Researchers are learning more about the baffling, deadly condition. Treatments are elusive, but one thing’s for certain: timing is everything.
At first, it looked like the flu. So doctors in Tolima, Colombia, advised Olga Peña’s 70-year-old father to rest and get plenty of fluids. Three days later, the elder Peña was sicker than ever. His skin stretched taut over swollen limbs and abdomen, and his fever raged on. Even as physicians in the local hospital labored to control his symptoms, he suffered heart attacks and organ failure. A little more than a week after he first got sick, Augustín Peña died of sepsis with his daughter by his side.
Researchers investigating sepsis—a serious life-threatening condition that often sends sufferers to the hospital—believe they are starting to see ways to get ahead of this fast-moving killer. Image courtesy of Shutterstock/Chaikom.
It was 2003, and as one way of coping with her loss, the then-23-year-old with an undergraduate degree in bacteriology tried to learn everything she could about the fast-moving syndrome that had killed her father. She found that this very abnormal response to an infection is nevertheless disturbingly common. There are now upward of 30 million sepsis cases a year worldwide, with 6 million deaths, and those figures are probably vast underestimates, experts say (1).
Sepsis is always an emergency, but it’s hard to identify with any certainty. “There’s no gold standard test, no X-ray, no lab test, no biopsy, no anything,” says critical care physician and researcher Clifford Deutschman of the Feinstein Institute for Medical Research. Fifteen years ago, scientists couldn’t even agree on what sepsis was. Without a solid understanding of what causes this disastrous cascade of body system failures, Peña knew that researchers couldn’t find reliable methods to diagnose or treat sepsis.
Much has changed since those early insights. Peña and others have been piecing together a new understanding of what causes sepsis. Their collective findings are challenging the long-established idea that sepsis was simply the product of an immune system in overdrive.
Instead, sepsis appears to be a systemic illness underpinned by a combination of both over- and underactivity by the immune system that leaves patients vulnerable to organ damage during the initial symptoms and prone to severe secondary infections later. There’s also new evidence that differing host responses to infection may explain who develops sepsis as well as the varying ways victims manifest the syndrome, which could shed light on why so many clinical trials of sepsis treatments have failed in the past.
Such insights offer promising steps toward better sepsis diagnostics, which in turn should facilitate better clinical trials of treatment protocols for each stage of the condition. Sepsis is far from being solved. But researchers believe they are starting to see ways to get ahead of this fast-moving killer.
Defining the Problem
“Sepsis is not a diagnosis. It’s a phenomenon,” says John Marshall, a hospital intensivist at St. Michael’s Hospital in Toronto and a member of the team that generated the first modern consensus criteria defining sepsis in the early 1990s (2).
Sepsis as a phenomenon had been recognized long before that. But millennia would pass before anyone began to understand what brought on the condition or how to treat it. With the advent of germ theory in the 19th and early 20th centuries, physicians realized that some type of infection almost always accompanied cases of sepsis. And by the middle of the 20th century, microbiologists and immunologists understood that many of the hallmarks of infectious diseases in general were caused not by the invading pathogen but by the body’s own immune response to it (3).
When our immune systems are fighting off an infection, we usually can tell that a war is being waged. The inflammatory response that causes fever also summons specialized immune cells to hunt and kill the invading pathogens. Virus-infected cells are programmed to kill themselves to prevent the release of more viruses. The runny nose, itchy eyes, and stuffed sinuses that bring misery during a cold are the body trying to flush out pathogens. On a microscopic level, signaling molecules that spur inflammation, such as IL-6 and NF-κB, can also cause problems with blood clotting and alter blood pressure (4). Other messengers, such as TNF and IL-1, can damage nearby cells as they tackle invading pathogens. The collateral damage from these battles makes us temporarily miserable but keeps us alive. Not so for sepsis patients.
