Abstract
Improved delivery of adeno-associated virus (AAV) vectors to the CNS will greatly enhance their clinical utility. Selection of AAV9 variants in a mouse model led to the isolation of a capsid called PHP.B, which resulted in remarkable transduction of the CNS following intravenous infusion. However, we now show here that this enhanced CNS tropism is restricted to the model in which it was selected, i.e., a Cre transgenic mouse in a C57BL/6J background, and was not found in nonhuman primates or the other commonly used mouse strain BALB/cJ. We also report the potential for serious acute toxicity in NHP after systemic administration of high dose of AAV.
Main Text
Identification of adeno-associated virus (AAV) capsids from natural isolates with CNS tropism has substantially enhanced the utility of this platform for basic studies of neurobiology and therapeutic applications of gene therapy of CNS diseases.1, 2, 3 The most widely used capsid for CNS applications has been AAV9, which we isolated from a human heart.3 A number of groups have demonstrated distribution of transduction following systemic delivery of AAV9 into heart, skeletal muscle, and the CNS.4, 5, 6 Clinical trials of systemically-delivered AAV9 are underway for targeting motor neurons of the spinal cord in patients with spinal muscular atrophy (ClinicalTrials.gov: NCT02122952) and in treating the neuropathic manifestations of the lysosomal storage disease Sanfilippo A (ClinicalTrials.gov: NCT02716246).
While AAV9 is unique compared to the many natural capsids that have been vectored in terms of CNS delivery, its efficiency is low even at very high systemic doses.7 Deverman et al.8 recently described an engineered variant of AAV9, called PHP.B, that exhibits much higher delivery to the CNS of mice following intravenous (i.v.) injection. This variant was isolated from a library of AAV9 capsids containing a randomized population of a 7 amino acid insert. A Cre transgenic line of C57BL/6J mice was used to select those capsid variants that efficiently trafficked to the CNS. PHP.B is being considered for the treatment of a wide range of CNS diseases.
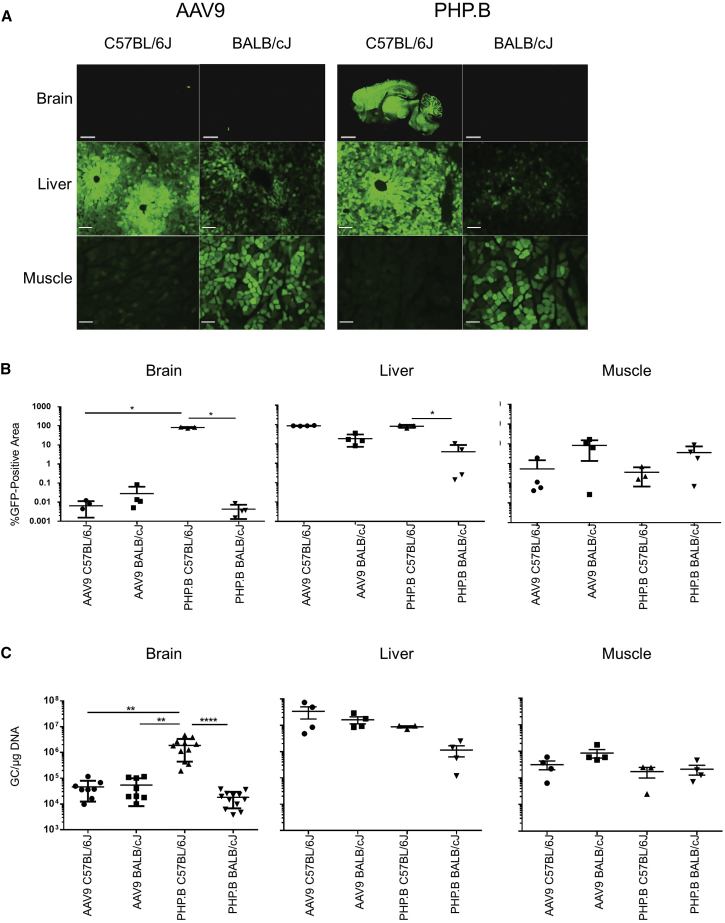
In an attempt to develop PHP.B for human gene therapy, we evaluated its efficacy and toxicity in nonhuman primates (NHPs). Prior to doing so, we confirmed the findings of Deverman et al.8 in C57BL/6J mice. Intravenous injection of 1E12 genome copies (GC) of PHP.B expressing GFP demonstrated remarkable transduction throughout the brain and the spinal cord, as measured by detection of GFP fluorescence, that was almost 4 logs higher than what was observed with AAV9 (Figures 1A and 1B; Figures S1 and S2). This high level