Abstract
Management of abdominal pain in a pregnant patient with a history of Roux-en-Y gastric bypass presents unique challenges. A misdiagnosis or delay in management can result in lethal maternal–fetal outcomes. We present a 30-year-old woman at 21 weeks of pregnancy presented with abdominal pain. She had a history of laparoscopic Roux-en-Y gastric bypass performed 3 years earlier. The clinical examination was remarkable for epigastric pain and tenderness. The vital signs and laboratory examinations were unremarkable. The CT scan was suggestive of an internal hernia. On an exploratory laparoscopy, the distal common small bowel was found to be herniating through the jejunojejunostomy mesenteric defect, causing intestinal obstruction with dilatation of the Roux limb and the biliopancreatic limb. The internal hernia was reduced, and no bowel resection was required. The mesenteric defect was closed with 3-0 silk sutures in a continuous fashion. The patient was discharged after 3 days and delivered a healthy baby at 40 weeks of gestation.
Keywords: gastrointestinal surgery, general Surgery, pregnancy, obesity (nutrition)
Background
Bariatric surgery remains the most clinically effective and cost-effective treatment for obesity.1 Among the bariatric surgeries, laparoscopic Roux-en-Y gastric bypass (LRYGB) is considered as the gold standard, accounting for 18.7% of the bariatric procedures in 2014.2 Women of childbearing age (18–45 years) constitute 49% of all bariatric procedures.3 The bariatric procedures increase fertility secondary to improvement in ovulation and sexual activity. Consequently, there is an increasing number of pregnant women with a history of bariatric surgeries. The abdominal pain in this cohort presents a unique challenge in management with the delay or misdiagnosis resulting in lethal effects. This case report describes the importance of the early and accurate diagnosis of an internal hernia in a 30-year-old pregnant patient with previous LRYGB.
Case presentation
A 30-year-old woman presented with 1 day of upper abdominal pain at 21 weeks of pregnancy. She had undergone LRYGB 3 years prior with 120 lbs lost since the surgery. She reported severe, colicky-type epigastric pain with nausea and vomiting. This pregnancy was her third, and her antenatal course prior to the presentation was uneventful. On physical examination, the vital signs were found to be within normal limits. Her abdomen was tender in the epigastric region with no guarding or rebound tenderness. The fetal heart and movements were normal.
Investigations
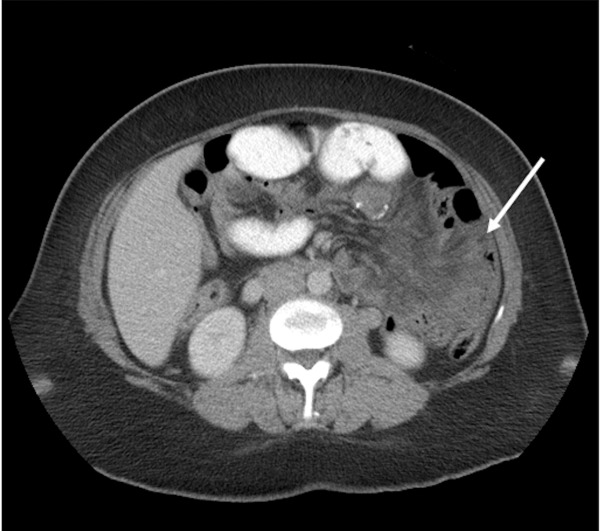
The laboratory evaluation was remarkable for a white count of 5.4 with 75% neutrophilia. An abdominal CT scan revealed dilated small bowel loops clustered in the left upper quadrant with congestion of the mesenteric vessels and fluid in the mesentery consistent with a diagnosis of an internal hernia (figure 1).
Figure 1.
CT scan of the abdomen showing clustering of intestines (white arrow) in the left upper quadrant of the abdomen.
Differential diagnosis
Small bowel obstruction from an internal hernia, adhesions, intussusception, volvulus and anastomotic strictures.
