Abstract
Taste allows animals to discriminate the value and potential toxicity of food prior to ingestion. Many tastants elicit an innate attractive or avoidance response that is modifiable with nutritional state and prior experience. A powerful genetic tool kit, well-characterized gustatory system, and standardized behavioral assays make the fruit fly, Drosophila melanogaster, an excellent system for investigating taste processing and memory. Recent studies have used this system to identify the neural basis for acquired taste preference. These studies have revealed a role for dopamine-mediated plasticity of the mushroom bodies that modulate the threshold of response to appetitive tastants. The identification of neural circuitry regulating taste memory provides a system to study the genetic and physiological processes that govern plasticity within a defined memory circuit.
Keywords: Feeding, dopamine, memory, neural circuitry, taste
Introduction
An animal’s response to food is dependent on sensory perception, neural processes governing reflexive feeding, and modulation of innate responses in accordance with past experience and internal state (Dethier 1976). In diverse insect species, contact with attractive tastants induces the proboscis extension reflex (PER), an innate feeding behavior that is modifiable with past experience. In the honey bee, Apis melifera, or fruit fly, Drosophila melanogaster, the pairing of neutral odorant with attractive sucrose results in classically conditioned memories where the odor alone is sufficient to elicit an attractive response (Duerr and Quinn 1982, Hammer and Menzel 1995). Conversely, the pairing of attractive sucrose with a punishing stimulus such as heat or a bitter tastant elicits an avoidance response and inhibits PER to (Masek and Scott 2010, Keene and Masek 2012). The modification of innate PER provides a system to investigate the neural principles underlying taste processing and memory formation. A powerful genetic tool kit, a relatively simple gustatory system, and standardized behavioral assays make Drosophila an excellent model organism for the study of taste processing (Ishimoto and Tanimura 2004, Vosshall and Stocker 2007). Drosophila displays robust innate feeding behavior following exposure to a variety of tastants. Current literature supports a model where the two predominant taste modalities that drive feeding response are sweet taste that mediates attraction, and bitter taste that mediates avoidance (Thorne et al. 2004, Marella et al. 2006, Masek and Scott 2010). The neural processes regulating detection of tastants and initiation of reflexive feeding are better understood than the neural mechanisms underlying taste memory and the modification of innate behavior. A complete understanding of taste processing will require identifying the neural basis for taste memory and higher-order processing of tastants. Here, we describe recent studies in Drosophila examining the neural circuitry required for aversive taste memories.
Taste processing in Drosophila
Drosophila discriminates all five distinct taste modalities that humans perceive including sweet, bitter, salt, acidity, and umami (Wang et al. 2004, Hiroi et al. 2008, Toshima and Tanimura 2012, Charlu et al. 2013, Zhang et al. 2013, Alves et al. 2014). In addition, Drosophila detects the taste of carbonation, water, fatty acids, and carboxylic acids, which may also represent independent taste modalities (Nakamura et al. 2002, Inoshita and Tanimura 2006, Fischler et al. 2007, Cameron et al. 2010, Chen et al. 2010, Charlu et al. 2013, Masek and Keene 2013a). Tastants are primarily detected by taste receptors on the mouth (proboscis) and legs (tarsi), as well as by additional taste receptors in the internal mouthparts, wing margins and female abdomen (Miyazaki and Ito 2010, Stafford et al. 2012, Ling et al. 2014, Yanagawa et al. 2014, Joseph and Carlson 2015). Tastants can be subdivided into two broad classes based on their valence in initiation of feeding response. They either facilitate attractive response resulting in feeding initiation (sugars, low salt, low fatty acids, carbonation, water) or an aversive response by inhibiting feeding (bitter, high salt, high acids, electrophilic substances) (Marella et al. 2006, Jiao et al. 2008, Kim et al. 2010). The innate response to many tastants appears to be conserved across distant species. For example, Drosophila is attracted to substances perceived as sweet by humans including some sugars, amino acids, sweet alcohols, the sweet salt PbCl2, and artificial sweeteners (Gordesky-Gold et al. 2008), and is repelled by compounds perceived as bitter by humans including quinine and denatonium (Marella et al. 2006, Meyerhof et al. 2009, Yarmolinsky et al. 2009). Therefore, the innate valance of many tastants is likely conserved between flies and mammals.
In flies, tastants are detected by G-protein coupled receptors, ionotropic receptors, ppk channels, or TRP ion channels (Freeman and Dahanukar 2015). Sweet and bitter represent the best studied taste modalities, and a number of receptor– ligand pairs have been identified including Gr5a as a trehalose receptor and Gr66a as a receptor for caffeine (Dahanukar et al. 2001, Jiao et al. 2008, Weiss et al. 2011, Miyamoto et al. 2013, Ling et al. 2014, Shim et al. 2015). A subset of gustatory receptor neurons (GRNs) in the tarsi and mouth-parts express gustatory receptors for sugars, and activation of these neurons promotes feeding (Thorne et al. 2004, Marella et al. 2006). Another, non-overlapping, subset of GRNs express receptors for bitter compounds and inhibits reflexive feeding (Thorne et al. 2004, Marella et al. 2006). The well-defined neural pathways regulating sweet and bitter taste, as well as the robust innate response resulting from activation of either pathway provides a system to investigate how competing tastants are integrated and modified.
