Significance
Recovery of cutaneous sensation after spinal cord injury relies on reestablishing effective somatotopic pathways from the skin to the cortex. We show that the recovery of cortical activity months after extensive damage to the primary touch pathway in the dorsal column depends on a few surviving primary axons, together with the greater number of preserved axons in a secondary touch pathway from spinal cord neurons. These surviving axons could be targeted for treatments that potentiate their roles in recovery after spinal cord injury.
Keywords: primate, spinal cord injury, somatosensory cortex, tactile
Abstract
Months after the occurrence of spinal cord dorsal column lesions (DCLs) at the cervical level, neural responses in the hand representation of somatosensory area 3b hand cortex recover, along with hand use. To examine whether the second-order spinal cord pathway contributes to this functional recovery, we injected cholera toxin subunit B (CTB) into the hand representation in the cuneate nucleus (Cu) to label the spinal cord neurons, and related results to cortical reactivation in four squirrel monkeys (Saimiri boliviensis) at least 7 months after DCL. In two monkeys with complete DCLs, few CTB-labeled neurons were present below the lesion, and few neurons in the affected hand region in area 3b responded to touch on the hand. In two other cases with large but incomplete DCLs, CTB-labeled neurons were abundant below the lesion, and the area 3b hand cortex responded well to tactile stimulation in a roughly somatotopic organization. The proportions of labeled neurons in the spinal cord hand region reflected the extent of cortical reactivation to the hand. Comparing monkeys with short and long recovery times suggests that the numbers of labeled neurons below the lesion increase with time following incomplete DCLs (<95%) but decrease with time after nearly complete DCLs (≥95%). Taken together, these results suggest that the second-order spinal cord pathway facilitates cortical reactivation, likely through the potentiation of persisting tactile inputs from the hand to the Cu over months of postlesion recovery.
Spinal cord injury often causes devastating deficits in motor and sensory functions, but considerable spontaneous recovery over weeks to months postlesion is common (1–6). The primary source of somatosensory cortex activation in response to touch on the hand is from afferents from the hand that enter the spinal cord and travel in the dorsal column to terminate directly in the cuneate nucleus (Cu). Many of these afferents branch to terminate on spinal cord neurons in the dorsal horn (7), and afferents from those neurons then terminate in the Cu. This is the second-order, or secondary spinal cord pathway. Neurons in the Cu relay to the contralateral somatosensory thalamus and then to the primary somatosensory cortex (area 3b), which projects to higher-order somatosensory areas.
To early investigators, the anatomy of the system suggested that a dorsal column lesion (DCL) in the upper cervical spinal cord would produce a profound loss of tactile hand function (8, 9). However, early studies of monkeys (10) and humans (11) with DCLs found little evidence for deficits in tactile abilities. It now seems likely that these discrepancies reflect differences in tactile abilities soon after DCLs or after months of recoveries. Here we present evidence that over time, a second-order pathway in the spinal cord, along with even a few preserved axons of the primary pathway, together mediate these recoveries. The results further indicate that the second order pathway alone can produce some cortical reactivation and a more limited functional recovery.
The secondary pathway has not been extensively studied but is considered a source of modulation that constrains receptive field sizes of neurons in the Cu and modifies the response characteristics driven by inputs from the primary axons in the dorsal column pathway (7, 12). Our studies in intact squirrel monkeys revealed that thousands of spinal cord neurons project to the Cu (13). These secondary neurons have the same tactile information as the primary afferents (12) and, in principle, can replace them, although with a synaptic delay. Because some of the secondary pathway axons travel more laterally within the spinal cord (14–16) or enter the dorsal column pathway above the lesion level, DCLs have a greater impact on the primary pathway than on the secondary pathway. The small numbers of surviving primary dorsal column axons, together with remaining second-order afferents to the Cu, are not initially capable of activating primary somatosensory cortex or other relays of the somatosensory system (17); however, reactivation and recovery of function occurs over weeks to months postlesion. Here we present evidence that this recovery results from an increasing and changing role for the second-order pathway.
Results
DCLs.