By the 1970s and 1980s, it was becoming clear that a patient’s own immune system response to infection was also responsible for the high fever, plummeting blood pressure, and organ dysfunction that characterized sepsis (5). The immune system seemed to be overzealous, ravaging the host before it could control the infection. A flurry of new clinical trials began testing immunosuppressive drugs to try to damp down inflammation in sepsis patients. In 1976, William Schumer of the University of Chicago treated septic patients with high doses of the steroid methylprednisolone or a placebo and found that the steroids reduced mortality from 39% to 11% (6). Subsequent trials weren’t nearly as convincing, though, and researchers speculated that inadequate definitions of sepsis itself might be muddying the waters.
“When I started treating sepsis patients as a young physician in the early 80s, I couldn’t compare two papers because they defined sepsis differently,” says Deutschman.
To facilitate better studies, the American College of Chest Physicians and the Society of Critical Care Medicine assembled a group of physicians in 1991, including Marshall, to create a clearer definition of sepsis (2). The new consensus criteria, published in 1992 in the journal Chest, differentiated the process of infection from the host response, noting that it was the host response that created sepsis, not the infection itself. The team also noted the wide range of symptoms and outcomes that fell under the sepsis umbrella, and the difficulties this heterogeneity posed, both for understanding the molecular basis of the condition and developing better ways to treat it.
Unfortunately, these criteria were still overly broad, Marshall says. Many hospitalized patients fit the description without having sepsis. Even when correctly identified, sepsis patients remained an extraordinarily diverse group, yet the protocols would treat them all in the same way. Perhaps not surprisingly, treatment trials continued to fail. Any hope of fighting sepsis would require a more fine-tuned analysis of the biochemical cascade that turned an ordinary response to infection into a life-threatening crisis.
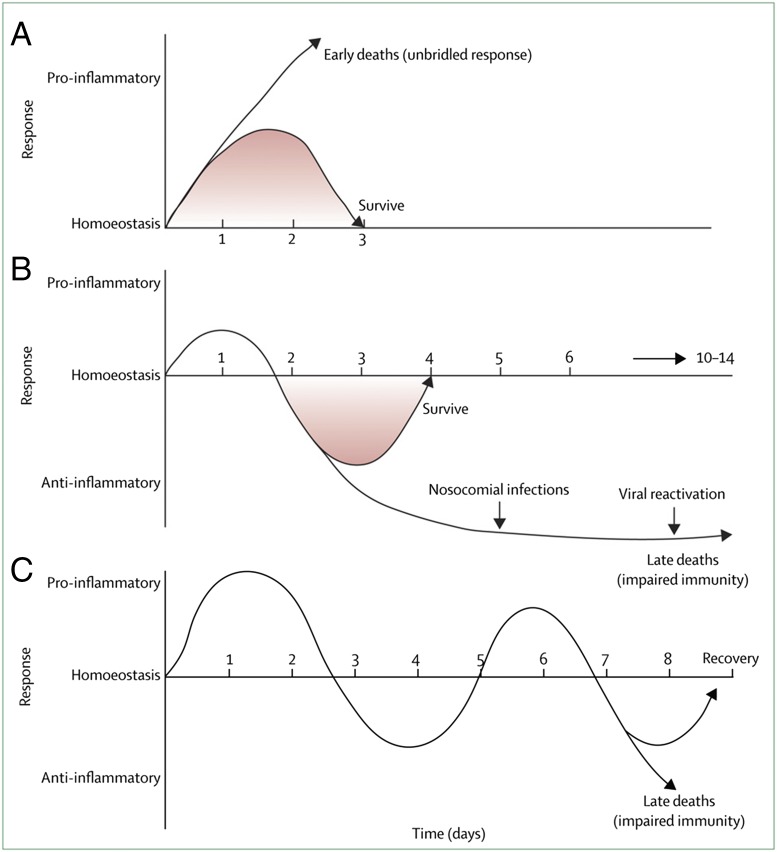
Sepsis is characterized by a complex interplay of both proinflammatory and antiinflammatory responses. In some cases, a hyperinflammatory phase can lead quickly to death (A). In others who are afflicted, compromised immune systems often result in little hyperinflammatory response but a strong antiinflammatory response (B). A third, theoretical scenario entails cycling between hyper- and hypoinflammatory responses, sometimes resulting in the development of a new, dangerous secondary infection (C). Reprinted from ref. 21, with permission from Elsevier.
