Abstract
Al-Induced secretion of organic acids from the roots has been considered as a mechanism of Al tolerance, but the processes leading to the secretion of organic acids are still unknown. In this study, the secretion pattern and alteration in the metabolism of organic acids under Al stress were examined in rye (Secale cereale L. cv King) and wheat (Triticum aestivum L. cv Atlas 66). Al induced rapid secretion of malate in the wheat, but a lag (6 and 10 h for malic and citric acids, respectively) between the exposure to Al and the secretion of organic acids was observed in the rye. The activities of isocitrate dehydrogenase, phosphoenolpyruvate carboxylase, and malate dehydrogenase were not affected by Al in either plant. The activity of citrate synthase was increased by the exposure to Al in the rye, but not in the wheat. The secretion of malate was not suppressed at low temperature in the wheat, but that of citrate was stopped in the rye. The Al-induced secretion of citrate from roots of the rye was inhibited by the inhibitors of a citrate carrier, which transports citrate from the mitochondria to the cytoplasm. All of these results suggest that alteration in the metabolism of organic acids is involved in the Al-induced secretion of organic acids in rye, but only activation of an anion channel seems to be responsible for the rapid secretion of malate in the wheat.
Although several mechanisms have been proposed for Al tolerance (for a review, see Delhaize and Ryan, 1995; Kochian, 1995; Ma, 2000; Matsumoto, 2000), recently secretion of organic acids from the roots has been shown to play an important role in the external Al detoxification (Ma et al., 1997b, 1997c). Some organic acids form a stable complex with ionic Al, thereby preventing the binding of Al with extra- and intracellular substances of the roots. Malic acid has been reported to be secreted from the roots of Al-tolerant cultivars of wheat (Triticum aestivum; Delhaize et al., 1993; Basu et al., 1994) in response to Al stress, citric acid from Al-tolerant cultivars of snapbean (Miyasaka et al., 1991) and maize (Pellet et al., 1995), and Cassia tora (Ma et al., 1997c), and oxalic acid from buckwheat (Ma et al., 1997b) and taro (Ma and Miyasaka, 1998). Al-induced secretion of organic acids has been characterized in several plant species or cultivars. For instance, the secretion is highly specific to Al; neither P deficiency nor other polyvalent cations causes the secretion of organic acids (Ryan et al., 1995a; Ma et al., 1997c; Zheng et al., 1998). The amount of organic acids secreted increases with increasing external Al concentrations (e.g. Delhaize et al., 1993; Ma et al., 1997c). The site of organic acid secretion has been localized to the apex of the roots in wheat (Delhaize et al., 1993), maize (Pellet et al., 1995), and buckwheat (Zheng et al., 1998), which is consistent with the targeting site of Al toxicity (Ryan et al., 1993). However, the processes leading to the secretion of organic acid under Al stress are still unknown.
The tribe Triticeae includes some of the most important grain cereal crops, such as wheat, barley, rye (Secale cereale), and triticale. Among them, rye is the most Al-tolerant species although cultivars within the same species vary in their Al tolerance (Aniol et al., 1980; Aniol and Gustafson, 1984). From intensive studies on the Al toxicity and tolerance in wheat, secretion of malic acid from the roots has been suggested as a general mechanism of Al tolerance (Ryan et al., 1995b). However, the mechanisms responsible for the high-Al tolerance in rye are not understood. Recently, Ma et al. (2000) reported that a triticale line secreted both malic and citric acids in response to Al. Furthermore, they found that the release of organic acids is linked to the genes on the short arm of chromosome 3R. Triticale is a synthetic hybrid between wheat and rye, and the Al tolerance of triticale is considered to be inherited from rye. Therefore, rye may also respond to Al by secretion of organic acids. In the present study, the secretion of organic acids from the roots of rye was investigated under Al stress. The pattern of organic acid secretion as well as the effect of Al on the metabolism of organic acids were compared between a rye cultivar and an Al-tolerant cultivar of wheat, cv Atlas 66.