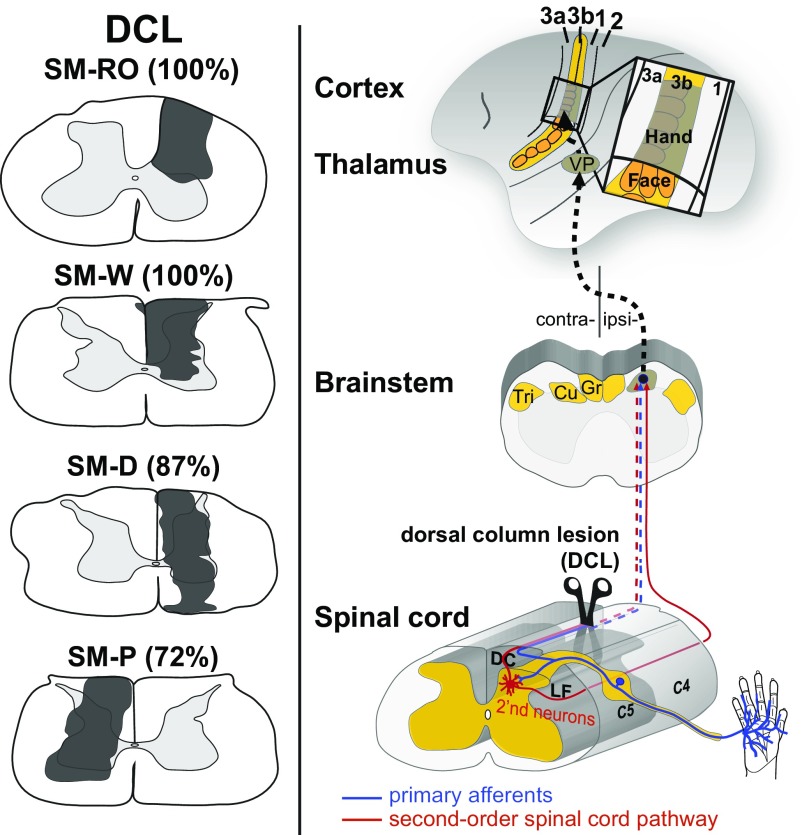
DCL sites were restricted unilaterally at the C4 level of the cervical spinal cord and were of varying severity in four squirrel monkeys (Fig. 1). To evaluate the extent of the DCLs, we injected cholera toxin subunit B conjugated to wheat germ agglutinin-horseradish peroxidase (B-HRP) into matching locations of digits 1, 3, and 5 of both hands. Lesion size was estimated based on the ratio of total labeled area on the lesioned side to that on the intact side (Fig. S2) (18, 19). Monkeys SM-RO and SM-W had the most extensive DCLs; their lesion sites were mostly restricted to the right side of the spinal cord and included the entire cuneate fasciculus and the dorsal and intermediate regions of the spinal gray matter. Patches of B-HRP–labeled axonal terminals were found in the expected locations of representations of digits 1, 3, and 5 in the Cu on the intact side. On the lesioned side, no B-HRP labeled patches were found throughout the rostrocaudal extent, suggesting that the extent of lesions was 100% complete. Monkeys SM-D and SM-P had large but incomplete DCLs that involved most of the cuneate fasciculus and spinal gray matter. The most medial and dorsolateral regions of the dorsal column were spared. B-HRP–labeled patches were found in the expected locations of digit representations in the Cu on both sides, but with far fewer labeled patches on the lesioned side. Quantitative comparisons of the labeled patches on both sides indicated that the lesion was 87% complete in monkey SM-D and 72% complete in monkey SM-P.
Fig. 1.
Transverse view of the DCL (dark-gray shading; estimated 72–100% complete) in four squirrel monkeys (Left) and diagram of how the DCL deprives the ascending primary afferents (blue) and second-order spinal cord pathway (red) from the dorsal column to the Cu in the brainstem, the ventroposterior nucleus (VP) in the thalamus, and the hand representation in primary somatosensory area 3b. Note that the second-order spinal cord pathway may continue to send cutaneous inputs from the hand to the Cu through the spared dorsal column (DC) and lateral funiculus (LF) of the spinal cord. C4–C5, cervical spinal segments; Gr, gracile nucleus; Tri, trigeminal nucleus.
Distributions of Labeled Neurons from the Cu Injection.
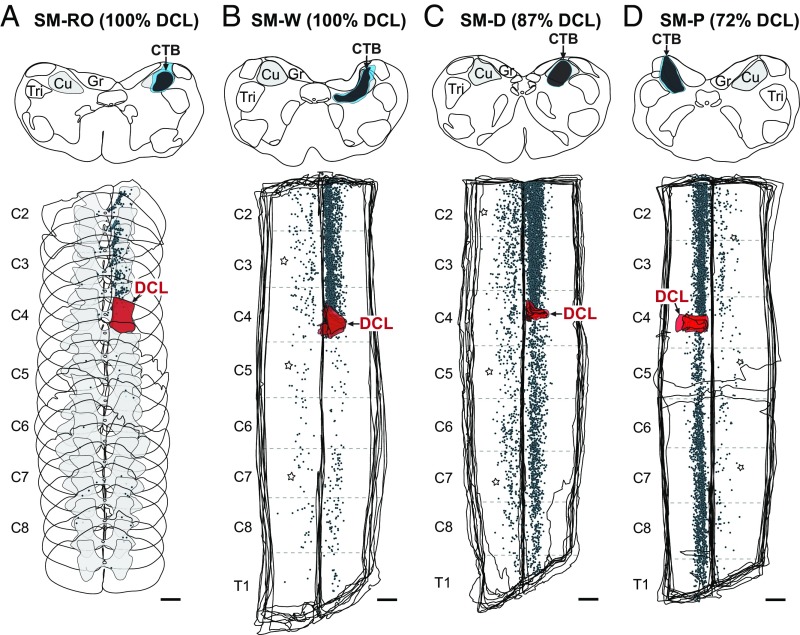
The organization of spinocuneate connections at ≥7 mo after DCL was revealed by labeling after cholera toxin subunit B (CTB) injection in the hand representation in Cu ipsilateral to the lesion site (Fig. 2 and Table S1). In all cases, the injection cores and dense uptake zones extended approximately 1–1.5 mm rostrocaudally and were confined primarily to the territory of the Cu, with slight spreading beyond its ventral border. CTB-labeled neurons were distributed mostly in the cervical spinal cord ipsilateral to the injection site, with differences in the distributions of labeled cells between monkeys with complete DCLs and those with incomplete DCLs. In the monkeys with estimated 100% complete DCLs, the majority of CTB-labeled neurons were located above the lesion (n = 1,554 in monkey SM-RO and 1,969 in monkey SM-W from the series of CTB sections); however, small numbers of labeled neurons (n = 63 in monkey SM-RO and 123 in monkey SM-W) were found below the lesion, within the hand representation in the spinal cord (Fig. 2 A and B). In the monkeys with incomplete DCLs, large numbers of labeled neurons were located both above (n = 2,599 in SM-D and 1,887 in SM-P) and below (n = 1,099 in SM-D and 1,457 in SM-P) the lesion(Fig. 2 C and D). As in normal monkeys (13), we found labeled neurons in the cervical spinal cord contralateral to the Cu injection in monkeys with complete DCLs (n = 147 in SM-RO and 241 in SM-W) and those with incomplete DCLs (n = 735 in SM-D and 256 in SM-P). Some CTB-labeled neurons were distributed on both sides of the thoracic, lumbar, and sacral spinal cord (Table S2).