Treatment
The patient was immediately taken for diagnostic laparoscopy. The alimentary or Roux limb was 100 cm in length approximately and was located antecolic and antegastric. The previous operative report mentioned closure of mesenteric defects with non-absorbable sutures, but the defects were noticed to be open and no sutures were seen, probably from suture absorption. The proximal alimentary, biliopancreatic and remnant stomach were dilated. On running the small bowel from the ileocaecal junction, a loop of common channel small bowel was found herniating through the mesenteric defect at the jejunojejunostomy site, causing intestinal obstruction. The bowel loops were viable and hernia was reduced. All the mesenteric defects and Petersen’s defect were then closed with non-absorbable sutures.
Outcome and follow-up
The postoperative period was uneventful. The patient had an uneventful delivery by caesarean section at 40 weeks of gestation.
Discussion
An internal hernia (IH) is defined as an intermittent or persistent herniation of the viscus through an opening in the peritoneal space. Internal hernias occur in up to 15% of patients after RYGB.4 5 The incidence of IHs increases with massive weight loss following weight reduction surgery due to the widening of the mesenteric defects, creating potential spaces for internal herniation. Potential spaces for IH after RYGB include the spaces behind the Roux limb (Petersen’s space) with incidence of about 18%, the mesentery at the jejunojejunostomy in 13% cases, and in the retrocolic route, the extra defect created in the mesocolon occurring in 69% cases.6 Other causes of small bowel obstruction after RYGB include abdominal wall hernias, adhesions, anastomotic strictures, volvulus and intussusception.
Rapid weight loss after the surgery results in improvement of conditions such as polycystic ovary syndrome, anovulation and irregular menses leading to fertility rebound.7 These improvements have resulted in an increasing number of pregnant women with weight loss surgeries. Compared with women without bariatric surgery, pregnancies following bariatric surgery is associated with decrease in incidence of pre-eclampsia, gestational diabetes mellitus, large neonates and increase in the incidence of small neonates, preterm birth, maternal anaemia and neonatal intensive care admissions.8 The best practice guidelines recommend waiting 12–24 months before conceiving to avoid exposure of the fetus to the rapid weight loss environment.7 As the pregnancy advances, the enlargement of the uterus and increased abdominal pressure with a cranial displacement of abdominal contents may play a role in the formation of IH.4
Abdominal pain in pregnancy is not always related to pregnancy. The surgical causes of acute abdominal pain in pregnant women include acute appendicitis, cholelithiasis and acute cholecystitis. In addition to the above, pregnant women with RYGB are at risk for IH, intussusception and anastomotic strictures. A delay in diagnosis can be catastrophic, resulting in maternal and fetal mortality. A multidisciplinary team involving the bariatric surgeons, maternal–fetal medicine and intensive care team should be involved from the beginning.
The American College of Obstetricians and Gynaecologists recommends a thorough evaluation of abdominal pain, nausea and vomiting, which are commonly seen in pregnant patients with a history of bariatric surgery.7
Gudbrand and his colleagues performed a retrospective study from the Danish health register on pregnant patients with RYGBs later admitted to the surgical department with the suspicion of IHs.9 Seventeen out of 423 women identified had IHs. The median age at presentation was 28 years (21–44). The median weight loss was 50 kg (23–78 kg). Neither a CT nor an MRI was performed during evaluation. An IH at Petersen’s space and jejunojejunostomy mesenteric defects were found in seven patients each. Caesarean section was performed on nine patients during the index operation, while in the non-caesarean group, the laparoscopic approach was used in 10 out of 14 patients. No maternal or fetal death was reported in this cohort.
Leal-González et al reviewed the literature involving internal hernias in pregnant patients following RYGB.10 The mean age was 31.2 years (range 22–41) with a SD of 5.2. The median gestational age was 30.5 weeks (range of 6–37), and the median time from the RYGB was 2 years. The most common presenting symptom was an abdominal pain (100%) with nausea and vomiting in 54.5% and 45.5% patients, respectively. The most common site of an internal hernia was in Petersen’s space (45.5%). Maternal and fetal death occurred in 9% and 13.6% cases, respectively.
