceptibility of B. burgdorferi are largely based on in vitro experiments. Herein, we present direct evidence that the presence of BBA57 is able to suppress the expression of AMPs in vivo. B. burgdorferi infection features a very transient bacteremia (59), avoiding colonization of highly vascularized tissues, although during transitions into tick vectors, the microbes are re-exposed to host blood in the tick gut (60). We also noted that bba57 mutants display only a transient defect in their survivability in tick gut during pathogen acquisition through host blood meal, but not during persistence in unfed ticks, or dissemination through tick tissues during transmission. Therefore, it is likely that BBA57 primarily facilitates spirochete evasion of innate immune responses in mammals but also supports B. burgdorferi survival in the tick via conferring defense against host immune cells like neutrophils or complement molecules in the blood meal that are translocated within tick gut.
The successful establishment of pathogen persistence is directly associated with the capacity to survive the initial immune offenses mounted by a host. For B. burgdorferi, it has been known that the spirochete is able to adapt to persist in the mammalian host and that this adaptation is associated with expression of specific genes (61). Our results show that the absence of BBA57 does not preclude the long-term survival of the bacterium when the infectious inoculum is large enough, but when the host is infected by tick attachment, which is probably a consequence of the difference in bacterial numbers being deposited in the skin. These results also show that under natural infectious conditions the function of this protein is crucial so the spirochete reaches a critical number and is allowed to disseminate. Notably, in addition to intragenic recombination in vlsE locus (6), multiplication of B. burgdorferi in vivo is known to generate a genetically and antigenically heterologous population of spirochetes with regulation of specific virulence determinants (62) that favor host survival (63, 64). Because bba57 mutants can induce expression of certain genes (bb0405, bb0090, and bb0598), this suggests their involvement in host persistence in adapted mutants. In fact, bb0405 encodes a surface-exposed outer membrane protein recently shown to support B. burgdorferi persistence in the mammalian hosts (20). On the other hand, bb0090 is part of a V-type ATPase operon, which supports proton efflux across the cell membrane facilitating rapid growth of the bacteria (65). Finally, bb0598 encodes a phosphate acetyltransferase required for cell wall biosynthesis and metabolism (66), which may facilitate their efficient survival and multiplication process in the host ensuring a long-term persistence. This remarkable ability of host adaptation has to be taken into account notably when studying B. burgdorferi antibiotic resistance observed in animals and in some patients (2, 3).
Materials and Methods
Bacteria, Mice, and Ticks.
An infectious isolate of B. burgdorferi, B31-A3, was used. The mutants of B. burgdorferi, bba57, ospC (67), bbh06 (38), and bba57-complemented strains, were obtained from our previous study (17). Four- to 6-wk-old female C3H/HeN, BalbC, and pathogen-free SCID mice were purchased from the National Cancer Institutes and Charles River Laboratories. Mice were infested with B. burgdorferi-infected ticks or inoculated with one s.c. injection of 105 bacteria per mouse (68). Human blood samples were collected following informed consents from healthy donors under approved clinical protocols (ClinicalTrials.gov Identifier: NCT00001539 and NCT00001846). Experiments involving human subjects received ethical approval from Institutional Review Board of the National Institute of Allergy and Infectious Diseases, whereas other studies including animals and pathogens were performed in accordance with the guidelines and approvals from the Institutional Animal Care and Use Committee and Institutional Bio-safety Committee of the University of Maryland, College Park. Ixodes scapularis ticks belong to a colony that has been reared and maintained in our laboratory.
Human Cell Culture.
Neutrophils and macrophages were obtained from blood collected from healthy humans. Primary human fibroblasts and keratinocytes were purchased from Thermo Fisher. Once plated, cells were incubated and processed as detailed in SI Appendix, Supplementary Materials and Methods.
Infection Studies.
For infection using ticks, larvae were fed on 21-d–infected C3H/HeN mice with spirochetes and retrieved after full repletion and either processed for B. burgdorferi burden analysis or kept at 23 °C in an environmental controlled chamber for molting. Newly molted nymphs were processed to analyze B. burgdorferi burden or used to infect mice (five ticks per mouse), which were killed between 5 and 14 d for analysis of spirochete burden using RT-qPCR and culture. During transmission, nymphs were collected and dissected to analyze the presence of B. burgdorferi in their salivary glands by confocal fluorescence microscopy as detailed (69).