Bitter and sweet sensing GRNs in the tarsi and proboscis project to non-overlapping regions of the gnathal ganglia (GNG). Functional imaging studies reveal different areas of this region are activated in accordance with taste modality and region of tastant application (Thorne et al. 2004, Marella et al. 2006, Harris et al. 2015). These findings suggest that both functional class and anatomical region encode tastants. A number of second-order neurons have been identified that receive input from the sweet sensing neurons and innervate the antennal mechanosensory motor area (AMMC) (Kain and Dahanukar 2015, Miyazaki et al. 2015). The AMMC has also been implicated in auditory processing suggesting this may be a generalized sensory processing center (Lai et al. 2012, Tootoonian et al. 2012, Vaughan et al. 2014). In addition, a number of interneurons have been identified that modulate feeding in accordance with other competing behaviors or satiation state, suggesting complex central brain circuitry underlies innate feeding behavior (Marella et al. 2012, Flood et al. 2013, Mann et al. 2013, Pool et al. 2014). While much is known about the coding of sweet and bitter tastants by GRNs, and how both motoneurons and interneurons govern behavioral response to tastants (Gordon and Scott 2009, Manzo et al. 2012), fewer studies have examined how gustatory information is processed and transferred to higher brain centers. A complete understanding of taste perception will require investigation of how tastants are integrated across multiple taste modalities, and modified according to past experience to generate feeding response.
Innate feeding behavior in Drosophila
Contact between GRNs on the tarsi or proboscis and attractive feeding substrates result in PER, where the fly extends its proboscis to initiate feeding (Dethier 1976, Duerr and Quinn 1982). PER response to attractive tastants is extremely robust and provides a quantifiable metric of feeding behavior (Dethier 1976). The co-application of bitter and sweet tastants inhibits proboscis extension or elicits retraction through direct inhibition of sugar-sensing GRNs or integration in the GNG and higher brain centers (Chu et al. 2014, French et al. 2015). Flies can integrate taste stimulations within the same taste organs (either tarsi or proboscis) or between them suggesting a multi-level taste system where cues are integrated between modalities and sensory organs prior to the initiation of a behavioral response (Medioni and Vaysse 1975, Keene and Masek 2012). This multi-level gustatory system is different from human taste organization and provides a mechanism to control the decision to ingest food. Experimentally, the application of different tastants to the proboscis and tarsi provides a system to investigate how different taste modalities are integrated and, more recently, describe the neural mechanisms of memory formation (Keene and Masek 2012, Kirkhart and Scott 2015, Masek et al. 2015).
Aversive taste memory in Drosophila
Flies can learn to pair neutral stimuli with a salient unconditioned stimulus in a number of learning assays (Duerr and Quinn 1982, Tully and Quinn 1985, Wolf et al. 1998, Mery and Kawecki 2002). The pairing of quinine with neutral or attractive cues including food, odor, light, or color results in the formation of associative memory where a less positive valence is assigned to the conditioned stimulus (Le Bourg and Buecher 2002, Honjo and Furukubo-Tokunaga 2009, Seugnet et al. 2011). Conversely, sugars can facilitate attraction to neutral or aversive olfactory, visual or motor stimuli (Huetteroth et al. 2015, Vogt et al. 2015, Yamagata et al. 2015). The reinforcing properties of quinine and sugars are present throughout development. The attraction of Drosophila larvae to a novel odor or darkness is reduced following quinine pairing, or increased following pairing with fructose (El-Keredy et al. 2012, Gerber et al. 2013). The effectiveness of quinine and sugars as reinforcers in adult and larval Drosophila highlights a prominent role for the taste system in modifying innate behavior.
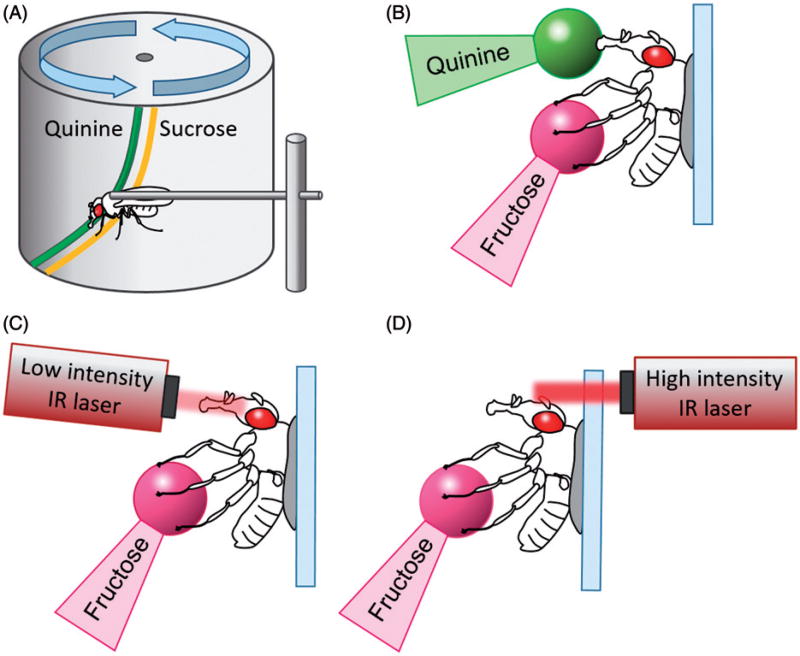
Building upon fly’s natural food searching behavior, Médioni and Vaysse (1975) developed an assay where a fly walks on a rotating cylinder containing a painted thin strip of sucrose that is accessible to the tarsi, but not the proboscis (Figure 1(A)) (Medioni and Vaysse 1975). A separate elevated strip of quinine was painted directly behind the sugar, so that it could only be reached by the proboscis. Sensing sucrose on tarsi induced PER that was followed by contact with quinine. Over several repetitions, the simultaneous pairing of sucrose and quinine resulted in suppression of PER in response to contact with the sucrose strip. Therefore, this assay allows taste memories to be measured in single freely moving Drosophila. Later, this assay was modified and used to measure habituation and the effects of aging on taste memory (DeJianne et al. 1985, Vaysse et al. 1988).
Figure 1.