Imaging in a pregnant patient is a concern due to fetal radiation exposure. Most of the reported literature advocates for the use of a CT scan.4 The sensitivity of CT scan to detect an IH is 76% (95% CI 54% to 90%), and the specificity is 60% (95% CI 39% to 78%).11 Mesenteric swirl sign in CT was shown to be the single best predictor of IH with a sensitivity of 61%–83% and a specificity of 67%–94%.12 The consensus minimal acceptable dose of radiation during the entire pregnancy is 5 rads (0.05 Gy) and the dose of CT of the abdomen and pelvis is 2.5 rads.13 In the study by Ahmed et al, the upper gastrointestinal series had a sensitivity of 65%, and when combined with CT, the sensitivity increased to 100%.14 Accurate diagnosis should take priority over ionising radiation because the consequences are catastrophic. Altieri et al report an algorithmic approach in using CT scans to evaluate pregnant bariatric patients. The authors advocate the immediate use of operative exploration with any concern of peritonitis at presentation.11 In other cases, they recommend beginning with an ultrasonogram to differentiate gynaecological and non-gynaecological causes. In non-gynaecological cases, an abdominal radiograph is used, and operative exploration is recommended in the case of any abnormalities. In patients with a normal radiograph, the authors recommend the use of MRI or CT depending on the trimester of pregnancy. If all imagings are normal without neutrophilia, a serial examination is recommended. If neutrophilia is present with normal imagings, serial examination or operative exploration is recommended depending on the clinical presentation.
Surgical exploration should be performed emergently because any delay can result in lethal outcomes. If there is any suspicion of IHs, a diagnostic procedure with or without C-section is recommended. Fetal monitoring is recommended preoperatively and postoperatively with the availability of the obstetricians to perform the caesarean section as dictated by the clinical situation. The approach depends on the expertise of the team. Laparoscopy has been shown to be safe in any trimester without any increased risk to the mother and the fetus.15 The lateral decubitus position is preferred to avoid compression of the inferior vena cava, causing decreased venous return and cardiac output. Intra-abdominal access can be accomplished with an open technique, Veress needle or optical trocar, adjusting for fundal height and previous incisions. CO2 insufflation of 10–15 mm Hg is considered safe in pregnancy.16 A thorough evaluation of the bowels is needed to identify the bowel necrosis and site of herniation and to rule out other competing pathologies. All the mesenteric defects need to be closed with non-absorbable sutures. Recently, closure of mesenteric defects with staplers has been reported to be safe and reduces the risk of IH.
Specific measures to decrease the incidence of IH include the routine closure of Petersen’s defect, mesenteric defects at jejunojejunostomy and closure of transverse mesocolonic defects with non-absorbable simple interrupted sutures.5 17 However, some authors do not favour routine closure of mesenteric defects as closure produces smaller holes increasing the risk of herniation, and increases operating time and cost.18 The antecolic technique decreases the incidence of IH because it eliminates the defect in the transverse mesocolon.5 19 20
Learning points.
Management of abdominal pain in pregnant women with a history of bariatric surgery needs multidisciplinary care for optimal outcomes.
In addition to acute appendicitis and cholecystitis, women with RYGB are at risk for an internal hernia, volvulus, and anastomotic strictures.
A delay in diagnosis can be catastrophic to both mother and the fetus. Accurate diagnosis should take precedence over ionising radiation.
Immediate surgical exploration is recommended. The surgical approach depends on the expertise of the team.
Footnotes
Contributors: Concept, design of work, critical revision: UK, BFG, VNK. Drafting: UK, RG. Final approval: UK, RG, BFG, VNK.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13:215–357. 10.3310/hta13410 [DOI] [PubMed] [Google Scholar]
- 2. American Society for Metabolic and Bariatric Society. Estimate of bariatric surgery numbers, 2011–16. 2016. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers (accessed 12 Dec 2017).