For infection and reinfection experiments using syringe-inoculated spirochetes, C3H/HeN (three animals per group) mice were infected by needle inoculation with spirochetes (105 cells per animal) and were killed after 2 h of injection or between 1 and 21 d of infection. The skin at the injection site and organs (skin, heart, joint, and bladder) were collected and processed for burden and/or culture analysis as detailed in SI Appendix, Supplementary Materials and Methods.
Artificial Tick Feeding System.
An artificial membrane feeder was developed using published procedures (37) and further detailed in SI Appendix, Supplementary Materials and Methods. For transmission experiments, we used infected nymphs molted from larvae fed on wild-type B. burgdorferi-infected and bba57-mutated B. burgdorferi-infected mice, respectively. Sixty ticks per capsule were used. A blood sample was collected after each change and stored at −80 °C. Total RNA from the blood sample was isolated by TRIzol (Ambion; Thermo Fisher Scientific). For acquisition experiments, wild-type or bba57 mutant B. burgdorferi (103 cells per mL) were inoculated in the feeder, and fully fed nymphs were collected and processed to analyze B. burgdorferi burden as detailed (18).
Quantitative PCR Analysis and qPCR Array.
Quantitative PCR (qPCR) analysis was performed as detailed (18). Total RNA was extracted from tissues using the TRIzol reagent (Invitrogen), processed with DNase, and transcribed into cDNA by using SuperScript VILO cDNA synthesis kit (Thermo Fisher Scientific). To detect levels of viable B. burgdorferi, we conducted RT-qPCR using SYBR Green master mix (Thermo Fisher Scientific) and targeting flaB gene, as developed, validated, and used in previous publications (18, 69). Transcript levels of individual genes were measured using specific primers (SI Appendix, Table S3) and calculated using the 2−ΔCt method (18) and presented as number of copies compared with control gene expression (β-actin for host genes or flaB for B. burgdorferi genes).
For qPCR array, the RT2 Profiler PCR Array (Qiagen PAMM-148Z) was used to measure the expression level of 84 antibacterial response genes, as detailed in SI Appendix, Supplementary Materials and Methods.
Next-Generation Sequencing and Read Mapping.
The original bba57 mutant (17) and the same clone isolated from mice after 3 wk of infection (host-adapted clone) were sequenced using a whole-genome shotgun sequencing approach on the Illumina MiSeq platform, as detailed in SI Appendix, Supplementary Materials and Methods.
In Vivo Depletion of Phagocytes.
The Balb/C and SCID mice (three animals per group) infected with wild-type or bba57 mutants were euthanized at day 5 of infection. Skin at the injection site was then processed for B. burgdorferi burden analysis. For macrophage depletion, C3H/HeN mice were injected s.c. with either PBS-liposome or Clodronate-liposome (purchased from ClodronateLiposomes) 48 and 24 h before infection. Mice were then infected with 105 wild-type or bba57 mutants. PBS-liposome or Clodronate-liposome were then injected s.c. 24 h after infection and I.P 24, 48, and 96 h after infection. Mice were euthanized at day 5 after infection, and skin injection site was retrieved to analyze B. burgdorferi burden. For depletion of neutrophils, the antibody Ly6-G (clone 1A8) or isotype control antibody (Bio-X-Cell) were used following published methods (7). The antibodies were injected to mice every 48 h, starting 48 h before infection and until 5 d of infection when mice were killed. Skin injection site was then processed for B. burgdorferi burden analysis.
Isolation of Murine Neutrophils and Activation.
Neutrophils were isolated from mouse peripheral blood and activated with either wild-type or bba57 mutant B. burgdorferi with MOI 1:50 for 12 h, as described (70), and further detailed in SI Appendix, Supplementary Materials and Methods.
Confocal Microscopy and Histopathological Analysis.
Histolopathological and confocal immunofluorescence microscopic analysis were performed as described (68) and detailed in SI Appendix, Supplementary Materials and Methods.
