Abstract
Metronidazole is a commonly used antimicrobial worldwide. The most common side effects that have been reported are nausea, vomiting and hypersensitivity reactions. However, neurotoxicity has been reported with the use of metronidazole but rather rare. The most common neurological manifestation is peripheral neuropathy involvement in the form of sensory loss. It is worth mentioning that central neurotoxicity is a rare side effect of metronidazole use but reversible. The manifestations vary from a headache, altered mental status to focal neurological deficits. The diagnosis is mainly by neuroimaging in the setting of acute neurological change in the patient status. Here, we report a case of metronidazole-induced neurotoxicity in a 38-year-old male patient who was admitted with a brain abscess and was started on metronidazole for more than 10 weeks.
Keywords: radiology (diagnostics), drugs and medicines, neurology (drugs and medicines)
Background
Metronidazole is widely used antimicrobial globally. Although neurotoxicity has been reported in many cases, however, most of the reported cases are peripheral rather than central neurotoxicity.1 Metronidazole induced-encephalopathy (MIE) is a rare complication of long-term metronidazole use, however, reversible. The likelihood of MIE occurrence is directly associated with the duration of therapy.2 However, the most reported neurotoxicity findings are related to cerebellar involvement.3 The presentation can range from a headache and altered mental status to seizures and focal neurological deficits.4 These symptoms are usually reversible after the cessation of metronidazole, yet, the needed duration for complete recovery vary from patient to the other. From the previous literature, few case reports revealed permanent encephalopathy secondary to metronidazole toxicity. The classical MRI findings are reversible signals in T2 and fluid attenuation inversion recovery (FLAIR) in the form of hyperintensities of dentate nucleus, brainstem and corpus callosum.5 Treatment is usually by cessation of the offending medication and in most of the cases, the symptoms are reversible.
Case presentation
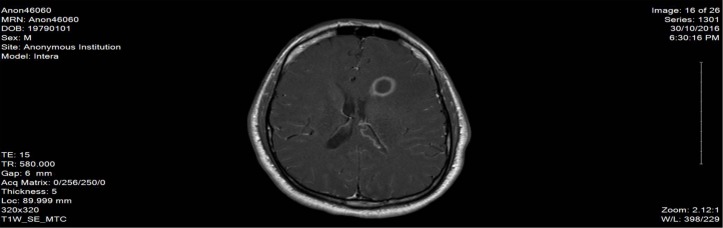
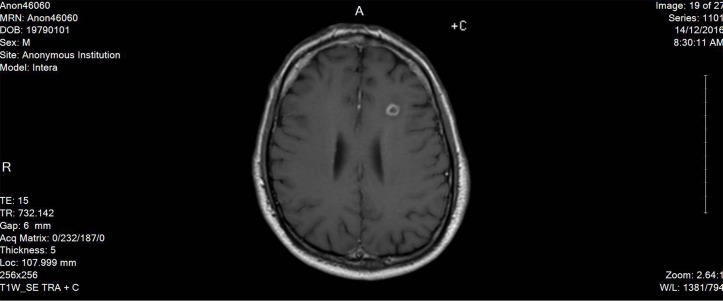
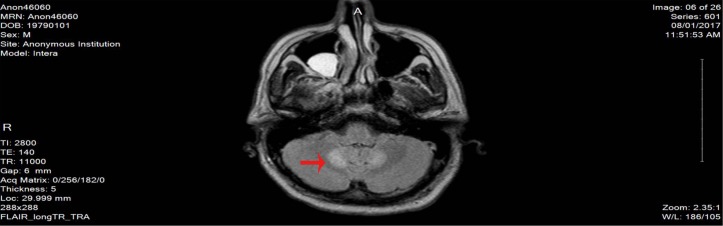
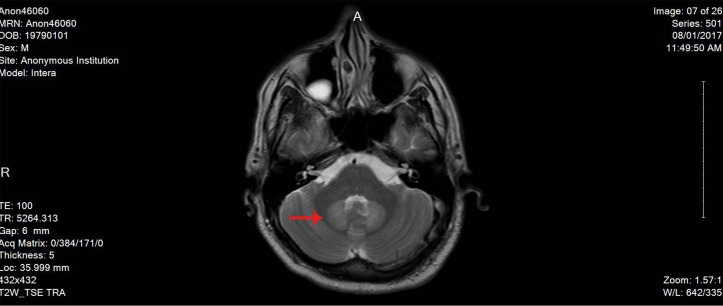
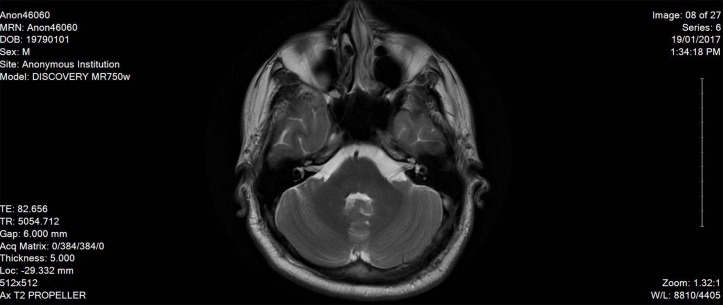
Here, we report a case of 38-year-old male patient, who is not known to have any medical history, not on any home medications, no smoking history and no reported substance or alcohol use, who presented with agitation and altered mental status of 1 day duration without any focal neurological deficits. The patient was started on levetiracetam 500 mg twice daily from the second day of admission and continued for over 2 months. He was found to have left frontal brain abscess which was seen on the CT scan without