Fig. 2.
Distributions of labeled neurons (blue dots) in the cervical spinal cord after CTB injection in the hand representation in Cu in four squirrel monkeys with DCLs (red; estimated 72–100% complete). Transverse sections are shown in monkey SM-RO (A), and aligned horizontal sections are shown in the other three monkeys (B–D). Dark-blue and light-blue shadings depict cores and halos of the injection site, respectively. C2–C8, cervical spinal segments 2–8; T1, thoracic spinal segment. (Scale bar: 1 mm.) Other conventions are as in Fig. 1.
Overall, our results show that with long recovery periods (≥7 mo), substantial numbers of cervical spinal cord neurons below the deactivating lesion continue to project to the Cu representation of the affected hand. The number of connections is affected by the extent of the lesion; significant numbers of connecting neurons are labeled after complete DCLs, but many more are labeled after large but incomplete lesions.
Cortical Reactivation of the Hand Region in Area 3b.
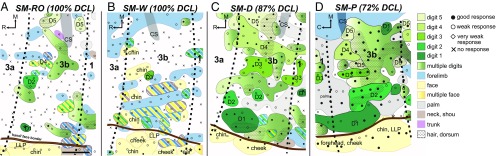
The regions of face, hand, and forelimb representations in area 3b are normally organized in a lateral-to-medial sequence in parietal cortex in squirrel monkeys (20). Interruption of dorsal column inputs from the upper cervical spinal cord temporarily silences the neurons in hand cortex and eventually alters somatotopic patterning (1, 2). We evaluated the somatotopic organization of the deprived hand cortex after long recovery times with multiunit microelectrode mapping (Fig. 3). Large-scale cortical reorganization was observed in monkeys SM-RO and SM-W with complete DCLs (Fig. 3 A and B). Approximately one-half of the neurons at recording sites in the deactivated hand cortex of area 3b (57.8% of recording sites in SM-RO and 42% in SM-W; Fig. S3) were totally unresponsive to touch on the hand, face, and forelimb. Variable percentages of hand cortex sites (24.5% and 1.4%) responded weakly to touch on the hand. However, neurons clustered in small, discontinuous patches of hand cortex (2% and 1.4%) were highly responsive to touch on the glabrous and dorsal regions of multiple digits or occasionally on a single digit. Other neurons in hand cortex became weakly responsive to touch on the face (0% and 13%), forearm (6.9% and 15.9%), or large surfaces of several body parts (8.8% and 24.6%).
Fig. 3.
Surface view of the somatotopic map of the hand region in area 3b after prolonged recovery periods from DCLs. In monkeys SM-RO (A) and SM-W (B), with estimated 100% complete DCLs, nearly one-half of the hand cortex in area 3b remained unresponsive, and some neurons responded weakly to touch on the face and forelimb, and occasionally on the hand. In monkeys SM-D (C) and SM-P (D) with large but incomplete DCLs (estimated 87% and 72% complete DCLs, respectively), the hand cortex in area 3b responded well to touch on the hand in a roughly somatotopic organization. Representations of the forelimb, hand, and face are color- coded in blue, green, and yellow, respectively; representations of multiple-site receptive fields involving the forelimb, hand, and face are shown in combinations of colored stripes accordingly. The rostral and caudal borders of area 3b (dashed lines) were estimated by the electrolytic lesions (stars) during electrophysiological mapping and from adjacent flattened sections immunostained for VGLUT2. C, caudal; CS, central sulcus; D, digit; LLP, lower lip; M, medial; R, rostral. (Scale bars: 1 mm.)
In contrast, in monkeys with incomplete DCLs (87% complete in SM-D and 72% complete in SM-P), neurons across many sites in the hand cortex were strongly activated by touch on the hand (74.2% of recording sites in SM-D and 96.5% in SM-P) and were arranged in a roughly somatotopic organization (Fig. 3 C and D). Complex receptive fields involving the hand and forelimb were rare (2% and 0%, respectively). Only 23.7% of neurons in the hand cortex in SM-D and 3.5% in SM-P remained unresponsive to touch on the hand, face, and forelimb. Our findings revealed that after large but incomplete lesions of dorsal column afferents and subsequent long recovery periods, most hand neurons in area 3b regain responsiveness to touch on the hand, whereas after complete lesions, only limited reactivations from the hand occur.
Potentiation of the Second-Order Spinal Cord Pathway and Cortical Reactivation.
