1. Introduction
This Topical Review considers the misalignment between outcome measures traditionally reported in animal models of neuropathic pain* and those employed for estimating pain intensity and the impact/burden of pain in clinical trials. In particular, we propose that traditional methods of assessing rodent sensory thresholds could have predictive utility for the sensory profiling approaches being explored for patient stratification in clinical trials. To initiate this process we propose a “research agenda” to develop and validate a protocol and normative values for sensory profiling in rodents which reflects the best established clinical methods. This could then be used to establish definitive sensory profiles of new and existing rodent neuropathic pain models.
In general, animal modelling of neuropathic pain has two main goals: Firstly, to identify pain mechanisms and thus potential targets for drug development. However, it is difficult to identify clear examples of the success of this approach in delivering new drugs for neuropathic pain, with the exception of high concentration topical capsaicin[20]. Secondly, animal models are used in an attempt to predict the clinical efficacy of a novel therapeutic and thus justify the initiation of clinical trials. We concentrate on the latter aspect and ask whether the drug response associated with specific sensory profiles in animal models might predict the most appropriate patients to examine in exploratory clinical trials?
2. The problem of homogeneity in current animal models of neuropathic pain
To date, animal modelling of neuropathic pain has been dominated by homogeneity in both the models created and outcomes (behavioural constructs) measured in those models. The predominant animal model reported is of traumatic injury to a rodent sciatic nerve. The predominant “pain” outcome measure is limb withdrawal evoked by applied sensory stimuli. (In passing, we note that experiments are usually conducted in genetically similar animals; although we do recognise that such homogeneity may be of relevance in the specific context of animal genetic studies. There has also been a tradition of homogeneity of sex and age [33], with the use of young male animals predominating). In contrast to the homogeneity of animal modelling, there is emerging evidence of the importance of clinical heterogeneity in neuropathic pain in terms of underlying disease, clinical presentations, pain mechanisms and treatment responses at the individual patient level. Recognising such heterogeneity is fundamental to developing the concept of precision (personalised) medicine for neuropathic pain[7]. Therefore, we argue that refinement of pre-clinical methods is required to achieve alignment with emerging concepts of clinical heterogeneity.
3. Potential biomarkers to reveal clinical heterogeneity in neuropathic pain
There are multiple potential biomarkers that might be hypothesised to predict efficacy of analgesic interventions in neuropathic pain at the individual patient level[54]. One example is the prediction of duloxetine efficacy in diabetic neuropathy by measuring endogenous pain modulation[72]. Others include symptom and epidermal innervation profiling[3], testing nociceptor function with capsaicin[12] and even ascertaining the characteristics of patient-derived stem cells[13]. However, here we focus on aligning sensory measurements made in animal models with current methods of clinical sensory assessment[4; 17; 18; 25]. In this context, we argue that the traditional evoked limb withdrawal outcome measures used in animal models of neuropathic pain should be redeployed as sensory profiling tools. We submit that their use should henceforth be interpreted not as a measure of “pain”, but rather as a sensory profile biomarker of that injury or disease model.
4. Neuropathic pain, associated sensory abnormalities and animal models
Neuropathic pain clinically manifests as spontaneous pain (ongoing or paroxysmal) and/or, more uncommonly, evoked pain. Neuropathic pain is usually accompanied by sensory abnormalities which are broadly categorised into modality-specific sensory gain (allodynia, hyperpathia and/or hyperalgesia) or sensory loss (anaesthesia dolorosa). Evoked limb withdrawal measures conventionally reported from animal model experiments reflect only the small group of patients characterised by sensory gain and then only the evoked pain component. Furthermore, these measures resemble a spinal cord reflex activation ignoring all other pain-related phenomena in the neuraxis. There are disparities between simple reflex withdrawal and operant escape responses which measure the cerebral-dependent component of the response in normal animals. In animals with traumatic nerve injury operant behaviours are more consistent with evidence from humans than evoked measures[59; 60]. Hyperpathia is not generally reported for animal models, possibly because of the inherent difficulty of quantifying an explosive pain response as opposed to a simple threshold[27].