RESULTS
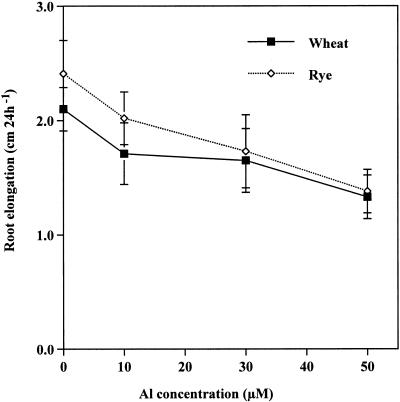
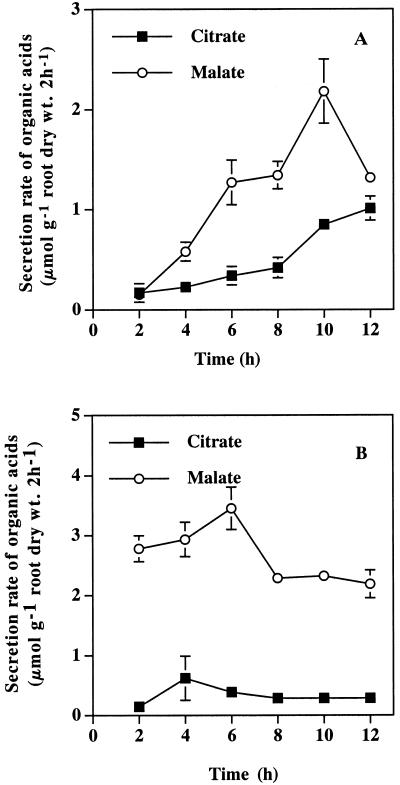
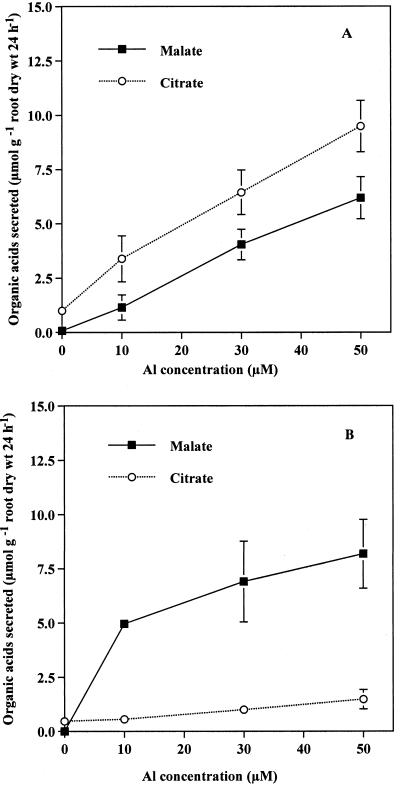
Root elongation of rye (cv King) during a 24-h period was inhibited by 16.2%, 28.2%, and 42.7% by the exposure to 10, 30, and 50 μm Al, respectively, whereas that of wheat (cv Atlas 66) was inhibited by 19.6%, 21.4%, and 37.7%, respectively (Fig. 1). Both malic and citric acids were secreted from the roots of the rye exposed to Al, whereas the major organic acid secreted from the root of the wheat was malic acid (Fig. 2). The secretion rate of malic acid (μmol 2 h−1 g−1 root dry weight) in the wheat was high during the first 2 h after the start of Al treatment (Fig. 2B) and kept at a high level thereafter. In contrast, the rate of secretion of malic and citric acids in the rye was very low during the first 4 and 8 h after the start of exposure to Al, respectively (Fig. 2A), but significantly increased at 6 and 10 h, respectively. The amount of both malic and citric acids secreted during the 24-h period increased with increasing external Al concentrations in the rye (Fig. 3A), and that of malic acid secreted from the roots of wheat also increased with increasing external Al concentrations (Fig. 3B). The amount of total organic acids secreted in the rye was higher than that in the wheat (Fig. 3). Phosphorous deficiency and the addition of polyvalent cations (lanthanum, lead, manganese, and cadmium) failed to induce the secretion of organic acids in both plants (data not shown).