- 3. Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA 2008;300:2286–96. 10.1001/jama.2008.641 [DOI] [PubMed] [Google Scholar]
- 4. Vannevel V, Jans G, Bialecka M, et al. Internal herniation in pregnancy after gastric bypass: a systematic review. Obstet Gynecol 2016;127:1013–20. 10.1097/AOG.0000000000001429 [DOI] [PubMed] [Google Scholar]
- 5. Geubbels N, Lijftogt N, Fiocco M, et al. Meta-analysis of internal herniation after gastric bypass surgery. Br J Surg 2015;102:451–60. 10.1002/bjs.9738 [DOI] [PubMed] [Google Scholar]
- 6. Iannelli A, Buratti MS, Novellas S, et al. Internal hernia as a complication of laparoscopic Roux-en-Y gastric bypass. Obes Surg 2007;17:1283–6. 10.1007/s11695-007-9229-5 [DOI] [PubMed] [Google Scholar]
- 7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 105: bariatric surgery and pregnancy. Obstet Gynecol 2009;113:1405–13. 10.1097/AOG.0b013e3181ac0544 [DOI] [PubMed] [Google Scholar]
- 8. Galazis N, Docheva N, Simillis C, et al. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014;181:45–53. 10.1016/j.ejogrb.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 9. Gudbrand C, Andreasen LA, Boilesen AE. Internal hernia in pregnant women after gastric bypass: a retrospective register-based cohort study. Obes Surg 2015;25:2257–62. 10.1007/s11695-015-1693-8 [DOI] [PubMed] [Google Scholar]
- 10. Leal-González R, De la Garza-Ramos R, Guajardo-Pérez H, et al. Internal hernias in pregnant women with history of gastric bypass surgery: case series and review of literature. Int J Surg Case Rep 2013;4:44–7. 10.1016/j.ijscr.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altieri MS, Pryor AD, Telem DA, et al. Algorithmic approach to utilization of CT scans for detection of internal hernia in the gastric bypass patient. Surg Obes Relat Dis 2015;11:1207–11. 10.1016/j.soard.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 12. Lockhart ME, Tessler FN, Canon CL, et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am J Roentgenol 2007;188:745–50. 10.2214/AJR.06.0541 [DOI] [PubMed] [Google Scholar]
- 13. Goodman TR, Amurao M. Medical imaging radiation safety for the female patient: rationale and implementation. Radiographics 2012;32:1829–37. 10.1148/rg.326125508 [DOI] [PubMed] [Google Scholar]
- 14. Ahmed AR, Rickards G, Johnson J, et al. Radiological findings in symptomatic internal hernias after laparoscopic gastric bypass. Obes Surg 2009;19:1530–5. 10.1007/s11695-009-9956-x [DOI] [PubMed] [Google Scholar]
- 15. Oelsner G, Stockheim D, Soriano D, et al. Pregnancy outcome after laparoscopy or laparotomy in pregnancy. J Am Assoc Gynecol Laparosc 2003;10:200–4. 10.1016/S1074-3804(05)60299-X [DOI] [PubMed] [Google Scholar]
- 16. Soper NJ. SAGES' guidelines for diagnosis, treatment, and use of laparoscopy for surgical problems during pregnancy. Surg Endosc 2011;25:3477–8. 10.1007/s00464-011-1928-2 [DOI] [PubMed] [Google Scholar]
- 17. Brolin RE, Kella VN. Impact of complete mesenteric closure on small bowel obstruction and internal mesenteric hernia after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2013;9:850–4. 10.1016/j.soard.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 18. Ortega J, Cassinello N, Sánchez-Antúnez D, et al. Anatomical basis for the low incidence of internal hernia after a laparoscopic Roux-en-Y gastric bypass without mesenteric closure. Obes Surg 2013;23:1273–80. 10.1007/s11695-013-0902-6 [DOI] [PubMed] [Google Scholar]
- 19. Al Harakeh AB, Kallies KJ, Borgert AJ, et al. Bowel obstruction rates in antecolic/antegastric versus retrocolic/retrogastric Roux limb gastric bypass: a meta-analysis. Surg Obes Relat Dis 2016;12:194–8. 10.1016/j.soard.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 20. Rondelli F, Bugiantella W, Desio M, et al. Antecolic or retrocolic alimentary limb in laparoscopic Roux-en-Y gastric bypass? A meta-analysis. Obes Surg 2016;26:182–95. 10.1007/s11695-015-1918-x [DOI] [PubMed] [Google Scholar]