Abstract
Many practicing biologists accept that nothing in their discipline makes sense except in the light of evolution, and that natural selection is evolution’s principal sense-maker. But what natural selection actually is (a force or a statistical outcome, for example) and the levels of the biological hierarchy (genes, organisms, species, or even ecosystems) at which it operates directly are still actively disputed among philosophers and theoretical biologists. Most formulations of evolution by natural selection emphasize the differential reproduction of entities at one or the other of these levels. Some also recognize differential persistence, but in either case the focus is on lineages of material things: even species can be thought of as spatiotemporally restricted, if dispersed, physical beings. Few consider—as “units of selection” in their own right—the processes implemented by genes, cells, species, or communities. “It’s the song not the singer” (ITSNTS) theory does that, also claiming that evolution by natural selection of processes is more easily understood and explained as differential persistence than as differential reproduction. ITSNTS was formulated as a response to the observation that the collective functions of microbial communities (the songs) are more stably conserved and ecologically relevant than are the taxa that implement them (the singers). It aims to serve as a useful corrective to claims that “holobionts” (microbes and their animal or plant hosts) are aggregate “units of selection,” claims that often conflate meanings of that latter term. But ITSNS also seems broadly applicable, for example, to the evolution of global biogeochemical cycles and the definition of ecosystem function.
Keywords: evolution, natural selection, process, persistence, microbiome
Our purpose here is to introduce and justify ITSNTS (“It’s the song, not the singer”) theory as a way of conceptualizing evolution by natural selection (ENS) that allows processes as well as things to be “units of selection,” selected for persistence and re-produced but not reproducing (1, 2). We see this as a legitimate and broadly useful application of Darwinian principles in a broad area (community evolution) currently in want of such theory. ITSNTS makes it sensible to talk about adaptations and functions as relevant to (if not properties of) multispecies collectives, while standard ENS formulations do not. Whenever what is remarkable about a biological or cultural phenomenon is the persistence of a process or pattern of interactions (a song) that can be carried out by a variety of cooperating entities (singers), each of these having evolved because individual participation has (as in standard ENS) favored its differential reproduction, that is ITSNTS.
Lewontin (3) summarized the collective wisdom of neoDarwinian theorists in a three-part recipe for what we here are calling “standard ENS.”
(i) There is variation in morphological, physiological, and behavioral traits among members of a species (the principle of variation). (ii) The variation is in part heritable, so that individuals resemble their relations more than they resemble unrelated individuals and, in particular, offspring resemble their parents (the principle of heredity). (iii) Different variants leave different numbers of offspring either in immediate or remote generations (the principle of differential fitness).
Lewontin (3) claimed that these conditions are necessary and sufficient for ENS, and here we take as one type of ENS that which will likely occur given them, regardless of the nature of the evolving entity. But as commonly understood, these principles entail differential reproduction of (arguably) material entities producing parent–offspring lineages. ITSNTS questions this requirement, seeing Lewontin’s conditions as sufficient but not necessary for ENS, which we take to be any reiterated causal process resulting in greater relative representation at time tn later than t1 of certain types of entities by virtue of those types’ possession of certain traits, those traits for that reason being adaptations (2).
Godfrey-Smith (4) describes the more limited, traditional view when he writes that:
Evolution by natural selection is change in a population owing to variation, heredity and differential reproductive success. This is usually seen as a micro-evolutionary process acting on organisms, but the criteria required are abstract; genes, cells, social groups and species can all, in principle, enter into change of this kind. For any objects to be units of selection in this sense, however, they must be connected by parent–offspring relations; they must have the capacity to reproduce. Units of selection in this sense can be called Darwinian individuals. An evolutionarily relevant case of reproduction can take many forms. There need not be replication, the faithful production of copies. Replicators are Darwinian individuals with high-fidelity, asexual reproduction, and it is possible to have evolution by natural selection on units where reproduction is sexual and heredity is weak (emphasis ours).
Although sexual heredity is weaker because each parent’s contribution is diluted by half, children usually resemble parents and siblings more than they do “unrelated individuals,” and this is all that Lewontin’s second condition actually demands. But the more parents, the more the dilution, and Godfrey-Smith (4) cautions that “… there must be some parent–offspring similarity, and the clarity of a ‘parent-offspring’ relation of the relevant kind is inversely related to the number of parents—if there are too many parents there are no parents at all.”
How There Can Be “Too Many Parents”
What ITSNTS theory aims to undermine and supplant are claims that multispecies groups comprise material units of selection even when in violation of Lewontin’s recipe, in violation because there are too many parents. Such claims (5–9) made with respect to microbial communities (symbioses, “holobionts,” biofilms, and circumscribed ecosystems) initially motivated ITSNTS, but the theory is more broadly applicable, as will be argued later.
Consider the following simple model. Imagine a constant number, c, of communities, each formed by the coming together at random (recruitment) of n individual asexual organisms (for example, bacteria) representing s species, where s << n. After formation of communities, individual organisms can replicate within them. We stipulate that no species’ lineage has a reproductive advantage over another during such in-community replication, so that differences in species composition at the end of a community’s life compared with its beginning are stochastic.
In this model, at the end of a community’s life the individual organisms contained in it are released into a pool, and n of these are chosen randomly from such a pool to form each community in the next generation of communities. What matters to the argument here is how many original communities (call them parental communities) contribute to the pool from which a new one is formed. Call that number p.
If p = 1, it is meaningful to think of communities as reproducing and forming parent–offspring lineages. The pool has a single contributing parent community and is the exclusive contributor to one or more “offspring” communities. The situation would be like that of a lichen, whose dispersal structures include individuals from both fungus and phycobiont or, as we envision here, of a multispecies biofilm that “reproduces” by budding off a fragment containing a random sample of the organisms present in it, this to reattach and grow at a new site. If fragments are large enough to overcome “ecological drift” (10), each offspring community will likely resemble its parent or siblings in species composition more than it resembles unrelated communities. Lewontin’s conditions would apply and community-level properties, such as the tendency to form more robust propagules more often (community-reproductive traits that might depend on species make-up) could be selected for.
However, suppose instead that all parental communities release the individual organisms they contain into a common pool, from which n are randomly chosen to form each community in the next generation of communities. In this case, the species compositions of the offspring communities will reflect only that of the common pool and will, sampling error aside, resemble each other. By the “law of large numbers,” such intercommunity resemblance will get closer and closer as n increases, just as the frequency of heads flipped with an honest coin gets closer and closer to 50% the longer one flips.
