Significance
Evolution of organismal complexity and species diversity depends on the emergence of novel gene functions. Nevertheless, evolution rarely produces novelties from scratch but works on the weak promiscuous preexisting activities or appears by genomic tinkering. We provide evidence of how rearrangement of conserved regulatory blocks can act as a driving force for gene cooption and evolution of novel developmental mechanisms at the base of important ecological adaptations. We gain insight into a crucial system for segregation of neuronal progenitors within the hindbrain: the evolutionary origin of the actomyosin-dependent cell-sorting mechanism, with rac3b as a main effector. We unveil that the rac3b/rfng/sgca regulatory cluster—specifically expressed at boundaries—emerged by establishment of novel long-range cis-regulatory interactions, allowing the evolution of a backup regulatory mechanism for cell segregation.
Keywords: hindbrain boundaries, regulatory landscape, segmentation, cis-regulatory elements, rhombomeres
Abstract
Developmental programs often rely on parallel morphogenetic mechanisms that guarantee precise tissue architecture. While redundancy constitutes an obvious selective advantage, little is known on how novel morphogenetic mechanisms emerge during evolution. In zebrafish, rhombomeric boundaries behave as an elastic barrier, preventing cell intermingling between adjacent compartments. Here, we identify the fundamental role of the small-GTPase Rac3b in actomyosin cable assembly at hindbrain boundaries. We show that the novel rac3b/rfng/sgca regulatory cluster, which is specifically expressed at the boundaries, emerged in the Ostariophysi superorder by chromosomal rearrangement that generated new cis-regulatory interactions. By combining 4C-seq, ATAC-seq, transgenesis, and CRISPR-induced deletions, we characterized this regulatory domain, identifying hindbrain boundary-specific cis-regulatory elements. Our results suggest that the capacity of boundaries to act as an elastic mesh for segregating rhombomeric cells evolved by cooption of critical genes to a novel regulatory block, refining the mechanisms for hindbrain segmentation.
Spatiotemporal changes in gene expression underlie many evolutionary novelties that impact the body plan and tissue specification. New patterns of gene expression may arise by a variety of mechanisms, involving changes in upstream gene networks, as well as modifications of individual cis-regulatory regions or entire regulatory landscapes. Thus, novel expression patterns can be generated by de novo genesis of enhancers (1), gain- and loss-of-function of enhancer regions (2–4), and cooption of latent activities from existing regulatory sequences (5–7). Individual enhancer–promoter interactions do not occur in isolation but in the context of topological associating chromosome domains (TADs), which organize the chromatin into discrete regulatory landscapes (8, 9). Disruption of TADs results in altered gene–enhancer interactions and deregulated gene expression, which may cause embryonic malformations (10, 11), cancer (12, 13), or dictate the virus integration sites (14). It has been proposed that changes affecting the organization of regulatory domains may have had a major influence in the evolutionary history of gene regulation (8). In fact, preliminary data support the notion that TADs can provide a structural basis for the maintenance of conserved syntenic blocks across species (9, 15). Despite this, there is little information on how the disruption of regulatory blocks during evolution may act as a positive force for the emergence of novel gene expression patterns and developmental mechanisms. Our report addresses the appearance of a novel gene regulatory landscape underlying hindbrain segmentation in zebrafish.
Embryonic segments are fundamental building blocks of the body plan. During animal development, proliferating cells are organized into segmental compartments with boundaries across which cells fail to intermingle, ensuring that their fates remain segregated as they proliferate and move. Thus, the establishment and maintenance of compartment boundaries are of critical importance in tissue segmentation and body plan organization (16). The vertebrate hindbrain is a good model to address the specification of segmental domains and the establishment of boundaries, since it is transiently segmented into rhombomeres (r1–r7) that constitute developmental units of gene expression and cell lineage compartments (17–19). The process of compartmentalization involves the sorting of cells from neighboring rhombomeres, which express Eph receptors or Ephrin ligands, and the formation of a cellular interface between adjacent segments named the hindbrain boundary (20). This cell population not only displays a different morphology than its neighbors (21) but it serves distinct functions as development proceeds. First, when morphological segments arise, boundary cells work as an elastic mesh, preventing cell intermingling between adjacent compartments. We have previously shown that, in zebrafish, this is due to the enrichment of actomyosin cable-like structures in their apical side (Movie S1), whose formation requires Eph/Ephrin signaling and downstream small GTPase effectors (22). During neurogenesis, hindbrain boundaries behave as a node for signaling pathways—such as Notch, Wnts, or semaphorins—instructing the differentiation and organization of neurons in the neighboring rhombomeres (23–26). Later, hindbrain boundaries provide proliferating progenitors and differentiating neurons to the hindbrain (27). Therefore, a fundamental question is how these cells coordinately unfold their distinct functional properties over the entire program of hindbrain morphogenesis. Hence, it is important to understand how morphogenetic genes are specifically activated in the boundary domain.
In this work, we aimed to identify the regulatory modules underlying the origin of a specific functional feature associated with hindbrain boundaries in zebrafish: the ability to assemble actomyosin structures. We show that the small GTPase Rac3b, which is specifically expressed at boundary cells, acts as an effector of Eph/Ephrin signaling within this cell population in the assembly of actomyosin structures, and thereby restricts cell mixing between neighboring compartments. Interestingly, in the genome, rac3b is placed in close proximity to two other genes, rfng and sgca, which are also expressed in hindbrain boundaries in zebrafish. We have identified and isolated several cis-regulatory regions driving gene expression specifically to hindbrain boundary cells and have shown the importance of other regulatory sequences, redundant enhancers, which provide regulatory robustness to the system. We show that the rac3b/rfng/sgca cluster appears within a regulatory block present in zebrafish (Danio rerio) and Astyanax mexicanus. These species are members of the superorder Ostariophysi, the second-largest superorder of fish, which contains 28% of fish species and nearly 70% of all freshwater fish species (28), and whose embryonic development is faster than other teleost fish (29). Moreover, in contrast to tetrapods and other teleosts, these two species display rac3b expression and actomyosin structures at the hindbrain boundaries. These results suggest that the capacity of hindbrain boundary cells to act as elastic barrier for segregating adjacent rhombomeric cells emerged in Ostariophysi superorder by an intrachromosomal rearrangement that allowed gene cooption to a novel regulatory domain. This additional mechanism may confer robustness to the hindbrain segmentation program.
Results
rac3b, rfng, and sgca Are Located in Genomic Synteny and Expressed in the Hindbrain Boundary Cells.
Increased cortical tension through contraction of actomyosin structures underlies rhombomeric cell segregation in zebrafish. Previously, we demonstrated the importance of RhoA-GTPase in the organization of actomyosin cable-like structures acting downstream of EphA/Ephrin signaling to segregate cells from different rhombomeres (22). Since RhoA is ubiquitously expressed within the embryo (30), we were interested in finding additional downstream effectors of Eph/Ephrin signaling that could be expressed in the hindbrain boundaries. To this end, we focused on the small GTPase gene family, and particularly on rac3b, which is highly enriched at the hindbrain boundaries, as shown by double staining with egr2a—a landmark for r3 and r5 (Fig. 1 B–E)—being expressed as well in the center of the rhombomeres (Fig. 1E) by 30 hours postfertilization (hpf).
Fig. 1.
rac3b, rfng, and sgca are closely positioned within chromosome 12 and expressed in hindbrain boundary cells. (A) Gene organization within the indicated region of chromosome 12. (B–M) Spatiotemporal profile of rac3b, rfng, and sgca in the hindbrain: double in situ hybridizations for rac3b, rfng, or sgca (blue) and egr2a (red) as a landmark of rhombomeres 3 and 5 (r3, r5). Note that rac3b, rfng, and sgca are expressed at the hindbrain boundaries with a similar onset of expression. All images are dorsal views of flat-mounted hindbrains with anterior at the top.
To study the regulation of rac3b expression in hindbrain boundary cells we analyzed the rac3b genomic landscape. Strikingly, we noticed that rac3b is located in chromosome 12 in synteny with two other genes known to be expressed in boundaries, such as rfng (31) and sgca (32) (Fig. 1A). In situ hybridization analyses revealed that rfng is similarly enriched at the hindbrain boundaries, as well as in other embryonic territories such as the midbrain-hindbrain boundary (31) (Fig. 1 F–I). sgca is also expressed at the hindbrain boundary regions (Fig. 1 J–M) and in the somites (32). The onset of expression of these three genes in hindbrain boundaries is very similar: expression starts at ∼17 hpf, just when actomyosin cables have been assembled (22), and is maintained in boundary cells at least until 30 hpf (Fig. 1 E, I, and M). None of the additional genes within this chromosomal region—wdr64, lrrc45, dcxr, and col1ab1—are specifically enriched in boundaries and, in turn, present a non-spatially restricted pattern of expression (32). This common spatiotemporal expression pattern led us to think that rac3b, rfng, and sgca may share cis-regulatory information responsible for driving their common expression to the hindbrain boundaries.