Figure 1.
Effect of Al on the root elongation in the rye (cv King) and the wheat (cv Atlas 66). The roots were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 0, 10, 30, or 50 μm Al for 24 h. Vertical bars represent sd (n = 12).
Figure 2.
Organic acids secreted from rye (A) and wheat (B) at different times in the presence of Al. Both rye (cv King) and wheat (cv Atlas 66) were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 50 μm Al. Root exudates were collected every 2 h after initiation of Al treatment. Organic acids were analyzed by HPLC. Vertical bars represent sd (n = 3).
Figure 3.
Effect of external Al concentration on the secretion of organic acids in the rye (A) and the wheat (B). Seedlings of the rye (cv King) and the wheat (cv Atlas 66) were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 0, 10, 30, or 50 μm Al. After a 24-h exposure, the root exudates were collected and organic acids were analyzed by HPLC. Vertical bars represent sd (n = 3).
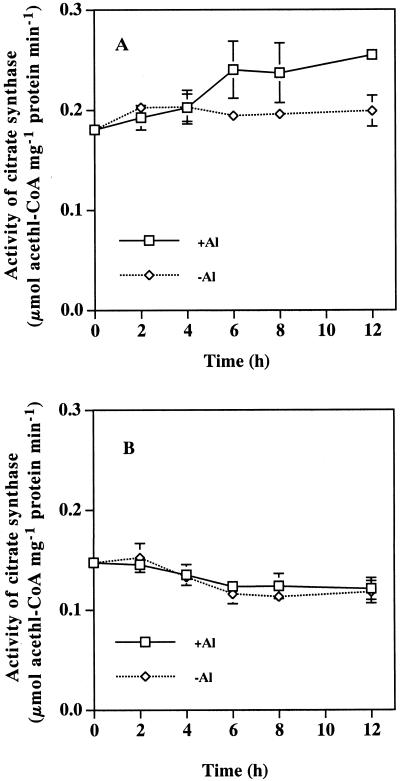
The effect of Al on the activities of several enzymes relevant to the metabolism of citric and malic acids was investigated in the root apices from both plants (Table I). The activity of phosphoenolpyruvate carboxylase (PEPCase), malate dehydrogenase (MDH), and NADP+-isocitrate dehydrogenase (NADP+-ICDH) was not significantly affected by a 12-h exposure to Al in either plant. However, the activity of citrate synthase (CS) in the rye was increased by about 30% by Al, whereas that in the wheat was not influenced. A time-course experiment showed that there was no difference in CS activity between the rye treated with and without Al until 6 h (Fig. 4A). However, from 6 h, a significant increase in the CS activity was observed in the rye exposed to Al. A similar experiment was conducted with the wheat (Fig. 4B), but the Al-induced increase in the CS activity was not observed throughout the 12-h experiment.
Table I.
Effect of Al on the activities of enzymes relevant to organic acid synthesis
| Treatment | Rye
|
Wheat
|
||||||
|---|---|---|---|---|---|---|---|---|
| PEPCase | MDH | NADP+-ICDH | CS | PEPCase | MDH | NADP+-ICDH | CS | |
| μmol mg−1 protein min−1 | ||||||||
| −Al | 0.09 ± 0.02 | 7.53 ± 0.51 | 0.27 ± 0.02 | 0.20 ± 0.02 | 0.11 ± 0.02 | 6.61 ± 0.69 | 0.32 ± 0.02 | 0.12 ± 0.01 |
| +Al | 0.08 ± 0.01 | 6.87 ± 0.37 | 0.31 ± 0.07 | 0.25 ± 0.01 | 0.12 ± 0.01 | 6.46 ± 0.30 | 0.32 ± 0.07 | 0.12 ± 0.01 |
The roots of rye (cv King) and wheat (cv Atlas 66) were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 0 (−Al) or 50 μm AlCl3 (+Al) for 12 h. The root apexes (1.0 cm) were then excised and assayed for the activity of CS, MDH, NADP+-ICDH, and PEPCase. Values are means ± sd of three replicates.