Training setup for aversive taste conditioning. (A) A tethered fly walks on a rotating cylinder. Sucrose stimulation of tarsi by strip of sucrose elicits proboscis extension that is punished by an aversive quinine ingested by the extended proboscis. The inhibition of such PER response can be conditioned over trials (Medioni and Vaysse 1975). (B) A tethered fly is attached to a microscopy slide with free legs and proboscis. A droplet of a fructose is applied on the tarsi to elicit proboscis extension. Droplet of quinine is then applied to proboscis and allowed to be ingested. This leads to subsequent reduction of PER response to fructose (Keene and Masek 2012). (C) Proboscis-targeted low-intensity infrared (IR) laser is used to activate TRPA1 expressed in bitter-sensing or dopamine neurons together with fructose presented to tarsi leading to suppression of PER (Keene and Masek, 2012). (D) High-intensity IR laser is used to heat up antennae as a aversive noxious stimulus, and paired with one sugar presented to tarsi was used for discriminatory associative learning (Masek and Scott 2010).
To enhance experimental control and generate a more robust assay, we built upon similar natural behavior by directly applying fructose to the tarsi and quinine solution to the proboscis of a tethered fly (Figure 1(B)). The repeated paired application of fructose to the tarsi and quinine to the proboscis resulted in a robust reduction of PER response compared to the application of fructose alone or unpaired application of the two stimuli (Keene and Masek 2012). Memory lasting several minutes or hours was induced following a single training trial. This single-fly assay is inexpensive and amenable to high throughput analysis. It is also accessible to optogenetic techniques that stimulate distinct populations of peripheral or central brain neurons (Keene and Masek 2012, Masek et al. 2015) and adaptable for functional imaging. Furthermore, this assay is unique from other classical conditioning assays because both the reinforcing cue (quinine) and the conditioned stimulus (fructose) are tastants processed as the same basic sensory modality. While aversive taste memory is predominantly classical conditioning, there are operant components to this assay. One of the requirements for association of the appetitive stimulus and the punishment in classical conditioning is that the conditioned stimuli must precede the unconditioned stimulus. Backward conditioning, by presentation of the unconditioned stimulus, bitter perception, prior to fructose application to the legs, does not induce memory, supporting the notion that aversive taste learning represents a form of classical conditioning (Keene and Masek 2012). However, PER threshold is increased in food-deprived animals, indicating there is a motivational and operant component to this behavior (Masek et al. 2014).
The aversive taste memory assay measures behavioral response in a single tethered fly, presenting a number of substantive advantages over memory assays measuring behaviors in groups of freely moving animals. PER can be measured throughout the training process, providing readouts of memory acquisition at multiple time points. This measurement is intrinsic to the assay and, therefore, does not contribute components that would alter the memory, such as active forgetting or habituation (Fois et al. 1991). This is critical because different neurons and molecular pathways have been shown to underlie memory acquisition and retention in Drosophila olfactory memory (Cervantes-Sandoval et al. 2013, Guven-Ozkan and Davis 2014, Oswald and Waddell 2015). In a number of studies, olfactory memory protocols have been simulated in tethered flies and changes in neural activity have been measured in response to odor and shock (Davis 2011). While these studies have been effective in measuring the progression of a memory trace, they do not allow for the ability to simultaneously measure the behavioral memory (e.g. moving away from an odor) and the neural changes associated with this behavior. The use of a tethered fly with freely moving proboscis allows for simultaneous imaging and measurements of PER, providing an ability to examine the relationship between neural and behavioral readouts of memory formation within a single individual.
Memories can be short-term and last for only seconds, or long-term and last for days or years (Mayford and Kandel 1999). Both short- and long-term memories require functional modifications of the synaptic connections between neurons (Davis 2011). In many memory assays, the strength and longevity of memory is dependent on the number of training trials and the spacing between these trials (Guven-Ozkan and Davis 2014). In flies, long-term protein synthesis-dependent olfactory memories are only formed when inter-trial training intervals of ~15 minutes are introduced (spaced training), while the same number of training trials without the intervals (massed training) results primarily in less stable, protein synthesis-independent, memories (Tully et al. 1994, Bouzaiane et al. 2015). Previous work on aversive taste memory has used three consecutive training trials that provide robust learning and memory lasting up to three hours (Keene and Masek 2012 and unpublished data), and single trial training that results in robust memory at 30 minutes following training (Seidner et al. 2015). This is consistent with protein synthesis-independent short-term and middle-term memories that have previously been characterized for olfactory taste memory (Tully and Quinn 1985, Bouzaiane et al. 2015). It is possible that modifications to the training protocol may allow for the induction of long-term protein synthesis-dependent memories by increasing the number of training trials and the inter-trial intervals. When used in combination with physiological imaging, this may allow for visualizing the structural changes in neural circuit connectivity throughout the memory process.
Optogenetic induction of taste memories
The use of a single fly assay allows for targeted optogenetic activation of neurons during memory formation, consolidation, or recall. A number of different systems including light activated channel rhodopsin, activation of caged ATP, and genetic expression of heat-activated channels have been used for optogenetic or thermogenetic manipulation of neural circuitry (Oswald et al. 2015). Neurons expressing the temperature sensitive cation channel, transient receptor potential channel A1 (TRPA1) become activated at temperatures of 28 °C or greater (Hamada et al. 2008, Pulver et al. 2009). We developed a system directly targeting a narrow beam of infrared laser light to the fly head to activate TRPA1-expressing neurons with high temporal and spatial resolution (Figure 1(C,D)) (Keene and Masek 2012). Targeting the proboscis or thoracic region of flies expressing the TRPA1 in sweet-sensing neurons labeled by Gr5a was sufficient to induce proboscis extension. However, targeting the abdomen in these same flies did not induce PER, demonstrating the laser is able to activate neurons within a localized region of the body (Keene and Masek 2012). Therefore, spatially restricted neuronal activation can be achieved by the combination of targeting a specific location with the laser and localized expression of TRPA1.
