Body size has widely been recognized as one of the most important determinants of organismal form and function (1). Extremes in size can be especially illuminating of the drivers and constraints in body size evolution, and marine mammals provide a remarkable set of test cases because their independent invasions of the sea have produced a stunning array of sizes, from sea otters to blue whales that span several orders of magnitude in body mass. Why should whales have become so huge and why aren’t they larger? One scale-dependent physiological process that may shed light on these questions relates to energy flux: how animals obtain energy from the environment and how they use stored energy (2). Although larger animals have higher absolute metabolic requirements than smaller ones, the rates are not proportional to body size (3). The negative allometry of metabolic rate, whereby larger animals exhibit lower mass-specific rates of metabolism, confers a suite of physiological and ecological benefits at greater body sizes. These advantages include a low cost of transport (4); enhanced fasting ability (5); and, for air-breathers in aquatic environments, the ability to dive longer and deeper in search of food (6). There is a tendency of many vertebrate lineages to increase body size through time, but the environmental drivers and physiological constraints of body size evolution remain poorly understood. In PNAS, Gearty et al. (7) analyze the evolution of body size in several independent mammalian lineages that underwent land-to-sea transitions in their evolutionary histories. They use comparative phylogenetic analyses and an examination of the fossil record to test several hypotheses that attempt to explain why mammals evolved larger size in aquatic ecosystems.
Physical and energetic constraints on maximum body size are a common theme in several previously proposed hypotheses regarding the phenomenon of large mammals in the ocean. However, the precise mechanisms invoked are fundamentally different and may not be mutually exclusive. Two hypotheses tested by Gearty et al. (7) focus on the potential limits of diet and how that may influence the energetic efficiency of foraging. For example, carnivores may require more energy to capture food, even if it is more energy-rich, and thus may be limited in their maximum body size compared with herbivores (8). However, most marine mammals are carnivores and evolved from carnivores, except for herbivorous dugongs and manatees, which evolved from herbivorous ancestors and represent only one of the four mammal lineages tested in this study. Although large filter-feeding baleen whales have often been referred to as grazers and likened to terrestrial herbivores due to their bulk filter-feeding strategies, recent research has shown that they exhibit high-cost foraging behaviors (9). Because marine ecosystems are more productive, and thus provide greater amounts of energy-rich food for consumers, terrestrial environments are considered limiting by comparison, and thus could limit maximum body size (8). Similarly, the vastness and 3D nature of ocean ecosystems may provide more opportunities for prey capture and enhanced foraging efficiency relative to largely 2D terrestrial environments (10). A terrestrial lifestyle may also limit maximum size because limbs must support the entire body weight, yielding increased bone strain with increasing body mass (11). Therefore, larger terrestrial animals require a change in limb posture to keep locomotor stresses low, which ultimately limits their locomotor performance (11).
In contrast to the impact of gravity and the potential limits to diet in the terrestrial environment, the thermoregulation hypothesis suggests that there is a minimum size needed to survive in cold water (12, 13). Based on this discrepancy, terrestrial constraints on maximum size versus aquatic requirements of minimum size, Gearty et al. (7) model different scenarios to predict the evolutionary outcome of body mass optima under an Orstein–Uhlenbeck (OU) adaptive process. This modeling framework estimates the “optimal body mass,” which can be thought of as the pinnacle of a peak on an adaptive landscape toward which these lineages evolve. The model also calculates a phylogenetic half-life, or the rate at which evolution pulls the lineage toward that peak, and the stationary variance (i.e., the maximum trait disparity allowed within a selective regime). Gearty et al. (7) suggest that if the transition from land to sea involved a release of constraints on maximum size, then an increase in stationary variance would be expected. Alternatively, if a fully aquatic existence required a minimum body size, then Gearty et al. (7) predict an equal or decreased stationary variance as well as an equal or decreased phylogenetic half-life that reflects strong, rapid selection toward larger size rather than a release from smaller size.