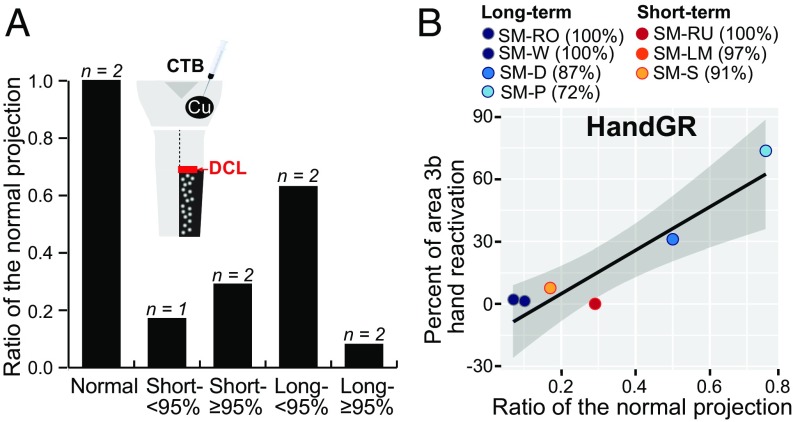
To examine whether injured second-order spinal cord projections to the Cu are potentiated after injuries, we tested if a longer time after the DCL altered the number of labeled neurons in the cervical spinal cord ipsilateral to and below the lesion (C5–C8). For comparison, data were reviewed from two normal monkeys and three monkeys shortly after DCLs (13) (Fig. 4A and Table S3). Given the possible variations in tracer injection size and transportation efficiency across cases, we used the “ratio of the normal proportion” to represent the number of surviving secondary projections (Methods). The monkeys with DCLs were categorized into two groups: complete plus nearly complete (estimated ≥95% complete) and incomplete (estimated <95% complete) (Fig. S4). Our previous study showed that the proportions of labeled neurons below the lesion are significantly decreased from normal 2 wk after DCLs (13). The proportions of surviving second-order connections were 0.29 in two monkeys following nearly complete DCLs, and 0.17 in one monkey with an incomplete DCL. However, in cases with long recovery times, the ratios were 0.75 and 0.5 in two monkeys with incomplete DCLs (87% and 72% complete, respectively), but only 0.11 and 0.07 in two monkeys with complete DCLs. Our findings suggest that the functional secondary projections to the Cu from neurons below the lesion increase after months of recovery when the DCL is <95% complete, but these connections may become sparser when the lesion is complete or nearly complete.
Fig. 4.
Alterations of second-order spinal cord projections over the DCL recovery periods and correlation with the cortical reactivation in the hand region in area 3b. (A) Bar graphs show that the ratio of labeled neurons below the lesion in the cervical spinal cord is decreased in monkeys with short-term DCLs. However, the ratio increases considerably in monkeys with incomplete DCLs (<95%; 0.65) following long recovery periods and decreases in those with nearly complete DCLs (≥95%; 0.09). (B) Nonparametric Spearman’s rho analysis indicates that the ratio of projection neurons for the affected hand tends to have a positive correlation with strong neural response to hand stimulation (HandGR). Points color-coded for individual monkeys represent one proportion value for each axis. The gray zone depicts the 95% confidence intervals, and the black line represents the trend line.
The contribution of the second-order spinal cord projection to reactivation of the area 3b hand cortex was analyzed in seven monkeys with injuries (Fig. 4B and Table S4). We divided the hand cortex in area 3b based on responsiveness to the hand, face, or forelimb or larger areas involving the hand and face and/or forelimb (hand and others), face and forelimb, or unresponsive. The percentage of cortical maps with these response fields was then related to the proportion of labeled neurons below the lesion. Although the number of data points might have been insufficient to achieve statistical significance, the proportion of labeled neurons tended to be positively correlated with the ratio of recording sites with strong neural responses to touch on the hand (Spearman’s ρ = 0.418; P = 0.3505) in the affected cortex. The proportion of labeled neurons had no clear correlation with the ratio of sites with weak responses to the hand (ρ = 0; P = 1.0000) and had negative correlations with touch on the face (ρ = −0.412; P = 0.3585); forelimb (ρ = −0.764; P = 0.0455); complex receptive fields involving the hand, face, and/or forelimb (ρ = −0.577; P = 0.1754); face and forelimb (ρ = −0.764, P = 0.0455); and the unresponsive sites (ρ = −0.342; P = 0.4523). These findings support the conclusion that the second-order spinal cord projections from the hand representation in spinal cord contribute to the cortical reactivation by inputs from the hand. Inputs from the face and forelimb may compete for cortical territory.
Discussion
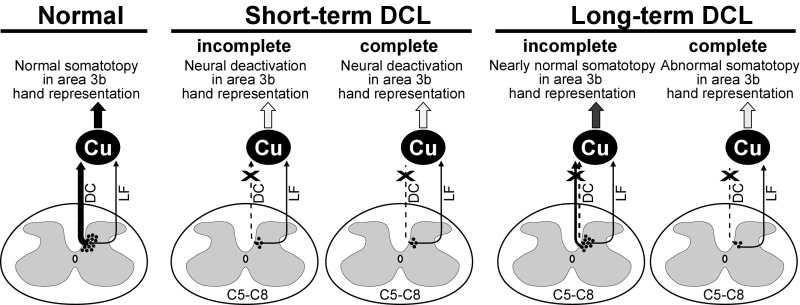
Here we provide anatomical and physiological evidence that the second-order pathway is critical to cortical reactivation and recovery after dorsal column injury. We show that the numbers of second-order spinal cord neurons below the lesion continue to project to the ipsilateral Cu over long recovery periods after extensive DCLs. These persisting projections accompany the variable extent of cortical reactivation to the hand in area 3b cortex. Through comparisons with data from normal monkeys and monkeys at 2 wk postlesion (13), we found that following complete DCLs, the number of spinal cord neurons from below the lesion to the ipsilateral Cu remained small or decreased with long recovery periods (Fig. 5), and yet some responses to touch on the hand recovered in deactivated area 3b hand cortex. After large but incomplete DCLs, the number of projection neurons increased with months of recovery, and the deactivated 3b hand cortex was extensively responsive to touch on the hand in a roughly somatotopic pattern. Importantly, the amount of the second-order spinal cord projections from the hand reflects the extent of cortical reactivation to touch on the hand, but not the responsiveness to other body parts in the hand region in area 3b.
Fig. 5.