The pairing of sugar and quinine during the conditioning process results in quinine ingestion, raising the possibility that post-ingestive feedback contributes to memory formation. We disassociated the effect of GRN activity from the post-ingestive effects of quinine during memory formation by direct neuronal activation of genetically labeled sensory neurons. The paired application of fructose and thermogenetic activation of bitter-sensing Gr66a neurons resulted in robust inhibition of PER, revealing that artificial activation of bitter-sensing neurons alone is sufficient for conferring aversive taste memory (Keene and Masek 2012). These findings suggest that aversive taste memories are exclusively dependent on bitter taste, rather than quinine ingestion. While it is possible that quinine ingestion may also induce memories through a mechanism independent of the taste system, this has not been tested directly. In mice, aversive taste conditioning is induced when lithium chloride is ingested orally or injected directly in the stomach, in both cases leading to acquired taste aversion (Welzl et al. 2001, Parker 2003). Assessing the reinforcing effects of bitter taste in flies lacking bitter taste receptors (Apostolopoulou et al. 2014) will address whether ingestion of the bitter substance alone could serves as a negative reinforcement.
In addition to spatial specificity, optogenetic or thermogenetic neural activation allows for precise temporal manipulation of neural activity (Keene and Masek 2012). During the pairing of quinine and fructose, residues from both tastants remain on the tarsi and proboscis and may signal the presence of the tastant following the intended training session. No changes in PER were detected in an optogenetic backward conditioning protocol, where laser activation of bitter sensing neurons precedes sugar presentation, confirming that the formation of aversive taste memory requires the conditioned stimulus (sugar) to precede, and therefore, be predictive of quinine punishment. Taken together, these studies demonstrate that the tastants required for memory formation can be replaced by optogenetic activation of sensory neurons.
Neuroanatomy of aversive taste memory
Dopamine is a potent reinforcer for both rewarding and punishing associative memories (Waddell 2010). The fly brain contains only ~300 dopamine neurons (Nassel and Elekes 1992), composed of 20 distinct clusters that have been implicated in numerous memory modalities (Friggi-Grelin et al. 2003) and innervate diverse brain regions including the mushroom bodies (MBs) and GNG (Marella et al. 2012, Pool et al. 2014). To identify whether dopamine neurons are required for aversive taste memory, Kirkhart and Scott (2015) silenced two populations of dopamine neurons, PAM and PPL1, through expression of the dominant negative GTPAse ShibireTS1. Silencing PPL1 neurons with tyrosine hydroxylase-GAL4 (TH-GAL4) abolishes taste memory (Kirkhart and Scott 2015, Masek et al. 2015). It appears that the PAM cluster of dopamine neurons is dispensable for aversive taste formation because silencing this cluster using Hl9-GAL4, an insertion in dopa decarboxylase, did not impair memory formation (Kirkhart and Scott 2015). Hl9-GAL4 labels 76 dopamine neurons, compared to 56 labeled by TH-GAL4 (Claridge-Chang et al. 2009), indicating that the difference in effectiveness between the drivers is due to expression pattern, rather than the number of neurons silenced.
The monoamines octopamine, dopamine, and serotonin convey the reinforcing cues for many different types of associative memory in Drosophila (Sitaraman et al. 2008, Guven-Ozkan and Davis 2014). Serotonin is required for both reward and punishment in associative place conditioning, while octopamine inputs to the MBs contribute to aversive and appetitive olfactory conditioning (Sitaraman et al. 2008, Waddell 2010). Silencing serotonin neurons with trh-GAL4 or octopamine neurons with tdc2-GAL4 did not impair taste memory (Kirkhart and Scott 2015). Therefore, aversive taste memory requires dopamine, but not octopamine or serotonin, revealing a difference in neural circuitry governing aversive taste memory and other memory modalities in the fruit fly.
Most GAL4 lines used to manipulate dopamine neurons drive expression in multiple clusters of dopamine neurons, or in non-dopamine cells, obscuring the ability to localize neurons contributing to behavior (Claridge-Chang et al. 2009, Liu et al. 2012) The PPL1 cluster is composed of 12 neuronal subtypes that innervate the vertical lobes and spur of the MBs, as well as the central complex (Aso et al. 2010). A number of recent genetic tools allow for refining neuronal populations labeled by GAL4 lines (Luan and White 2007). The Split-GAL4 system allows for manipulation of overlapping populations of neurons labeled by two promoters (Luan et al. 2006). Recently, the generation of split-GAL4 collections labeling small populations of dopamine neurons provided the ability to localize specific clusters regulating aversive taste memory (Jenett et al. 2012). Screening a collection of 29 split-GAL4 lines that label small clusters of dopamine neurons revealed three lines with overlapping expression in the PPL1α2 and PPL1α’2 subset of the PPL1 cluster that were required for aversive taste memory (Masek et al. 2015). This cluster consists of five neurons that innervate the upper segment of the vertical MB lobes, implicating the α/β neurons in formation of aversive taste memory.
The identification of MB-innervating PPL1 dopamine neurons as regulators of taste memory raises the possibility that these neurons are downstream of GRNs and transmit taste information to the MBs. This notion would be supported if the activation of PPL1 dopamine neurons would be sufficient to substitute for the tastant in memory formation. Indeed, activation of PPL1α2 and PPL1α’2 dopamine neurons was sufficient to substitute for quinine during training, phenocopying activation of bitter tasting neurons with quinine or thermogenetic activation of bitter neurons alone (Masek et al. 2015). The finding that the simultaneous pairing of PPL1α2 and PPL1α’2 activation with fructose presentation is sufficient to induce taste memory suggests these neurons convey bitter taste information to the MBs.
The mushroom bodies are required for aversive taste memory
Abundant evidence suggests the MBs are a center for fly memory. Many of the first isolated memory mutants are preferentially expressed in the MBs including the cAMP phosphodiesterase dunce and the adenylyl cyclase rutabaga (Davis 2005, Margulies et al. 2005). Further, flies with pharmacologically ablated MBs or mutants with maldeveloped MBs fail to form olfactory memories (Heisenberg et al. 1985, de Belle and Heisenberg 1994). More recently, experiments using ShibireTS1 revealed the MBs to be critical for both consolidation and retrieval of olfactory memory (Dubnau et al. 2001, McGuire et al. 2001, Krashes et al. 2007). While the role of the MBs has been best studied for olfactory memory, they are also required for a number of memory modalities including associative visual memory, courtship conditioning, and aversive phototaxis suppression (McBride et al. 1999, Seugnet et al. 2011, Vogt et al. 2014). Therefore, the MBs appear to represent a memory center in the fly brain that underlies many types of associative memory.