The results by Gearty et al. (7) reveal common body mass optima of ∼500 kg in multiple lineages within aquatic ecosystems. The notable exceptions include baleen whales (Mysticeti) and otters (Musteloidea), which either evolved gigantism or retained the small body sizes that are similar to their ancestors, respectively. The estimates for phylogenetic half-life and stationary variance for aquatic mammals were less than those for terrestrial mammals within all examined lineages, except for cetaceans and their relatives. Therefore, rather than demonstrating a release from terrestrial constraints on maximum body size, the analyses by Gearty et al. (7) support the thermoregulation hypothesis for a minimum body size for mammals in water. However, if there is an energetic limit on maximum body size in the ocean, as Gearty et al. (7) and others (14, 15) have suggested, there may be only a limited size range of potential body sizes that can evolve. This may be why the stationary variance estimated for baleen whales was not much different from their ancestors. Nevertheless, the evidence indicates that thermoregulatory stresses rapidly selected for increased body size in water. However, this hypothesis does not completely explain the apparent convergence in optimal body size, and it does not predict the entire size distribution of mammals in the aquatic realm.
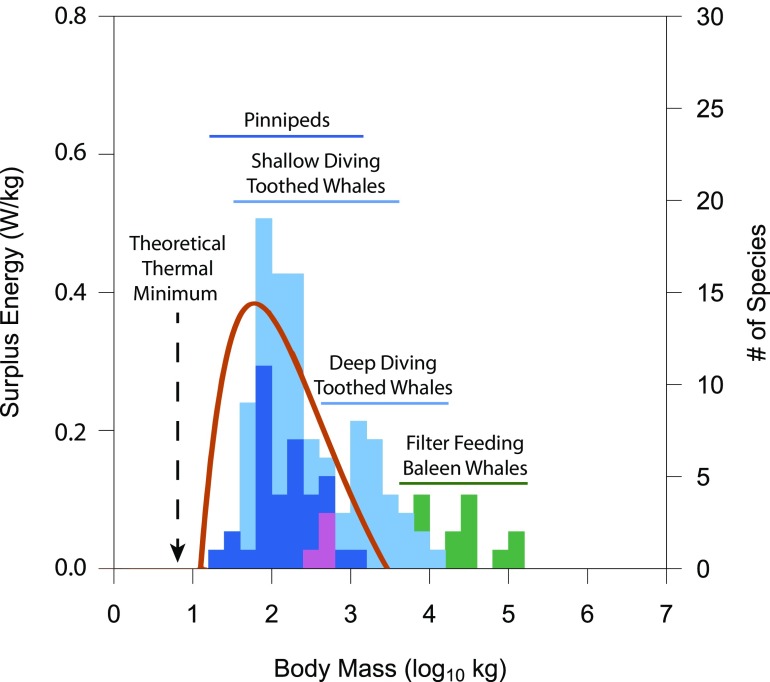
Why should a particular body size be selected for in marine mammals? To tackle this question, Gearty et al. (7) provide a simple model that predicts the energetic optimal size for a given set of intake (i.e., feeding rates) and cost (i.e., heat loss, basal metabolism) functions. These functions are most well-known for pinnipeds, which can be studied in captivity under controlled laboratory conditions (16, 17). Using allometric equations for pinnipeds, Gearty et al. (7) calculate a mass-specific energy surplus curve that matches well with the distribution of most extant marine mammals, as well as with the estimates for optimal body size from the OU adaptive model (Fig. 1). However, the body sizes of the largest cetacean species (beaked whales, sperm whales, and baleen whales) extend well beyond the predicted maxima. Gearty et al. (7) note that by tuning the feeding function exponent from 0.71 to 0.78, the energy surplus curve maintains its peak around most of the calculated body mass optima (except for mysticetes, which are far right-shifted), but also includes the largest extant cetaceans. By comparison, a cladogenic diffusion model, driven by a tradeoff between short-term selective advantages and long-term extinction risks (with no tunable parameters except a minimum viable size), also predicts similar body size distributions that include the largest whales (18, 19).
Fig. 1.
Theoretical scaling of surplus energy and body size distributions of extant marine mammals. Gearty et al. (7) calculated the scaling of surplus energy (orange curved line) from heat loss, feeding rate, and metabolic rate functions. Extant marine mammal body mass distributions are shown for seals and sea lions (Pinnipedia; dark blue), toothed whales and dolphins (Odontoceti; light blue), manatees and dugongs (Sirenia; pink), and baleen whales (Mysticeti; green). The theoretical thermal minimum predicted by a previous heat loss model (13) is shown by the vertical dashed arrow. Filter-feeding baleen whales and the largest, deepest diving toothed whales extend beyond the body size maximum predicted by the energy surplus model. Modified from ref. 7.