Of necessity, n cannot be less than p, the number of parents contributing at least one individual to a new community. In many real microbial communities assembled by de novo recruitment from multiple sources, such as multispecies biofilms, the microbiomes of many animals and plants, and environmental sites like hydrothermal vents or whale carcasses on the sea bottom, p and n will both often be very large—dozens to thousands, perhaps—and new recruits can also come from lineages of organisms previously only loosely associated with circumscribable communities. (It is important to remember that it is the number of parent communities that contribute individuals to each offspring community that matters in this model, not the number of different species that these individuals might represent.)
So, the relevance of Godfrey-Smith’s (4) “too many parents” warning is this. When p is large, n is as large and usually larger. It might in principle be possible to identify all parental communities contributing at least one individual organism to any particular offspring community: complex parent–offspring relations could then still be defined. But individual offspring communities will not resemble their “parents” or relations significantly more than they resemble “unrelated” communities. Lewontin’s second condition does not hold and ENS as normally understood cannot affect communities as communities. So it seems appropriate to say that at some value of p there come to be too many parents. Past this point we should consider that, although the collection of communities is re-produced (created again), communities do not individually reproduce. Communities cannot be units of selection in the sense of Godfrey-Smith (4).
The Hole in Holobiosis
Proponents of holobiosis, however, often do consider animals and plants together with their respective microbiota to be units of selection (5–9), even when the latter are randomly recruited (re-produced) at each reproductive generation of the former. There is some ambiguity in this literature about whether designating an entity as a unit of selection means that it takes part in an ENS process or is simply an assertion that it (like an organism) is a circumscribable thing subject to environmental forces (11, 12). But it is in the former sense that holobiosis theory has attracted most interest and criticism (13–16).
Sometimes, of course, unit of selection claims might be sensible. The value of p could be 1.0, for example when we are dealing with an obligate maternally transmitted symbiosis, or lichens and biofilms as imagined above. The value of p could also be fairly low for symbioses of coevolved species in which recruitment of new partners is local (as for humans and their pets) (17). But, as noted above, the value of p (and thus n) could be very large for complex ephemeral communities, such as those that establish themselves independently on the sea bottom or in our guts after a course of antibiotics. Thus, Lewontin’s recipe should not apply. For something like the “community” of microbes that, as free-living lineages or symbionts, collectively perform the nitrogen cycle, p is incalculably large, if a community can be defined at all. And it is around the problematics of collective differential reproduction that invocations of even more all-embracing global organism-like evolved entities, such as “Gaia,” have foundered (2, 18).
It is for the same reason problematic to speak of adaptation of multiparental communities formed by recruitment or re-production. Sober and Wilson (19) recommend what they call “William’s Principle”—that “adaptation at a level requires that there was selection at that level”—noting that “[t]he fact that a trait now benefits groups does not entail that it evolved because it was beneficial to groups.” Indeed, it is not clear that any property can be considered “beneficial” to impermanent and nonreproducing communities. These might come to show traits of interest to or valued by us as researchers or citizens, such as functional stability, or resilience, or “eubiosis” (in the case of the “holobiont” that is us and our gut microbiomes), these being the indirect byproducts of “lower-level” processes. But without some form of differential reproduction or differential and continuous physical persistence, these traits cannot be considered adaptations for communities if anything resembling Lewontin’s recipe is to be applied.
ITSNTS Theory
ITSNTS theory seeks to rationalize (or “Darwinize”) such indirect “beneficial” outcomes, casting these not as adaptations for the individuals or collectives that implement a process, but for the process itself. Indeed, it was initially motivated by the now frequent claim that microbial community activities (“functions”) are more stable or ecologically resilient than are the taxonomic compositions of the assemblages carrying them out, a phenomenon demanding evolutionary explanation. Early in the development of microbiomics as a science, Turnbaugh et al. (20) for example, used an appealing ecological metaphor to describe this phenomenon as it was observed in human gut microbiota:
This conservation suggests a high degree of redundancy in the gut microbiome and supports an ecological view of each individual as an “island’ inhabited by unique collections of microbial phylotypes: as in actual islands, different species assemblages converge on shared core functions provided by distinctive components.
How fine-scaled the mapping of genomic diversity to ecological diversity is remains an active question (21, 22). We must ask how often taxa redundant with respect to participation in a given process are specifically adapted to other, unaccounted-for, environmental constraints. In the context of ITSNTS, this will translate to the individuation of processes: Is a given set of biochemical or developmental steps performed under (even very slightly) different conditions to be counted as the same or different? However, when intermediates are freely and globally diffusible (as is the case for many intermediates in major biogeochemical cycles), it makes no sense to attempt any such differentiation. It seems reasonable to regard redundancy as real and irreducible, and the stability and change over time of the more broadly defined processes implemented by redundant taxa as that which a complete theory of ENS needs to encompass and explain.
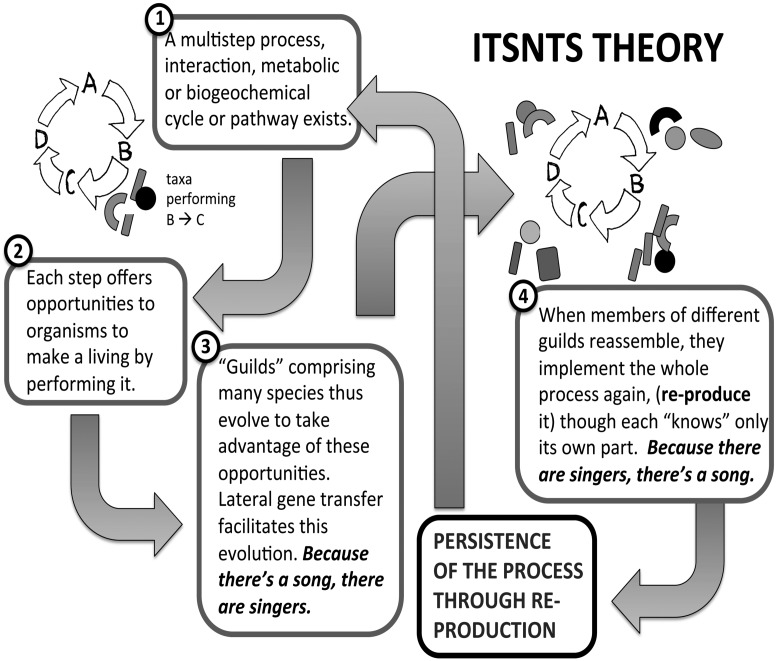
ITSNTS is cartooned in Fig. 1. Consider a long-standing interconnected metabolic interaction, the individual steps or components of which are implemented by individuals from different microbial or multicellular taxa recruited from the environment, none being able to perform all of the steps. These individuals might be physically associated or exchange metabolites over long distances and time periods. The bobtail squid and its recruited vibrios (23) meet our criteria, but again the global nitrogen cycle may be a better example (24). No individual of any species involved in that cycle performs its role self-sacrificially. Each has coevolved with other participants serving as its environment, or against a background of the products of these other participants. No alleles have been fixed just because they promote continuance of the nitrogen cycle as a cycle, for all that alleles that have been fixed may do that: the time scales are too long and there are “too many parents.” Thus, a completed nitrogen cycle (or the squid–vibrio symbiotic relationship) is only a “fortuitous benefit” of the coevolution of separate species, not an adaptation of some interspecies aggregate. Such properties might be seen as beneficial for life in the very long run (2), but have never been adaptations as traditionally defined by a history of differential reproduction within populations. Standard notions of ENS by differential reproduction, and associated concepts of adaptation of things, cannot apply.