5. The proposition
We advocate that animal models should not only be classified by the lesion or disease of the somatosensory system which they purport to reflect, but also by a phenotype-based classification defined by sensory profile. This will align animal modelling with the cross-disease sensory heterogeneity of sensory profiles in patients with neuropathic pain[7; 62] and the associated stratification approaches implicit in precision medicine[6]. Mitchell Max was amongst the first to hypothesise that the efficacy of neuropathic pain treatments might be predicted using measurable characteristics[40]. It was later reported that patients with postherpetic neuralgia could be stratified on the basis of three broad sensory profiles which likely reflect distinct pain mechanisms[19]. Using large multi-aetiology clinical cohorts we have shown that most neuropathic pain patients can be classified, irrespective of underlying disease, into one of these three distinct sensory subgroups[7; 62]. These are strikingly similar to those originally reported in postherpetic neuralgia[19] (Figure 1). There are now encouraging examples of how such sensory profiles can represent heterogeneous pharmacologically tractable pain mechanisms[3; 12; 15; 38; 53]. However, a caveat is that this effect was reported to have only limited usefulness in a retrospective analysis of data from clinical trials of painful polyneuropathy[29]. A strengthening of the clinical evidence is required before definitive statements can be made about the value and robustness of sensory biomarkers for precision pain medicine. Nevertheless, as clinical medicine moves towards stratification of patients based on individual sensory characteristics, we posit that animal modelling be refined to inform that approach. This, in turn, could predict the logical sensory profile to recruit in a sensory profile-stratified clinical trial.
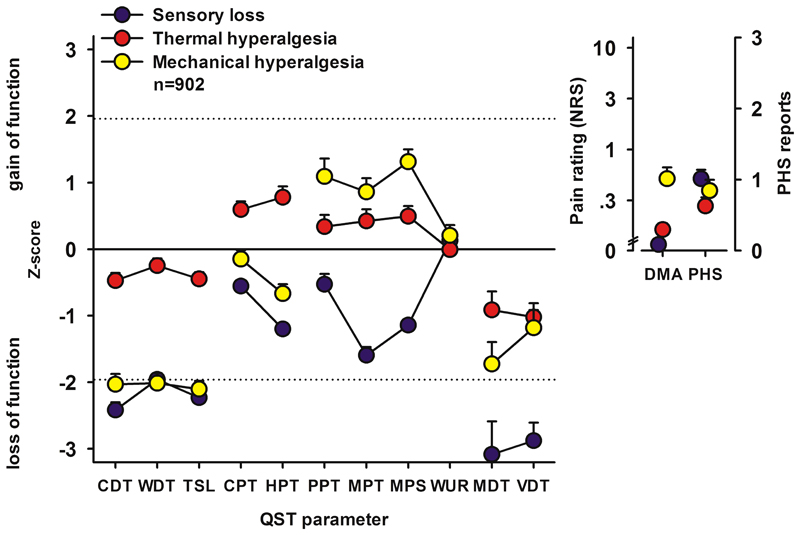
Figure 1.
The three major subgroups of sensory profiles reported in neuropathic pain patients across a range of underlying diseases. Positive z scores indicate positive sensory signs (hyperalgesia), whereas negative z values indicate negative sensory signs (hypoaesthesia and hypoalgesia). Dashed lines: 95% confidence interval for healthy subjects (−1.96 < z < +1.96). The inset graphs represent the reported pain intensity on a 0-10 numerical rating scale for dynamic mechanical allodynia and the number of episodes of paradoxical heat sensations reported during cooling stimulation, respectively. Blue symbols: cluster 1 “sensory loss” (42%) are distinguished by a loss of small and large fiber function in combination with paradoxical heat sensations. Red symbols: cluster 2 “thermal hyperalgesia” (33%) is characterized by preserved sensory functions in combination with heat and cold hyperalgesia and mild dynamic mechanical allodynia. Yellow symbols: cluster 3 “mechanical hyperalgesia” (24%) which is characterized by a loss of small fiber function in combination with pinprick hyperalgesia and dynamic mechanical allodynia.
CDT, cold detection threshold; CPT, cold pain threshold; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio; DMA, dynamic mechanical allodynia; PHS, paradoxical heat sensations.
Adapted with permission from Baron R, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. PAIN 2017;158(2):261-272.