Interestingly, when comparing the chromosomal organization of rac3b, rfng, and sgca across different vertebrates, we found that this microsyntenic block is conserved in zebrafish and A. mexicanus (Fig. 2A), both from the Ostariophysi superorder, as well as in all other ostariophysian species according to the available genomic information (Fig. S1). In contrast, this microsyntenic block is absent in tetrapods and nonostariophysian fish, which show an alternative genomic organization, with sgca orthologs lying next to ppp1r9b and separated from the rac3-rfng region (Fig. 2A and Fig. S2). This led us to test whether this specific chromosomal organization was relevant for gene expression at the boundaries. If this assumption is true, A. mexicanus embryos should display rac3b, rfng, and sgca expression in these same territories. To test this hypothesis, we cloned the corresponding A. mexicanus genes and performed in situ hybridization experiments. As shown in Fig. 2 B and C, both rac3b and rfng are expressed at the boundary cells in A. mexicanus surface fish embryos at 24 hpf. In contrast, although the expression of sgca in the somites is maintained, no expression was observed in boundaries (Fig. 2D). Interestingly, the onset of rac3b and rfng expression in hindbrain boundaries is at 18 hpf, which is the developmental stage equivalent to the zebrafish expression (Fig. 1). It is important to note that rac3b, rfng, and sgca orthologous genes are not expressed in the hindbrain boundaries in vertebrate species that do not share this specific chromosomal organization, including Actinopterygii fish, such as medaka (Fig. 2 E–G), and tetrapods, such as chickens and mice (33). Moreover, rac3a, the sister paralog of rac3b, is not expressed in the zebrafish hindbrain boundaries (32) and, rather, shows an expression pattern highly similar to that of the medaka rac3b (Fig. S3).
Fig. 2.
Expression of rac3b, rfng, and sgca in hindbrain boundaries is related to their chromosomal organization. (A) A phylogenetic tree displaying the microsynteny arrangements around rac3b, rfng, and sgca in different vertebrate species; genes are represented by arrows showing their transcriptional orientation. Note that conservation of the rac3b/rfng/sgca cluster is present in zebrafish and A. mexicanus Pachón cavefish genomes. Orange boxes indicate the conservation of the synteny within the 5′ region, and gray boxes indicate the conservation within the 3′ region. (B–D) In situ hybridization experiments in A. mexicanus surface fish embryos with rac3b, rfng, and sgca probes. Note that rac3b and rfng are expressed within the hindbrain boundary domains. Although the expression of sgca in the somites is maintained, no expression was observed in the boundaries. (E–G) Expression of the orthologous rac3b, rfng, and sgca genes in medaka embryos; note that there is no expression of any of these genes in the hindbrain boundaries, although sgca expression within the somites is conserved. All pictures are dorsal views with the anterior to the left.
These results suggest that, thanks to the tight genomic linkage of rac3b-rfng with sgca, regulatory elements within this region are specifically combined to drive rac3b expression to the rhombomeric boundaries, allowing actomyosin assembly in the boundary cells. This prompted us to compare the presence of hindbrain actomyosin structures in species displaying the same genomic organization as zebrafish, such as A. mexicanus, and with different genomic organization such as medaka. Indeed, anti–phosphomyosin-light-chain (anti-PMLC) immunostainings revealed enrichment of these structures within the hindbrain of A. mexicanus but not in medaka at equivalent embryonic stages (18–19ss; Fig. S4). This suggests that Rac3b function in the hindbrain boundaries is conserved across the Ostariophysi superorder.
Rac3b Induces the Formation of Actomyosin Structures in Hindbrain Boundaries.
We observed that the enriched expression of rac3b in boundary cells (Figs. 1 and 3 A and A′) was diminished upon EphA4 down-regulation (Fig. 3 B and B′), as previously shown for RhoA (22). To confirm the putative role of Rac3b in assembling apical actomyosin cables within the boundary cells to prevent cell intermingling, we undertook distinct functional approaches. Loss-of-function of rac3b was assessed either by its down-regulation using splice-blocking morpholinos (MO-Rac3b in Fig. 3 D and G and Fig. S5 A and C), by generating a mutant allele using CRISPR-Cas9 genome-editing technology (Fig. 3 E and H and Fig. S5 B and D–I), or by the clonal mosaic expression of a dominant negative form of Rac3b (DN-Rac3b-Myc; Fig. 3 I–I′′). Morpholino oligomer (MO)-Rac3b–injected embryos displayed a disruption of the actomyosin cables compared with control embryos [Fig. 3 C and D; control (n = 0/10) vs. MO-Rac3bSBI4E5 (n = 21/25) and MO-Rac3bSBE4I4 (n = 10/13)]. Accordingly, this disruption resulted in rhombomeric cell intermingling at 20 hpf [Fig. 3 F and G; control (n = 1/16) vs. MO-Rac3bSBI4E5 (n = 10/15)]. rac3b−/− embryos displayed defective actomyosin structures within the boundaries, although phenotypes were milder than in rac3b morphants [Fig. 3E; control (n = 1/25) vs. rac3b−/− (n = 3/7)]. Furthermore, rhombomeric cell sorting was also compromised at 24 hpf [Fig. 3H; control (n = 1/20) vs. rac3b−/− (n = 11/22)]. The mutation of the rac3b locus by CRISPR-Cas9 did not compromise embryo survival, resulting only in mild phenotypic abnormalities at the standard temperature of 28 °C (Fig. S5 D and E). However, curled body axis and increased lethality were observed when mutant embryos were incubated at temperatures of ≥36 °C (Fig. S5 F–I). The mild phenotypes displayed by rac3b loss-of-function in actomyosin disruption could be explained either by genetic compensation induced by deleterious mutations (34, 35) or by the functional redundancy of the ubiquitously expressed RhoA (22).
Fig. 3.
Expression and function of Rac3b in the hindbrain boundary cells. (A–B) Whole-mount in situ hybridization with rac3b in Tg[elA:GFP] embryos injected at the 1-cell stage with MO-Control (A and A′) or MO-EphA4 (B and B′), followed by anti-GFP staining. Note the expression of rac3b in boundary cells (white arrowheads) and how this expression diminishes upon EphA4 down-regulation. (A–B) Dorsal views and corresponding (A′–B′) sagittal views displaying only the red channel. (C–H) Loss of function of Rac3b, either by splicing blocking morpholino MO-Rac3b (D and G), or by CRISPR-Cas9 induced mutation (rac3b−/−) (E and H), results in the disruption of actomyosin cables (D and E) and cell mixing (G and H), compared with control embryos (C and F). White arrowheads in G and H point to ectopic r3/r5 rhombomeric cells. MO-Rac3b figures correspond to MO-Rac3bSBI4E5–injected Mü4127 embryos (Fig. S5C). Rhombomeric cell mixing in morphants was observed by expression of mCherry in r3 and r5 cells upon injecting the Mü4127 transgenic line. Cell mixing in rac3b−/− hindbrains was assessed by in situ hybridization with egr2a. (I–J) Sagittal views of representative examples of Tg[myosinII:GFP] embryos injected with the corresponding Rac3b construct, displaying either the merge (I–J) or the separate (I′–J′′) channels. (I–I′′) hs:DN-Rac3b-Myc clone (in magenta) hitting the rhombomeric boundary, with the subsequent disruption of the actomyosin cable; (J–J′′) hs:CA-Rac3b-Myc clone (in magenta) in rhombomere 5 generating ectopic actomyosin II structures. Yellow arrowheads in I–I′′ point to disrupted cables, and white arrowheads in J–J′′ indicate ectopic actomyosin structures. Anterior is to the left in all images.