Fig. 1.
A cartoon illustrating ITSNTS. Hand-drawn arrows are meant to represent the steps or components of a cyclical process with intermediate metabolites or stages A–D. Shaded symbols (rectangles, ellipses, and so forth) are microbial taxa comprising guilds that implement each step. The process or interaction, by existing, encourages the evolution of entities (taxa) that implement its steps, and these in turn reconstruct the process or interaction as a whole. The process thus persists, and changes in the mode of implementation that promote persistence are selected for. (As discussed briefly in the text, a similar scheme could be developed for developmental or regulatory interactions and the genes that carry them out.)
Nevertheless, because each step of the nitrogen cycle in isolation can benefit a taxon that implements it, energetically or in the provision of metabolites, and because the cycle has long been in operation, many taxa (sometimes from all three domains and forming ecological “guilds”) have evolved that can and now do, collectively, perform it (Fig. 1). Lateral gene transfer, whose various mechanisms are adaptations for the subcellular agents of transfer, facilitates the expansion of guilds, enabling operation of the cycle under many environmental conditions. Because there’s a song, there are singers.
Reciprocally, because there are many taxa capable of implementing each step, and intermediates are variously diffusible, the cycle would spontaneously resume operation if disrupted. The responsible taxa would re-produce it. Such disruption is no doubt unimaginable in the case of the nitrogen cycle globally, but analogous re-production occurs locally (and daily for sunlight-dependent processes) and periodically for others assembled by recruitment, such as the squid–vibrio pair, decaying whale carcass microbiota, and our own gut microbiomes. Because there are singers, there’s a song.
Together, evolutionary recruitment of capable taxa and their periodic reassembly, re-producing the nitrogen cycle or any of these less global processes, promote their persistence and evolution as processes. All else being equal, we might imagine that the persistence of a process would depend on the taxa that have evolved to perform its various steps, specifically on their number, population sizes, diversity (ecological and biochemical), and geographic dispersal. And the properties of a process will affect these variables and thus the persistence of that process, in a variety of ways. Among relevant properties would be: (i) the advantage in energetic or metabolic gain offered to taxa that can evolve to perform a given step; (ii) the genetic underpinning of such performance, for example close genetic linkage of the required genes, facilitating simultaneous lateral gene transfer and the formation of taxonomically and ecologically diverse “guilds”; (iii) sustainability, in not requiring abiotic components available only in limiting supply; (iv) the range of physical conditions under which the process can be implemented; and (v) ability to integrate with other processes, either through interchangeable products or the availability or evolvability of taxa participating in multiple processes. Comprising a cycle (rather than a linear pathway leading to the accumulation of an unmetabolizable end product) should also promote process persistence, and Life’s existence and continuity might depend on the prevalence and interconnectedness of many biogeochemical cycles (24). Processes will change over time as a consequence of changes (driven by ENS as usually understood) among the taxa that collectively implement them, and changes that promote process persistence will preferentially accumulate among the survivors. To the extent that changes promoting persistence buy time for further persistence-promoting changes, “complex adaptations” will be progressively elaborated (25–27).
Interestingly, some investigators now treat the history and development of biogeochemical cycles in the way one would treat those of organismal lineages (28, 29), showing both stability and change over time. Both are “complex adaptive systems” exhibiting “aggregation” (hierarchical organization), “nonlinearity” (path-dependant evolvability), “diversity” (redundant singers), and “flows” (implementations of process) as described, for example, by Levin (30). Their evolutionary trajectories are constrained by the laws of physics and chemistry but also by their own prior histories: we do not know if Life’s tape were rewound how similar its processes would be (31).
Parallels to ITSNTS
ITSNTS thus offers an alternative to luck and the anthropic principle (2) for rationalizing Life’s long tenure on this planet, recasting “fortuitous benefits” as persistence-promoting adaptations of processes, not of collectives of things. It links process evolution by differential re-production to ENS through the differential reproduction of process-implementing individual organisms, addressing deficiencies of claims about holobionts and attributions of function to nonmicrobial ecosystems (see below). The theory nevertheless has similarities, some only apparent but some quite relevant, to other areas of biological theory and philosophy.
Group Selection.
Group selection—as in popular models of the evolution of altruism within species (for example, see ref. 32)—might be seen as relevant to the evolution of microbial collectives. For example, a minor modification of our model still consistent with standard ENS would allow that groups with more of species X produce more individuals overall (of any species). The frequency of X individuals in pools would thus rise, as would the frequency of communities dominated by species X formed from them. But this would be a direct effect of group composition on reproduction of individuals within groups, not on the differential reproduction of groups as groups (33). By Lewontin’s recipe, or “William’s Principle,” only the latter can generate group-level adaptations (19). Interspecies cooperation, whether or not taken to be a kind of altruism, counts as merely a “fortuitous benefit” of group selection of this kind.
Endosymbiosis and the Black Queen Hypothesis.
The Black Queen Hypothesis was developed to accommodate the increasingly common finding of natural microbial communities in which genes necessary for life are distributed among cellular lineages, such that some depend on the leaked metabolites of others, seen as the result of a self-limiting process driven by selection for reduced genome size (34). This seems more surprising when it involves separate cellular lineages rather than endosymbiont and nuclear lineages in a single cell, but the evolutionary strategy is the same: a biochemical pathway remains in operation while the genes underwriting it are distributed among genomic lineages that are henceforth bound to evolve together. The singers divide up the parts, but the song goes on (35).
With independent cellular lineages, one might speak of a “population pan-genome,” comprising all genes present in all lineages at a given site and, as Fullmer et al. (36) suggest:
… seriously consider populations as the operative units in which genes are selected … rather than exclusively individual organisms. Similar to how Richard Dawkins advocated thinking of an organism as a collection of generally agreeable, but selfish, genes perhaps we should be thinking of lineages and populations as the collections of genes, i.e., pan-genomes, rather than the individual cells.