6. Evolution and current status of animal models of neuropathic pain
In one of the first reports of an animal model of neuropathic pain (sciatic nerve axotomy), the importance of reflecting pain occurring in the context of the sensory loss was recognised[64; 65]. This sensory loss profile is reported in the majority of patients with common polyneuropathies for example: diabetic[47; 55], chemotherapy-induced[58] and HIV-associated[46] polyneuropathies, rarer conditions such as non-freezing cold injury[56] and in some patients with spinal cord injury[21] and postherpetic neuralgia[19]. Wall and colleagues also recognised that to assess the impact of pain in such a model required the measurement of complex behavioural paradigms; although the autotomy measure they described is no longer in widespread use for ethical reasons and because of uncertainty about exactly which sensory construct it reflects[64].
Later, the animal modelling of neuropathic pain was refined by a sciatic nerve constriction method[8]. This partial nerve trauma approach has, with many subtle variations, since dominated the literature. Partial nerve trauma models are generally characterised by an ipsilateral gain in sensory function measured as hypersensitivity of reflex limb withdrawal evoked by mechanical and sometimes heat or cold stimuli[8]. This perhaps reflects the mechano- or thermal sensory profile of allodynia observed in some, but by no means all, patients with neuropathic pain, notably some of those with peripheral nerve injury, Complex Regional Pain Syndrome, inherited channelopathies, postherpetic neuralgia, some aspects of pain after spinal cord injury and the mixed picture reported in epidermolysis bullosa[13; 16; 21; 23; 37; 45; 63]. An important caveat is that sometimes patients can report symptoms of sensory gain, such as allodynia, but it can be difficult to show the corresponding signs by cutaneous quantitative sensory testing in a circumscribed test area[61].
The reporting of sensory gain outcome measures in animal models has become ubiquitous. They are usually, and we suggest incorrectly, described in terms of a “pain” outcome measure. In an ongoing systematic review of animal models of drug-induced neuropathy, 348 (95.6%) of publications report such outcomes (data on file). The reason for the historical focus on such measures might be that they appear robust, reliable and relatively easy to consistently measure[60]. However, is this a case of measuring what can be measured and not what should be measured? We argue that these measures of sensory gain remain useful, but should be primarily interpreted as reflecting one modality of the model´s global sensory profile and not as implying the presence of non-evoked pain for predicting the efficacy of putative therapeutic interventions.
For many years the neuropathic pain animal model literature has been dominated by traumatic nerve injury (~66% of publications from our ongoing animal models meta-analysis– protocol at www.dcn.ed.ac.uk/camarades/research.html#protocols); a condition examined in only ~8% of clinical trials; the majority of which recruit patients with postherpetic neuralgia or polyneuropathy[20]. Traumatic peripheral nerve injury patients are most often characterised by a loss of sensitivity to non-painful stimuli and moderate gain in sensitivity to painful stimuli[23]. However, recently other relevant disease models have been developed and validated, notable examples being those related to drug-induced neuropathy and diabetic and HIV-associated neuropathy and herpes zoster infection[2; 11; 22; 26; 28; 31; 32; 66; 67]. Establishing a portfolio of diverse disease–based animal models affords the opportunity not only to document a range of sensory profiles, but also to explore heterogeneity in the pathophysiological responses to nerve damage. Examples from our own work reveal that features consistently observed in animal models of traumatic nerve injury are not necessarily prominent in other conditions. These include changes in dorsal root ganglion gene and protein expression[10; 39], spinal cord microgliosis[9] and pharmacological responses[26; 66].
7. The problem of opposing sensory profiles in animal models and patients
Another difficulty with some existing animal models is that the sensory gain reported[31; 35; 66; 67] is the precise opposite of the sensory loss profile described in the corresponding disease,; for example, HIV-associated or diabetic polyneuropathy[46; 47; 55]. This could be related to the fact that the animal models reflect the early initiation phase (up to 28 days) of the neuropathy when sensory gain could conceivably be a clinical feature; whereas the clinical profiles were reported in patients with an established neuropathy, often of several years standing. It is conceivable that if such animal models were followed up for extended periods then the sensory profile could switch to sensory loss, as has been demonstrated for diabetic neuropathy[11]. It has also been shown that following traumatic nerve injury in animals, alterations in operant behaviours persist beyond the resolution of enhanced limb withdrawal reflexes evoked by sensory stimuli[60]. Another explanation could possibly be that animals with a sensory loss profile are indeed observed in such experiments, but through reporting bias are excluded from the analysis as “outliers” or “non-responders” which did not show sensory gain[30]. It is important that long-term behavioural and other biological outcomes are assessed across multiple time points in both animal models and in patients participating in clinical research.