contrast initially and confirmed using MRI with contrast (figure 1). The patient was treated empirically (as the size of the abscess was not ideal for biopsy from the neurosurgery point of view) with intravenous metronidazole (500 mg every 8 hour) for 10 weeks together with vancomycin (according to the trough level) for 7 weeks, ceftriaxone 2 g for 5 weeks and dexamethasone tapering dose 6 mg to 4 mg over 1 month. The duration of therapy was extended beyond 8 weeks due to gradual resolution of the initial brain abscess at week 8 (figure 2). On day 72 of hospital stay, the patient developed hoarseness of voice and dysphagia without peripheral symptoms; the neurological examination revealed dysarthria and ataxic gait. The initial workup revealed normal electrolyte levels and other investigations can be found in the table below (table 1). The MRI brain did not show any sign of acute stroke or demyelinating disease features. However, it revealed bilateral symmetrical confluent bright T2 and FLAIR at both cerebellar hemispheres of dentate nucleus (figures 3 and 4), which is highly suggestive of metronidazole-induced central nervous system (CNS) toxicity. Metronidazole was stopped at the same day (day 72 after the MRI report) and the patient was started on amoxicillin-clavulanate instead of metronidazole for additional 2 weeks (the overall duration of therapy was 12 weeks). The symptoms gradually improved within 4 days after cessation of metronidazole. Moreover, the repeated MRI after 2 weeks showed complete resolution of the previously seen metronidazole-induced neurotoxicity features (figure 5).
Figure 1.
The MRI image at initial presentation.
Figure 2.
The MRI image after 8 weeks of treatment.
Table 1.
Laboratory findings
| Test | Result |
| Ammonia | 46 μmol/L |
| Total protein | 76 g/L |
| Albumin | 36 g/L |
| Total bilirubin | 17.6 μmol/L |
| Direct bilirubin | 4.0 μmol/L |
| Alkaline phosphatase | 73 IU/L |
| Aspartate aminotransferase | 22 IU/L |
| Alanine aminotransferase | 30 IU/L |
| Glucose | 7.7 mmol/L |
| HIV | Negative |
| Cerebrospinal fluid (CSF) analysis | |
| Appearance | Cloudy |
| Red blood cells CSF | 4×106/L |
| Nucleated cells CSF | 510×106/L |
| Neutrophils CSF | 86% |
| Lymphocytes CSF | 9% |
| Monocytes CSF | 4% |
| Eosinophils CSF | 1% |
| Protein CSF | 1.15 g/L |
| Glucose CSF | 2 mmol/L |
| Lactic acid CSF | 8.17 mmol/L |
| Lactate dehydrogenase CSF | 550 IU/L |
| CSF culture | Negative |
| Mycobacterium PCR CSF | Negative |
| Thyroid stimulation hormone | 0.954 milli IU/L |
| Free T4 | 17.8 pmol/L |
| Vitamin B12 | 325 pmol/L |
| Syphilis | Negative |
| Venous blood gas | |
| pH | 7.38 |
| Partial pressure of carbon dioxide | 47.4 mm Hg |
| PO2 | 29.9 mm Hg |
| Bicarbonate | 28.2 mmol/L |
| Base Excess | 2.7 mmol/L |
Figure 3.
The MRI image on day 72 on diagnosing metronidazole-induced neurotoxicity.
Figure 4.