The problem here, again, is that so long as lineages and populations behave as in our model with large p and n, Lewontin’s recipe does not apply. ITSNTS does.
Types, Tokens, and Genes.
In an essay on types and tokens, Wetzel (37) uses Gertrude Stein’s “Rose is a rose is a rose is a rose” as an example. If “word” is taken as a type, there are three words in this sentence, while as tokens there are 10. Whatever biological processes are made of, it’s not the same kind of stuff as the things (genes, cells, organisms, species) that implement them. The difference seems like the philosophical one between types and tokens.
Dawkins (38), in his popular explication of emerging theory (The Selfish Gene), also had something like this in mind. His genes as “immortal coils” are informational types and their embodiments in DNA are tokens. In the same vein and explicitly, Haig (39) writes of this way of thinking that:
… material genes were physical objects but informational genes were the abstract sequences of which material genes were temporary vehicles. Material genes were identified with gene tokens and informational genes with gene types … Continuity resides in the recursive representation of immortal pattern by ephemeral avatars (emphasis ours).
For ITSNTS, too, the continuity of process “resides in the recursive representation of immortal pattern by ephemeral avatars (collectively implementing taxa)” (39). Arguably unlike genes, the pattern as a type need never be represented in one place at one time: the taxa implementing it (the singers) know only their own parts and can, in the case of something like the nitrogen cycle, be on opposite parts of the globe in different centuries (29).
Although the focus here is on community-level processes, it is also possible to apply such reasoning to within-lineage developmental evolution at the organismal, cellular, and molecular levels. Wagner (40) argues, for example, that “it is the historical continuity of gene regulatory networks rather than the expression of individual homologous genes that underlies the homology of morphological characters.” In a sense, preexisting developmental processes (regulatory networks) are the types maintained by (persisting through) ENS. These processes recruit genes, and not always the same ones, needed for their implementation (as tokens), just as biogeochemical cycles recruit microbial taxa.
Memes.
There are equally obvious parallels between ITSNTS and the notion of meme selection, and objections to it might take a similar form (4, 41). Dennett (42) recently reviewed and rebutted some of these objections in the case of meme theory. He dismisses out of hand the complaint that memes are not “real.” We venture that this complaint is ontological, having to do with the differing kinds of stuff of which types and tokens are made. It seems incontrovertible that something like ITSNS happens in the exemplary case of words. Words as tokens do not reproduce, but through the intermediary of speaking or writing (indeed remarkably, through many media) they are re-produced, and as a consequence individual words persist as represented types. We suggest that ITSNTS can similarly dismiss the reality concern: processes implemented by organisms are as real (or unreal) as words spoken by people.
Godfrey-Smith (4) articulates a second concern about memetics, writing: “As far as theory goes, recent work does establish substantial links between cultural and biological [ENS] mechanisms. It gives us ‘how-possibly’ explanations for the maintenance of various behaviours. Its empirical application—its ability to give us ‘how actually’ explanations—is another matter.”
Indeed, even meme skeptics accept that a coherent selective process can be imagined. The situation with ITSNTS seems similar. It is easier to argue that persistence is a legitimate target for ENS, in principle, than to show that this matters in practice. And the former was ITNTS’s initial purpose: to legitimize discussions of selection, adaptation, and function for community activities that seem inexplicable to ENS, as long as this is focused on the differential reproduction of things (1, 2). Whether or not such selection, function, and adaptation will prove to be important in explaining what we see in the world is a separate, if not uninteresting, matter. Similarly one might uncouple Darwin’s formulation of the principle that reiterated acts of selection and amplification of variants will produce complexity [which Sober (43) calls an a priori] from the empirically testable claim that this process explains biological adaptedness and diversity overall, or even any particular instance thereof. But ITSNTS does provide a specific mechanism (sketched in Fig. 1) for what might otherwise seem a mere logical necessity.
For the cultural domain, Godfrey-Smith (4) insists that the means by which memes are reproduced must mimic organismal reproduction (not entailing too many extraneous influences or, especially, too many parents) for ENS to be the applicable model. Direct imitation will do it for him [as for the critic Sperber (41)], while more diffuse mixtures of group learning and idiosyncratic creativity will not. For the evolution of microbial communities, ITSNTS relaxes our understanding of what ENS requires, allowing re-production to stand in for reproduction, and such a relaxation may also make sense in the cultural context, on the condition that there remains a causal relationship between the properties of populations over time, as noted below (44, 45).
Major Transitions in Evolution.
A longstanding problem with Maynard Smith and Szathmàry’s “Major Transitions in Evolution” formalism, in for example its 1995 version (46), is that the transition from “Solitary individuals to colonies (non-reproductive classes)” is followed by “Primate societies to human societies (language),” and these seem to be different in kind. The first (and several before it) are “transitions in individuality,” successive steps in the hierarchy of life in which lower-level units are combined into higher-level collectives. Languages, however, are not comprised of the individuals that speak them, although each affects ENS of the other. ITSNTS thinking offers a way, analogous to gene-culture coevolution (47), to understand interacting evolutionary process that are not nested, one within another, but comprise separate though interacting domains.
Process Ontology.
Dupré (48) argues that much of contemporary biology embraces a generally mechanistic and reductionist “thing” (or “substance”) ontology. According to such a metaphysics, what the universe holds are material entities, while processes are secondary: just what happens to, or are performed by, things. Conversely, process ontologists see processes as primary, things as their manifestations. For ITSNTS this would mean, in effect, that taxa and the communities they form are adaptations of the processes they implement, not the other way around. And of course we, as multicellular individuals, are processes: few of our cells are “the same” as those with which we were born, and our identity is sustained throughout our lives by the continuity of developmental and regenerative processes, not the atoms or cells in our bodies at birth. Dupré makes a similar case for sexual (not asexual) species, linking that to the widely accepted notion that such species are spatio-temporally continuous biological individuals, not essence-defined classes. Processes can be individuals, although not all phenomena we call processes (erosion, inflation, evolution itself) have the necessary coherence. ITSNTS envisions that some community-level processes (which Dupré would call “stabilized”) do have the necessary coherence. Some individual processes (the squid–vibrio interaction) will be relatively easy to circumscribe, while others (many biogeochemical cycles or the metabolic interactions in our gut) will be highly interconnected.
ITSNTS and Niche Construction.