Spinning Wheels
As a young researcher, Richard Hotchkiss, now an anesthesiologist and sepsis expert at Washington University in St. Louis, was performing necropsies on mice that had died of sepsis when he noticed large numbers of lymphocytes, a type of white blood cell, dying by apoptosis. This 1999 finding was puzzling, because it contradicted the assumption that sepsis was caused by an overactive immune system. So many dead lymphocytes meant the immune system should be severely impaired. Hotchkiss decided to examine specimens from patients who had died from sepsis and found the same signature: large numbers of dead white blood cells in the spleen and intestine (7).
Despite growing dissatisfaction with the overactive immune theory, no one had a better idea to replace it. Now Hotchkiss did. His theory about immune system impairment in protracted sepsis also suggested a reason for one of the most peculiar aspects of the syndrome: many patients survived the initial crisis with the help of advanced life support and antibiotics only to die from a secondary infection. “This could help explain what was driving the immune impairment, why patients can’t clear the bacteria and develop secondary infections,” Hotchkiss says.
A 2000 report in Critical Care Medicine noted that, even at the first signs of disease, the majority of sepsis patients showed signs not of inflammation but of immune suppression (8). Lymphocytes and monocytes, types of pathogen-fighting white blood cells, are deactivated or undergoing cellular suicide. In 2008, Spanish physicians documented cases of sepsis in rheumatoid arthritis patients treated with etanercept, a drug that blocks the action of TNF-α, one of the inflammation-inducing signaling molecules, known as cytokines, that summon immune cells to battle pathogens. This surprising finding reinforced the idea that immune paralysis was just as important, if not more important, as immune overstimulation in the physiology of sepsis (9). Not only would immune suppressive medications fail to improve health, they could make things worse.
But then the relationship between immune activation and suppression grew murkier. Longitudinal studies showed that some patients could start in the former group and make a transition to the latter. Peña, who had moved to Vancouver in November 2006 to begin her graduate work in microbiology at the University of British Columbia, focused on figuring out what caused this shift. At first, the problem seemed intractable. The data appeared too noisy, and she couldn’t find a guiding principle to make sense of the chaos. Then, late on one sleepless night, Peña remembered the idea of endotoxin tolerance. “If the body continually comes into contact with bacteria or a bacterial product, it can eventually stop responding to it,” Peña explains. “The body develops a type of immune amnesia.”
This odd phenomenon had been observed at the beginning of the 20th century, when researchers experimenting with so-called fever therapy found that the pathogens they used to induce fever in their human subjects would lose effectiveness (10). In 1946, physician Paul Beeson at Emory University picked up that thread and similarly found that rabbits injected every day with the typhoid vaccine, which contained killed bacteria, soon stopped showing signs of fever (3). Subsequent work revealed that the animals’ immune systems had learned to tolerate the regular exposure to a molecule called LPS, also known as endotoxin, that studs the membranes of gram-negative bacteria such as Salmonella typhi (11, 12).
LPS normally provokes such a vigorous immune response that a synthetic version of the molecule is sometimes used as an adjuvant to turbocharge vaccines (13). Yet Beeson found the rabbits’ immune systems started simply ignoring its presence. Researchers had reasoned that endotoxin tolerance must be an ancient evolutionary mechanism to keep the infected host from self-destructing while fighting off an infection. To Peña, it sounded remarkably similar to what happened in sepsis patients.