To further confirm the role of Rac3b in actomyosin contractile structures in boundary cells, we conditionally and clonally modulated Rac3b expression in Tg[myosinII:GFP] embryos at 14 hpf and scored the phenotype at 18 hpf. DN-Rac3b-Myc clones result in disruption of actomyosin structures when the clone hits the interrhombomeric actomyosin cable (n = 44/54; see yellow arrowheads in sagittal views, Fig. 3 I–I′′) in comparison with control clones expressing Myc alone (n = 1/32). On the contrary, using a constitutively active form of Rac3b (CA-Rac3b-Myc), ectopic myosin II enrichment is obtained at sites of induction (n = 54/74; see white arrowheads in sagittal views, Fig. 3 J–J′′) but not in control clones (n = 14/81). Interestingly, this enrichment of myosin II structures can be observed in all rhombomeres, independently of their odd/even identity, and in nonapical locations, suggesting that constitutive Rac3b activity is able to recruit myosin II to these sites, as previously described for RhoA (22). These results support the role of Rac3b as a new small-GTPase player in boundary cells inducing the assembly of actomyosin structures to prevent cell mixing.
Analysis of the rac3b/rfng/sgca Regulatory Landscape and Identification of Hindbrain Boundary Enhancers.
To gain insight into the evolutionary emergence of the rac3b/rfng/sgca cluster present in zebrafish and A. mexicanus, we performed a comparative analysis of the chromosome landscapes containing the ancestral gene blocks rac3/dcxr/rfng/gps1 (Fig. S2A) and ppp1r9b/sgca/col1a1 (Fig. S2B) along the fish evolutionary tree. We observed that these conserved syntenic regions present in bony vertebrates (Fig. 2) are also conserved in spotted gar, whose lineage diverged from other teleost fish before their specific whole-genome duplication (36, 37). Whole-genome analyses of chromatin interactions by Hi-C support the notion that these two blocks are each contained within their respective TADs in mammals (38), and thus it is very likely that they constitute distinct regulatory domains. In contrast, after the genome duplication in teleosts, the architecture of these ancestral blocks became more flexible and, for each of the two generated paralogs, gene losses and chromosomal rearrangements are frequently observed in the different lineages (Fig. S2). In particular, we observed that the rac3b/rfng/sgca conformation found only in Ostariophysi (i.e., zebrafish and A. mexicanus) entailed the fragmentation and fusion of the two ancestral blocks: rac3/dcxr/rfng/gps1 and ppp1r9b/sgca/col1a1 (Fig. 2A and Fig. S2). The presence of these two blocks in the same chromosome in zebrafish as well as in other vertebrate species, such as medaka (Fig. S2), northern pike, and humans, suggests that intrachromosomal rearrangements were the causative events that gave rise to this new gene organization in Ostariophysi.
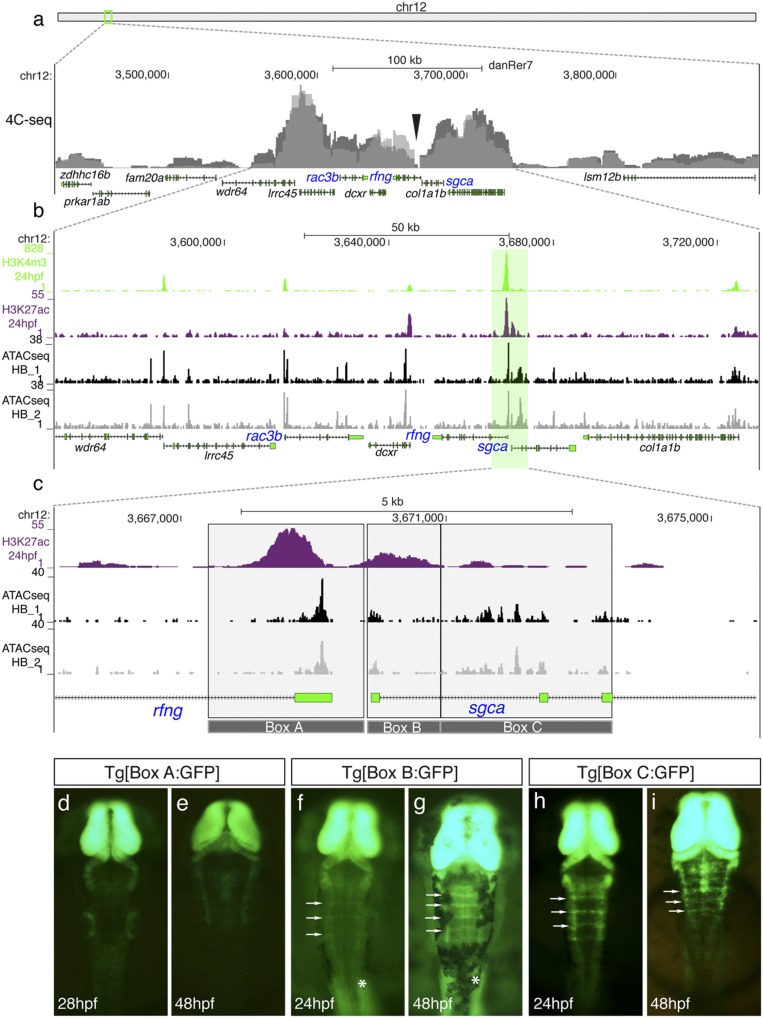
The conservation of the syntenic arrangement rac3b/rfng/sgca in Ostariophysi (Fig. S1), together with the shared expression pattern of these genes within this locus in zebrafish, suggest the emergence of a new common regulatory landscape. To investigate chromatin interactions in this region, we performed Chromosome Conformation Capture combined with high-throughput sequencing (39) (4C-seq) in 24 hpf zebrafish embryos using the rfng/sgca fusion border as reference bait. This analysis showed that rac3b, rfng, and sgca genes are all part of the same interaction domain, with most of the chromatin interactions occurring within a region of 170kb, between the genes wdr64 and col1a1b (Fig. 4A). Next, we focused on this region to identify the putative cis-regulatory elements responsible for the expression of the syntenic genes in hindbrain boundaries. To this end, we analyzed the distribution of predictive promoter and active enhancer epigenetic marks available for whole zebrafish embryos at 24 hpf (40) in the previously described locus (green/magenta peaks in Fig. 4B). To further refine these analyses, we performed a genome-wide comparative study of open-chromatin domains by ATAC-seq (41) using zebrafish 24 hpf dissected hindbrains as starting material. A small collection of ATAC-seq peaks was identified through this procedure within the 170kb window previously defined (gray and black peak profiles in Fig. 4B). Interestingly, some of the most prominent ATAC-seq peaks were located at the junction between the two ancestral syntenic blocks, at the rfng/sgca border, partially overlapping with predictive marks for active enhancers (Fig. 4B). Thus, to pinpoint cis-regulatory regions driving expression to boundary cells we focused our attention on this border using a classical transgenesis approach. To dissect the region, we generated stable transgenic lines harboring mainly nonoverlapping genomic fragments (see gray boxes in Fig. 4C). Three regions—Box A (2 kb), Box B (1.1 kb), and Box C (2.5 kb) (see Table 1 for chromosomal coordinates)—were cloned into a vector carrying the GFP reporter and an internal (midbrain) transgenesis control (42) and injected into 1-cell-stage zebrafish embryos. Embryos deriving from F1 crosses showed no enhancer activity in the hindbrain boundaries for Box A (Fig. 4 D and E), whereas Box B and Box C were able to drive GFP expression to the boundaries (Fig. 4 F–I). In addition, Box B, which contains the proximal promoter region of sgca, was also able to drive expression to the developing somites (see asterisks in Fig. 4 F and G). Boxes B and C display the same spatial but different temporal activity at the hindbrain boundaries: Box B is active only from 24 hpf onward (Fig. 4 F and G and Fig. S6 A–C), and Box C is already active at 19 hpf (Fig. 4 H and I and Fig. S6 D–F). This suggests that initiation and maintenance of gene expression might be under the control of different enhancer elements. Additional transgenesis experiments allowed further dissection of Box B and Box C (Fig. S6 G–N), narrowing the enhancer activity to Box B2 and Boxes C2 and C3 (Table 1), consistent with the distribution of the ATAC-seq signal in this area.
Fig. 4.