The MBs comprise a central brain neuropil consisting of ~200 intrinsic neurons (Kenyon cells) with three primary neural subtypes. The axons of Kenyon cells form five lobes that are critical sites for synaptic plasticity (Margulies et al. 2005, Guven-Ozkan and Davis 2014). The lobes are formed by α/β and α′/β′ neurons, which branch to form vertical and horizontal lobes, while γ neurons form horizontal lobes. Many studies have identified distinct functions for individual lobes in olfactory memory formation, however, more recently this view has been challenged (Claridge-Chang et al. 2009). While less is known about the role of MB subtypes in the formation of aversive taste memory, three studies have shown that silencing either γ or α′/β′ by expression of ShiTs1 disrupts short-term aversive taste memory (Masek and Scott 2010, Kirkhart and Scott 2015, Masek et al. 2015). These findings are consistent with a previously reported role for diverse MB lobes in olfactory memory, and support the notion that the MBs are a central site for chemosensory memory in Drosophila.
The intrinsic neurons of the MBs converge onto only 34 identifiable MB output neurons (MBONs) that are anatomically grouped into 21 neuronal subtypes (Figure 2) (Aso et al. 2014a). A collection of recently annotated GAL4 lines provided the ability to manipulate distinct subtypes of MB output neurons (Aso et al. 2014b). Screening of this collection revealed that blocking synaptic output from the MB-V2α output neurons disrupts aversive taste memory. Axons of the α2/α3 population of PPL1 neurons and dendrites of the MB-V2α innervate overlapping regions of the MB vertical lobes, fortifying the notion that these neurons form a functional circuit modulating taste memory (Aso et al. 2014a, Masek et al. 2015).
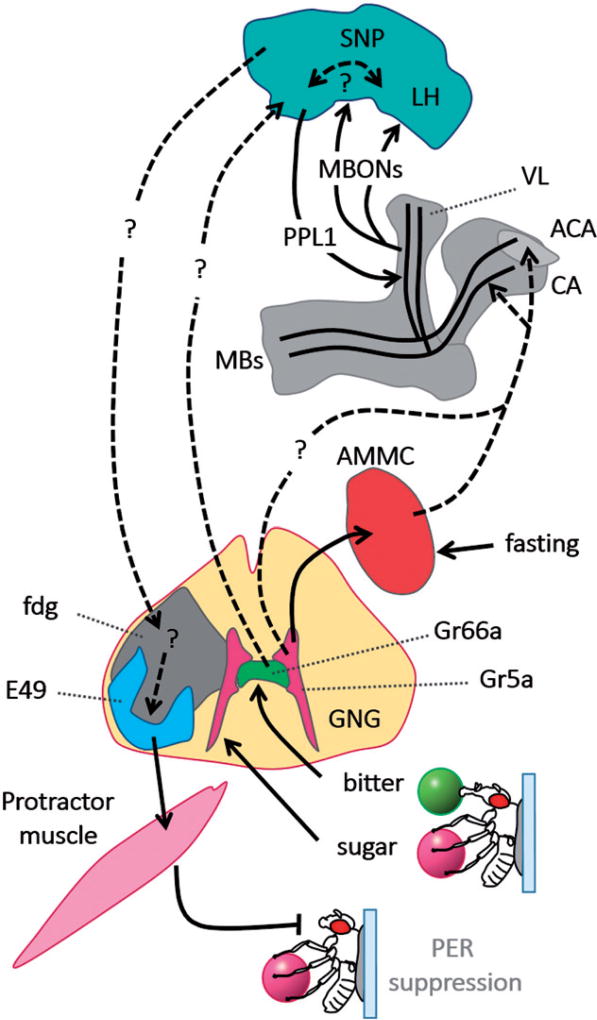
Figure 2.
Model of aversive taste conditioning pathway. Sugar is sensed by the tarsi and paired with bitter quinine applied on proboscis. Both group of sensory neurons project to gnathal ganglia (GNG), the initial processing gustatory center. The second-order taste neurons project from the area of sweet-sensing neurons in GNG to accessory antennal mechanosensory and motor center (AMMC). Sugar taste activates neurons in the calyx (CA) and accessory calyx (ACA) of mushroom bodies (MBs), via the AMMC and/or an unidentified pathway. Information encoding bitter taste signals through bitter-sensing neurons to the CA, ACA, and likely superior neuropil regions (SNP) where it may connect to PPL1 dopamine neurons. A subset of PPL1 neurons then innervates the vertical lobes (VL) of MBs. The information encoding sugar tastant is then likely conveyed from CA and ACA through MB α/β neurons. The simultaneous activation of these neurons together with specific PPL1 neurons leads to modulation of MB output neurons (MBONs) projecting from the same area of MBs to several regions of SNP and the lateral horn (LH). It is possible that there is recurrent feedback loop between MBONs and dopamine neurons within SNP. The connectivity from SNP to other brain region and back to GNG is not known. Specific pair of dopamine neurons in the GNG (fgn) controls proboscis extension reflex (PER) including motor neurons (E49) synapsing on Protractor muscle M10 extending the rostrum. Inhibition of this pathway will result in suppression of naïve PER following the sugar stimulation.