Bactericidal and Complement-Mediated Killing Assays.
Bactericidal and complement-mediated killing assays were performed using published procedures (20, 71). Briefly, 105 midlog cells (wild-type or bba57 mutant B. burgdorferi) were incubated in 20 µL of PBS at 37 °C with various concentrations of the recombinant form of the antimicrobial peptide, BPI (Athens Research and Technology), an antibacterial molecule present in azurophilic granules of neutrophils. For bactericidal experiments using mouse complement, aliquots of fresh blood were collected from mice and immediately used in the assays. After 2 h of incubation, 1 µL aliquot was transferred to 1 mL of BSK, and the bacteria regrowth was analyzed for 10 d (72). Besides, 10 µL were stained with propidium iodide and Syto 9 from BacLight bacterial viability kit (Thermo Fisher Scientific) to differentiate dead B. burgdorferi (red) from living ones (green) using a LSM 510 confocal microscope (Zeiss). Same protocol was used for serum killing activity with an incubation time of 48 h.
Flow Cytometry.
Flow cytometry assay was performed as detailed (7) and further detailed in SI Appendix, Supplementary Materials and Methods.
Antibodies and Immunoblotting.
Immunoblotting was performed as described elsewhere (71) and further detailed in SI Appendix, Supplementary Materials and Methods.
Statistical Analysis.
Data are presented as means and SEM of at least two independent experiments. Statistical significance was analyzed with the Prism 5.0 statistical program (GraphPad Software). Comparisons between more than two groups were performed with one-way analysis of variance (ANOVA) followed by a Tukey posttest, whereas comparisons between two experimental groups were analyzed by two-tailed Student’s t test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Kamoltip Promnares for assistance. This research was supported by the National Institute of Allergy and Infectious Diseases (NIAID) Awards AI080615 and AI116620 (to U.P.) and AI128232 (to X.Y.); the Intramural Research Programs of the Center for Cancer Research, National Cancer Institute (to S.D.C. and J.E.D.), as well as NIAID (to A.M.); and a grant from the Spanish Ministry of Economy and Competitiveness, SAF2015-65327 (to J.A.). X.Z. is the recipient of the Deborah and Mark Blackman Postdoctoral Fellowship from Global Lyme Alliance. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718595115/-/DCSupplemental.
References
- 1.Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One. 2014;9:e86907. doi: 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother. 2015;59:4616–4624. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embers ME, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One. 2012;7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramamoorthi N, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco SE, et al. Outer surface protein OspC is an antiphagocytic factor that protects Borrelia burgdorferi from phagocytosis by macrophages. Infect Immun. 2015;83:4848–4860. doi: 10.1128/IAI.01215-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks CS, et al. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- 9.Silva MT. Classical labeling of bacterial pathogens according to their lifestyle in the host: inconsistencies and alternatives. Front Microbiol. 2012;3:71. doi: 10.3389/fmicb.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seifert SN, Khatchikian CE, Zhou W, Brisson D. Evolution and population genomics of the Lyme borreliosis pathogen, Borrelia burgdorferi. Trends Genet. 2015;31:201–207. doi: 10.1016/j.tig.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris SJ. vls antigenic variation systems of Lyme disease borrelia: Eluding host immunity through both random, segmental gene conversion and framework heterogeneity. Microbiol Spectr. 2014;2 doi: 10.1128/microbiolspec.MDNA3-0038-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Önder Ö, et al. OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J Biol Chem. 2012;287:16860–16868. doi: 10.1074/jbc.M111.290775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar A, et al. Borrelia burgdorferi resistance to a major skin antimicrobial peptide is independent of outer surface lipoprotein content. Antimicrob Agents Chemother. 2009;53:4490–4494. doi: 10.1128/AAC.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard Q, Jaulhac B, Boulanger N. Smuggling across the border: how arthropod-borne pathogens evade and exploit the host defense system of the skin. J Invest Dermatol. 2014;134:1211–1219. doi: 10.1038/jid.2014.36. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, et al. Novel microbial virulence factor triggers murine lyme arthritis. J Infect Dis. 2013;207:907–918. doi: 10.1093/infdis/jis930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Coleman AS, Anguita J, Pal U. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 2009;5:e1000326. doi: 10.1371/journal.ppat.