Figure 4.
Effect of Al on the activity of CS in root apexes of the rye (A) and the wheat (B). The roots were exposed to 0.5 mm CaCl2 solution with (+Al) or without (−Al) 50 μm Al for 0, 2, 4, 6, 8, and 12 h, respectively, then the root apices (1 cm) were excised. CS activity was assayed as described in “Materials and Methods.” Bar represents sd (n = 3).
The effect of low temperature on the Al-induced secretion was compared in the two plants. The Al-induced secretion of citric acid from the roots of rye was not found after low-temperature treatment (Table II). However, the Al-induced secretion of malic acid was not suppressed by low-temperature treatment for 12 h in the wheat (Table II). Organic acids were not detected in the root exudates in the absence of Al at a low temperature (data not shown).
Table II.
Effect of low temperature on the Al-induced secretion of organic acids in rye (cv King) and wheat (cv Atlas 66)
| Plant | 25°C
|
4°C
|
||
|---|---|---|---|---|
| Malate | Citrate | Malate | Citrate | |
| μmol g−1 root fresh wt 12 h−1 | ||||
| Rye | 0.15 ± 0.03 | 0.20 ± 0.05 | 0.05 ± 0.01 | 0 ± 0 |
| Wheat | 0.72 ± 0.14 | 0 ± 0 | 0.80 ± 0.09 | 0 ± 0 |
The seedlings were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 50 μm Al at 20/25°C and 4°C. After a 12-h exposure, the root exudates were collected and the organic acids were analyzed by HPLC. Data are means ± sd (n = 3).
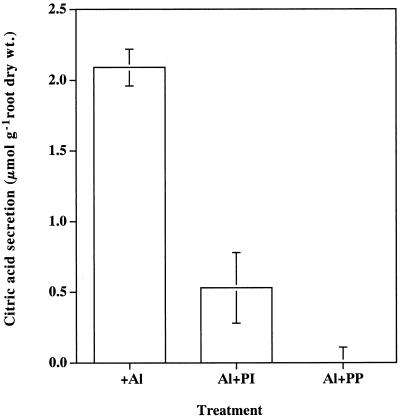
Pyridoxal 5′-P (PP) and phenylisothiocyanate (PI) have been reported as inhibitors for citrate carrier on mitochondrial membrane (Genchi et al., 1999). The effect of these inhibitors on the Al-induced secretion of organic acids was investigated in the rye. The presence of PI and PP significantly inhibited the Al-induced secretion of citric acid (Fig. 5). The secretion of citric acid was completely inhibited by PP.
Figure 5.
Effect of citrate-carrier inhibitor on the Al-induced secretion of citric acid in rye. Seedlings were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 25 μm of PP and PI in the presence of 50 μm Al. After a 12-h exposure, the root exudates were collected and citric acid was analyzed by HPLC. Bar represents sd (n = 3).