Identification of hindbrain boundary cell cis-regulatory elements by the analysis of the rac3b/rfng/sgca cluster regulatory landscape. (A) Chromosomal localization of the rac3b/rfng/sgca cluster and chromatin interaction profile by 4C-seq at 24 hpf (n = 2; overlaid gray peaks correspond to both replicates). The viewpoint used for both replicates is showed with a black arrowhead. (B) Enlarged view from A of a 170kb window of chromosome 12 where most of the chromatin interactions, unveiled by 4C-seq, occur. Epigenetic marks of putative promoters (H3K4me3; green peaks) and active enhancers (H3K27ac; magenta peaks) (40) are shown along with ATAC-seq profiles from dissected hindbrains at 24 hpf (n = 2; black and gray profiles). (C) Magnification of the region framed in B showing the H3K27ac profile and hindbrain-specific ATAC-seq signatures. The ∼5.6kb region (gray-shaded region) was divided in three fragments associated with hindbrain ATAC-seq peaks (Boxes A–C), which were cloned in an enhancer reporter vector to generate stable transgenic lines. (D–I) Dorsal views of embryonic hindbrains from Tg[Box A:GFP] (D and E), Tg[Box B:GFP] (F and G), and Tg[Box C:GFP] (H and I) stable transgenic lines at indicated stages. Note that Box B and Box C sequences (but not Box A) are able to drive GFP expression to the hindbrain boundaries (white arrows in F–I). White asterisks in F and G show the expression of GFP in the somites. In all images, the anterior is at the top.
Table 1.
Coordinates of the tested cis-genomic regions within chromosome 12
| Regulatory regions (Boxes A–F) and subboxes tested | Chr 12 coordinates Zv9 |
| Box A | 3,667,421–3,669,761 |
| Box B | 3,669,721–3,670,933 |
| Box B1 | 3,670,109–3,670,930 |
| Box B2 | 3,669,736–3,670,543 |
| Box C | 3,670,907–3,673,475 |
| Box C1 | 3,670,907–3,671,756 |
| Box C2 | 3,671,311–3,672,120 |
| Box C3 | 3,671,691–3,672,541 |
| Box C4 | 3,672,113–3,672,905 |
| Box C5 | 3,672,485–3,673,475 |
| Box D | 3,614,971–3,615,450 |
| Box E | 3,627,182–3,627,782 |
| Box F | 3,643,538–3,644,345 |
Corresponding coordinates in the zebrafish genome annotation Zv9/danRer7 of the different regulatory regions (Boxes A–F) and subboxes are shown.
Unveiling Enhancers with Highly Correlated Spatial Activity.
To investigate the functional contribution of the identified enhancers to the regulation of expression of the neighboring genes, we deleted a 2.5kb genomic region comprised between position 3,669,916 and 3,672,466 by CRISPR-Cas9 genome editing (CRISPR-Δ2.5kb; Fig. 5A). This deletion contains both Box B2, Box C2, and Box C3 (Fig. 5A and Fig. S6G). Founders were screened for this 2.5kb deletion and the F1 was generated and crossed to obtain embryos homozygous for the deletion. When the expression of rac3b, rfng, and sgca was compared between mutants and WT siblings, no main changes in their spatiotemporal profile were observed at the hindbrain boundaries (Fig. 5 B–G). Since the 2.5kb CRISPR-deletion contained the promoter of sgca, which acts as a somite proximal enhancer (Fig. 4C), the expression of this gene in the somites was abolished at 24 hpf and thus we could use it as an internal control of the deletion (compare Fig. 5 D and G). In agreement with the mild expression changes observed, the CRISPR-Δ2.5kb mutant line is viable in homozygosis. However, the survival of the mutants is compromised, as only a sub-Mendelian proportion of adults obtained from a heterozygous cross are mutants (4/43 = 9.3%). These data strongly suggest that other shadow enhancer regions might act redundantly, either totally or partially, to define the precise spatial and temporal activity of the interrogated genes.
Fig. 5.
CRISPR-Cas9 deletion of hindbrain boundary cis-regulatory elements revealed the existence of redundant enhancers. (A) Scheme depicting the 2.5kb deletion induced by CRISPR-Cas9 technology (Δ2.5kb; gray-shadowed stretch) containing Box B2 and Box C2/3, along with ATAC-seq profiles from 24 hpf dissected hindbrains (black and gray profiles correspond to two different replicates). The position of the sgRNAs (in blue) used to generate the 2.5kb deletion and the two pairs of primers (in magenta) used for genotyping the mutant line are displayed. (B–G) In situ hybridization analyses of rac3b, rfng, and sgca in WT siblings (B–D) and CRISPR-Δ2.5kb homozygous mutant embryos (E–G) at 22 hpf. Note that rac3b and rfng expression does not considerably change within the hindbrain boundaries between WT and homozygous mutant embryos. sgca expression in the somites is abolished in mutant embryos (black asterisk in G), due to the deletion of the main sgca promoter. All images are dorsal views of flat-mounted hindbrains with anterior to the left. (H) Epigenetic profiles of putative promoters (H3K4me3; green peaks) and active enhancers (H3K27ac; magenta peaks) are shown within the chromosomal region containing rac3b and dcxr along with ATAC-seq signatures (black and gray profiles) from dissected hindbrains at 24 hpf. Three regions associated with the most prominent ATAC-seq peaks were selected (shadowed in gray, Boxes D–F) and each of the fragments was cloned in an enhancer reporter vector to generate a stable transgenic line. (I–N) Dorsal views of embryonic hindbrains from Box D (I and J), Box E (K and L), and Box F (M and N) stable transgenic lines at indicated stages. Note that Tg[Box D:GFP] embryos display GFP expression in the hindbrain boundaries starting at 48 hpf and that Box F is able to drive GFP expression to the hindbrain boundaries before 28 hpf (white arrows in J, M, and N). Box E did not drive GFP to the boundaries. In all images, anterior is at the top. Overall, the expression of the rac3b/rfng/sgca microsyntenic group at the hindbrain boundaries is regulated by multiple enhancers. We have identified at least two early-activated (Boxes C and F) and two late-activated regulatory elements (Boxes B and D).
Regulatory elements with overlapping functions may provide robustness to gene expression during embryonic development. Thus, we explored whether other putative regulatory elements identified by our ATAC-seq analysis may function as redundant additional shadow enhancers driving gene expression at the hindbrain boundaries. We cloned in our enhancer reporter vector some of the most prominent peaks within the contact region revealed by 4C-seq on zebrafish hindbrains (Fig. 4A), here termed Boxes D, E, and F (Fig. 5H and Table 1). After the generation of the corresponding stable zebrafish lines, embryos were assayed for GFP expression; indeed, two of the analyzed peaks drive GFP expression to the hindbrain boundaries, acting as partially redundant enhancers (Fig. 5 I–N). These zebrafish enhancer regions display different temporal activity: Box D activates only late in the hindbrain boundaries (Fig. 5 I and J), whereas Box F is already active at 28 hpf (Fig. 5 M and N). Finally, we failed to detect GFP expression at the boundaries associated with Box E (Fig. 5 K and L). Overall, multiple enhancers regulate the expression of the rac3b/rfng/sgca microsyntenic group in hindbrain boundaries. We identified at least two early-activated regulatory elements (Boxes C and F) and two late-activating regulatory elements (Boxes B and D).
Since boundary cells unfold distinct functions at different developmental stages, we hypothesized that such a sophisticated regulation using multiple enhancers was devoted to ensuring the proper compartmentalization of the tissue. With this in mind, we examined the impact of the 2.5kb enhancer deletion in actomyosin cable formation and cell intermingling. Therefore, we placed WT and homozygous CRISPR-Δ2.5kb embryos both under control and stress conditions, namely disrupting the actomyosin cables using a pharmacological treatment with para-nitroblebbistatin (Fig. S7). We observed that an increased percentage of embryos display cells violating the boundary upon deletion of the enhancer, both under normal environmental conditions (10% in control vs. 19% in CRISPR-Δ2.5kb; Fig. S7 A, C, and E) and when the boundaries are challenged with para-nitroblebbistatin (Fig. S7 B, D, and E; 38% in control vs. 50% in CRISPR-Δ2.5kb). Although mild, this increase was statistically significant (P < 0.048), as revealed by two-way ANOVA. Along the same lines, a higher proportion of CRISPR-Δ2.5kb embryos display disrupted actomyosin cables in control conditions (0% in control vs. 9% in CRISPR-Δ2.5kb; Fig. S7 F, H, and J) as well as upon para-nitroblebbistatin treatment (56% in control vs. 69% in CRISPR-Δ2.5kb; Fig. S7 G, I, and J). Again, this increase was statistically significant (P < 0.037), as revealed by χ2 test. Thus, although rac3b/rfng/sgca expression at the hindbrain boundaries is not substantially altered in the CRISPR-Δ2.5kb embryos, the elimination of these cis-regulatory elements results in reduced viability and enhanced boundary violation. These results suggest that multiple enhancers are required to provide robustness to genetic variation within a cell population, allowing hindbrain development to proceed unperturbed.