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaultney RA, Gonzalez T, Floden AM, Brissette CA. BB0347, from the lyme disease spirochete Borrelia burgdorferi, is surface exposed and interacts with the CS1 heparin-binding domain of human fibronectin. PLoS One. 2013;8:e75643. doi: 10.1371/journal.pone.0075643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kung F, et al. A Borrelia burgdorferi surface-exposed transmembrane protein lacking detectable immune responses supports pathogen persistence and constitutes a vaccine target. J Infect Dis. 2016;213:1786–1795. doi: 10.1093/infdis/jiw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y-P, et al. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS Pathog. 2014;10:e1004238. doi: 10.1371/journal.ppat.1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 23.Hyde JA, et al. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol Microbiol. 2011;82:99–113. doi: 10.1111/j.1365-2958.2011.07801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brissette CA, Gaultney RA. That’s my story, and I’m sticking to it–an update on B. burgdorferi adhesins. Front Cell Infect Microbiol. 2014;4:41. doi: 10.3389/fcimb.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhi H, et al. The BBA33 lipoprotein binds collagen and impacts Borrelia burgdorferi pathogenesis. Mol Microbiol. 2015;96:68–83. doi: 10.1111/mmi.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallström T, et al. Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis. 2010;202:490–498. doi: 10.1086/653825. [DOI] [PubMed] [Google Scholar]
- 27.Verma A, Brissette CA, Bowman A, Stevenson B. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun. 2009;77:4940–4946. doi: 10.1128/IAI.01420-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koenigs A, et al. BBA70 of Borrelia burgdorferi is a novel plasminogen-binding protein. J Biol Chem. 2013;288:25229–25243. doi: 10.1074/jbc.M112.413872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toledo A, Coleman JL, Kuhlow CJ, Crowley JT, Benach JL. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect Immun. 2012;80:359–368. doi: 10.1128/IAI.05836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueira SV, Smith AA, Qin J-H, Pal U. A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks. Infect Immun. 2012;80:82–90. doi: 10.1128/IAI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Floden AM, Watt JA, Brissette CA. Borrelia burgdorferi enolase is a surface-exposed plasminogen binding protein. PLoS One. 2011;6:e27502. doi: 10.1371/journal.pone.0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earnhart CG, et al. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol. 2010;76:393–408. doi: 10.1111/j.1365-2958.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caine JA, Coburn J. Multifunctional and redundant roles of Borrelia burgdorferi outer surface proteins in tissue adhesion, colonization, and complement evasion. Front Immunol. 2016;7:442. doi: 10.3389/fimmu.2016.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caine JA, et al. Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol. 2017;19:e12786. doi: 10.1111/cmi.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pistor S, Chakraborty T, Niebuhr K, Domann E, Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 1994;13:758–763. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Promnares K, et al. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol Microbiol. 2009;74:112–125. doi: 10.1111/j.1365-2958.2009.06853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver JD, et al. Infection of immature Ixodes scapularis (Acari: Ixodidae) by membrane feeding. J Med Entomol. 2016;53:409–415. doi: 10.1093/jme/tjv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman AS, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One. 2008;3:3010e. doi: 10.1371/journal.pone.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowalk AJ, Gilmore RD, Jr, Carroll JA. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun. 2006;74:3864–3873. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraiczy P, et al. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur J Immunol. 2003;33:697–707. doi: 10.1002/eji.200323571. [DOI] [PubMed] [Google Scholar]
- 41.Kraiczy P, Stevenson B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis. 2013;4:26–34. doi: 10.1016/j.ttbdis.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montgomery RR, Malawista SE. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect Immun. 1996;64:2867–2872. doi: 10.1128/iai.64.7.2867-2872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz H, Weiss JP. The bactericidal/permeability-increasing protein (BPI) in infection and inflammatory disease. Clin Chim Acta. 2007;384:12–23. doi: 10.1016/j.cca.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petzke MM, et al. Borrelia burgdorferi induces a type I interferon response during early stages of disseminated infection in mice. BMC Microbiol. 2016;16:29. doi: 10.1186/s12866-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petzke M, et al. Early type I interferon response correlates with Borrelia burgdorferi dissemination in a mouse model of Lyme disease. J Immunol. 2015;194(Suppl 1):127.22. [Google Scholar]