8. Developing a portfolio of non-evoked pain-related outcome measures for use in rodents
Although evoked limb withdrawal measures will retain utility in the specific scenario of sensory gain, rebadging their use as profiling tools will require alternative measures of the impact of pain in rodents to be developed. Self-evidently, such measures will also be required for assessment of pain in the proposed rodent models of neuropathic pain in the context of a sensory loss profile. This research-active area is now reaching sufficient maturity for this to be plausible – several groups have risen to this challenge and are developing complex behavioural constructs that crucially do not require an evoked response, for example[41; 44; 48; 49]. In doing so it is important to understand the ethological response to pain in a prey species and to avoid anthropomorphising human emotions to rodents[5; 57]. To illustrate this approach we have, for example, shown that animals with purportedly painful surgical, viral or drug induced neuropathies and inflammation show a pharmacologically sensitive increase in a predator avoidance behavioural construct (thigmotaxis)[26; 31; 32; 42; 66–69]. Similarly, an innate rodent social behaviour (burrowing) is pharmacologically-sensitive in pain models[1; 14; 24; 34; 43; 51; 52; 70]. Another approach not requiring evoked responses is pharmacologically induced place preference[36] It will be important to demonstrate that such measures are robust, reliable and reproducible across centres and have face, construct and pharmacological validity. This is not a trivial task and to facilitate this we have demonstrated the value of a prospective multicentre validation approach[71] and advocated the open publication of individual animal level data for further analysis[42].
9. Conclusions
We propose classifying animal models of neuropathic pain by sensory profile. This presents major challenges which will require a collaborative approach. We suggest prioritising the following key aspects of a research agenda to address these:
The development and validation of a range of stable, reproducible, ethologically–relevant, and pharmacologically tractable behavioural constructs which can be used to assess the impact of pain, especially in those models characterised by a sensory loss profile[43]. In all likelihood, such behavioural paradigms will reflect the general wellbeing of an animal rather than be pain specific (and thus akin to “global impression” measures used in humans). Their pain relevance in any particular situation could be revealed by pharmacological validation using responses to known analgesic interventions. There may also be scope for cross fertilisation with inflammatory pain research, if it can be shown that behavioural findings are consistently replicated between neuropathic and inflammatory pain models because in the latter the presence of ongoing pain can be more reasonably purported[41; 72]. Clearly, developing and validating pain related outcome measures reflecting neuropathic pain in the specific context of sensory loss could also benefit from additional surrogate non-behavioural techniques such as electrophysiology and in vivo imaging.
The development and validation of multimodal sensory profiling protocols and normative data for use in rodents. These can then be used to establish the definitive sensory profiles of new and existing models of neuropathic pain, including models characterised by sensory loss. Presently the sensory profile of animal models is often only reported by evoking limb withdrawal to one or two sensory modalities, yet clinical research shows the importance of pan-sensory modality profiling[7; 62], especially in conditions such as postherpetic neuralgia and epidermolysis bullosa where differential responses to various sensory modalities are observed within the same patient[19; 63]. The best validated multimodal clinical sensory profiling protocol is that developed by the German Neuropathic Pain Network (DFNS)[50] which was used to define sensory subgroups[7; 62] and demonstrate the heterogeneity of pharmacological responses[15]. To facilitate this discussion, we have suggested (Table 1) aspects of the DFNS Quantitative Sensory Testing protocol and associated measures that might conceivably be deployed for sensory profiling in animals.
The development and validation of a portfolio of animal models which accurately reflect at least the three major sensory profiles reported in neuropathic pain patients including, crucially, aspects of sensory loss[8; 60].
Table 1.