Discussion
Cortical tension at compartment boundaries based on actomyosin-driven mechanical forces has been shown to play a key role in several systems, from Drosophila to vertebrates (22, 43–45). This mechanism allows the tissue to keep cells from adjacent compartments sorted, meanwhile undergoing extensive growth and morphogenesis. Here we show that the small GTPase Rac3b plays an important role in assembling actomyosin cables at the hindbrain boundaries, thus restricting cell intermingling. The expression of small GTPase regulators, including GAPs (Rho GTPase-activating proteins such as arhgap29b) and GEFs (guanine nucleotide exchange factors such as rasgef1ba), in hindbrain boundaries (Fig. S8) suggests that the assembly of cables is under the control of a tissue-specific genetic program.
Here we have shown that interrhombomeric actomyosin cables are present in zebrafish and A. mexicanus, two deeply diverged species of the Ostariophysi superorder (46), suggesting that this boundary formation mechanism was also present in the last common ancestor of this extremely diverse fish supergroup. In contrast, these cables are absent in the Actinopterygii medaka, and similarly, little actomyosin has been found at the rhombomeric boundaries in tetrapods (47). This suggests that the use of mechanical forces is not a universal mechanism for segregating cells from adjacent rhombomeres within the hindbrain. Cell segregation in adjacent rhombomeres depends critically on interactions between Eph receptors and Ephrin ligands expressed in odd and even segments, respectively (25, 48), and this Eph/Ephrin code, which is tightly coupled to hindbrain patterning, would be sufficient for cell sorting in many vertebrates. Here, we identify rac3b as an effector of Eph/Ephrin activity in the assembly of the actomyosin structures, which will play a role in increasing cortical tension at the borders (22). This complementary mechanism may be particularly important to confer robustness to the system when the cell segregation process is challenged.
The adaptive reasons behind the emergence of a cell-sorting backup mechanism are currently unclear. A possible explanation may come from the faster embryonic development in Ostariophysi compared with other fish (Fig. S9; www.fishbase.org). The short hatching period in zebrafish (∼48 hpf) and A. mexicanus (∼28 hpf) (49) is shared by other orders of the Ostariophysi clade. This is in contrast to other teleost species such as members of the Euteleostei supergroup, Ostariophysi sister orders, or even basal actinopterygian lineages, for which embryos take a week longer to hatch (Fig. S9). Zebrafish embryos are able to swim and display a full hindbrain-wired scape response as early as 48 hpf (50). In agreement, the heterochronic development of the muscles and the nervous system is particularly noticeable when the zebrafish transcriptome is compared with that of medaka (29). Thus, a possibility is that the machinery for actomyosin cables had evolved under the requirements imposed by the accelerated development of the hindbrain. An alternative hypothesis comes from the presence of a particular adaptation in Ostariophysi, the weberian apparatus. This is a set of small bones connecting the swim bladder with the auditory system that plays a role as a sound amplifier (51). The acquisition of novel sensory inputs into the hindbrain vestibular nuclei may have contributed to an increased selective pressure for rhombomeric cell segregation in Ostariophysi. Importantly, these two scenarios are not incompatible. A rapid development and novel hearing capabilities may have both contributed to the preeminence of the ostariophysian lineage in freshwater environments, and both would have set specific evolutionary pressures on hindbrain developmental processes. In agreement with this, it has been recently shown that the zebrafish hindbrain has experienced an intense process of recent genetic innovation, being the only brain region with a highly significant recruitment of zebrafish- and ostariophysian-specific genes (52).
The study of the regulation of the small GTPase rac3b brought us to the identification of the block rac3b/rfng/sgca as a microsyntenic group expressed at the hindbrain boundaries. Our functional and comparative genomic analyses outline a possible evolutionary scenario for the emergence of this regulatory block (Fig. 6). The two ancestral gene blocks rac3/dcxr/rfng/gps1 and ppp1r9b/sgca/col1a1 would be initially present in the same chromosome in the last common ancestor of all bony vertebrates (e.g., they are separated ∼32 Mb in the human chromosome 17). The specific whole-genome duplication in fish has been postulated as a positive force for teleosts’ diversity and evolutionary success (53). This duplication may have led to a relaxation of some evolutionary constraints, releasing enough evolutionary pressure to allow the breakage of the two ancestral blocks. Then, the new block, rac3b/rfng/sgca/col1a1, would have emerged by intrachromosomal rearrangement specifically in the Ostariophysi lineage (Fig. 6). Through this rearrangement, sgca could have brought preexisting regulatory elements into the proximity of the rac3b locus, allowing the establishment of new long-range cis-regulatory interactions and the emergence of novel rac3b expression domains. In fact, one of the boundary enhancers we identified, Box B2, lies precisely at the junction of this genomic rearrangement, at the sgca promoter, and this enhancer element contains a conserved sequence present in all teleost species examined, including all Ostariophysi outgroups. This indicates that the evolutionary origin of the Box B2 enhancer predates the chromosomal rearrangement of Ostariophysi, the emergence of rac3b expression in hindbrain boundaries, and the appearance of the actomyosin cables (Fig. S10). Thus, it is likely that the evolution of these pioneer regulatory interactions with the ancestral Box B2 element facilitated the emergence of additional redundant enhancers within the locus (Fig. 6). Consistent with this scenario, the sequences of the two additional boundary enhancers located outside the junction genomic region with sgca, Box D and Box F, are conserved only among cypriniform species (Fig. S11), suggesting that these two shadow enhancers evolved long after the origin of Ostariophysi and the emergence of the rac3b/rfng/sgca regulatory block.
Fig. 6.
Evolutionary model for the emergence of a novel hindbrain boundary mechanism in Ostariophysi. (A) Diagram showing the arrangement of the two ancestral blocks rac3/dcxr/rfng/gps1 (shaded in orange) and ppp1r9b/sgca/col1a1 (shaded in gray) in the common ancestor of all bony vertebrates. The new rac3b/rfng/sgca/col1a1 cluster emerged by intrachromosomal rearrangement in the Ostariophysi lineage [1]. This new regulatory space allowed the gain of long-range regulatory contacts [2] and the acquisition of shadow enhancers to provide regulatory robustness [3]. (B) The new topology allowed the emergence of a novel gene expression domain and, subsequently, the formation of actomyosin cables at the rhombomeric boundaries. Images in B are dorsal views except for the actomyosin cables image, which is a sagittal view. Anterior is always to the left. CRE, cis-regulatory elements.
The partial overlapping activity of redundant enhancers appears to be a common regulatory theme, as they ensure robust development by suppressing transcriptional noise (54–56). As it has been shown in several systems, and is the case in our study, deletion of a redundant enhancer does not cause major phenotypic alterations, at least in a given environmental condition, as one or more redundant elements can compensate for its loss. In the same line, recent work in Drosophila has shown how pervasive redundant enhancers are, with >70% of loci having three or more enhancers (57). In addition, genes with redundant enhancers also tend to initiate their expression more synchronously during very rapid cell divisions (58).
Hindbrain boundary cis-regulatory regions seem to interact selectively with genes within the rac3b/rfng/sgca locus. Although they are contained within the 4C-delineated domain, some genes, such as wdr64, lrrc45, and dcxr, are not expressed at the boundaries and, rather, show a non-spatially restricted expression. While it happens often that genes within a genomic domain are coordinately regulated, this is not invariably the case and it depends on multiple factors. Neighbor genes are not always coexpressed, as enhancers and their target core promoters are not necessarily adjacent and collinear in the genomic sequence (59). This may be due to the reliance of their promoters on other tethering elements, as is the case for two neighboring genes, Scr and ftz, in Drosophila, in which the enhancer of Scr is located 3′ of ftz and the enhancer of ftz lies between the two genes; here, the selectivity of the enhancer for the Scr promoter depends on a promoter-proximal tethering element (60). For the rac3b/rfng/sgca locus, we show a differential behavior for sgca as it is recruited to the boundaries in zebrafish but not in A. mexicanus.