DISCUSSION
The cultivar (cv King) of rye used in the present study is commercial and usually cultivated in Japan. However, its Al tolerance is comparable to that of cv Atlas 66 (Fig. 1), which is one of the most Al-tolerant cultivars of wheat (Ryan et al., 1995b). The secretion of malic acid has been reported as an Al-tolerance mechanism in cv Atlas 66 (Basu et al., 1994; Ryan et al., 1995b). However, it is unknown whether the Al-induced secretion of organic acids occurs in rye. We found that both malic and citric acids were secreted from the roots of rye in response to Al (Fig. 2A). Furthermore, the amount of organic acids secreted from the rye increased with increasing external Al concentrations (Fig. 3A) and the secretion was highly specific to Al (neither P deficiency nor other polyvalent cations caused the secretion of organic acids). These characteristics of the Al-induced secretion of organic acids are similar to those found in the Al-tolerant wheat (Fig. 3; Delhaize et al., 1993), maize (Pellet et al., 1995), C. tora (Ma et al., 1997c), and buckwheat (Zheng et al., 1998), suggesting that the secretion of organic acids also participates in Al tolerance of the rye. However, the secretion pattern is obviously different between the two plants (Fig. 2).
Ma et al. (2000) has classified the Al-induced secretion of organic acids into two patterns. In pattern I, there was no discernible delay between the addition of Al and the onset of the release of organic acids. The secretion pattern in the wheat (cv Atlas 66) belongs to this pattern (Fig. 2B). This pattern has been reported in other Al-tolerant wheat cultivars and in buckwheat. For example, in an Al-tolerant genotype of wheat, ET3, Al-stimulated secretion of malate from both intact roots and excised root apexes was observed within 20 min after the exposure to Al (Delhaize et al., 1993; Ryan et al., 1995a). In buckwheat the secretion of oxalic acid occurred within 30 min after the exposure to Al (Ma et al., 1997b). In pattern II, there is a marked lag phase between the addition of Al and the onset of organic acid release. The secretion of organic acid in the rye belongs to this pattern (Fig. 2A). The lag in the rye was 6 and 10 h for malic and citric acids, respectively (Fig. 2A). This secretion pattern has also been observed in other plant species and cultivars. In C. tora, the secretion of citrate in response to Al was increased after 4 h (Ma et al., 1997c). In an Al-resistant cultivar of maize, a considerable lag phase before the maximal citrate efflux is observed (Pellet et al., 1995; Jorge and Arruda, 1997). Al-Induced secretion of malic and citric acids was recently found to be significantly increased after 6 and 12 h, respectively, in a triticale line (Ma et al., 2000).
Different mechanisms for the two secretion patterns have been proposed (Ma et al., 2000). The rapid secretion of organic acids upon Al exposure in pattern I has been suggested to be caused by the activation of an anion channel for organic acids, and not by gene induction. In contrast, gene induction may be involved in the pattern II secretion. The gene(s) may be related to the metabolism (biosynthesis and decomposition) of organic acids, anion channel on plasma membrane and/or tonoplast, or transport of organic acids from mitochondria (Ma et al., 2000). However, evidence supporting these speculations is lacking. We compared the effects of Al on the activity of enzymes related to organic acid metabolism, and the effect of low temperature and the citrate-carrier inhibitors on the Al-induced secretion of organic acids between the two plants. The activities of four enzymes related to the biosynthesis and degradation of malic and citric acids in the root apex of the wheat were not affected by Al (Table I). This is in agreement with previous finding by Ryan et al. (1995a) that the activities of PEPCase and NAD-MDH did not differ between Al-sensitive and -tolerant cultivars of wheat and between the plants treated and not treated with Al. The internal malic acid content was not changed by the exposure to Al during a short time (Delhaize et al., 1993). All of these facts suggest that in vivo the synthesis of organic acids is not altered by Al in wheat. In contrast, the activity of CS in the root apices of the rye was increased by the exposure to Al, although that of the other three enzymes was not affected (Table I). Results from the time course experiment showed that significant increase in the CS activity occurs from 6 h after the start of the exposure to Al (Fig. 4). The significant secretion of citric acid in the roots of rye was observed at 10 h after the exposure to Al (Fig. 2A). These results suggest that CS activity increased by Al causes the secretion of citric acid in the rye. de la Fuente et al. (1997) introduced a Pseudomonas aureginosa CS gene into tobacco and papaya. As a result, the transgenic plants showed enhanced Al tolerance, which was associated with an increase in CS activity and citric acid secretion. Recently, an increase in the specific activity of CS was also reported in a P-deficiency-tolerant cell line of carrot (Takita et al., 1999), which secretes citric acid that enables the acquisition of insoluble Al-P. Over expression of a mitochondrial CS gene from Arabidopsis in carrot cells resulted in higher CS activity and higher secretion of citric acid compared with wild-type cells (Koyama et al., 1999). All these findings indicate that the secretion of citric acid could be altered by manipulation of citric acid metabolism.