Key parameters of the German Network on Neuropathic Pain (DFNS) protocol for Quantitative Sensory Testing[47].
| Sensory Stimulus | Fibre Type | Putative Animal Model Correlate | Example of Behavioural Method |
|---|---|---|---|
| - | - | ||
| Cold Detection Threshold (CDT) | Aδ | Electrophysiological surrogate? | |
| Warm Detection Threshold (WDT) | C | Electrophysiological surrogate? | |
| Thermal Sensory Limen (TSL) & Paradoxical Heat Sensation | Aδ & C | ? | |
| Cold Pain Threshold (CPT) | Aδ? C? | Established - behavioural | Plantar cold assay (Brenner)1 |
| Heat Pain Threshold (HPT) | Aδ? C? | Established - behavioural | Plantar radiant heat assay (Hargreaves)2 |
| Mechanical Detection Threshold (MDT) | Aβ | Electrophysiological surrogate? | |
| Mechanical Pain Threshold (MPT) | Aδ | Established - behavioural | |
| Mechanical Pain Sensitivity (MPS) | Aδ Stimulus - Response | Electrophysiological surrogate? Behavioural -possible, needs development | Monofilament (von Frey) or pinprick |
| Dynamic Mechanical Allodynia (ALL) | Aβ Stimulus - Response | Electrophysiological surrogate? Behavioural -possible, needs development | Brush-evoked limb withdrawal3 |
| Windup Ratio (WUR) | Aδ or C Temporal Summation | Electrophysiological surrogate | |
| Vibration Detection Threshold (VDT) | Aβ | Electrophysiological surrogate? | |
| Pressure Pain Threshold (PPT) | Aδ? C? | Established - behavioural | Randall-Sellito device4 |
| Additional items | |||
| Hyperpathia | ? | Electrophysiological surrogate? | |
| Conditioned Pain Modulation | Diffuse Noxious Inhibitory Control | Electrophysiological surrogate |
Brenner DS, Golden JP, Gereau RWIV. A novel behavioral assay for measuring cold sensation in mice. PLOS ONE 2012;7(6):e39765.
Field MJ, Bramwell S, Hughes J, Singh L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: are they signalled by distinct primary sensory neurones? Pain 1999;83(2):303-311.
Hargreaves KM, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;32(1):77-88.
Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Archives internationales de pharmacodynamie et de therapie 1957;111(4):409-419.
Column three indicates the correlate for each parameter that could be measured in an animal model, and the current status thereof. For the purpose of this review, additional domains representing hyperpathia and conditioned pain modulation have been added.
Footnotes
For brevity we will adhere to convention and use the shorthand “animal model of neuropathic pain”. However, we suggest that a more accurate classification is in terms of the disease they purportedly mimic (e.g. traumatic nerve injury, diabetic neuropathy etc) rather than as a model of “pain”.
The authors report no conflict of interest directly relevant to this review
References
- [1].Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice ASC. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. European Journal of Pain. 2012;16(4):485–495. doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- [2].Apfel SC, Lipton RB, Arezzo JC, Kessler JA. Nerve growth factor prevents toxic neuropathy in mice. Ann Neurol. 1991;29(1):87–90. doi: 10.1002/ana.410290115. [DOI] [PubMed] [Google Scholar]
- [3].Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, Raicher I, Üçeyler N, Sommer C, Bouhassira D. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. The Lancet Neurology. 2016;15(6):555–565. doi: 10.1016/S1474-4422(16)00017-X. [DOI] [PubMed] [Google Scholar]
- [4].Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154(9):1807–1819. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- [5].Barnett SA, Whilshaw IQ, Kolb B. The behaviour of the laboratory rat. Oxford: Oxford University Press; 2006. [Google Scholar]
- [6].Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11(11):999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- [7].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- [9].Blackbeard J, Wallace VC, O'Dea KP, Hasnie F, Segerdahl A, Pheby T, Field MJ, Takata M, Rice AS. The correlation between pain-related behaviour and spinal microgliosis in four distinct models of peripheral neuropathy. Eur J Pain. 2012;16(10):1357–1367. doi: 10.1002/j.1532-2149.2012.00140.x. [DOI] [PubMed] [Google Scholar]