Several examples of changes in regulatory sequences that provide the basis for evolving species-specific traits have been shown (3, 4, 61). In this study, we reveal a specific example linking the appearance of a new regulatory domain to the emergence of a novel morphogenetic mechanism in the Ostariophysi superorder: the formation of actomyosin cables at the rhombomeric boundaries. We have shown that this mechanism depends on the cooption of rac3b to the boundaries. However, the appearance of the rac3b/rfng/sgca regulatory space may have facilitated the evolution of more than one mechanism for hindbrain morphogenesis. In addition to rac3b, rfng, which is not specifically expressed in this territory in other vertebrates (62, 63), was also coopted to this rhombomeric domain in Ostariophysi. It has been suggested that activation of Notch in boundary cells promotes cell segregation, in a process dependent on rfng (23). Moreover, rfng plays a signaling role in hindbrain boundaries in the patterning of neurogenesis (26). Overall, our observation suggests that the umbrella of the novel rac3b/rfng/sgca regulatory block allowed the emergence of an entire morphogenetic program providing robustness to the hindbrain boundary cells to unfold their different functions upon morphogenesis. Thus, this seemingly serendipitous mutational event may have been crucial for adapting hindbrain morphogenesis to novel developmental traits of the ostariophysian lineage.
Materials and Methods
Conditional Overexpression.
CA-Rac3 (Q61L mutation) and DN-Rac3 (T71N mutation) constructs were generated by site-directed mutagenesis (QuikChange Site-Directed Mutagenesis Kit; Stratagene #200518), and cloned into the multicloning site of a Tol2-based custom vector containing a heat shock promoter and a Myc-tag. Tg[myosinII:GFP] embryos were injected at the 1-cell stage, grown at 28.5 °C, and heat-shocked at 14 hpf. All embryos were fixed at 18–20 hpf, coimmunostained for Myc and GFP, and imaged for further analysis. For the phenotypic analysis: (i) the integrity of the actomyosin cable was assessed in Myc or DN-Rac3b-Myc clones hitting the boundary and (ii) ectopic actomyosin structures were scored in Myc or CA-Rac3b-Myc clones located within the rhombomere.
Whole-Mount in Situ Hybridization.
Zebrafish whole-mount in situ hybridization was adapted from ref. 64. The following riboprobes were generated by in vitro transcription from cloned cDNAs: egr2a/krx20 (65), rfng (23), gfp (22), rac3b, and sgca. For arghap29 and rasgef1ba, in vitro transcription was performed by RT-PCR using the following primers: arhgap29b_forward (Fw): 5′-GTG GAG CTG CTC ATC AAA CA-3′; arhgap29b_t7- reverse (Rv): 5′-TAA TAC GAC TCA CTA TAG GGT GAT TTT GCC AGC AAG TCA G-3′; rasgef1ba_Fw: 5′-CTC AGC TCG CGT CTC TTT CT-3′; and rasgef1ba_t7_Rv: 5′-TAA TAC GAC TCA CTA TAG GGT TTG GCG GTT TTA ACT TTG G-3′. The chromogenic in situ hybridizations were developed with Nitro-Blue Tetrazolium chloride 5-Bromo-4-chloro-3-indolyl phosphate toluidine salt (NBT/BCIP) (blue) and FastRed (red) substrates. For fluorescent in situ hybridization, digoxigenin (DIG)-labeled riboprobes were developed with fluorescein-tyramide substrate (TSA system). After staining, embryos were either flat-mounted and imaged under a Leica DM6000B fluorescence microscope or whole-mounted in agarose and imaged under SP5 or SP8 Leica confocal microscopes. For A. mexicanus whole-mount in situ hybridization, DIG riboprobes were synthesized from PCR templates. Embryos were progressively rehydrated before being incubated overnight at 68 °C in hybridization buffer containing the appropriate probe. After stringent washes, the chromogenic in situ hybridizations were developed with NBT/BCIP. After staining, the dissected embryos were mounted in glycerol and photographed with a Nikon Eclipse E800 microscope. In medaka, whole-mount in situ hybridization was performed using DIG riboprobes as described (66). After staining, the dissected embryos were mounted in glycerol and photographed with a Leica DM6000B fluorescence microscope.
In Toto Embryo Immunostainings.
For immunostaining, embryos were blocked in 5% goat serum in phosphate buffer saline (GS/PBS) 1 h at room temperature and incubated overnight at 4 °C with primary antibody. Primary policlonal Abs (pAbs) were the following: anti-DsRed (1:500; Clontech), anti-GFP (1:200; Torrey Pines), and anti-Myc (1:200; Clontech). After extensive washings with PBS, embryos were incubated with secondary Ab conjugated with Alexa Fluor488 or Alexa Fluor555 (1:500; Invitrogen). Embryos were flat-mounted or whole-mounted in agarose and imaged under a Leica SP5 or an SP8 confocal microscope.
Assessment of Actomyosin Cable-Like Structures.
Live embryos from Tg[myosinII:mCherry/GFP] lines were anesthetized with 0.4% Tricaine (#A-5040; Sigma) and mounted as previously described (22). In some cases, cables were imaged in fixed embryos immunostained for the reporter protein or anti-PMLC. For actomyosin cable-structure analysis, whole-mounted embryos were imaged in the SP8 confocal microscope, and 0.6-µm z stacks were acquired in dorsal view and resliced to generate YZ confocal cross-sections. Images were resliced in XZ, and finally, a maximal projection of the XZ sections corresponding to the apical side of cells in the neural tube was generated (22). Animations of cable-like structures were generated using ImageJ (Movie S1). For scoring the disruption of actomyosin cable-like structures, the integrity of these structures in each embryo was analyzed, and when at least two of the cables were disrupted embryos were considered affected.
Analysis of Cell Mixing.
Confocal images of live or fixed embryos were acquired in dorsal view covering the r3–r5 region with 1-µm z distance. Images were resliced in XZ axes and analyses were performed along the whole DV axis for the presence of red ectopic cells either in Mü4127 or embryos in situ hybridized with egr2a. These stacks were then projected into a single dorsal view image for display (22). When ectopic cells were observed, the embryo was scored as positive, regardless of the number of ectopic cells observed. Ectopic cells are the result of cells undergoing mitosis, which incurred into the neighboring territory and due to the disruption of cables cannot be brought back to the territory of origin (22). Therefore, the number of ectopic cells usually varies between one and three, since within this time period cells do not undergo more than two cell cycles. This makes it difficult to use the number of total ectopic cells to measure the expressivity of the phenotype. Thus, we prefer to score for ectopic cells.
ATAC-Seq Experiments.
ATAC-seq experiments in zebrafish embryos were performed as described (41). Dissected hindbrains from 30 embryos at 24 hpf were collected in cold 1× PBS, centrifuged at 500g for 5 min at 4 °C and resuspended in cold lysis buffer (10 mM Tris pH 7.4, 10 mM NaCl, 3 mM MgCl2 and 0.1% Igepal). Samples were centrifuged at 500g for 10 min at 4 °C, resuspended with 50 μL of transposition reaction mix (Catalog No. FC-121-1030; Illumina), incubated for 30 min at 37 °C and purified with a Qiagen MinElute kit. For generation of ATAC-seq libraries a PCR was performed with 13 cycles using 10 μM Ad1F and Ad2.1R/Ad2.2R primers (41) and KAPA HiFi Hot-Start enzyme (Kapa Biosystems). The resulting library was multiplexed and sequenced in a HiSEq 2000 lane. Reads were aligned using the Zv9/danRer7 assembly. ATAC-seq data are available under the GEO accession number GSE109219.
4C-Seq.
4C-Seq assays were performed as recently reported (67) using ∼500 zebrafish embryos at 24 hpf as starting material. The following primers were used: rfng_4C_R: 5′-CGA ATC TTA TAA ACT TGA TGA ATG TGA TC-3′and rfng_4C_NR: 5′-TCA TTG CAA AGC TGA CAA CG-3′. The DNA was digested with DpnII (R0543M; New England BioLabs) and Csp6I (FD0214; Fermentas, Thermo Scientific) as primary and secondary enzymes, respectively. Two libraries from different biological replicates were generated. These libraries were purified with a PCR Product Purification kit (No. 11732668001; Roche), their concentrations were measured using the Qubit dsDNA BR assay kit (No. Q32850; Molecular Probes), and they were sent for deep sequencing. Raw sequencing data were demultiplexed and aligned using the zebrafish Zv9/danRer7 reference genome. 4C-Seq data are available under the Gene Expression Omnibus (GEO) accession number GSE109219.
CRISPR-Cas9 Genomic Edition.
Preparation of sgRNA.
CRISPR target sites were identified using the CRISPRscan online tool (68). Small guide RNAs (sgRNAs) used were as follows:
-
i)
rac3−/− mutant in chr12; sgRNA1: 5′-GTT GTG CTT TTC TCC AGG GCG G-3′; and sgRNA2: 5′-CCT TCC CCG GCG AGT ACA TCC C-3′. The CRISPR-Cas9 genomic edition generated an insertion of 11 bp and a deletion of 6 bp in exon 2 resulting in a truncated protein of 22AA (Fig. S5 A and B).