Determining how a small number of MBONs are capable of relaying a diverse array of MB-dependent memories and behaviors is critical for understanding behavioral plasticity in Drosophila. To investigate this question, a large consortium examined the role of outputs in diverse types of memory (Aso et al. 2014b). This study found the MBONs in V2 cluster are required for appetitive visual, short-term olfactory and ethanol-induced memory but also for aversive long-term olfactory memory, besides the aversive taste memory (Aso et al. 2014b). Supporting this notion, MBONs of M4/M6 cluster appear to determine the valence of response to both conditioned odorants and naturally aversive CO2, suggesting these neurons integrate signals from multiple behaviors (Lewis et al. 2015, Owald et al. 2015). Therefore, it appears that the same MBONs may govern different memories and behaviors. Dopamine neuron dendrites overlap with axons of the MBONs in a number of neuropil regions, raising the possibility that MBONs form a recurrent loop with dopamine neurons that innervate the MBs (Aso et al. 2014b, Lewis et al. 2015, Owald et al. 2015). In addition, some MBONs receive input from multiple regions of the MB lobes, or even from different lobes, which may provide a mechanism for connecting memory phases that reside in different lobes (Aso et al. 2014a). Therefore, the targets of MBONs, or the MBONs themselves, may function as an integration site for different types of memory and provide positive and negative feedback that increases the computational capacity of the MBs. Identifying the MB circuits that are required for aversive taste memory may provide cellular-level resolution of interactions between aversive taste memory and other MB-V2α-dependent behaviors.
The formation of associative memories is accompanied by changes in Ca2+ activity within the MB lobes, suggesting alterations in synaptic strength (Davis 2011). The MBs are dispensable for reflexive feeding, but required for conditioned responses, suggesting that taste conditioning may alter the naive feeding response (Masek and Scott 2010, Masek et al. 2015). Aso et al. (2014a) proposed the taste center may localize to a number of different brain regions that are targets of MBON projections and innervated by dopamine neurons. They postulate a circuit in the MBON convergence zones, in which US inputs activate motor command neurons to elicit innate responses. Convergence of the US signal and the modified learned response toward CS in this region then leads to final modulated behavior (Aso et al. 2014a). To test this hypothesis, MB-V2α output neurons were activated or silenced and PER was measured in response to increasing sugar concentrations. Activation of the MB-V2α output neurons increased the response threshold to sugar, while silencing these neurons reduced the response threshold, suggesting the MBONs can modify the innate response to sugars (Masek et al. 2015). The finding that MBONs can modulate the innate response to sensory stimuli is not specific to taste. The MB-β′2 is required for olfactory memory, and activity is altered bi-directionally following odor-shock or odor-taste memory (Owald et al. 2015). Further, these neurons are activated by CO2 and required for innate CO2 avoidance (Lewis et al. 2015), fortifying the notion that MBON activity regulates the behavioral response to sensory cues. Taken together, these findings provide insight into how the modification of sensory information within the MB-circuits modify innate responses to sensory cues to alter behavior. Understanding the neurotransmitter expressed by the MB-V2α neurons and their connectivity to the reflexive feeding circuit will be critical for elucidating the mechanism of aversive taste conditioning.
Neural mechanisms of synaptic plasticity
A central question in sensory processing is how inputs from different taste modalities modulate MB physiology during memory formation. In aversive taste memory, value appears to be assigned to a number of memory components including the tastants and body location. Kirkhart and Scott (2015) expressed the Ca2+ sensor GCaMP in the MB Kenyon cells and monitored activity during exposure to sugar and quinine. Distinct populations of Kenyon cells were activated by each taste modality, suggesting different tastes are integrated within the MBs or by downstream neurons. Interestingly, different populations of Kenyon cells are activated following sucrose application to the leg or proboscis indicating that qualitative, as well as spatial, representation is retained in the MBs (Kirkhart and Scott 2015). Equally intriguing, different MB neurons are activated following sucrose application to the right or left leg suggesting laterality of sensory processing (Kirkhart and Scott 2015). Consistent with this notion, selectively training flies on the right or left leg results in aversive taste memory only on one side, suggesting distinct neural circuits specify the conditioning stimuli and region of stimulation (Kirkhart and Scott 2015). These findings highlight the ability of a single fly to distinguish between localized activation of taste neurons, and reveal additional layers of complexity in the central brain circuitry involved in processing this information.
Integration of aversive taste memory and additional behavioral processes
Memory is influenced by a number of environmental and life history traits including age, sleep, circadian time, and feeding state (Dissel et al. 2015a). Both short-term and long-term memories are impaired in sleep deprived animals in multiple memory modalities revealing interactions between these processes (Seugnet et al. 2011). Further, in an aversive phototaxis suppression assay, the memory deficits of a number of memory mutant flies can be restored by feeding flies the sleep inducing drug Gaboxadol, and the formation of long-term memories can be potentiated by activating the fan-shaped body, which contains a population of sleep promoting neurons (Dissel et al. 2015b). To investigate the relationship between sleep homeostasis and memory, flies were sleep deprived by activating wake-promoting neural circuits and assaying for memory before or after, following recovery. Similar to other memory assays, acute sleep deprivation impaired aversive taste memory (Li et al. 2009, Seidner et al. 2015). Memory was not impaired when flies were left undisturbed for two hours following deprivation indicating that homeostatic sleep rebound is critical for memory following sleep deprivation (Seidner et al. 2015). Interestingly, memory did not recover in flies sleep deprived through the activation of neural circuits that do not induce rebound, fortifying the notion that maintenance of sleep homeostasis is critical for memory formation (Seidner et al. 2015). Therefore, aversive taste memory is sensitive to changes in sleep, and this assay provides a system to examine interactions between sleep and memory formation.