- [10].Boateng EK, Novejarque A, Pheby T, Rice AS, Huang W. Heterogeneous responses of dorsal root ganglion neurons in neuropathies induced by peripheral nerve trauma and the antiretroviral drug stavudine. Eur J Pain. 2015;19(2):236–245. doi: 10.1002/ejp.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Calcutt NA, Freshwater JD, Mizisin AP. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia. 2004;47(4):718–724. doi: 10.1007/s00125-004-1354-2. [DOI] [PubMed] [Google Scholar]
- [12].Campbell CM, Kipnes MS, Stouch BC, Brady KL, Kelly M, Schmidt WK, Petersen KL, Rowbotham MC, Campbell JN. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153(9):1815–1823. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao L, McDonnell A, Nitzsche A, Alexandrou A, Saintot PP, Loucif AJ, Brown AR, Young G, Mis M, Randall A, Waxman SG, et al. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med. 2016;8(335):335–356. doi: 10.1126/scitranslmed.aad7653. [DOI] [PubMed] [Google Scholar]
- [14].Deacon RMJ. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. NatProtocols. 2006;1(1):118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- [15].Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155:2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- [16].Dib-Hajj SD, Rush AM, Cummins TR, Hisama FM, Novella S, Tyrrell L, Marshall L, Waxman SG. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain. 2005;128(Pt 8):1847–1854. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- [17].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- [18].Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157(9):1851–1871. doi: 10.1097/j.pain.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fields HL, Rowbotham MC, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. NeurobiolDis. 1998;5(4):209–227. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- [20].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW, Jensen TS. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126(Pt 1):57–70. doi: 10.1093/brain/awg007. [DOI] [PubMed] [Google Scholar]
- [22].Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, Nash AA, Dalziel RG. Behavioural changes in the rat following infection with varicella zoster virus. J Gen Virol. 1999;80(9):2433–2436. doi: 10.1099/0022-1317-80-9-2433. [DOI] [PubMed] [Google Scholar]
- [23].Gierthmuhlen J, Maier C, Baron R, Tolle T, Treede RD, Birbaumer N, Huge V, Koroschetz J, Krumova EK, Lauchart M, Maihofner C, et al. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain. 2012;153(4):765–774. doi: 10.1016/j.pain.2011.11.009. [DOI] [PubMed] [Google Scholar]
- [24].Gould SA, Doods H, Lamla T, Pekcec A. Pharmacological characterization of intraplantar Complete Freund's Adjuvant-induced burrowing deficits. Behavioural Brain Research. 2016;301:142–151. doi: 10.1016/j.bbr.2015.12.019. [DOI] [PubMed] [Google Scholar]
- [25].Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- [26].Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington PR, Dickenson AH, Pheby T, Rice ASC. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144(4):1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Helme R, Finnerup NB, Jensen TS. Hyperpathia: "To be or not to be: that is the question". Pain. 2018 doi: 10.1097/j.pain.0000000000001149. in press. [DOI] [PubMed] [Google Scholar]
- [28].Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116(1):29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- [29].Holbech JV, Bach FW, Finnerup NB, Jensen TS, Sindrup SH. Pain phenotype as a predictor for drug response in painful polyneuropathy-a retrospective analysis of data from controlled clinical trials. Pain. 2016;157(6):1305–1313. doi: 10.1097/j.pain.0000000000000563. [DOI] [PubMed] [Google Scholar]
- [30].Holman C, Piper SK, Grittner U, Diamantaras AA, Kimmelman J, Siegerink B, Dirnagl U. Where Have All the Rodents Gone? The Effects of Attrition in Experimental Research on Cancer and Stroke. PLoS Biol. 2016;14(1):e1002331. doi: 10.1371/journal.pbio.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang W, Calvo M, Karu K, Olausen HR, Bathgate G, Okuse K, Bennett DLH, Rice ASC. A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain. 2013;154(4):560–575. doi: 10.1016/j.pain.2012.12.023. [DOI] [PubMed] [Google Scholar]
- [32].Huang W, Calvo M, Pheby T, Bennett DLH, Rice ASC. A rodent model of HIV protease inhibitor indinavir induced peripheral neuropathy. Pain. 2017;158(1):75–85. doi: 10.1097/j.pain.0000000000000727. [DOI] [PubMed] [Google Scholar]