If the increased CS activity contributes to the secretion of citric acid in rye, the activity of CS and the secretion of citric acid may be suppressed at a low temperature. At low temperature, the Al-induced secretion of malic acid in the wheat was not suppressed during 12 h of the treatment, whereas that of citric acid in the rye was stopped (Table II). This result further indicates that alternation of organic acid metabolism is involved in the Al-induced secretion of citric acid in the rye, but not in the wheat. The low-temperature-induced increase in the secretion of malic acid was found after 24 h of treatment (data not shown). The mechanism of the increase in the secretion of malic acid may be due to the decomposition of malic acid by microbial activity at 25°C, failure of regulation of anion channels at low temperature, low-temperature-induced increase in the Al-induced depolarization of the membrane, or a change in the structure of the anion channel protein at low temperature. The mechanism is not attributable to the leakage from the roots, because no malic acid was detected in the absence of Al at low temperature (data not shown).
Citric acid synthesized in the mitochondria is transported via a citrate-carrier on the membrane. PI and PP are lysyl-specific reagents and inhibit the transport of citric acid from the mitochondria (Genchi et al., 1999). Fifteen micromolar PI or PP had no effect or an only slightly negative effect on root elongation of the rye in the absence of Al (data not shown). In the presence of PI and PP, the secretion of citric acid from the roots of rye was significantly decreased (Fig. 5). This result suggests that the transport process of citric acid from mitochondria is inhibited, resulting in the reduced secretion of citric acid.
The secretion of malic acid was also increased at 6 h in the rye after the exposure to Al (Fig. 2A). However, the activities of MDH and PECase were not affected by Al (Table I) in the rye. The Al-induced secretion of malic acid was also found in the rye at low temperature (Table II), whereas no malic acid was detected in the absence of Al at same temperature (data not shown). These results suggest that different from citric acid, the synthesis of malic acid may not be affected by Al. One possible mechanism is that an anion channel for malic acid secretion is induced by Al, but this needs to be examined in future.
In conclusion the mechanism involved in the Al-induced secretion of organic acids is different between rye and wheat. Al-Induced increase in the synthesis of citric acid seems to be responsible for the increased secretion of citric acid in rye.
MATERIALS AND METHODS
Plant Materials
Seeds of rye (Secale cereale L. cv King) and wheat (Triticum aestivum L. cv Atlas 66) were soaked in water for 3 and 8 h, respectively. Then the seeds were placed on a net tray, which was floated on a 0.5 mm CaCl2 solution at pH 5.6 in a plastic container. After kept in the dark for 4 d at 25°C, the seedlings with similar size were transplanted into 1-L plastic pots (16–20 seedlings per pot) containing aerated nutrient solution. The nutrient solution contained 1.0 mm CaNO3, 1.0 mm KNO3, 0.4 mm MgSO4, 0.2 mm NH4H2PO4, 10 μm Fe-EDTA, 3 μm H3BO3, 0.5 μm MnCl2, 0.2 μm CuSO4, 0.4 μm ZnSO4, and 1 μm (NH4)6Mo7O24. The nutrient solution was adjusted to pH 4.5 with 1.0 m HCl and replaced every 2 d. After 12 to 15 d of culture, the seedlings were used in the following experiments. Plants were grown in a controlled-environment growth cabinet (TGE-9H-S, TABAI ESPEC, TABAI, Osaka) with a 14-h/25°C day at a light intensity of 40 W m−2 and 10-h/20°C night regime. Each experiment was repeated at least twice.