Summary diagram illustrating the plasticity of second-order spinal cord pathways after DCLs at C4 and the cortical reactivation in the hand cortex in area 3b. In normal monkeys, abundant numbers of secondary neurons project to the Cu, and the area 3b hand cortex is somatotopically organized. The numbers of projection neurons decrease significantly at 2 wk after incomplete or complete DCLs (shown by  ), and the corresponding hand cortex in area 3b is deactivated. However, the number of projection neurons increases considerably in monkeys with incomplete DCLs over time through the potentiation of the surviving dorsal column pathway and the preserved dorsolateral pathway. Most of the affected area 3b cortex becomes responsive to touch on the hand in a nearly normal pattern. In monkeys with complete DCLs, the number of projection neurons is small, and the affected area 3b cortex remains unresponsive or abnormal. Line thickness indicates the strength of projections, arrowheads represent the existence of inputs, dashed lines denote the injured pathway, and the dark- to light-gray shading in arrows differentiates the strengths of ascending inputs. Other conventions are as in Fig. 1.
), and the corresponding hand cortex in area 3b is deactivated. However, the number of projection neurons increases considerably in monkeys with incomplete DCLs over time through the potentiation of the surviving dorsal column pathway and the preserved dorsolateral pathway. Most of the affected area 3b cortex becomes responsive to touch on the hand in a nearly normal pattern. In monkeys with complete DCLs, the number of projection neurons is small, and the affected area 3b cortex remains unresponsive or abnormal. Line thickness indicates the strength of projections, arrowheads represent the existence of inputs, dashed lines denote the injured pathway, and the dark- to light-gray shading in arrows differentiates the strengths of ascending inputs. Other conventions are as in Fig. 1.
Secondary-Order Spinal Cord Pathway and Cortical Reactivation After the DCLs.
At weeks to months after the DCLs at a high level of the cervical spinal cord, whether incomplete or complete, a variable percentage of the affected hand region in primary somatosensory cortex and higher-order cortex becomes responsive to touch on the hand (2, 18, 19, 21, 22). Such somatotopically organized cortical reactivation suggests the existence of spinal cord projections that circumvent the lesion to convey low-threshold tactile inputs from the hand to the Cu after injuries. As no other spinal cord afferents have these characteristics, the few (if any) surviving primary afferents, together with a variable population of glabrous hand inputs relayed by spinal cord neurons, appear to combine to reactivate the Cu and, in turn, the somatosensory cortex (23).
The contribution of the secondary spinal cord inputs is most clearly shown after complete DCLs, which disrupt all the direct primary afferents but spare a small portion of secondary spinal cord projections traveling in the dorsolateral funiculus (13, 16, 24). In monkeys SM-RO and SM-W with estimated 100% complete DCLs, the Cu injection labeled small numbers of neurons (7% and 10% of the normal projection, respectively) in the hand representation below the lesion, which most likely connected through the secondary pathway through the dorsolateral funiculus. Functional evidence for this secondary pathway has been presented for rats (25) and cats (26).
Our present results support the conclusion that a small amount of secondary inputs from axons within the dorsolateral funiculus can effectively excite the Cu neurons, possibly through rearrangement and increased production of synapses and potentiation of subthreshold inputs that remained in place to become more effective (5, 27). In turn, the resulting activation of Cu neurons activates the hand representation in the contralateral ventroposterior nucleus, cortical area 3b, and areas of higher-order somatosensory cortex. Indeed, we found that a small percentage of area 3b cortex was responsive to touch on hand in the two monkeys with complete DCLs (31.4% in SM-RO and 11.5% in SM-W). In mice, as few as 3% of the corticospinal axons reestablished motor functions after the spinal cord injury (28). Likewise, we attribute the cortical reactivation in area 3b after complete DCLs to the surviving secondary spinal cord inputs.
The role of second-order spinal cord inputs in functional recovery appears to be even greater in monkeys with incomplete DCLs, which spare some of the dorsal column fibers and the dorsolateral funiculus. In monkeys SM-D and SM-P, with 87% and 72% complete DCLs, respectively, large areas in the hand region in area 3b (76.2% and 96.5%, respectively) regained responsiveness to touch on the hand in a roughly somatotopic pattern. While the incomplete DCLs allowed more inputs from the spinal cord neurons below the lesion to the Cu (50% and 75% of the normal projection, respectively), both spared primary and secondary projections that remained topographically connected to neurons in the Cu likely contributed to this extensive, somatotopic reactivation. These connections would remain roughly topographic even if surviving axons sprouted to activate nearby neurons. As the reactivated neurons in area 3b tended to respond to the skin indentation with longer latencies (average, ∼31 ms) than normal neurons (average, ∼21 ms) (29), much of the cortical reactivation may depend on the secondary pathway.
Relationship Between Somatotopically Normal and Abnormal Reactivation and Second-Order Spinal Cord Projection to the Cu.
We also found that cases with greater numbers of surviving second-order neurons labeled by injections in the Cu tended to have greater extents of the area 3b hand representation strongly reactivated by touch on the hand. The correlation was positive but only suggestive, as statistical analysis was limited by the number of cases. Correspondingly, the correlation was negative for other parts of the body (face and/or forelimb) that reactivated sites in the hand representations, as these activations reflect incomplete reactivation of the hand cortex by inputs from the hand. Such abnormal activations have been reported previously, especially after longer recovery times (5, 17). Surprisingly, there was no correlation between the number of second-order neurons projecting to the Cu and cortical sites that were weakly activated by touch on the hand. These weak responses may reflect intrinsic horizontal connections in area 3b (30, 31), rather than thalamic inputs activated by hand neurons in the Cu. Overall, the weak and somatotopically abnormal activation patterns likely reflect the formation and potentiation of atypical connections within the Cu and cortex that are possible only when reactivation of the Cu by hand inputs is incomplete (18, 32, 33).
Plasticity of the Second-Order Spinal Cord Projection and the Cu.