-
ii)
CRISPR-Δ2.5kb genomic deletion covering the chromosomal region chr12 3,669,901–3,672,546 (from Zv9/danRer7 coordinates) resulted in a deletion of 2,564 bp plus an insertion of 205 bp; sgRNA1: 5′-GGG AGT CTC TGT GAT GCT GTC GG-3′ and sgRNA2: 5′-AGG TCT GCT GTA TTT AGG ATG GG -3′ (Fig. 5A).
Injection and genotyping.
One-cell stage zebrafish embryos were coinjected with 2–3 nL of a solution containing 250 ng/μL Cas9 protein and 40 ng/μL sgRNAs. For screening of the edited genome, gDNA was obtained by incubating the samples in TE buffer supplemented with 5% Chelex-100 (BioRad) and 10 μg/mL Proteinase K (Roche) for 4 h at 55 °C and 10 min at 95 °C and then stored at 4 °C. One microliter of the supernatant was used as a template in a standard 25-μL PCR. Screening of the CRISPR-rac3b mutants was performed by PCR amplification using the following primers: rac3b-Fw: 5′-GAA CTC CCC CAA TAA TGT GAT G-3′ and rac3b-Rv: 5′-TGC AGT GTA TGT AAA CTT CTG CTT T-3′. The WT PCR product of 289 bp undergoes digestion with BstXI leading to two bands, and a single band is detected after digestion in the mutant by loss of the BstXI restriction site. Screening of CRISPR-Δ2.5kb deletion was performed by PCR amplification with the following primers: P1-Fw: 5′-TAA CAT TTG TCA TGC CAA CAA AG-3′ and P1-Rv: 5′-GAA ATA AAG CAA ATA AGA CTT TCT CCA-3′. P2-Fw: 5′-TTG TCA GCT TTG CAA TGA ATT AAG-3′, P2-Rv: 5′-ATT GTT TTG AAA TAA ATG CTG CAA-3′. P1 primers align outside the deletion; thus, WT fish display a 2.9kb band and heterozygous fish amplify the 2.9kb band, and a 535-bp band associated to the deletion. P2 primers are designed to be inside and outside the deletion; therefore, mutant fish do not display the 764-bp band (Fig. 5A).
Supplementary Material
Acknowledgments
We thank the individuals who kindly provided us with transgenic fish lines and reagents, especially P. Charnay, C. P. Heisenberg, R. Koster, A. Málnási-Csizmadia, and D. Wilkinson. We also thank those who critically read the manuscript such as E. Jimenez-Guri and C. Cortes-Campos. We thank S. Calatayud for technical assistance and the J.R.M.-M. and C.P. laboratories for critical insights. This work was supported by La Marató-TV3 Grant 345/C/2014 (to C.P.), “Equipe FRM” (to S.R.), and the Spanish Ministry of Economy and Competitiveness [MINECO-FEDER; Grants BFU2012-31994 and BFU2015-67400-P (to C.P.)], Unidad de Excelencia María de Maetzu [Grants MDM-2014-0370 to DCEXS-UPF (Department of Experimental and Health Sciences-Universitat Pompeu Fabra); BFU2011-22916, P11-CVI-7256, BFU2014-53765, and BFU2014-55738-REDT (to J.R.M.-M.); and BFU2016-81887-REDT/AEI (to J.R.M.-M. and C.P.)]. J.L. and C.A.U. were funded by postdoctoral fellowships from the Becas Chile program (CONICYT, Chile). J.T. was a recipient of a postdoctoral Beatriu de Pinos fellowship (AGAUR, Generalitat de Catalunya), A.V. is a recipient of a predoctoral fellowship from LaCaixa, and I.B. holds a predoctoral Fromació d’Investigadors (FI) fellowship (AGAUR, Generalitat de Catalunya). C.P. is a recipient of an ICREA Academia Award (Institució Catalana per la Recerca i Estudis Avançats, Generalitat de Catalunya). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie Grant 658521 (to I.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The ATAC-seq and 4C-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE109219).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719885115/-/DCSupplemental.
References
- 1.Eichenlaub MP, Ettwiller L. De novo genesis of enhancers in vertebrates. PLoS Biol. 2011;9:e1001188. doi: 10.1371/journal.pbio.1001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Rios J, et al. Attenuated sensing of SHH by Ptch1 underlies evolution of bovine limbs. Nature. 2014;511:46–51. doi: 10.1038/nature13289. [DOI] [PubMed] [Google Scholar]
- 3.Indjeian VB, et al. Evolving new skeletal traits by cis-regulatory changes in bone morphogenetic proteins. Cell. 2016;164:45–56. doi: 10.1016/j.cell.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kvon EZ, et al. Progressive loss of function in a limb enhancer during snake evolution. Cell. 2016;167:633–642, e11. doi: 10.1016/j.cell.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 6.Rebeiz M, Jikomes N, Kassner VA, Carroll SB. Evolutionary origin of a novel gene expression pattern through co-option of the latent activities of existing regulatory sequences. Proc Natl Acad Sci USA. 2011;108:10036–10043. doi: 10.1073/pnas.1105937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koshikawa S, et al. Gain of cis-regulatory activities underlies novel domains of wingless gene expression in Drosophila. Proc Natl Acad Sci USA. 2015;112:7524–7529. doi: 10.1073/pnas.1509022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nora EP, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon JR, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupiáñez DG, et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke M, et al. Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature. 2016;538:265–269. doi: 10.1038/nature19800. [DOI] [PubMed] [Google Scholar]
- 12.Hnisz D, et al. Convergence of developmental and oncogenic signaling pathways at transcriptional super-enhancers. Mol Cell. 2015;58:362–370. doi: 10.1016/j.molcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavahan WA, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini B, et al. Nuclear architecture dictates HIV-1 integration site selection. Nature. 2015;521:227–231. doi: 10.1038/nature14226. [DOI] [PubMed] [Google Scholar]
- 15.Ahituv N, Prabhakar S, Poulin F, Rubin EM, Couronne O. Mapping cis-regulatory domains in the human genome using multi-species conservation of synteny. Hum Mol Genet. 2005;14:3057–3063. doi: 10.1093/hmg/ddi338. [DOI] [PubMed] [Google Scholar]
- 16.Dahmann C, Oates AC, Brand M. Boundary formation and maintenance in tissue development. Nat Rev Genet. 2011;12:43–55. doi: 10.1038/nrg2902. [DOI] [PubMed] [Google Scholar]
- 17.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 18.Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Guri E, et al. Clonal analysis in mice underlines the importance of rhombomeric boundaries in cell movement restriction during hindbrain segmentation. PLoS One. 2010;5:e10112. doi: 10.1371/journal.pone.0010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthrie S, Lumsden A. Formation and regeneration of rhombomere boundaries in the developing chick hindbrain. Development. 1991;112:221–229. doi: 10.1242/dev.112.1.221. [DOI] [PubMed] [Google Scholar]
- 21.Gutzman JH, Sive H. Epithelial relaxation mediated by the myosin phosphatase regulator Mypt1 is required for brain ventricle lumen expansion and hindbrain morphogenesis. Development. 2010;137:795–804. doi: 10.1242/dev.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calzolari S, Terriente J, Pujades C. Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J. 2014;33:686–701. doi: 10.1002/embj.201386003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y-C, et al. Notch activation regulates the segregation and differentiation of rhombomere boundary cells in the zebrafish hindbrain. Dev Cell. 2004;6:539–550. doi: 10.1016/s1534-5807(04)00097-8. [DOI] [PubMed] [Google Scholar]
- 24.Riley BB, et al. Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev Dyn. 2004;231:278–291. doi: 10.1002/dvdy.20133. [DOI] [PubMed] [Google Scholar]
- 25.Cooke JE, Kemp HA, Moens CB. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr Biol. 2005;15:536–542. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Terriente J, Gerety SS, Watanabe-Asaka T, Gonzalez-Quevedo R, Wilkinson DG. Signalling from hindbrain boundaries regulates neuronal clustering that patterns neurogenesis. Development. 2012;139:2978–2987. doi: 10.1242/dev.080135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peretz Y, et al. A new role of hindbrain boundaries as pools of neural stem/progenitor cells regulated by Sox2. BMC Biol. 2016;14:57. doi: 10.1186/s12915-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson JS, Grande T, Wilson MVH. Fishes of the World. 5th Ed John Wiley & Sons; Hoboken, NJ: 2016. [Google Scholar]
- 29.Tena JJ, et al. Comparative epigenomics in distantly related teleost species identifies conserved cis-regulatory nodes active during the vertebrate phylotypic period. Genome Res. 2014;24:1075–1085. doi: 10.1101/gr.163915.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Liu L, Korzh V, Gong Z, Low BC. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: Use of zebrafish as an in vivo model for GTPase signaling. Cell Signal. 2006;18:359–372. doi: 10.1016/j.cellsig.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Skromne I, Thorsen D, Hale M, Prince VE, Ho RK. Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development. 2007;134:2147–2158. doi: 10.1242/dev.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thisse B, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 2004;77:505–519. doi: 10.1016/s0091-679x(04)77027-2. [DOI] [PubMed] [Google Scholar]