There are especially obvious parallels to formulations of niche construction theory (49, 50), taken as an evolutionary process in which “organisms, through their metabolism, their activities, and their choices, modify their own and/or each other’s niches” (50) in such a way as to affect further ENS. By prioritizing processes as units of selection, accepting as adaptations characteristics that have promoted differential process persistence, and allowing re-production to stand in for reproduction, ITSNTS may offer additional resistance to the skeptical view that niche construction is about nothing more than “extended phenotypes” (51). Indeed, Haig (52) sees these as usefully distinguished, writing that “[t]he ‘extended phenotype’ and ‘niche construction’ are sometimes presented as alternative labels for a single concept, but I prefer a division of labor in which the former term refers to differences under selection and the latter to evolved parts of the environment. From this perspective, bodies and genomes are constructed niches that select among genetic differences.” ITSNTS similarly admits that “evolution by natural selection converts that which is selected (phenotype) into that which selects (environment)” (52), but sees this conversion, too, as part of a larger process, selectable among alternatives for persistence. As well, processes as patterns of interaction are not committed to any single type of material implementation: the same “immortal pattern” can be realized in fundamentally distinct “ephemeral avatars.” Thus, we might allow bridging of awkward transitions in retelling the history of life, such as that between unicells and multicellular organisms, the RNA and DNA-protein worlds, and indeed prebiotic nucleic acid-independent chemical processes and the RNA world (53, 54).
Rewriting Lewontin’s Recipe
If ITSNTS is accepted as a form of ENS, then Lewontin’s three conditions, as commonly interpreted, are sufficient but in fact not necessary. We must allow that ENS can address differential persistence as well as differential reproduction, and that re-production can be how the former is underwritten. Arguments have been made for both elements of such a synthesis: ITSNTS integrates them.
Differential Persistence and Differential Reproduction.
Claims that ENS addresses “survival and reproduction” are commonplace in textbooks and general folk understandings, but most evolutionists view differential reproduction (of alleles in particular) as the fundamental feature of ENS, with survival (of individual organisms) being of value only because it allows more time for reproduction. Possibly, it is the contingent history of the modern synthesis and population genetics as its dominant formalism that make us think like this. But as Bouchard (26) has pointed out:
… persistence (viability, survival, etc.) is always in the picture. To put this a different way, differential persistence seems to be necessary to have evolution by natural selection while differential reproductive success seems to be a contingent strategy for a lineage to survive. The irony is that this account was always in our face: Darwinism is usually understood, not as “reproduction of the fittest” but as “survival of the fittest.”
Indeed, and in line with a suggestion by Dennett (55) in a review of Godfrey-Smith’s 2009 book Darwinian Populations and Natural Selection (56), ITSNTS theory might integrate persistence-based and reproduction-based ENS as extremes on a seamless spectrum. It is not so much that Nature holds two distinct kinds of evolving entities in either the domain of things or the domain of processes: there is a spectrum in both. But there are two distinct ways of thinking about variation-and-selection, one emphasizing reproduction and the other persistence. “The nitrogen cycle,” for example, might be individuated as a single persistent process evolving by the ITSNTS mechanism, being re-produced rather than reproduced. But equally, one might regard nitrogen cycles performed at different temperatures or values of pH, or by different microbial guilds, as multiple different progeny of some original cycle whose reproductive success is thereby demonstrated. Reproduction in this case might be a gradual divergence, as in some cases of speciation. Note that for ITSNTS it would be types (processes) and not tokens (implementations of processes by things) whose persistence or reproduction is inferred.
As Godfrey-Smith (4) stresses in his essay on cultural evolution, the “grain” of the analysis matters. With fine-grained individuation (many types of nitrogen cycles, individual organisms in a species) differential reproduction makes sense, but as the grain gets coarser (nitrogen versus sulfur cycles, for example, or entire species or higher clades in the domain of things), differential persistence of processes becomes more satisfying as an explanation, and the role of reproduction as just one mechanism for promoting it becomes more obvious (25–27, 57–59). Having more (or more diverse) progeny benefits individuals within a species through differential reproduction but can benefit the species through differential persistence (relative immunity to extinction). Similarly, having nitrogen cycles implemented in many places under many conditions favor the persistence of the cycle, broadly construed.
As reproduction becomes persistence, the need for identifying a relevant “Darwinian population” becomes less obvious: there is already a literature that makes this point (26, 27, 57–59). What ITNSTS has additionally to offer, we think, is a mechanism (evolutionary recruitment) by which the processes that are re-produced evolve, as do persistent clonal organisms (like aspen groves) or clades above species (57). ENS indeed is about “survival and reproduction,” but the relationship is more interesting than that organisms that don’t survive won’t reproduce.
Re-Production and Reproduction.
Persistence of a process of necessity entails continual or continuous re-production, and vice versa. Sperber (41) and other meme-skeptics complain of memes that they don’t reproduce except when mindlessly imitated: memes are otherwise re-produced by a diversity of influences and do not show parent–offspring lineages. This is true but not fatal to ENS (for either memes or community metabolisms) as long as there is some causal connection between populations over time, a relationship between implementations of a process now and in the future such that the former can be seen as necessary for the latter. There are currently several competing formulations bearing on this possibility. Charbonneau (58) argues that population-level processes of ENS (“generation” and “memory”) do not require “local-level lineages.” Bourrat (59) shows that evolution for persistence without reproduction is consistent with the Price equation. And De Monte and Rainey (53) claim that reproduction and “heritable variation in fitness” are unnecessary as long as genealogies of collectives are traceable.
One might also refer to re-production as recurrence (60), as is done for organs like the heart that are reconstructed during development but do not reproduce or generate parent–offspring lineages: I am my father’s son, but my heart is not the offspring of my father’s heart. But hearts are encoded in genomes that are reproduced, while re-production in ITSNTS theory requires no comparable single information repository nor any single lineage of such repositories unless, as very expanded forms of developmental systems theory (61) or niche construction might hold, one regards the larger biosphere and associated abiotic components as that repository.
Still, for ITSNTS there is a causal relationship between implementations of a process now and in the future such that the former are in the long run necessary for (and determine the properties of) the latter: if a process dies, taxa that benefit from performing its components likely will as well. That is, successive re-productions are necessary for the persistence of a process in the same way that continued reproduction of its members is necessary for the persistence of a species or continual replacement of cells is necessary for our survival as individual organisms. Godfrey-Smith (45) has stressed that it is the complexity of any causal chain that determines the appropriateness of “replicator” language in such situations: “persistence language” may be more forgiving.
ITNSTS Beyond Microbial Communities
ITSNTS is, as Sober argues in the case of some other biological models, a priori true in the sense that no experiment could disprove it (43, 62). The empirical question then is about range of application and utility as explanation. One obvious extension beyond microbial ecology and biogeochemistry is into traditional (animal and plant) community ecology and the field of biodiversity-ecosystem function research (63). Indeed, Turnbaugh et al. (20) (quoted above) likened the stability of microbial community functions across sites (guts) to the stability of functional types of nonmicrobial species across islands.