Al Tolerance in Rye and Wheat
The effect of Al on the root elongation was compared between the rye and the wheat. Four-day-old seedlings, prepared as described above, were exposed to 0.5 mm CaCl2 solution, pH 4.5, containing 0, 10, 30, or 50 μm AlCl3 (Wako, Tokyo) for 24 h. Twelve replicates were made for each treatment. Root length was measured with a ruler before and after the Al treatment.
Collection of Root Exudates Solution and Organic Acid Analysis
Before collection of root exudates, the roots were placed in a 0.5 mm CaCl2 solution at pH 4.5 overnight. The seedlings (12-d-old) were then exposed to 0.5 mm CaCl2 (pH 4.5) containing 50 μm AlCl3. The Al solution was freshly prepared prior to use. Root exudates were collected at different times. In a dose-response experiment, the seedlings were exposed to a 0.5 mm CaCl2 solution (pH 4.5) containing 0, 10, 30, or 50 μm AlCl3. After a 24-h exposure to Al, the Al solution containing root exudate was collected. For analysis of organic acids, the solution was first passed through a cation exchange column (16 mm × 14 cm) filled with 5 g of Amerlite IR-120B (H+ form) resin (Muromachi Chemical, Toyko), and then through an anion-exchange column filled with 2 g of Dowex 1× 8 resin (100–200 mesh, formate form) in a cold room. Organic acids retained on the anion-exchange resin were eluted with 2 m HCl and the eluent was concentrated using rotary evaporator at 40°C. The residue was then dissolved in dilute HClO4 solution, pH 2.1, and analyzed with HPLC according to Ma et al. (1997a). The detection was at 425 nm after reaction with 0.2 mm bromthymol blue.
Enzyme Assay
After exposure to 0.5 mm CaCl2 (pH 4.5) containing 50 μm AlCl3 for 2, 4, 6, 8, or 12 h, 20 root apexes (1.0 cm) were excised for the enzyme extraction. The root apexes were homogenized for 30 s in a cold 50 mm HEPES-NaOH buffer (pH 7.5) containing 5 mm MgCl2, 5 mm EDTA, 10% (v/v) glycerol and 0.1% (v/v) Triton X-100, with a micro-homogenizer (NS-310E, Niti-on, Chiba, Japan). The homogenate was then centrifuged at 20,000g for 5 min, and the supernatant was used to assay CS, MDH, PEPCase, and NADP+-ICDH. The activity CS was assayed spectrophotometrically by monitoring the reduction of acetyl coenzyme A with 5,5′-dithio-bis-2-nitrobenzonic acid at 412 nm for 3 min according to Johnson et al. (1994). The reaction mixture contained 100 mm Tris-HCl buffer (pH 8.0), 5 mm MgCl2, 100 mm 5,5′-dithio-bis-2-nitrobenzonic acid, 0.3 mm acetyl coenzyme A and 0.5 mm oxalacetic acid. MDH and PEPCase were assayed spectrophotometically by monitoring the disappearance of NADH at 340 nm in a direct and coupled assay, respectively (Johnson et al., 1994). NADP+-ICDH was assayed spectrophotometically by monitoring the disappearance of NADPH at 340 nm in a direct and coupled assay. Amounts of protein in the supernatants were determined with bovine serum albumin as a standard (Bradford, 1976).
Low Temperature and Inhibitor Treatment
Seedlings (15-d-old) were exposed to 0.5 mm CaCl2 (pH 4.5) solution containing 50 μm AlCl3 at 4°C and 25°C. After 12 h, the solution was collected and the organic acids were analyzed as described above. The effect of citrate carrier inhibitors on the secretion of organic acids was also investigated by exposing the seedlings to 0.5 mm CaCl2 (pH 4.5) containing 50 μm AlCl3 in the presence of PP and PI at 25 μm.