We have provided evidence that the contribution of second-order spinal cord afferents to the Cu may decrease over recovery time after nearly complete DCLs and increase after incomplete DCLs. Previously, we found that 91–100% of complete lesions greatly reduced second-order spinal cord inputs to the Cu to 17–29% of the normal projection when measured within 2 wk of the spinal cord lesion (13). Following ≥7 mo of recovery in the monkeys with complete DCLs, considerably smaller (7% and 10%) proportions of secondary neurons were labeled below the lesion, suggesting that some secondary inputs may be lost over time. Reasons for this possible loss are unclear, but the loss of secondary pathway neurons and/or inputs may result from inflammation, edema, ischemia, and excitotoxicity (34). Alternatively, inputs related to the hand may be outcompeted by inputs from the forelimb and face (18, 32), especially if the remaining afferents are damaged and impaired. In contrast, the numbers of labeled neurons below the lesion, when incomplete, were greater after long recoveries than expected, such that 50% and 75% of the normal numbers of secondary neurons were labeled below the lesion (87% and 72% complete DCLs). This greater number of labeled secondary neurons could reflect the reestablishment of functional connections of damaged axons or the wider distributions of axonal arbors in the Cu due to postlesion sprouting of axons and potentiation by remaining primary afferents so that more neurons would be labeled by similar injections.
Conclusion
We conclude that the recovery of activation of the somatosensory system via afferents from the hand that occurs months following extensive lesions of the spinal cord dorsal column pathway depends on small populations of primary afferents and secondary spinal cord afferent inputs to the Cu. While recovery is less extensive after complete lesions of the dorsal column pathway, the reduced second-order pathway is capable of mediating considerable recovery. Importantly, the reduced primary and secondary pathways are not capable of activating the somatosensory cortex immediately after lesions, as the return of cortical activation takes weeks to months. Thus, the effectiveness of preserved connections increases and promotes reactivation over time (4). Since the extent of cortical reactivation is often considered an index of behavioral recovery (23), surviving spinal cord afferents likely contribute to the recovery of hand use after long recovery times. Clinical efforts to promote recovery from spinal cord (34) and other nervous system injuries might focus on treatments to increase and promote the functions of preserved axons in damaged pathways. To this end, treating the Cu to promote the sprouting of axons, for example, with the enzyme chondroitinase ABC (35), increases the activation of somatosensory cortex by preserved inputs after DCLs (21).
Methods
Four New World squirrel monkeys (Saimiri boliviensis) were used in this study. All surgical procedures and animal care were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and Vanderbilt University’s guidelines. The monkeys were initially tranquilized with an i.m. injection of ketamine hydrochloride (10–25 mg/kg), and anesthesia was maintained by isoflurane (1–2% mixed in O2) during the surgical procedures. All procedures were performed under aseptic conditions, and vital signs were monitored throughout the experimental period. The anesthesia was switched to i.v. infusion of ketamine hydrochloride (10–25 mg/kg) during electrophysiological recordings.
In survival surgeries involving the Cu and cervical spinal cord, the monkey’s head was rotated ventrally to fully expose the magnum foramen and cervical vertebrae (13, 21). Each monkey’s recovery from anesthesia was closely monitored. In terminal surgeries, the monkey’s head was fixed in a standard stereotaxic position to access somatosensory area 3b in the parietal cortex.
Unilateral Dorsal Column Lesion at C4.
After an incision was made in the skin at the midline over the neck, we retracted the muscle layers above the C3-C5 vertebrae, removed the dorsal arch of the C4 vertebra, and displaced the dura and pia that covered the exposed area. We used fine-tipped forceps (no. 4) to crush the unilateral dorsal column at C4 for 2 min, followed by a cut with surgical microscissors at the same location. The exposed spinal cord was then protected by a piece of Gelfilm and Gelfoam (Pfizer) before the opening was closed.
Microelectrode Mapping and Tracer Injection in the Cu.
Retrograde tracer CTB (Sigma-Aldrich) was injected into the electrophysiologically defined hand representation in the Cu ipsilateral to the DCL to label the second-order spinal cord neurons after long recovery times (SM-RO, 231 d; SM-W, 251 d; SM-D, 209 d; SM-P, 245 d). Once the brainstem was exposed, a low-impedance microelectrode (1 MΩ) was inserted perpendicularly into the Cu ipsilateral to the lesion to identify the locations of hand representation (19). A small amount (0.01 μL) of 1% CTB (in dH2O) was injected at two depths, 800 and 600 μm. After the micropipette was withdrawn, the exposed area was covered by Gelfilm and Gelfoam, and the opening was closed.
Digit Injection.
To evaluate the extent of lesion, CTB conjugated with B-HRP (5 μL, 0.2% in distilled water; List Biological) was injected into matching parts of digits 1, 3, and 5 of both hands at 5 d before the terminal mapping procedure, to allow for tracer transportation.
Microelectrode Mapping in Area 3b.
At 3 wk after the Cu injections, the hand cortex of area 3b contralateral to the lesion was examined by multiunit microelectrode recordings. A low-impedance tungsten microelectrode was lowered perpendicularly through the brain surface to a depth of 650 μm, where layer IV is located. Standard mapping approaches, including light touching and brushing the skin, tapping the muscles, and moving the joints, were used to define the neuronal receptive fields. We systematically placed the microelectrode every 300–400 μm. Small electrolytic lesions (2 mm deep, 10 μA for 10 s) were made at borders of hand regions in areas 3b, 3a, and 1 for identification after tissue processing.
Perfusion and Histology.