of transduction was observed in multiple areas of the brain (Figure S2). Similar transduction was observed with both vectors in non-CNS tissues of C57BL/6J mice, such as liver and muscle (Figures 1A and 1B). Quantitation of vector genomes in tissue confirmed the substantially higher CNS gene transfer with PHP.B as described by Deverman et al.8 (Figure 1C). We were surprised, however, with the results of the same experiment conducted in BALB/cJ mice, which showed very low transduction and gene transfer in the CNS that was no different than what was observed with AAV9 (Figures 1A–1C; Figures S1 and S2). Transduction and gene transfer of PHP.B was slightly lower than AAV9 in liver and muscle (Figures 1A–1C). For both vectors, transduction of liver and CNS was higher in C57BL/6J than in BALB/cJ, whereas muscle transduction was higher in BALB/cJ (Figures 1A–1C).
Figure 1.
Comparison of AAV9- and PHP.B-Mediated Gene Transfer in Mice
(A) Direct GFP fluorescence in the brain, liver, and gastrocnemius muscle. Scale bars, 2 mm (brain) and 100 μm (liver, muscle). (B) GFP quantification of whole-slide scans. (C) Vector biodistribution in tissues. After systemic administration of 5E13 GC/kg to adult mice, PHP.B’s ability to target the brain with high efficiency is limited to the C57BL/6J strain. In BALB/cJ adult mice, AAV9 and PHP.B are equivalent and display low blood-brain-barrier crossing. PHP.B-mediated brain transduction is intermediate in CB6F1 hybrids. *p < 0.05; **p < 0.01; ****p < 0.0001. Error bars represent SD.
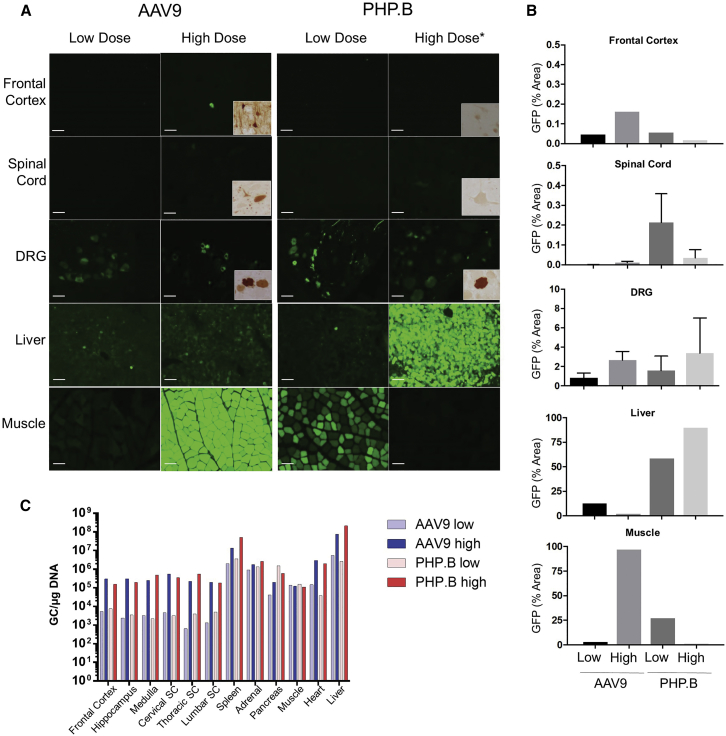
Despite the disappointing results obtained in BALB/cJ mice, we proceeded with pilot studies in NHPs (Table S1). Adult rhesus macaques (n = 1 per vector) that were negative for neutralizing antibodies to the respective capsids were injected with 2E13 GC/kg of the same GFP versions of AAV9 and PHP.B studied in mice; this represents a 2.5-fold lower vector per kg dose than we studied in mice, but it was also 4-fold higher than the low dose evaluated by Deverman et al.8 that showed high-level CNS transduction in C57BL/6J mice. Both NHPs tolerated the vector well, with only minor abnormalities in clinical pathology (data not shown); both animals were necropsied at the planned time of 21 days. GFP expression was detected by direct fluorescence of GFP and quantified in most non-CNS tissues, including liver and muscle (Figures 2A and 2B), kidney, pancreas, heart, spleen, and pituitary (Figure S3) at equivalent levels in the AAV9- and PHP.B-treated animals, except for in skeletal muscle, where transduction was higher for PHP.B. However, very low transduction was observed in the CNS based on GFP fluorescence, including in