- [33].Jackson SJ, Andrews N, Ball D, Bellantuono I, Gray J, Hachoumi L, Holmes A, Latcham J, Petrie A, Potter P, Rice ASC, et al. Does age matter? The impact of rodent age on study outcomes. Lab Anim. 2017;51(2):160–169. doi: 10.1177/0023677216653984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165. doi: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jolivalt CG, Frizzi KE, Guernsey L, Marquez A, Ochoa J, Rodriguez M, Calcutt NA. Current Protocols in Mouse Biology. John Wiley & Sons, Inc.; 2011. Peripheral Neuropathy in Mouse Models of Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maier C, Baron R, Toelle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [38].Mainka T, Malewicz NM, Baron R, Enax-Krumova EK, Treede RD, Maier C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur J Pain. 2016;20(1):116–129. doi: 10.1002/ejp.703. [DOI] [PubMed] [Google Scholar]
- [39].Maratou K, Wallace VC, Hasnie FS, Okuse K, Hosseini R, Jina N, Blackbeard J, Pheby T, Orengo C, Dickenson AH, McMahon SB, et al. Comparison of dorsal root ganglion gene expression in rat models of traumatic and HIV-associated neuropathic pain. Eur J Pain. 2009;13(387):398. doi: 10.1016/j.ejpain.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Max MB. Towards physiologically based treatment of patients with neuropathic pain. Pain. 1990;42(2):131–137. doi: 10.1016/0304-3959(90)91156-D. [DOI] [PubMed] [Google Scholar]
- [41].Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- [42].Morland RH, Novejarque A, Huang W, Wodarski R, Denk F, Dawes JD, Pheby T, McMahon SB, Rice AS. Short-term effect of acute and repeated urinary bladder inflammation on thigmotactic behaviour in the laboratory rat. F1000Res. 2015;4:109. doi: 10.12688/f1000research.6255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Muralidharan A, Kuo A, Jacob M, Lourdesamy JS, Carvalho LM, Nicholson JR, Corradini L, Smith MT. Comparison of Burrowing and Stimuli-Evoked Pain Behaviors as End-Points in Rat Models of Inflammatory Pain and Peripheral Neuropathic Pain. Front Behav Neurosci. 2016;10:88. doi: 10.3389/fnbeh.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Percie du Sert N, Rice AS. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014;171(12):2951–2963. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pfau DB, Krumova EK, Treede RD, Baron R, Toelle T, Birklein F, Eich W, Geber C, Gerhardt A, Weiss T, Magerl W, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain. 2014;155(5):1002–1015. doi: 10.1016/j.pain.2014.02.004. [DOI] [PubMed] [Google Scholar]
- [46].Phillips TJC, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, Williams ACdC, Orengo C, Bennett DLH, Bodi I, Cox S, Maier C, et al. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: A cross-sectional deep profiling study. Pain. 2014;155(9):1846–1860. doi: 10.1016/j.pain.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Raputova J, Srotova I, Vlckova E, Sommer C, Üçeyler N, Birklein F, Rittner HL, Rebhorn C, Adamova B, Kovalova I, Kralickova Nekvapilova E, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. PAIN. 2017 doi: 10.1097/j.pain.0000000000001034. Articles in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rice ASC. Predicting Analgesic Efficacy from Animal Models of Peripheral Neuropathy and Nerve Injury: A Critical View from the Clinic. In: Mogil JS, editor. Pain 2010-An Updated Review: Refresher Course Syllabus. Seattle: IASP Press; 2010. pp. 415–426. [Google Scholar]
- [49].Rice ASC, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: A critical appraisal and call for uniform reporting standards. Pain. 2008;139(2):243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- [50].Rolke R, Baron R, Maier C, Toelle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- [51].Rutten K, Robens A, Read SJ, Christoph T. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18(2):213–222. doi: 10.1002/j.1532-2149.2013.00359.x. [DOI] [PubMed] [Google Scholar]