By 1988, experiments in mice by a pair of German scientists had shown that inaction by macrophages created endotoxin tolerance (12). In the 1990s and early 2000s, French microbiologist Jean-Marc Cavaillon filled in more details. In a state of endotoxin tolerance, macrophage production of TNF-α and other cytokines plummets. At the same time, production of signals that douse the inflammatory response rises. Cavaillon referred to the process as a type of cellular reprogramming that collectively switched off many of the disease-fighting functions of white blood cells (14).
Peña wanted to go one step further, using the genetic and chemical signatures of this cellular reprogramming to create a molecular fingerprint for sepsis. Using data from 583 patients from 11 different sepsis studies, she and her colleagues analyzed gene expression in lymphocytes and monocytes—collectively known as peripheral mononuclear blood cells (PMBCs)—and identified a set of 99 genes that were expressed differently in endotoxin-tolerant human PMBCs. Then, using blood drawn from 72 patients hospitalized with suspected sepsis, Peña found that this genetic signature not only predicted which patients actually had sepsis, but it also predicted subsequent organ dysfunction (15). “Knowing that sepsis is not just extreme inflammation but also immune suppression, and the competition between these two lines, this will give us a better idea of what is happening to each patient,” Peña says.
Digging further into this gene expression profile, Peña discovered that the immune dysfunction in sepsis appears to be a tug of war between two different types of white blood cells: monocytes, proinflammatory cells that can differentiate into macrophages, and dendritic cells, and neutrophils, the most common white blood cell. Whereas monocytes can survive for 1 to 2 weeks, neutrophils are short-lived, with an average lifespan of just 5.4 days. Long-lived monocytes lead the development of endotoxin tolerance, but the body continues to churn out new neutrophils that lack this tolerance. Circulating neutrophils encounter circulating bacteria and generate an inflammatory response. Without the help of monocytes, however, the infection-fighting machinery fails to gain traction (16). “Think of it like a car spinning its wheels,” Deutschman says. “It looks like it’s working hard, but it’s not getting anywhere.”
Less Is More
Gene studies have provided further clues. In 2015, a genome-wide association study, or GWAS, uncovered different variants of the FER gene, involved in intercellular signaling, that was strongly associated with 28-day survival in sepsis patients (17). A separate 2016 GWAS study identified three regions of the genome, which include two immune system genes, VPS13A and CRISPLD2, that also seemed to influence 28-day survival (18). These underlying host differences interact with the infecting organism, which can lead to a range of organ dysfunctions and disease outcomes, according to Peter Pickkers, an intensive care physician at Radboud University in The Netherlands.
Still, he warns against getting too focused on any single factor or pathway. “There’s so much going on [in sepsis] that targeting just one pathway probably won’t have much of an effect,” Pickkers says.
Not only might sepsis involve multiple physiological pathways, Deutschman says, but treating such a heterogeneous condition is like tackling different diseases simultaneously. “We treat sepsis like it’s this monolithic thing, and it’s really not,” he says. “There are aspects of the immune system that look like they are overactive and others that look like they are underactive and some that don’t look like anything you’ve ever see anywhere else ever before.” Currently, sepsis treatment consists primarily of helping patients weather the immunological storm rampaging through their bodies until they can recover on their own.
But one principle has become abundantly clear: The earlier doctors see the storm coming, the better the patient’s chances. That’s why attention has increasingly turned to more sophisticated diagnostics in treating sepsis. New diagnostics are analyzing host factors to help identify which parts of the immune system are over- or underactive. Inflammatix, led by Timothy Sweeney, formerly of Stanford University, has created a tool that identifies the immune system genes switched on and off in response to infection to predict the likelihood sepsis will develop and the chances it will be severe. This diagnostic has been through clinical trials and is currently waiting on US Food and Drug Administration (FDA) approval.