- 33.Moran JL, et al. Manic fringe is not required for embryonic development, and fringe family members do not exhibit redundant functions in the axial skeleton, limb, or hindbrain. Dev Dyn. 2009;238:1803–1812. doi: 10.1002/dvdy.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi A, et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015;524:230–233. doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 35.Cerikan B, et al. Cell-intrinsic adaptation arising from chronic ablation of a key Rho GTPase regulator. Dev Cell. 2016;39:28–43. doi: 10.1016/j.devcel.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Amores A, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 37.Braasch I, et al. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet. 2016;48:427–437. doi: 10.1038/ng.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao SSP, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Werken HJG, et al. Robust 4C-seq data analysis to screen for regulatory DNA interactions. Nat Methods. 2012;9:969–972. doi: 10.1038/nmeth.2173. [DOI] [PubMed] [Google Scholar]
- 40.Bogdanović O, et al. Dynamics of enhancer chromatin signatures mark the transition from pluripotency to cell specification during embryogenesis. Genome Res. 2012;22:2043–2053. doi: 10.1101/gr.134833.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acemel RD, et al. A single three-dimensional chromatin compartment in amphioxus indicates a stepwise evolution of vertebrate Hox bimodal regulation. Nat Genet. 2016;48:336–341. doi: 10.1038/ng.3497. [DOI] [PubMed] [Google Scholar]
- 43.Monier B, Pélissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–69. doi: 10.1038/ncb2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terriente J, Pujades C. Cell segregation in the vertebrate hindbrain: A matter of boundaries. Cell Mol Life Sci. 2015;72:3721–3730. doi: 10.1007/s00018-015-1953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Neill AK, et al. Unidirectional Eph/ephrin signaling creates a cortical actomyosin differential to drive cell segregation. J Cell Biol. 2016;215:217–229. doi: 10.1083/jcb.201604097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011;11:177. doi: 10.1186/1471-2148-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filas BA, et al. Regional differences in actomyosin contraction shape the primary vesicles in the embryonic chicken brain. Phys Biol. 2012;9:066007–066019. doi: 10.1088/1478-3975/9/6/066007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooke JE, Moens CB. Boundary formation in the hindbrain: Eph only it were simple. Trends Neurosci. 2002;25:260–267. doi: 10.1016/s0166-2236(02)02134-3. [DOI] [PubMed] [Google Scholar]
- 49.Hinaux H, et al. A developmental staging table for Astyanax mexicanus surface fish and Pachón cavefish. Zebrafish. 2011;8:155–165. doi: 10.1089/zeb.2011.0713. [DOI] [PubMed] [Google Scholar]
- 50.Eaton RC, Nissanov J, Wieland CM. Differential activation of Mauthner and non-Mauthner startle circuits in the zebrafish: Implications for functional substitution. J Comp Physiol. 1984;155:813–820. [Google Scholar]
- 51.Bird NC, Hernandez LP. Building an evolutionary innovation: Differential growth in the modified vertebral elements of the zebrafish Weberian apparatus. Zoology (Jena) 2009;112:97–112. doi: 10.1016/j.zool.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Šestak MS, Domazet-Lošo T. Phylostratigraphic profiles in zebrafish uncover chordate origins of the vertebrate brain. Mol Biol Evol. 2015;32:299–312. doi: 10.1093/molbev/msu319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer A, Van de Peer Y. From 2R to 3R: Evidence for a fish-specific genome duplication (FSGD) BioEssays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- 54.Hong J-W, Hendrix DA, Levine MS. Shadow enhancers as a source of evolutionary novelty. Science. 2008;321:1314. doi: 10.1126/science.1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perry MW, Boettiger AN, Bothma JP, Levine M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol. 2010;20:1562–1567. doi: 10.1016/j.cub.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frankel N, et al. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature. 2010;466:490–493. doi: 10.1038/nature09158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannavò E, et al. Shadow enhancers are pervasive features of developmental regulatory networks. Curr Biol. 2016;26:38–51. doi: 10.1016/j.cub.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perry MW, Boettiger AN, Levine M. Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc Natl Acad Sci USA. 2011;108:13570–13575. doi: 10.1073/pnas.1109873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zabidi MA, Stark A. Regulatory enhancer-core-promoter communication via transcription factors and cofactors. Trends Genet. 2016;32:801–814. doi: 10.1016/j.tig.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calhoun VC, Stathopoulos A, Levine M. Promoter-proximal tethering elements regulate enhancer-promoter specificity in the Drosophila Antennapedia complex. Proc Natl Acad Sci USA. 2002;99:9243–9247. doi: 10.1073/pnas.142291299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kvon EZ, et al. Genome-scale functional characterization of Drosophila developmental enhancers in vivo. Nature. 2014;512:91–95. doi: 10.1038/nature13395. [DOI] [PubMed] [Google Scholar]
- 62.Johnston SH, et al. A family of mammalian Fringe genes implicated in boundary determination and the Notch pathway. Development. 1997;124:2245–2254. doi: 10.1242/dev.124.11.2245. [DOI] [PubMed] [Google Scholar]
- 63.Tossell K, Kiecker C, Wizenmann A, Lang E, Irving C. Notch signalling stabilises boundary formation at the midbrain-hindbrain organiser. Development. 2011;138:3745–3757. doi: 10.1242/dev.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- 65.Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez-Morales J-R, et al. Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev Cell. 2005;8:565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Fernández-Miñán A, Bessa J, Tena JJ, Gómez-Skarmeta JL. Assay for transposase-accessible chromatin and circularized chromosome conformation capture, two methods to explore the regulatory landscapes of genes in zebrafish. Methods Cell Biol. 2016;135:413–430. doi: 10.1016/bs.mcb.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Moreno-Mateos MA, et al. CRISPRscan: Designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods. 2015;12:982–988. doi: 10.1038/nmeth.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Distel M, Wullimann MF, Köster RW. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci USA. 2009;106:13365–13370. doi: 10.1073/pnas.0903060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Labalette C, et al. Hindbrain patterning requires fine-tuning of early krox20 transcription by Sprouty 4. Development. 2011;138:317–326. doi: 10.1242/dev.057299. [DOI] [PubMed] [Google Scholar]
- 71.Behrndt M, et al. Forces driving epithelial spreading in zebrafish gastrulation. Science. 2012;338:257–260. doi: 10.1126/science.1224143. [DOI] [PubMed] [Google Scholar]
- 72.Maître J-L, et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338:253–256. doi: 10.1126/science.1225399. [DOI] [PubMed] [Google Scholar]
- 73.Elipot Y, Legendre L, Père S, Sohm F, Rétaux S. Astyanax transgenesis and husbandry: How cavefish enters the laboratory. Zebrafish. 2014;11:291–299. doi: 10.1089/zeb.2014.1005. [DOI] [PubMed] [Google Scholar]
- 74.Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech Dev. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 75.McGaugh SE, et al. The cavefish genome reveals candidate genes for eye loss. Nat Commun. 2014;5:5307. doi: 10.1038/ncomms6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hinaux H, et al. De novo sequencing of Astyanax mexicanus surface fish and Pachón cavefish transcriptomes reveals enrichment of mutations in cavefish putative eye genes. PLoS One. 2013;8:e53553. doi: 10.1371/journal.pone.0053553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol. 2002;12:2023–2028. doi: 10.1016/s0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- 78.Yang L, et al. Phylogeny and polyploidy: Resolving the classification of cyprinine fishes (Teleostei: Cypriniformes) Mol Phylogenet Evol. 2015;85:97–116. doi: 10.1016/j.ympev.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 79.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Képiró M, et al. para-Nitroblebbistatin, the non-cytotoxic and photostable myosin II inhibitor. Angew Chem Int Ed Engl. 2014;53:8211–8215. doi: 10.1002/anie.201403540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.