the frontal cortex and spinal cord (Figures 2A and 2B) and hippocampus and cerebellum (Figure S3). Interestingly, the dorsal root ganglia (DRG), which lie outside of the CNS, were transduced at equally high levels with both AAV9 and PHP.B than were cells within the CNS (Figures 2A and 2B). The quantity of vector genomes in various tissues paralleled GFP expression (Figure 2C). Notably, vector genomes were 3 logs lower in tissues of the spinal cord and brain than in liver and spleen, with no differences between AAV9 and PHP.B.
Figure 2.
Comparison of AAV9- and PHP.B-Mediated Gene Transfer in Nonhuman Primates
(A) Direct GFP fluorescence in the cortex, spinal cord, dorsal root ganglia (DRG), liver, and quadriceps muscle. Insets show GFP immunohistochemistry in high-dose animals. *Euthanized 5 days post injection; all others were euthanized 21 days post injection. Scale bars, 100 μm. (B) GFP quantification showing values for whole-slide scans (cortex, liver, muscle) or averages and SD from multiple images taken with a microscope (10 images for DRGs, 2 images for lumbar spinal cord). (C) Vector biodistribution in tissues. After systemic administration of 2E13 GC/kg (low dose) or 7.5E13 GC/kg (high dose) to adult rhesus macaques, both AAV9 and PHP.B vectors are equivalent with low to mild CNS targeting. Organs unprotected by the blood-brain barrier, such as dorsal root ganglia, liver, and muscles, are efficiently transduced. Note that GFP expression is not directly comparable in the PHP.B high-dose monkey that was euthanized 5 days post injection due to vector-related clinical condition. Vector biodistribution is similar for dose-matched AAV9 and PHP.B vectors, showing that both vectors share the same tropism and properties in NHP.
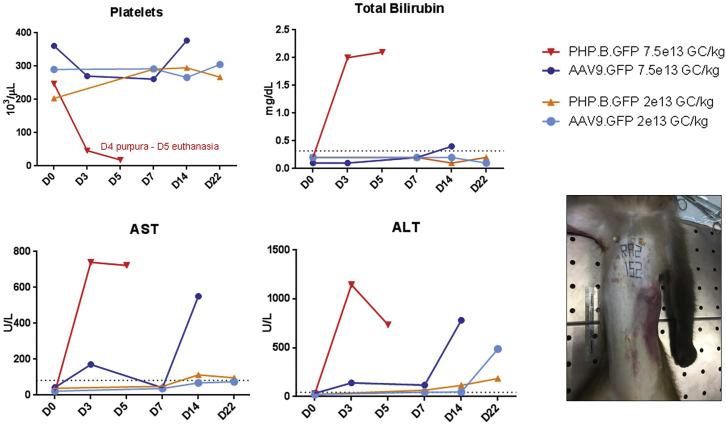
Having demonstrated that both vectors were safe at 2E13 GC/kg, we injected animals (n = 1 per group) at a higher dose of 7.5E13 GC/kg. The animal injected with PHP.B vector developed marked elevations of serum transaminases along with profound thrombocytopenia and diffuse bleeding, which required euthanasia on day 5 (Figure 3; Table S2). Pathology at necropsy was consistent with diffuse hemorrhage (Figure S4). The AAV9-injected animal tolerated the vector well without clinical sequelae or laboratory abnormalities except biphasic elevations in transaminases, which were higher on day 14, presumably due to a T cell response to GFP (Figure 3; Table S2). Tissues were harvested on day 21 as planned and showed mild mononuclear cell infiltrates (Figure S5). A more thorough description of the clinical and laboratory abnormalities observed at high doses of vector can be found in the Supplemental Information.