After mapping, the monkey was euthanized with a high dose of sodium pentobarbital (120 mg/kg). Perfusion was performed through the ascending aorta with 0.01 M PBS (pH 7.4), followed by 2–4% paraformaldehyde in 0.1 M PB and 10% sucrose-containing fixative. The brainstem and spinal cord (C2–C8) were removed and placed in 30% sucrose-PB for cryoprotection. We placed pins at boundaries of the cervical spinal cord segments based on the rostrocaudal arrangement of dorsal rootlets to identify the cervical segments after the spinal cord was cut. The brainstem was cut in the transverse plane at a thickness of 50 μm. In monkey SM-RO, the cervical spinal cord was cut in the transverse plane (50 μm thickness) to reveal the laminar distribution of labeled neurons. In the other three monkeys, the cervical spinal cord was cut in the horizontal plane (40 μm thickness). The sections were divided into series to reveal the B-HRP and CTB labeling along with architectural structures by cytochrome oxidase (CO), vesicular glutamate transporter 2 (VGLUT2), and NeuN. Histology has been described in detail previously (36).
Data Analysis.
The DCL level was identified based on the pin placements between the cervical segments and was augmented by the B-HRP label at C5, C6, and C7 from injections in digits 1, 3, and 5 (19). In the three monkeys in which the spinal cord was cut in the horizontal plane, a transverse view of the DCL was reconstructed from a series of horizontal sections. In addition, we measured the area of B-HRP–labeled patches in the Cu on both sides across the rostrocaudal brainstem sections using ImageJ 64 software (National Institutes of Health). The ratio of total B-HRP–labeled area on the lesioned side to that on the intact side was used as a quantitative estimate of DCL extent in each monkey (18, 19). The location of the CTB injection site in the brainstem and the distributions of CTB-labeled neurons in the spinal cord were depicted systematically using the Neurolucida system (MBF Bioscience).
In monkey SM-RO, the plot of labeled cells was aligned to the adjacent NeuN sections (Adobe Illustrator; Adobe Systems) to reveal the labeled neurons in the spinal laminae. In the other three monkeys, we aligned the plots of horizontal cervical sections based on the pinholes. The numbers of labeled neurons above and below the lesion in the cervical spinal cord were quantified. Based on the receptive fields and response modalities identified by the electrophysiological recordings, the somatotopic map in the hand representation of area 3b was reconstructed (18). We estimated borders of the area 3b hand region based on the mapping results and the architectural information revealed by the adjacent VGLUT2-stained sections.
To assess whether the second-order spinal cord projection varied in terms of the number of connections with time after the DCL, we compared the numbers of labeled neurons in the cervical spinal cord after the Cu injection in the present study (n = 4 long-term DCLs) with the data obtained from normal monkeys (n = 2) and monkeys after short-term DCL (n = 3) in our previous study (13). Because tracer injection size and transportation efficiency could differ across cases, we normalized these data to the ratio of the normal projection to represent the second-order spinal cord projection in monkeys with DCLs. For each case, we calculated the proportion of labeled neurons in the two subdivisions—above the lesion (or C2–C4 in normal monkeys) and below the lesion (or C5–C8)—relative to the number of total labeled neurons in the cervical spinal cord ipsilateral to the lesion (C2–C8). The “normal projection” was the mean value from the two normal monkeys. The secondary projection below the lesion in each monkey with a DCL was assessed with the ratio of the proportion of labeled neurons below the lesion to the proportion of normal projection. Similarly, the neuron responses to touch on the hand, face, and/or forelimb were represented as proportions for each monkey, because the total number of microelectrode penetrations collected differed. We categorized the monkeys into four groups based on the survival periods (short- or long-term) and lesion extent [incomplete (<95%) or nearly complete (≥95%)] by the complete linkage hierarchical clustering of scaled factors (z-score, using SPSS; Fig. S4). We estimated the relationship of second-order spinal cord projections and the cortical reactivation in the hand cortex using nonparametric Spearman’s correlations (two-sided, with 95% confidence interval, using R).
Supplementary Material
Acknowledgments
We thank Drs. Emily C. Turner and Daniel J. Miller for data collection; Mary Feurtado, veterinary technician; Laura Trice, histological technician; Ya-Chen Lisa Lin (Biostatistics Graduate Program and Biostatistics Clinic, Vanderbilt University) for data analysis; and Dr. Pooja Balaram for helpful comments on the manuscript. This work was supported by National Institutes of Health Grants NS16446 (to J.H.K.) and NS067017 (to H.-X.Q.) and the Craig H. Neilsen Foundation (J.H.K. and J.L.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718826115/-/DCSupplemental.