Future application for taste memory in Drosophila
The identification of a central brain circuitry underlying aversive taste memory, combined with a detailed understanding of taste coding, presents the foundation for future studies examining physiological changes associated with aversive taste memory. Functional imaging has been used to identify physiological changes in defined populations of neurons during the formation of olfactory memory. Dynamic changes within the MBs and MB-associated neurons during memory formation, retention, and recall suggest that transient plasticity underlies the formation and maintenance of olfactory memory (Davis 2011). The application of functional imaging to the aversive taste memory paradigm will provide the ability to image MB physiology throughout training and consolidation. Further, adapting the current training protocols to induce long-term protein synthesis-dependent memory may allow for real-time imaging of structural changes resulting in formation of long-term memory. Therefore, the combination of this single fly memory assay with functional imaging will provide a unique opportunity to associate functional changes in neural circuits with resulting behavioral plasticity.
In addition to its utility in examining synaptic plasticity, the aversive taste memory assay provides the ability to dissect the nuances of gustatory processing or coding that are difficult to resolve with simple gustatory assays. Taste may represent an analytical sense with single modalities that are processed independently. On the other hand, overlapping neural mechanisms may contribute to the detection and processing of distinct taste modalities (Reiter et al. 2015). Determining whether flies discriminate between different sugars by pairing one sugar with heat-punishment and testing with an alternative sugar revealed that flies are unable to distinguish between different sugars, and instead rely on relative sweetness or palatability (Masek and Scott 2010). In addition to sugars, the sweet sensing neurons are necessary and sufficient for response to fatty acids (Masek and Keene 2013b). The pairing of fatty acids or sugars with quinine, and then testing flies with the other sweet substance can be used to determine whether flies are capable of differentiating between fatty acids and sugars. These experiments would shed light on whether the taste of fatty acid represents independent taste modality in flies, or is a subset of a more general appetitive taste modality. Because many taste receptors express in only partially overlapping populations of neurons, discriminative aversive taste conditioning provides a method to examine whether animals can differentiate between similar tastants.
Conclusions
A powerful genetic toolkit, the growing understanding of neural circuits regulating memory and the relatively simple taste system makes Drosophila aversive taste memory a powerful system for investigating neural mechanisms underlying behavioral plasticity and sensory processing. Defined populations of sweet and bitter sensing neurons convey reflexive acceptance or avoidance behavior, and modifications of these innate responses are dependent on the MBs. Aversive taste memory requires PPL1α2 and PPL1α’2 dopamine neurons to convey the reinforcing aversive stimulus to MBs. The MBs likely modulate the response of defined output neurons that alter the naïve response to sugars. These findings provide components of a central brain circuit for investigating memory formation. This system can be applied in future studies investigating diverse biological principles ranging from synaptic plasticity to gustatory processing.
Acknowledgments
Funding information
This work was supported by NSF-IOS 1551136 to ACK and PM.
Footnotes
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alves G, Sallé J, Chaudy S, Dupas S, Manière G. High-NaCl perception in Drosophila melanogaster. Journal of Neuroscience. 2014;34:10884–10891. doi: 10.1523/JNEUROSCI.4795-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulou AA, Mazija L, Wüst A, Thum AS. The neuronal and molecular basis of quinine-dependent bitter taste signaling in Drosophila larvae. Frontiers in Behaviour Neuroscience. 2014;8:6. doi: 10.3389/fnbeh.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Siwanowicz I, Bräcker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Current biology. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo, Rubin GM. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014a;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Gurin G, Rubin GM. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014b;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Animal Learning & Behaviour. 2002;30:330–341. doi: 10.3758/bf03195958. [DOI] [PubMed] [Google Scholar]
- Bouzaiane E, Trannoy S, Scheunemann L, Placais P, Preat T. Two independent mushroom body output circuits retrieve the six discrete components of Drosophila aversive memory. Cell Reports. 2015;11:1280–1292. doi: 10.1016/j.celrep.2015.04.044. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I, Martin-Pena a, Berry Ja, Davis RL, Martin-Peña A, Berry Ja, Davis RL. System-like consolidation of olfactory memories in Drosophila. Journal of Neuroscience. 2013;33:9846–9854. doi: 10.1523/JNEUROSCI.0451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlu S, Wisotsky Z, Medina A, Dahanukar A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nature Communications. 2013;4:2042. doi: 10.1038/ncomms3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wang Q, Wang Z. The amiloride-sensitive epithelial Na + channel PPK28 is essential for drosophila gustatory water reception. Journal of Neuroscience. 2010;30:6247–6252. doi: 10.1523/JNEUROSCI.0627-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Chui V, Mann K, Gordon MD. Presynaptic gain control drives sweet and bitter taste integration in Drosophila. Current Biology. 2014;24:1978–1984. doi: 10.1016/j.cub.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenböck G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nature Neuroscience. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annual Review of Neuroscience. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJianne D, McGuire TR, Pruzan-Hotchkiss A. Conditioned suppression of proboscis extension in Drosophila melanogaster. Journal of Comparative Psychology. 1985;99:74–80. [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding. Cambridge, MA; London, England: Harvard University Press; 1976. [Google Scholar]
- Dissel S, Melnattur K, Shaw P. Sleep, performance, and memory in flies. Current Sleep Medicine Reports. 2015a;1:47–54. doi: 10.1007/s40675-014-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissel S, Angadi V, Kirszenblat L, Suzuki Y, Donlea J, Klose M, Shaw PJ. Sleep restores behavioral plasticity to Drosophila mutants. Current Biology. 2015b;25:1270–1281. doi: 10.1016/j.cub.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Duerr JS, Quinn WG. Three Drosophila mutations that block associative learning also affect habituation and sensitization. Proceedings of National Academic Sciences of United States of America. 1982;79:3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Keredy A, Schleyer M, König C, Ekim A, Gerber B. Behavioural analyses of quinine processing in choice, feeding and learning of larval Drosophila. PLoS One. 2012;7:e40525. doi: 10.1371/journal.pone.0040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- Flood TF, Iguchi S, Gorczyca M, White B, Ito K, Yoshihara M. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013;499:83–87. doi: 10.1038/nature12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fois C, Medioni J, Le Bourg E. Habituation of the proboscis extension response as a function of age in Drosophila melanogaster. Gerontology. 1991;37:187–192. doi: 10.1159/000213259. [DOI] [PubMed] [Google Scholar]