Figure 3.
Toxicity Event in the High-Dose-Treated PHP.B NHP
Platelet counts, total bilirubin, transaminase levels, and gross picture of the cutaneous petechiae and bruises on the thorax and abdomen of the NHP treated with 7.5E13 GC/kg of PHP.B-GFP vector. The toxicity event was characterized by thrombocytopenia, hyperbilirubinemia, and increased transaminases on day 3 and day 5 post vector administration. Massive subcutaneous and internal bleeding led to humane euthanasia of the animal on day 5.
The development of pathology in the PHP.B-treated animal and the different necropsy times make it difficult to compare the two high-dose animals in terms of transduction efficiency, although the amount of vector genomes measured in tissues were the same between the two animals and 2 to 3 logs higher in spleen and liver than in brain and spinal cord (Figure 2C). Very low to undetectable transduction, as measured by GFP fluorescence, was observed in CNS tissues, including frontal cortex and spinal cord (Figures 2A and 2B) and hippocampus and cerebellum (Figure S3). Like the low-dose animals, both high-dose animals demonstrated GFP expression in DRG (Figures 2A and 2B; Figure S6). Brain tissues from high-dose animals were also analyzed for GFP protein by immunohistochemistry, which confirmed the low transduction achieved with both vectors (high magnification insets in Figures 2 and S3 and low magnification micrographs in Figure S6). Expression of GFP outside of the CNS was high, with substantial differences between the two vectors possibly reflecting the different times of necropsy and the influence of CTLs to transduce cells in the AAV9-treated animal that was necropsied at day 22 (Figures 2A and 2B; Figure S3). The level of transduction of skeletal muscle and heart achieved with the high-dose AAV9 vector was impressive, supporting its use in primary muscle diseases (Figures 2A and 2B; Figure S3). It is interesting that the amount of vector genomes was similar between the CNS and muscle in the AAV9 animal, although expression of GFP was logs higher in muscle.
We have never observed such a profound difference in AAV vector performance between two strains of mice. In vivo transduction of vectors based on natural isolates of AAV have generally tracked between strains of mice and different species, including larger animals, with the notable exception of liver, where mice are more efficiently transduced than primates.9, 10 The lack of translation from mice to NHPs and to humans is often explained by stronger immune responses in primates. Another team recently reported the lack of significant blood-brain barrier (BBB) penetration of PHP.B vector in a smaller NHP, the marmoset.11 We do not think that stronger cell-mediated response in BALBc/J mice can explain the lack of GFP expression in the brain, as the liver was efficiently transduced and C57BL/6 and BALB/c mice are prototypical Th1- and Th2-type mouse strains, respectively, accounting for weak T cell responses in BALB/c.12 The apparent restriction of high-CNS transduction with PHP.B to C57BL/6J mice may reflect the fact that it was selected for high delivery to the CNS in this strain of mice. This remarkable strain-specific difference in vector delivery to the CNS may facilitate the identification of factors that enhance CNS delivery.