The Duke University laboratory of Dennis Ko, meanwhile, has identified a biomarker called methylthioadenosine that is involved in the body’s inflammatory response and at high levels is associated with high rates of fever-induced host-cell death, which can predict sepsis death (19). Peña created Sepset Biosciences to refine a genetic and immunological signature of sepsis that can rapidly distinguish it from other illnesses with similar symptoms. She is currently whittling down the number of factors needed to make a diagnosis before embarking on clinical trials. And in February 2017, the FDA approved SeptiCyte LAB from Seattle-based startup Immunexpress. The RNA-based blood test looks for particular immune biomarkers to distinguish sepsis from a systemic inflammatory syndrome with similar symptoms, providing results in about 4 hours (20).
The newest insights into the molecular biology of sepsis have not affected treatment protocols yet, but Deutschman and Marshall believe that understanding interactions between the disease-causing pathogen, host genetics, and the precise nature of the host’s immune response will yield better outcomes. What physicians need, Pickkers says, is not so much batteries of newer, better drugs (though he acknowledges they’ll likely play a role), but better ways to deploy existing ones. “When you look at what we used to put patients with sepsis through, it was like torture,” he says, referring to the drugs used to increase the amount of blood pumped by the heart to above normal levels and the high settings on ventilators. “It may be that less is more.”
“These new approaches being investigated are the complete opposite of what we have been doing,” Pickkers concludes. “But I think in the next 5 to 10 years, we’ll see some breakthroughs.”
References
- 1.Reinhart K, et al. Recognizing sepsis as a global health priority—A WHO resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 3.Beeson PB. Technical Assistance of Elizabeth Roberts Tolerance to bacterial pyrogens: I. Factors influencing its development. J Exp Med. 1947;86:29–38. doi: 10.1084/jem.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Keller TT, van Gorp E, ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. doi: 10.1016/s0008-6363(02)00857-x. [DOI] [PubMed] [Google Scholar]
- 5.Michalek SM, Moore RN, McGhee JR, Rosenstreich DL, Mergenhagen SE. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980;141:55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184:333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling–Regulation of the immune response in normal and critically ill states. Crit Care Med. 2000;28(Suppl):N3–N12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Nuñez-Cornejo C, et al. Septic shock and community-acquired pneumonia associated with etanercept therapy. Int J Clin Pharmacol Ther. 2008;46:193–197. doi: 10.5414/cpp46193. [DOI] [PubMed] [Google Scholar]
- 10.Van Epps HL. Ignoring endotoxin. J Exp Med. 2006;203:1137. doi: 10.1084/jem.2035fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peña OM, Pistolic J, Raj D, Fjell CD, Hancock RE. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–7254. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg MA, Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-d-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988;56:1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zariri A, van der Ley P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev Vaccines. 2015;14:861–876. doi: 10.1586/14760584.2015.1026808. [DOI] [PubMed] [Google Scholar]
- 14.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: Endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peña OM, et al. An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. EBioMedicine. 2014;1:64–71. doi: 10.1016/j.ebiom.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour SC, Pena OM, Hancock RE. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014;35:443–450. doi: 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Rautanen A, et al. ESICM/ECCRN GenOSept Investigators Genome-wide association study of survival from sepsis due to pneumonia: An observational cohort study. Lancet Respir Med. 2015;3:53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherag A, et al. Genetic factors of the disease course after sepsis: A genome-wide study for 28day mortality. EBioMedicine. 2016;12:239–246. doi: 10.1016/j.ebiom.2016.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, et al. Wellcome Trust Case Control Consortium 2; Kenyan Bacteraemia Study Group Human genetic and metabolite variation reveals that methylthioadenosine is a prognostic biomarker and an inflammatory regulator in sepsis. Sci Adv. 2017;3:e1602096. doi: 10.1126/sciadv.1602096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHugh L, et al. A molecular host response assay to discriminate between sepsis and infection-negative systemic inflammation in critically ill patients: Discovery and Validation in independent cohorts. PLoS Med. 2015;12:e1001916. doi: 10.1371/journal.pmed.1001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]