Footnotes
This study was supported in part by the Program for Promotion of Basic Research Activities for Innovative Bioresources, by a Grant-in-Aid for Encouragement of Young Scientists (grant no. 09760058 to J.F.M.) from the Ministry of Education, Science, Sports and Culture of Japan, by the Agriculture Science and Education Foundation, by Grants-in-Aid for General Scientific Research (A and B) (nos. 09460038 and 11306006) from the Ministry of Education, Science, Sports and Culture of Japan, by the Joint Research Project Program under the Japan-Korea, Basic Scientific Cooperation Program, by the Research for the Future Program from Japan Society for the Promotion of Science, and by the Ohara Foundation for Agricultural Science.
LITERATURE CITED
- Aniol A, Gustafson JP. Chromosome location of genes controlling aluminum tolerance in wheat, rye and triticale. Can J Genet Cytol. 1984;26:701–705. [Google Scholar]
- Aniol A, Hill RD, Larter EN. Aluminum tolerance of spring inbred lines. Crop Sci. 1980;20:205–208. [Google Scholar]
- Basu U, Godbold D, Taylor GJ. Aluminum resistance in Triticum aestivum L. associated with enhanced exudation of malate. J Plant Physiol. 1994;144:747–753. [Google Scholar]
- Bradford MM. A rapid and sensitive methods for the quantitation of microgram of proteins utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- de la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.)? Aluminum stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi G, Spagnoletta A, Santis AD, Stefanizzi L, Palmieri F. Purification and characterization of the reconstitutively active citrate carrier from maize mitochondria. Plant Physiol. 1999;120:841–848. doi: 10.1104/pp.120.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JF, Allan DL, Vance CP. Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiol. 1994;104:657–665. doi: 10.1104/pp.104.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RA, Arruda P. Aluminum-induced organic acids exudation by roots of an aluminum-tolerant tropical maize. Phytochemistry. 1997;45:675–681. [Google Scholar]
- Kochian LV. Cellular mechanism of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Koyama H, Takita E, Kawamura A, Hara T, Shibata D. Over expression of mitochondria citrate synthase gene improves the growth of carrot cells in Al-phosphate medium. Plant Cell Physiol. 1999;40:482–488. doi: 10.1093/oxfordjournals.pcp.a029568. [DOI] [PubMed] [Google Scholar]
- Ma JF. Role of organic acids in detoxification of Al in higher plant. Plant Cell Physiol. 2000;44:383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. Internal detoxification mechanism of Al in hydrangea. Identification of Al form in the leaves. Plant Physiol. 1997a;113:1033–1039. doi: 10.1104/pp.113.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Taketa S, Yang ZM. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiol. 2000;122:687–694. doi: 10.1104/pp.122.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Hiradate S, Matsumoto H. Detoxifying aluminum with buckwheat. Nature. 1997b;390:569–570. [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H. Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol. 1997c;38:1019–1025. [Google Scholar]
- Ma Z, Miyasaka SC. Oxalate exudation by taro in response to Al. Plant Physoil. 1998;118:861–865. doi: 10.1104/pp.118.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H. Int Rev Cytol in press. 2000. Cell biology of aluminum toxicity and tolerance in higher plants. [DOI] [PubMed] [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbean: root exudation of citric acid. Plant Physiol. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995a;196:103–110. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices: evidence for a general mechanism of Al-tolerance in wheat. Aust J Plant Physiol. 1995b;22:531–536. [Google Scholar]
- Ryan PR, Ditomaso JM, Kochain LV. Aluminum toxicity in roots: an investigation of spatial sensitive. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Takita E, Koyama H, Hara T. Organic acid metabolism in aluminum-phosphate utilizing cells of carrot (Daucus carota L.) Plant Cell Physiol. 1999;40:489–495. [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat: Ι. Al-induced special secretion of oxalic acid from root tips. Plant Physiol. 1998;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]