Another MRI image showing metronidazole-induced neurotoxicity features.
Figure 5.
The MRI image after cessation of metronidazole and resolution of changes.
Differential diagnosis
Metabolic (hypoglycaemia, electrolyte Imbalance, uraemic encephalopathy).
CNS (normal pressure hydrocephalus, closed-head injury, space occupying lesion (brain abscess, tumour)).
Vascular events (cerebrovascular accident, transient ischaemic attack).
Chronic hypoxia.
Hepaticencephalopathy.
Postictal state.
Psychiatry conditions (psychosis).
Infectious (tuberculosis, toxoplasmosis, HIV, syphilis).
Substance abuse or withdrawal.
Endocrine (myxoedema coma, thyrotoxicosis storm).
Treatment
The cessation of metronidazole after establishing the diagnosis was the mainstay measure for the encephalopathy. To continue treating the brain abscess, additional 2 weeks of amoxicillin-clavulanate was added.
Outcome and follow-up
The patient was discharged home with minimal burning sensation in both feet and imbalance while walking. These symptoms were followed by the writers over the phone, as the patient’s insurance was not covered in the out-patient setting. The patient reported gradual improvement in his symptoms without the occurrence of major long-term complications over a period of 10 months since the discharge date. He reported being able to resume his life as before without any major difficulties.
Discussion
Metronidazole is one of the mainstay drugs for the treatment of anaerobic and protozoal infections.1 It is well tolerated and fairly safe, yet, the most common adverse effects associated with systemic metronidazole therapy are gastrointestinal effects, hypersensitivity reactions and can also cause nervous system effects, for instance, mental status changes, weakness, peripheral neuropathy, sensory losses, vertigo, nausea and vomiting. However, less reported symptoms were ataxia and dysarthria.1 2
Different hypothesis were believed to contribute to metronidazole-induced neurotoxicity, yet the exact mechanism is not totally understood. However, multiple theories have been proposed in the literature to clarify the most possible mechanism. Its extracellular space concentration contributes to its toxicity when therapeutic level is achieved in the cerebrospinal fluid.6 7 The main pathophysiology behind the encephalopathy is the vascular spasm that might produce mild reversible ischaemia.6 7 This occurs when metronidazole induces oxidation of catecholamines to form radicals that reduce tissue oxygenation leading to increase in the nerve axonal water concentration which eventually results in axonal swelling; which is mostly evident on the MRI. Roy et al and Knorr et al also suggested that the binding of metronidazole metabolites to DNA or RNA of the neural cells could result in MIE.6 7 Furthermore, they proposed that the modulation of the gamma-aminobutyric acid receptors within the cerebellar and vestibular systems contributed to MIE.6 7
Antibiotic-associated encephalopathy is evident within 5 days of onset of treatment, except for isoniazid and metronidazole, in which the effect can be delayed for up to 3 weeks.4 Metronidazole-induced neurotoxicity has been reported in the literature within few days to weeks after initiation of therapy.8 The MRI abnormality that is typically seen in metronidazole-induced neurotoxicity is increased signal changes on MRI T2 in the dentate nucleus, brainstem and corpus callosum. The long-term high cumulative dose treatment of metronidazole considered to cause neurotoxicity ranges from 21 to 182 g across different case series.7 9
The presented study patient was previously healthy and metronidazole naive, which contributed to the late presentation of neurotoxicity symptoms (more than 7 weeks), while according to Kuriyama et al and Kim et al, the neurotoxicity symptoms development was within 2 hours and 7 days of metronidazole administration, respectively.10 11 From the previous literature, the longest duration recorded was 4 months,1 12 which also can be associated with liver cirrhosis.4 7 It is worth mentioning that the reported mean age group was 64.7 years13; however, our study patient’s age was younger than the mean age group reported. The study patient developed the commonly reported symptoms as dysarthria and voice hoarseness,7 11 while he also developed dysphagia that was rarely reported. Moreover, the cumulative dose from the course of treatment was 108 g in the study patient, while the average cumulative dose in Sonthalia et al was 44 g.4 The lesion seen on the study patient’s MRI was typical to the reported cases in the previous literature, showing the involvement of dentate nuclei bilaterally.4 9 14 The most common condition treated with metronidazole was brain abscess which was around 35.3% in Kato et al13 and Yokoyama et al,5 which was also the same indication in our study patient. It is worth mentioning that our patient scored seven on the Naranjo Adverse Drug Reaction Probability Scale, which reflects probable reaction that is recognised by the response to the offending drug and confirmed by the drug withdrawal.15
This case report will add to the reported cases in the literature; our case highlights the fact that metronidazole-induced central neurotoxicity is a serious side effect of the medication, although rarely reported but clinicians should consider such adverse effect in patients with prolonged use of metronidazole therapy.