- 47.Müllegger RR, et al. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–4628. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheckelhoff MR, Telford SR, Wesley M, Hu LT. Borrelia burgdorferi intercepts host hormonal signals to regulate expression of outer surface protein A. Proc Natl Acad Sci USA. 2007;104:7247–7252. doi: 10.1073/pnas.0607263104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gherardini F, Boylan J, Lawrence K, Skare J. Borrelia: Molecular biology, host interaction and pathogenesis. In: Samuels DS, Radolf JD, editors. Metabolism and Physiology of Borrelia. Caister Academic Press; Norfolk, UK: 2010. pp. 103–138. [Google Scholar]
- 50.Page R, Peti W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol. 2016;12:208–214. doi: 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- 51.Grimm D, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci USA. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nestle FO, Di Meglio P, Qin J-Z, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peniche AG, et al. A secondary wave of neutrophil infiltration causes necrosis and ulceration in lesions of experimental American cutaneous leishmaniasis. PLoS One. 2017;12:e0179084. doi: 10.1371/journal.pone.0179084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lusitani D, Malawista SE, Montgomery RR. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J Infect Dis. 2002;185:797–804. doi: 10.1086/339341. [DOI] [PubMed] [Google Scholar]
- 56.Menten-Dedoyart C, et al. Neutrophil extracellular traps entrap and kill Borrelia burgdorferi sensu stricto spirochetes and are not affected by Ixodes ricinus tick saliva. J Immunol. 2012;189:5393–5401. doi: 10.4049/jimmunol.1103771. [DOI] [PubMed] [Google Scholar]
- 57.Bernard Q, Wang Z, Di Nardo A, Boulanger N. Interaction of primary mast cells with Borrelia burgdorferi (sensu stricto): role in transmission and dissemination in C57BL/6 mice. Parasit Vectors. 2017;10:313. doi: 10.1186/s13071-017-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isogai E, Isogai H, Takahashi K, Kobayashi-Sakamoto M, Okumura K. Antimicrobial activity of three tick defensins and four mammalian cathelicidin-derived synthetic peptides against Lyme disease spirochetes and bacteria isolated from the midgut. Exp Appl Acarol. 2009;49:221–228. doi: 10.1007/s10493-009-9251-5. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz I, et al. Polymerase chain reaction amplification of culture supernatants for rapid detection of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1993;12:879–882. doi: 10.1007/BF02000415. [DOI] [PubMed] [Google Scholar]
- 60.De Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 61.Pal U, Fikrig E. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect. 2003;5:659–666. doi: 10.1016/s1286-4579(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 62.Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci USA. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rego ROM, Bestor A, Stefka J, Rosa PA. Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PLoS One. 2014;9:e101009. doi: 10.1371/journal.pone.0101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Troy EB, et al. Understanding barriers to Borrelia burgdorferi dissemination during infection using massively parallel sequencing. Infect Immun. 2013;81:2347–2357. doi: 10.1128/IAI.00266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci USA. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richards CL, et al. Acetyl-phosphate is not a global regulatory bridge between virulence and central metabolism in Borrelia burgdorferi. PLoS One. 2015;10:e0144472. doi: 10.1371/journal.pone.0144472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pal U, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith AA, et al. Cross-species interferon signaling boosts microbicidal activity within the tick vector. Cell Host Microbe. 2016;20:91–98. doi: 10.1016/j.chom.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis. 2010;201:1084–1095. doi: 10.1086/651172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of mouse neutrophils. Curr Protoc Immunol. 2015;110:3.20.1–3.20.15. doi: 10.1002/0471142735.im0320s110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, et al. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun. 2010;78:4477–4487. doi: 10.1128/IAI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pal U, et al. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J Immunol. 2001;166:7398–7403. doi: 10.4049/jimmunol.166.12.7398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.