- [52].Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, Christoph T. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. European Journal of Pain. 2014;18(2):204–212. doi: 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- [53].Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R. Pregabalin for painful HIV neuropathy: A randomized, double-blind, placebo-controlled trial. Neurology. 2010;74(5):413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smith SM, Dworkin RH, Turk DC, Baron R, Polydefkis M, Tracey I, Borsook D, Edwards RR, Harris RE, Wager TD, Arendt-Nielsen L, et al. The Potential Role of Sensory Testing, Skin Biopsy, and Functional Brain Imaging as Biomarkers in Chronic Pain Clinical Trials: IMMPACT Considerations. J Pain. 2017;18(7):757–777. doi: 10.1016/j.jpain.2017.02.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice ASC, Bennett DLH. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132–1145. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vale TA, Symmonds M, Polydefkis M, Byrnes K, Rice ASC, Themistocleous AC, Bennett DLH. Chronic non-freezing cold injury results in neuropathic pain due to a sensory neuropathy. Brain. 2017;140:2557–2569. doi: 10.1093/brain/awx215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vasconcelos M, Hollis K, Nowbahari E, Kacelnik A. Pro-sociality without empathy. Biology Letters. 2012;8(6):910–912. doi: 10.1098/rsbl.2012.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ventzel L, Madsen CS, Karlsson P, Tankisi H, Isak B, Fuglsang-Frederiksen A, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chronic pain and neuropathy following adjuvant chemotherapy: A clinical study. Pain Medicine. 2017 doi: 10.1093/pm/pnx231. in press. [DOI] [PubMed] [Google Scholar]
- [59].Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135(1–2):7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- [60].Vierck CJ, Yezierski RP. Comparison of operant escape and reflex tests of nociceptive sensitivity. Neuroscience and biobehavioral reviews. 2015;51:223–242. doi: 10.1016/j.neubiorev.2015.01.022. [DOI] [PubMed] [Google Scholar]
- [61].Vollert J, Kramer M, Barroso A, Freynhagen R, Haanpaa M, Hansson P, Jensen TS, Kuehler BM, Maier C, Mainka T, Reimer M, et al. Symptom profiles in the painDETECT Questionnaire in patients with peripheral neuropathic pain stratified according to sensory loss in quantitative sensory testing. Pain. 2016;157(8):1810–1818. doi: 10.1097/j.pain.0000000000000588. [DOI] [PubMed] [Google Scholar]
- [62].Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmuhlen J, Haanpaa M, Hansson P, et al. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain. 2017;158(8):1446–1455. doi: 10.1097/j.pain.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].von Bischhoffshausen S, Ivulic D, Alvarez P, Schuffeneger VC, Idiaquez J, Fuentes C, Morande P, Fuentes I, Palisson F, Bennett DLH, Calvo M. Recessive dystrophic epidermolysis bullosa results in painful small fibre neuropathy. Brain. 2017;140(5):1238–1251. doi: 10.1093/brain/awx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7(2):103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- [65].Wall PD, Scadding JW, Tomkiewicz MM. The production and prevention of experimental anesthesia dolorosa. Pain. 1979;6(2):175–182. doi: 10.1016/0304-3959(79)90124-6. [DOI] [PubMed] [Google Scholar]
- [66].Wallace VCJ, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice ASC. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133(1–3):47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wallace VCJ, Blackbeard J, Segerdahl A, Hasnie FS, Pheby T, McMahon SB, Rice ASC. Characterisation of rodent models of HIV-gp120 and anti-retroviral associated neuropathic pain. Brain. 2007;130(10):2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wallace VCJ, Segerdahl AR, Blackbeard J, Pheby T, Rice ASC. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neuroscience Letters. 2008;448(1):153–156. doi: 10.1016/j.neulet.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wallace VCJ, Segerdahl AR, Lambert DM, Vandevoorde S, Blackbeard J, Pheby T, Hasnie F, Rice ASC. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. Br J Pharmacol. 2007;151(7):1117–1128. doi: 10.1038/sj.bjp.0707326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Whittaker AL, Lymn KA, Nicholson A, Howarth GS. The assessment of general well-being using spontaneous burrowing behaviour in a short-term model of chemotherapy-induced mucositis in the rat. Lab Anim. 2015;49(1):30–39. doi: 10.1177/0023677214546913. [DOI] [PubMed] [Google Scholar]
- [71].Wodarski R, Delaney A, Ultenius C, Morland R, Andrews N, Baastrup C, Bryden LA, Caspani O, Christoph T, Gardiner NJ, Huang W, et al. Cross-centre replication of suppressed burrowing behaviour as an ethologically relevant pain outcome measure in the rat: a prospective multicentre study. Pain. 2016;157(10):2350–2365. doi: 10.1097/j.pain.0000000000000657. [DOI] [PubMed] [Google Scholar]
- [72].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]