We report an unprecedented acute toxicity event leading to the euthanasia of an NHP 5 days after injection. Clinical pathology, gross findings, and histology were compatible with acute tissue injury and systemic activation of innate immunity with endothelial cells and/or platelet activation, leading to consumption thrombocytopenia and secondary hemorrhages. While the event reported here has to be interpreted with caution due to the limited number of animals, the acute timing (within days after injection) suggests a direct AAV-mediated toxicity when administered i.v. at high doses that is different in timing, quality, and severity to the liver toxicity previously reported in systemic human AAV studies. In the systemic human AAV studies, the liver toxicity is believed to be caused by adaptive immune responses to the capsid or the transgene product. Additional NHP studies are needed to determinate the precise pathological and molecular mechanisms involved as well as the potential implication for clinical trials using comparable doses of systemic vectors. In the meantime, it seems prudent for sponsors to monitor acute manifestations of innate immunity, such as thrombocytopenia, in clinical trials involving high dose systemic AAV.
The low CNS transduction of PHP.B at doses of vector associated with dose-limiting toxicity in NHPs will limit its utility in treating human diseases beyond that which could be achieved with AAV9.
Author Contributions
J.H. designed and conducted mice and nonhuman primate studies and interpreted all the data. Q.W. produced the initial AAV-PHP.B vector and validated it in vitro and in vivo. N.K. conducted the initial experiment in BALB/cJ mice. E.L.B. analyzed histopathological and clinical data in nonhuman primates. P.B. supervised and analyzed GFP expression and quantification. J.M.W. conceived the studies, was responsible for research coordination and strategy, and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
J.M.W. is an advisor to, a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO; he is a former consultant to and holds stock in Solid Biosciences; he also has a sponsored research agreement with Ultragenyx; in addition, he is a consultant to several biopharmaceutical companies. J.M.W. is an inventor on patents licensed to various biopharmaceutical companies.
Acknowledgments
We thank Cecilia Dyer, Mohamad Nayal, Vanitha Ramachandran, Tahsin Jahan, Liying Han, and Miao Jiang for invaluable technical assistance as well as the Program in Comparative Medicine and the Nonhuman Primate Research Program of the Gene Therapy Program at the University of Pennsylvania and the Human Immunology Core (P30-CA016520) for study support. Vectors were produced by the Penn Vector Core. We also thank Jennifer Stewart for editorial assistance with this manuscript.
Footnotes
Supplemental Information includes six figures, two tables, a clinical case description, and Supplemental Materials and Methods and can be found with article online at https://doi.org/10.1016/j.ymthe.2018.01.018.
Supplemental Information
References
- 1.Gao G., Vandenberghe L.H., Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 2.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duque S., Joussemet B., Riviere C., Marais T., Dubreil L., Douar A.M., Fyfe J., Moullier P., Colle M.A., Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacak C.A., Mah C.S., Thattaliyath B.D., Conlon T.J., Lewis M.A., Cloutier D.E., Zolotukhin I., Tarantal A.F., Byrne B.J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 6.Gray S.J., Matagne V., Bachaboina L., Yadav S., Ojeda S.R., Samulski R.J. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevan A.K., Duque S., Foust K.D., Morales P.R., Braun L., Schmelzer L., Chan C.M., McCrate M., Chicoine L.G., Coley B.D. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W.L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Calcedo R., Wang H., Bell P., Grant R., Vandenberghe L.H., Sanmiguel J., Morizono H., Batshaw M.L., Wilson J.M. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Bell P., Somanathan S., Wang Q., He Z., Yu H., McMenamin D., Goode T., Calcedo R., Wilson J.M. Comparative Study of Liver Gene Transfer With AAV Vectors Based on Natural and Engineered AAV Capsids. Mol. Ther. 2015;23:1877–1887. doi: 10.1038/mt.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzaki Y., Konno A., Mochizuki R., Shinohara Y., Nitta K., Okada Y., Hirai H. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 2017;665:182–188. doi: 10.1016/j.neulet.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Mills, C.D., Kincaid, K., Alt, J.M., Heilman, M.J., and Hill, A.M. (2000). M-1/M-2 macrophages and the [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.