ITSNTS offers a sensible way of discussing adaptations and functions relevant to communities and ecosystems generally, concepts also tied to recent discussions of the “health” of such systems, and about which there is arguably “an urgent need for a general framework” (64). Particularly germane is the long history of attempts in community and ecosystem ecology to discover and justify the conditions for stable persistence or resilience of assemblages through time (indeed, of complex adaptive systems as collectives more generally) (30, 65–67). Recent authors have argued that these conditions involve at least functional redundancy, response diversity, spatial heterogeneity, and modularity (68). Such a move away from traditional explanations that treat communities as defined by a list of member species (as things) is at least implicit in trait-based (or “process-focused”) accounts of biodiversity and functional biogeography, now a standard approach in literature on resilience (69, 70). Jax (71) writes that “In this type … the processes are the very focus of the system’s definition. In a conservation and resource management context, it is often the processes (and their bearers) selected as ecosystem services that are of concern; they guide the idea of what constitutes a functioning ecosystem.”
On the advantage of this approach, Gagic et al. (72) write that “[i]ndices solely based on the numbers and abundances of species [i.e., taxonomic diversity measures] were consistently poor at predicting ecosystem functioning… Moreover, they performed worse than indices using a trait-based approach, both in previous studies of plants …and in our current analysis of animals.”
ITSNTS provides an evolutionary framework for rationalizing collective community properties—such as redundancy or diversity of guilds—as pattern or process adaptations, rather than adaptations of communities themselves or mere fortuitous byproducts of the “selfish” interactions and adaptations of their individual members. Selection for the persistence (or stability) of these patterns as types is underwritten by their re-production as repeatable tokens—by implementing species assemblages—such as the longleaf pine forest (73). ITSNTS thus rationalizes and redefines ecological “community function,” potentially in one (or both) of two ways (74, 75).
The first is forward-looking, casting functions as contributions to current or future persistence-promoting traits of processes, implemented by a current collection of taxa. Such an approach was recently suggested by Dussault and Bouchard (76), who nevertheless see the community itself as adaptation-bearing. For ITSNTS, if a property, such as being implementable by diverse taxa under diverse conditions, contributes to the persistence of a pattern, then it is ascribed a forward-looking function. Such functions are explanatory in the sense that they provide answers to “why?” questions about the contribution of pattern properties to observable pattern persistence.
A backward-looking account, equally consistent with ITSNTS thinking and perhaps more in line with something like William’s Principle (19) can also be entertained. Such explanations answer a different explanatory question: they explain why the pattern has the properties it has, and they do so by referring to the function of those properties as past selected effects (77). The evolution of patterns of interaction is premised on some patterns persisting longer than others as a result of certain properties. The function of a property, on this account, is that of its effects that contributed to the property’s maintenance under selection for persistence, regardless of how the diversity of a pattern or process arose. Either or both of these ITSNTS understandings of function may prove helpful for current discussions of ecosystem functioning and services (71), or for helping to clearly delineate different explanatory projects in different branches of ecology.
Another point of connection between ITSNTS and ecological theory are notions of niche-based environmental filtering and species sorting, which have been well-developed as part of the theory of community assembly, both in microbial and nonmicrobial ecology (66, 78). These theories add rigor and substance to our claim that “because there’s a song, there are singers” by explaining the process through which species are “selected” from larger pools to join as members of smaller communities, as in Fig. 1.
Finally, ITSNTS side-steps an important problem that has been raised for ecosystem evolution and function: the inclusion of abiotic components. Odenbaugh (79) has argued that a backward-looking (selected effects) definition of function cannot apply to abiotic components of ecosystems because those components are not “reproduced” with the rest of the ecosystem. Heritability seems to be a stumbling block. More recently, Bouchard (80) has argued that the inclusion of abiotic components in ecosystems causes problems for ecosystem evolution in general, because,
the inclusion of abiotic material as parts of an evolving system makes the evolution of whole ecosystems a nonstarter for most evolutionary approaches: ecosystems do not have a unified genome, and even a meta-genome will leave out essential parts of ecosystems, such as the abiotic factors that maintain much of the emergent properties of said ecosystems. Evolution as change in allelic frequencies does not seem to apply to systems that have a motley crew of alleles and abiotic material interacting in a systematic way.
ITSNTS provides a way of eliding these issues by focusing attention instead on the processes that evolve, not on the things (biotic or abiotic) that implement them. Reproduction is not required.
ITSNTS and Evolutionary Explanation
Darwin argued, first, that the diversity of living organisms can be understood historically and, second, that a law-like principle, natural selection, was a significant (for him the principal) cause of such diversification. What biologists have seen over the last century is an expansion of levels in the biological hierarchy, from stretches of “selfish DNA” to species, at which this law-like process is thought to operate. We might also embrace ENS in the cultural domain: surely dairying practices both explain and are explained by the frequencies of lactose intolerance in dairying and nondairying populations (81). But cultures are not just another level in the hierarchy, so this might be seen as bidirectional “sideways,” rather than “upward” and “downward” causation (82).
ITSNTS is similar and could play a similar role in explaining the prevalence of taxa and their activities. What we need is identification and understanding of factors that make some biologically mediated processes more persistent than others, and how these affect and are affected by differential reproduction of taxa that implement them. This understanding could answer “why?” questions that are left unanswered by Lewontin’s recipe, at whatever level it is focused, questions such as: “Why has nitrogen metabolism evolved towards completion as a cycle?” (29), “What drives genome reduction in cross-feeding microbial communities?” (34), and “Why has life on this planet lasted long enough for us to be asking anything about it?” (2). These are questions about biological systems, generously defined, and search for commonalities across systems as addressed by systems biologists seems to require some definition of the subject matter (83, 84). ITNTS provides a framework in which biological systems are those “stabilized” (48) processes that can be individuated as units of selection for persistence. Indeed, in a spirit of explanatory pluralism (85), ITSNTS encourages thinking about process/thing coevolution quite broadly.