The study by Gearty et al. (7) clearly shows that the largest cetaceans cannot be explained by the same energetic tradeoffs that predict the body size distribution of other marine mammals. Their energetics model was parameterized with feeding and metabolic rate data from pinnipeds in captivity (16, 17); thus, the predictions may not completely reflect the dynamic vital rates of wild animals that must dive and hunt to forage (20), or the variability in diet (21). Furthermore, other taxa exhibit specialized feeding modes that may disproportionally increase energy intake at greater body sizes, especially filter feeders that exhibit positively allometry of the engulfment apparatus (14). However, net energy gain is dependent on a number of factors, including prey energy density (energy per unit prey mass), prey patch quality (number of prey per unit search volume), and costs associated with locomotion and diving. The largest cetaceans have evolved extreme foraging strategies that are thought to enhance energetic efficiency by accessing unique habitats or the batch processing of specific trophic levels, both of which require large body size. The largest toothed whales, such as beaked whales (Ziphiidae) and sperm whales (Physeteridae), dive to extreme depths to exploit vast resources composed largely of energy-rich squid (21). Baleen whales target densely packed aggregations of zooplankton (krill or copepods) or forage fish to enhance foraging efficiency, but the highest quality prey patches are often found at significant depths (22). Because odontocetes and mysticetes must dive to find the highest quality prey, their diving capacity, which is generally limited by physiological scaling (6), may constrain the energetic scope of foraging (14, 23).
The energy limitations imposed by the need to dive for food is one of many factors that could modify the size optima estimated by Gearty et al. (7) Other factors include the abundance and distribution of food, predator–prey interactions, competition, and different life history strategies. Moreover, energetic optima may not equate to fitness optima if the transfer of surplus energy to offspring is limited by a different scale-dependent factor (24). For example, the largest baleen whales exhibit intensive feeding bouts in the summer months at higher latitudes, followed by transoceanic migrations to breeding grounds at lower latitudes. The energy lipids developed during these feeding months must not only fuel long-distance migrations but also be used for reproduction during 11- and 7-mo gestation and lactation periods, respectively (25). Such a life history may differentially select for enhanced diving capacity, fasting ability, cost of transport, and the efficient exploitation of ephemeral resources. Although these physiological factors are known to scale with body size in some form (1–6, 14, 23, 26), how they manifest at the largest scale and reflect fitness is not known. Unlike many other lineages whose giants are represented only in the fossil record, we are living in a time of giants with respect to modern marine mammals (27, 28). Therefore, researchers have the unique opportunity to study how animals function at the upper extreme of body mass (9), and Gearty et al. (7) provide a rich evolutionary context for this work.
Footnotes
The author declares no conflict of interest.
See companion article on page 4194.
References
- 1.Schmidt-Nielsen K. Scaling: Why Is Animal Size So Important. Cambridge Univ Press; Cambridge, UK: 1984. [Google Scholar]
- 2.Alexander RM. Energy for Animal Life. Oxford Univ Press; New York: 1999. [Google Scholar]
- 3.White CR, Blackburn TM, Seymour RS. Phylogenetically informed analysis of the allometry of Mammalian Basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution. 2009;63:2658–2667. doi: 10.1111/j.1558-5646.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams TM. The evolution of cost efficient swimming in marine mammals: Limits to energetic optimization. Philos Trans R Soc Lond B Biol Sci. 1999;354:193–201. [Google Scholar]
- 5.Millar J, Hickling G. Fasting endurance and the evolution of mammalian body size. Funct Ecol. 1990;4:5–12. [Google Scholar]
- 6.Halsey LG, Butler PJ, Blackburn TM. A phylogenetic analysis of the allometry of diving. Am Nat. 2006;167:276–287. doi: 10.1086/499439. [DOI] [PubMed] [Google Scholar]