Patient’s perspective.
This experience has been interesting as I didn’t experience any symptoms before this happened, it was all sudden in onset, luckily it was managed properly. The hospital stay was pleasant and I was positive throughout the whole experience.
My only concern that I still have burning sensation in my feet and minimal imbalance, which is getting better slowly with time.
Learning points.
The sudden change in the patient’s clinical status should be taken seriously; many differential diagnoses can be considered including the side effect of the medications, which is seen in our case.
Targeted therapy is the optimal approach to brain abscess treatment, therefore, we encourage the use of definitive diagnostic measures to avoid blind therapy.
Prompt evaluation of altered mental status by excluding the most common differential diagnosis is important to effectively treat any reversible causes by simple measures like the cessation of the causative medication as in our case.
Footnotes
Contributors: WA found the case and read the literature review about its prevalence. AA was involved in the direct care of the patient and wrote the case. NG worked on the introduction and the discussion parts.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Agarwal A, Kanekar S, Sabat S, et al. Metronidazole-induced cerebellar toxicity. Neurol Int 2016;8 10.4081/ni.2016.6365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senthilkumaran S, Shah S, Balamurugan N, et al. Metronidazole encephalopathy: uncommon reaction to a common drug. Int J Crit Illn Inj Sci 2015;5:123 10.4103/2229-5151.158422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SU, Jung IE, Kim HJ, et al. Metronidazole-induced combined peripheral and central vestibulopathy. J Neurol Sci 2016;365:31–3. 10.1016/j.jns.2016.03.043 [DOI] [PubMed] [Google Scholar]
- 4.Sonthalia N, Pawar SV, Mohite AR, et al. Metronidazole-induced encephalopathy in alcoholic liver disease: a diagnostic and therapeutic challenge. J Emerg Med 2016;51:e79–e83. 10.1016/j.jemermed.2016.05.038 [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama Y, Asaoka K, Sugiyama T, et al. [Metronidazole-induced encephalopathy during brain abscess treatment:two case reports]. No Shinkei Geka 2015;43:927–32. 10.11477/mf.1436203151 [DOI] [PubMed] [Google Scholar]
- 6.Roy U, Panwar A, Pandit A, et al. Clinical and neuroradiological spectrum of metronidazole induced encephalopathy: our experience and the review of literature. J Clin Diagn Res 2016;10 10.7860/JCDR/2016/19032.8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knorr JP, Javed I, Sahni N, et al. Metronidazole-induced encephalopathy in a patient with end-stage liver disease. Case Reports Hepatol 2012;2012:1–4. 10.1155/2012/209258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuriyama A, Jackson JL, Doi A, et al. Metronidazole-induced central nervous system toxicity. Clin Neuropharmacol 2011;34:241–7. 10.1097/WNF.0b013e3182334b35 [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Kim YW, Kim SR, et al. Metronidazole-induced encephalopathy in a patient with infectious colitis: a case report. J Med Case Rep 2011;5:63 10.1186/1752-1947-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuriyama A. Chestnut sign: metronidazole-induced encephalopathy. J Emerg Med 2017;52:101–2. 10.1016/j.jemermed.2016.07.091 [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Chun J, Park JY, et al. Metronidazole-induced encephalopathy in a patient with Crohn’s disease. Intest Res 2017;15:124–9. 10.5217/ir.2017.15.1.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hari A, Srikanth BA, Lakshmi GS. Metronidazole induced cerebellar ataxia. Indian J Pharmacol 2013;45:295–7. 10.4103/0253-7613.111903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato H, Sosa H, Mori M, et al. [Clinical characteristics of metronidazole-induced encephalopathy: a report of two cases and a review of 32 Japanese cases in the literature]. Kansenshogaku Zasshi 2015;89:559–66. 10.11150/kansenshogakuzasshi.89.559 [DOI] [PubMed] [Google Scholar]
- 14.Farmakiotis D, Zeluff B. Metronidazole-associated encephalopathy. N Engl J Med Overseas Ed 2016;374:1465 10.1056/NEJMicm1505174 [DOI] [PubMed] [Google Scholar]
- 15.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]