Acknowledgments
We thank Austin Booth, Carlos Mariscal, Tyler Brunet, Scott McCain, Letitia Meynell, Richmond Campbell, Elliott Sober, and Andrew Hendry for comments on earlier drafts. This work was supported by Grant GLDSU447989 from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Doolittle WF, Booth A. It’s the song, not the singer: An exploration of holobiosis and evolutionary theory. Biol Philos. 2017;32:5–24. [Google Scholar]
- 2.Doolittle WF. Darwinizing Gaia. J Theor Biol. 2017;434:11–19. doi: 10.1016/j.jtbi.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Lewontin RC. Population genetics. Annu Rev Genet. 1985;19:81–102. doi: 10.1146/annurev.ge.19.120185.000501. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey-Smith P. Darwinism and cultural change. Philos Trans R Soc Lond B Biol Sci. 2012;367:2160–2170. doi: 10.1098/rstb.2012.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plans: The hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert SF, Sapp J, Tauber AI. A symbiotic view of life: We have never been individuals. Q Rev Biol. 2012;87:325–341. doi: 10.1086/668166. [DOI] [PubMed] [Google Scholar]
- 7.Gordon J, Knowlton N, Relman DA, Rohwer F, Youle M. Superorganisms and holobionts. Microbe. 2013;8:152–153. [Google Scholar]
- 8.Bordenstein SR, Theis KR. Host biology in the light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015;13:e1002226. doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemanceau P, Blouin M, Muller D, Moënne-Loccoz Y. Let the core microbiota be functional. Trends Plant Sci. 2017;22:583–595. doi: 10.1016/j.tplants.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Vellend M. Conceptual synthesis in community ecology. Q Rev Biol. 2010;85:183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 11.Ereshefsky M, Pedroso M. Rethinking evolutionary individuality. Proc Natl Acad Sci USA. 2015;112:10126–10132. doi: 10.1073/pnas.1421377112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roughgarden J, Gilbert JF, Rosenberg E, Zilber-Rosenberg I, Lloyd EA. Holobionts as units of selection and a model of their population dynamics and evolution. Biol Theory. 2017;13:44–65. [Google Scholar]
- 13.Moran NA, Sloan DB. The hologenome concept: Helpful or hollow? PLoS Biol. 2015;13:e1002311. doi: 10.1371/journal.pbio.1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas AE, Werren JH. Holes in the hologenome: Why host-microbe symbioses are not holobionts. MBio. 2016;7:e02099. doi: 10.1128/mBio.02099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skillings D. Holobionts and the ecology of organisms: Multi-species communities or integrated individuals. Biol Philos. 2016;31:875–892. [Google Scholar]
- 16.Clarke E. Levels of selection in biofilms: Multispecies biofilms are not evolutionary individuals. Biol Philos. 2016;31:191–212. [Google Scholar]
- 17.Song SJ, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfrey-Smith P. The ant and the steam engine. Lond Rev Books. 2015;37:18–20. [Google Scholar]
- 19.Sober E, Wilson DS. Adaptation and natural selection revisited. J Evol Biol. 2011;24:462–468. doi: 10.1111/j.1420-9101.2010.02162.x. [DOI] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordero OX, Polz MF. Explaining microbial genomic diversity in light of evolutionary ecology. Nat Rev Microbiol. 2014;12:263–273. doi: 10.1038/nrmicro3218. [DOI] [PubMed] [Google Scholar]
- 22.Louca S, et al. High taxonomic variability despite stable functional structure across microbial communities. Nat Ecol Evol. 2016;1:15. doi: 10.1038/s41559-016-0015. [DOI] [PubMed] [Google Scholar]
- 23.Nyholm SV, McFall-Ngai MJ. The winnowing: Establishing the squid-vibrio symbiosis. Nat Rev Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- 24.Falkowski PG, Fenchel T, Delong EF. The microbial engines that drive Earth’s biogeochemical cycles. Science. 2008;320:1034–1039. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- 25.Doolittle WF. Natural selection through survival alone, and the possibility of Gaia. Biol Philos. 2013;29:415–423. [Google Scholar]
- 26.Bouchard F. Darwinism without populations: A more inclusive understanding of the “survival of the fittest”. Stud Hist Philos Biol Biomed Sci. 2011;42:106–114. doi: 10.1016/j.shpsc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Bouchard F. Causal processes, fitness, and the differential persistence of lineages. Philos Sci. 2008;75:560–570. [Google Scholar]
- 28.Braakman R, Follows MJ, Chisholm SW. Metabolic evolution and the self-organization of ecosystems. Proc Natl Acad Sci USA. 2017;114:E3091–E3100. doi: 10.1073/pnas.1619573114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klotz MG, Stein LY. Nitrifier genomics and evolution of the nitrogen cycle. FEMS Microbiol Lett. 2008;278:146–156. doi: 10.1111/j.1574-6968.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 30.Levin SA. Ecosystems and the biosphere as complex adaptive systems. Ecosystems. 1998;1:431–436. [Google Scholar]
- 31.Falkowski P. Life’s Engines: How Microbes Made Earth Habitable. Princeton Univ Press; Princeton: 2015. [Google Scholar]
- 32.Sober E, Wilson DS. Unto Others: The Evolution and Psychology of Unselfish Behavior. Harvard Univ Press; Cambridge, MA: 1999. [Google Scholar]
- 33.Okasha S. Evolution and the Levels of Selection. Oxford Univ Press; Oxford: 2006. [Google Scholar]
- 34.Morris JJ, Lenski RE, Zinser ER. The Black Queen Hypothesis: Evolution of dependencies through adaptive gene loss. MBio. 2012;3:e00036-12. doi: 10.1128/mBio.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estrela S, Kerr B, Morris JJ. Transitions in individuality through symbiosis. Curr Opin Microbiol. 2016;31:191–198. doi: 10.1016/j.mib.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Fullmer MS, Soucy SM, Gogarten JP. The pan-genome as a shared genomic resource: Mutual cheating, cooperation and the black queen hypothesis. Front Microbiol. 2015;6:728. doi: 10.3389/fmicb.2015.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetzel L. 2014 Types and tokens. The Stanford Encyclopedia of Philosophy, ed Zalta EN, Spring 2014 Ed. Available at https://plato.stanford.edu/archives/spr2014/entries/types-tokens/. Accessed December 22, 2017.