- 7.Gearty W, McClain CR, Payne JL. Energetic tradeoffs control the size distribution of aquatic mammals. Proc Natl Acad Sci USA. 2018;115:4194–4199. doi: 10.1073/pnas.1712629115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucker MA, Rogers TL. Examining predator-prey body size, trophic level and body mass across marine and terrestrial mammals. Proc Biol Sci. 2014;281:1–9. doi: 10.1098/rspb.2014.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldbogen JA, et al. How baleen whales feed: The biomechanics of engulfment and filtration. Annu Rev Mar Sci. 2017;9:367–386. doi: 10.1146/annurev-marine-122414-033905. [DOI] [PubMed] [Google Scholar]
- 10.Pawar S, Dell AI, Savage VM. Dimensionality of consumer search space drives trophic interaction strengths. Nature. 2012;486:485–489. doi: 10.1038/nature11131. [DOI] [PubMed] [Google Scholar]
- 11.Biewener AA. Scaling body support in mammals: Limb posture and muscle mechanics. Science. 1989;245:45–48. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- 12.Ahlborn BK, Blake RW. Lower size limit of aquatic mammals. Am J Phys. 1999;67:920–922. [Google Scholar]
- 13.Downhower JF, Bulmer LS. Calculating just how small a whale can be. Nature. 1988;335:675. [Google Scholar]
- 14.Goldbogen JA, Potvin J, Shadwick RE. Skull and buccal cavity allometry increase mass-specific engulfment capacity in fin whales. Proc Biol Sci. 2010;277:861–868. doi: 10.1098/rspb.2009.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potvin J, Goldbogen JA, Shadwick RE. Metabolic expenditures of lunge feeding rorquals across scale: Implications for the evolution of filter feeding and the limits to maximum body size. PLoS One. 2012;7:e44854. doi: 10.1371/journal.pone.0044854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Innes S, Lavigne D, Earle W, Kovacs K. Feeding rates of seals and whales. J Anim Ecol. 1987;56:115–130. [Google Scholar]
- 17.Lavigne D, et al. Metabolic rates of seals and whales. Can J Zool. 1986;64:279–284. [Google Scholar]
- 18.Clauset A. How large should whales be? PLoS One. 2013;8:e53967. doi: 10.1371/journal.pone.0053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clauset A, Erwin DH. The evolution and distribution of species body size. Science. 2008;321:399–401. doi: 10.1126/science.1157534. [DOI] [PubMed] [Google Scholar]
- 20.Maresh JL, et al. Free-swimming northern elephant seals have low field metabolic rates that are sensitive to an increased cost of transport. J Exp Biol. 2014;217:1485–1495. doi: 10.1242/jeb.094201. [DOI] [PubMed] [Google Scholar]
- 21.Clarke MR. Cephalopods as prey. III. Cetaceans. Philos Trans R Soc Lond B Biol Sci. 1996;351:1053–1065. [Google Scholar]
- 22.Hazen EL, Friedlaender AS, Goldbogen JA. Blue whales (Balaenoptera musculus) optimize foraging efficiency by balancing oxygen use and energy gain as a function of prey density. Sci Adv. 2015;1:e1500469. doi: 10.1126/sciadv.1500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldbogen JA, et al. Scaling of lunge feeding performance in rorqual whales: Mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct Ecol. 2012;26:216–226. [Google Scholar]
- 24.Sebens KP. Energetic constraints, size gradients, and size limits in benthic marine invertebrates. Integr Comp Biol. 2002;42:853–861. doi: 10.1093/icb/42.4.853. [DOI] [PubMed] [Google Scholar]
- 25.Pirotta E, et al. A dynamic state model of migratory behavior and physiology to assess the consequences of environmental variation and anthropogenic disturbance on marine vertebrates. Am Nat. 2018;191:E40–E56. doi: 10.1086/695135. [DOI] [PubMed] [Google Scholar]
- 26.Friedlaender AS, et al. Feeding rates and under-ice foraging strategies of the smallest lunge filter feeder, the Antarctic minke whale (Balaenoptera bonaerensis) J Exp Biol. 2014;217:2851–2854. doi: 10.1242/jeb.106682. [DOI] [PubMed] [Google Scholar]
- 27.Slater GJ, Goldbogen JA, Pyenson ND. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proc Biol Sci. 2017;284:20170546. doi: 10.1098/rspb.2017.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyenson ND, Vermeij GJ. The rise of ocean giants: Maximum body size in Cenozoic marine mammals as an indicator for productivity in the Pacific and Atlantic Oceans. Biol Lett. 2016;12:20160186. doi: 10.1098/rsbl.2016.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]