References
- 1.Kaas JH, et al. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi HX, Kaas JH, Reed JL. The reactivation of somatosensory cortex and behavioral recovery after sensory loss in mature primates. Front Syst Neurosci. 2014;8:84. doi: 10.3389/fnsys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoichet MS, Tate CC, Baumann MD, LaPlaca MC. Strategies for regeneration and repair in the injured central nervous system. In: Reichert WM, editor. Indwelling Neural Implants: Strategies for Contending with the in Vivo Environment. CRC Press/Taylor & Francis; Boca Raton, FL: 2008. [PubMed] [Google Scholar]
- 4.Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darian-Smith C, Ciferri M. Cuneate nucleus reorganization following cervical dorsal rhizotomy in the macaque monkey: Its role in the recovery of manual dexterity. J Comp Neurol. 2006;498:552–565. doi: 10.1002/cne.21088. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett JW, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 7.Dykes RW, Craig AD. Control of size and excitability of mechanosensory receptive fields in dorsal column nuclei by homolateral dorsal horn neurons. J Neurophysiol. 1998;80:120–129. doi: 10.1152/jn.1998.80.1.120. [DOI] [PubMed] [Google Scholar]
- 8.Mountcastle VB, Darian-Smith I. Neural mechanisms is somesthesia. In: Mountcastle VB, editor. Medical Physiology. Mosby; St. Louis, MO: 1968. pp. 1372–1423. [Google Scholar]
- 9.Rose JE, Mountcastle VB. Activity of single neurons in the tactile thalamic region of the cat in response to a transient peripheral stimulus. Bull Johns Hopkins Hosp. 1954;94:238–282. [PubMed] [Google Scholar]
- 10.Azulay A, Schwartz AS. The role of the dorsal funiculus of the primate in tactile discrimination. Exp Neurol. 1975;46:315–332. doi: 10.1016/0014-4886(75)90138-7. [DOI] [PubMed] [Google Scholar]
- 11.Wall PD. The sensory and motor role of impulses travelling in the dorsal columns towards cerebral cortex. Brain. 1970;93:505–524. doi: 10.1093/brain/93.3.505. [DOI] [PubMed] [Google Scholar]
- 12.Dick SH, French AS, Rasmusson DD. Postsynaptic dorsal column and cuneate neurons in raccoon: Comparison of response properties and cross-correlation analysis. Brain Res. 2001;914:134–148. doi: 10.1016/s0006-8993(01)02787-1. [DOI] [PubMed] [Google Scholar]
- 13.Liao CC, DiCarlo GE, Gharbawie OA, Qi HX, Kaas JH. Spinal cord neuron inputs to the cuneate nucleus that partially survive dorsal column lesions: A pathway that could contribute to recovery after spinal cord injury. J Comp Neurol. 2015;523:2138–2160. doi: 10.1002/cne.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cliffer KD, Giesler GJ., Jr Postsynaptic dorsal column pathway of the rat, III: Distribution of ascending afferent fibers. J Neurosci. 1989;9:3146–3168. doi: 10.1523/JNEUROSCI.09-09-03146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enevoldson TP, Gordon G. Spinocervical neurons and dorsal horn neurons projecting to the dorsal column nuclei through the dorsolateral fascicle: A retrograde HRP study in the cat. Exp Brain Res. 1989;75:621–630. doi: 10.1007/BF00249913. [DOI] [PubMed] [Google Scholar]
- 16.Rustioni A, Hayes NL, O’Neill S. Dorsal column nuclei and ascending spinal afferents in macaques. Brain. 1979;102:95–125. doi: 10.1093/brain/102.1.95. [DOI] [PubMed] [Google Scholar]
- 17.Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- 18.Liao CC, Reed JL, Kaas JH, Qi HX. Intracortical connections are altered after long-standing deprivation of dorsal column inputs in the hand region of area 3b in squirrel monkeys. J Comp Neurol. 2016;524:1494–1526. doi: 10.1002/cne.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi HX, Chen LM, Kaas JH. Reorganization of somatosensory cortical areas 3b and 1 after unilateral section of dorsal columns of the spinal cord in squirrel monkeys. J Neurosci. 2011;31:13662–13675. doi: 10.1523/JNEUROSCI.2366-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sur M, Nelson RJ, Kaas JH. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: Comparisons with other primates. J Comp Neurol. 1982;211:177–192. doi: 10.1002/cne.902110207. [DOI] [PubMed] [Google Scholar]
- 21.Bowes C, Massey JM, Burish M, Cerkevich CM, Kaas JH. Chondroitinase ABC promotes selective reactivation of somatosensory cortex in squirrel monkeys after a cervical dorsal column lesion. Proc Natl Acad Sci USA. 2012;109:2595–2600. doi: 10.1073/pnas.1121604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi HX, et al. Spatiotemporal trajectories of reactivation of somatosensory cortex by direct and secondary pathways after dorsal column lesions in squirrel monkeys. Neuroimage. 2016;142:431–453. doi: 10.1016/j.neuroimage.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi HX, Reed JL, Gharbawie OA, Burish MJ, Kaas JH. Cortical neuron response properties are related to lesion extent and behavioral recovery after sensory loss from spinal cord injury in monkeys. J Neurosci. 2014;34:4345–4363. doi: 10.1523/JNEUROSCI.4954-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cliffer KD, Willis WD. Distribution of the postsynaptic dorsal column projection in the cuneate nucleus of monkeys. J Comp Neurol. 1994;345:84–93. doi: 10.1002/cne.903450106. [DOI] [PubMed] [Google Scholar]
- 25.Tomasulo KC, Emmers R. Activation of neurons in the gracile nucleus by two afferent pathways in the rat. Exp Neurol. 1972;36:197–206. doi: 10.1016/0014-4886(72)90146-x. [DOI] [PubMed] [Google Scholar]
- 26.Dart AM, Gordon G. Some properties of spinal connections of the cat’s dorsal column nuclei which do not involve the dorsal columns. Brain Res. 1973;58:61–68. doi: 10.1016/0006-8993(73)90823-8. [DOI] [PubMed] [Google Scholar]
- 27.Bradbury EJ, McMahon SB. Spinal cord repair strategies: Why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 28.Hilton BJ, et al. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci. 2016;36:4080–4092. doi: 10.1523/JNEUROSCI.3386-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JL, et al. Response properties of neurons in primary somatosensory cortex of owl monkeys reflect widespread spatiotemporal integration. J Neurophysiol. 2010;103:2139–2157. doi: 10.1152/jn.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das A, Gilbert CD. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature. 1995;375:780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- 32.Jain N, Florence SL, Qi HX, Kaas JH. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci USA. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kambi N, et al. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun. 2014;5:3602. doi: 10.1038/ncomms4602. [DOI] [PubMed] [Google Scholar]
- 34.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 35.Massey JM, et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao CC, Gharbawie OA, Qi H, Kaas JH. Cortical connections to single digit representations in area 3b of somatosensory cortex in squirrel monkeys and prosimian galagos. J Comp Neurol. 2013;521:3768–3790. doi: 10.1002/cne.23377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.