- 38.Dawkins R. The Selfish Gene. Oxford Univ Press; Oxford: 1976. [Google Scholar]
- 39.Haig D. Fighting the good cause: Meaning, purpose, difference, and choice. Biol Philos. 2014;29:675–697. [Google Scholar]
- 40.Wagner GP. The developmental genetics of homology. Nat Rev Genet. 2007;8:473–479. doi: 10.1038/nrg2099. [DOI] [PubMed] [Google Scholar]
- 41.Sperber D. An objection to the memetic approach to culture. In: Aunger R, editor. Darwinizing Culture: The Status of Memetics as a Science. Oxford Univ Press; Oxford: 2000. pp. 143–162. [Google Scholar]
- 42.Dennett D. From Bacteria to Bach and Back: The Evolution of Minds. W. W. Norton; New York: 2017. [Google Scholar]
- 43.Sober E. A priori causal models of natural selection. Australas J Philos. 2011;89:571–589. [Google Scholar]
- 44.Claidière N, Scott-Phillips TC, Sperber D. How Darwinian is cultural evolution? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130368. doi: 10.1098/rstb.2013.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godfrey-Smith P. The replicator in retrospect. Biol Philos. 2000;15:403–423. [Google Scholar]
- 46.Szathmáry E, Smith JM. The major evolutionary transitions. Nature. 1995;374:227–232. doi: 10.1038/374227a0. [DOI] [PubMed] [Google Scholar]
- 47.Richerson PJ, Boyd R, Henrich J. Colloquium paper: Gene-culture coevolution in the age of genomics. Proc Natl Acad Sci USA. 2010;107:8985–8992. doi: 10.1073/pnas.0914631107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dupré J. The metaphysics of evolution. Interface Focus. 2017;7:20160148. doi: 10.1098/rsfs.2016.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laland K, Matthews B, Feldman MW. An introduction to niche construction theory. Evol Ecol. 2016;30:191–202. doi: 10.1007/s10682-016-9821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton Univ Press; Princeton: 2003. [Google Scholar]
- 51.Wells DA. The extended phenotype(s): A comparison with niche construction theory. Biol Philos. 2015;30:547–567. [Google Scholar]
- 52.Haig D. The extended reach of the selfish gene. Evol Anthropol. 2017;26:95–97. doi: 10.1002/evan.21528. [DOI] [PubMed] [Google Scholar]
- 53.De Monte S, Rainey PB. Nascent multicellular life and the emergence of individuality. J Biosci. 2014;39:237–248. doi: 10.1007/s12038-014-9420-5. [DOI] [PubMed] [Google Scholar]
- 54.Pross A. The evolutionary origin of biological function and complexity. J Mol Evol. 2013;76:185–191. doi: 10.1007/s00239-013-9556-1. [DOI] [PubMed] [Google Scholar]
- 55.Dennett DC. Homunculi rule: Reflections on Darwinian populations and natural selection by Peter Godfrey Smith. Biol Philos. 2011;26:475–488. [Google Scholar]
- 56.Godfrey-Smith P. Darwinian Populations and Natural Selection. Oxford Univ Press; Oxford: 2009. [Google Scholar]
- 57.Doolittle WF. Making the most of clade selection. Philos Sci. 2017;84:275–295. [Google Scholar]
- 58.Charbonneau M. Populations without reproduction. Philos Sci. 2014;81:727–740. [Google Scholar]
- 59.Bourrat P. How to read “heritability” in the recipe approach to natural selection. Br J Philos Sci. 2015;66:883–903. [Google Scholar]
- 60.Godfrey-Smith P. Reproduction, symbiosis, and the eukaryotic cell. Proc Natl Acad Sci USA. 2015;112:10120–10125. doi: 10.1073/pnas.1421378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffiths PE, Gray RD. Developmental systems and evolutionary explanation. J Philos. 1994;91:277–304. [Google Scholar]
- 62.McCain K, Weslake B. Evolutionary theory and the epistemology of science. In: Kampourakis K, editor. The Philosophy of Biology. Springer; Dordrecht, The Netherlands: 2013. pp. 101–119. [Google Scholar]
- 63.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 64.Palmer MA, Febria CM. Ecology. The heartbeat of ecosystems. Science. 2012;336:1393–1394. doi: 10.1126/science.1223250. [DOI] [PubMed] [Google Scholar]
- 65.Diamond JM. Assembly of species communities. In: Cody ML, Diamond JM, editors. Ecology and Evolution of Communities. Harvard Univ Press; Cambridge, MA: 1975. pp. 342–444. [Google Scholar]
- 66.Weiher E, et al. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos Trans R Soc Lond B Biol Sci. 2011;366:2403–2413. doi: 10.1098/rstb.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Folke C, et al. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- 68.Desjardins E, Barker G, Lindo Z, Dieleman C, Dussault AC. Promoting resilience. Q Rev Biol. 2015;90:147–165. doi: 10.1086/681439. [DOI] [PubMed] [Google Scholar]
- 69.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. The emergence and promise of functional biogeography. Proc Natl Acad Sci USA. 2014;111:13690–13696. doi: 10.1073/pnas.1415442111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jax K. Ecosystem Functioning. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 72.Gagic V, et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc Biol Sci. 2015;282:20142620. doi: 10.1098/rspb.2014.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steen DA, Barrett KE, Guyer C. Conceptualizing communities as natural entities: A philosophical argument with basic and applied implications. Biol Philos. 2017;32:1019–1034. [Google Scholar]
- 74.Mitchell S. Dispositions or etiologies? A comment on Bigelow and Pargetter. J Philos. 1993;90:249–259. [Google Scholar]
- 75.Godfrey-Smith P. A modern history theory of functions. Noûs. 1994;28:344–362. [Google Scholar]
- 76.Dussault A, Bouchard F. A persistence enhancing propensity account of ecological function to explain ecosystem evolution. Synthese. 2017;194:1115–1145. [Google Scholar]
- 77.Garson J. A generalized selected effects theory of function. Philos Sci. 2017;84:523–543. [Google Scholar]
- 78.Robinson CJ, Bohannan BJ, Young VB. From structure to function: The ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74:453–476. doi: 10.1128/MMBR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Odenbaugh J. On the very idea of an ecosystem. In: Hazlett A, editor. New Waves in Metaphysics. Palgrave; London: 2010. [Google Scholar]
- 80.Bouchard F. Ecosystem evolution is about variation and persistence, not populations and reproduction. Biol Theory. 2014;9:382–391. [Google Scholar]
- 81.Laland KN, Odling-Smee J, Myles S. How culture shaped the human genome: Bringing genetics and the human sciences together. Nat Rev Genet. 2010;11:137–148. doi: 10.1038/nrg2734. [DOI] [PubMed] [Google Scholar]
- 82.Jablonski D. Species selection: Theory and data. Annu Rev Ecol Evol Syst. 2008;39:501–524. [Google Scholar]
- 83.Bizzarri M, Palombo A, Cucina A. Theoretical aspects of systems biology. Prog Biophys Mol Biol. 2013;112:33–43. doi: 10.1016/j.pbiomolbio.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 84.O’Malley MA, Dupré J. Fundamental issues in systems biology. BioEssays. 2005;27:1270–1276. doi: 10.1002/bies.20323. [DOI] [PubMed] [Google Scholar]
- 85.Sterelny K. Explanatory pluralism in evolutionary biology. Biol Philos. 1996;11:193–214. [Google Scholar]