- Freeman EG, Dahanukar A. Molecular neurobiology of Drosophila taste. Current Opinion in Neurobiology. 2015;34:140–148. doi: 10.1016/j.conb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AS, Sellier M-J, Moutaz AA, Guigue A, Chabaud M-A, Reeb PD, Marion-Poll F. Dual mechanism for bitter avoidance in Drosophila. Journal of Neuroscience. 2015;35:3990–4004. doi: 10.1523/JNEUROSCI.1312-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. Journal of Neurobiology. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gerber B, Biernacki R, Thum J. Odor-taste learning assays in Drosophila larvae. Cold Spring Harbor Protocols. 2013;8:213–223. doi: 10.1101/pdb.prot071639. [DOI] [PubMed] [Google Scholar]
- Gordesky-Gold B, Rivers N, Ahmed OM, Breslin PAS. Drosophila melanogaster prefers compounds perceived sweet by humans. Chemical Senses. 2008;33:301–309. doi: 10.1093/chemse/bjm088. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learning Memory. 2014;21:519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Learning and memory in the honey-bee. Journal of Neuroscience. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DT, Kallman BR, Mullaney BC, Scott K. Representations of taste modality in the Drosophila brain. Neuron. 2015;86:1449–1460. doi: 10.1016/j.neuron.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M, Borst A, Wagner S, Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. Journal of Neurogenetics. 1985;2:1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Tanimura T, Marion-Poll F. Hedonic taste in Drosophila revealed by olfactory receptors expressed in taste neurons. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Furukubo-Tokunaga K. Distinctive neuronal networks and biochemical pathways for appetitive and aversive memory in Drosophila larvae. Journal of Neuroscience. 2009;29:852–862. doi: 10.1523/JNEUROSCI.1315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Current Biology. 2015;25:751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita T, Tanimura T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proceedings of National Academic Sciences of United States of America. 2006;103:1094–1099. doi: 10.1073/pnas.0502376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Tanimura T. Molecular neurophysiology of taste in Drosophila. Cellular and Molecular Life Science. 2004;61:10–18. doi: 10.1007/s00018-003-3182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TTB, Shepherd D, Murphy C, Dionne H, Zugates CT. A GAL4-driver line resource for Drosophila neurobiology. Cell Reports. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Current Biology. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R, Carlson J. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends in Genetics. 2015;12:683–695. doi: 10.1016/j.tig.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain P, Dahanukar A. Secondary taste neurons that convey sweet taste and starvation in the Drosophila brain. Neuron. 2015;85:819–832. doi: 10.1016/j.neuron.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Keene AC, Masek P. Optogenetic induction of aversive taste memory. Neuroscience. 2012;222:173–180. doi: 10.1016/j.neuroscience.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proceedings of National Academic Sciences of United States of America. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkhart C, Scott K. Gustatory learning and processing in the Drosophila. Journal of neuroscience. 2015;15:5950–5958. doi: 10.1523/JNEUROSCI.3930-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS-Y, Lo S-J, Dickson BJ, Chiang A-SY. Auditory circuit in the Drosophila brain. Proceedings of National Academic Sciences of United States of America. 2012;109:2607–2612. doi: 10.1073/pnas.1117307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LPC, Siju KP, Aso Y, Friedrich AB, Bulteel AJB, Rubin GM, Grunwald Kadow IC. A higher brain circuit for immediate integration of conflicting sensory information in Drosophila. Current Biology. 2015;25:2203–2214. doi: 10.1016/j.cub.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Li X, Yu F, Guo A. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 2009;32:1417–1424. doi: 10.1093/sleep/32.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Dahanukar A, Weiss LA, Kwon JY, Carlson JR. The molecular and cellular basis of taste coding in the legs of Drosophila. Journal of Neuroscience. 2014;34:7148–7164. doi: 10.1523/JNEUROSCI.0649-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Current Biology. 2012;22:2114–2123. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, White BH. Combinatorial methods for refined neuronal gene targeting. Current Opinion in Neurobiology. 2007;17:572–580. doi: 10.1016/j.conb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Gordon M, Scott K. A pair of interneurons influences the choice between feeding and locomotion in Drosophila. Neuron. 2013;79:754–765. doi: 10.1016/j.neuron.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo K, Silies M, Gohl D, Scott K. Motor neurons controlling fluid ingestion in Drosophila. Proceedings of National Academic Sciences of United States of America. 2012;109:6307–6312. doi: 10.1073/pnas.1120305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Current Biology. 2005;15:700–713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Keene AC. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genetics. 2013a;9:e1003710. doi: 10.1371/journal.pgen.1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Keene AC. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genetics. 2013b;9:e1003710. doi: 10.1371/journal.pgen.1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Scott K. Limited taste discrimination in Drosophila. Proceedings of National Academic Sciences of United States of America. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Reynolds LA, Bollinger WL, Moody C, Mehta A, Murakami K, Keene AC, et al. Altered regulation of sleep and feeding contribute to starvation resistance in Drosophila. Journal of Experimental Biology. 2014;217(Pt 17):3122–3132. doi: 10.1242/jeb.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Worden K, Aso Y, Rubin G, Keene A. A dopamine-modulated neural circuit regulating aversive taste memory in Drosophila. Current biology. 2015;25:1535–1541. doi: 10.1016/j.cub.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Kandel ER. Genetic approaches to memory storage. Trends in Genetics. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Siwicki KK. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- Medioni J, Vaysse G. [Conditional suppression of a reflex in Drosophila melanogaster: acquisition and extinction] Comptes rendus des séances de la Société de biologie et de ses filiales. 1975;169:1386–1391. [PubMed] [Google Scholar]
- Mery F, Kawecki TJ. Experimental evolution of learning ability in fruit flies. Proceedings of National Academic Sciences of United States of America. 2002;99:14274–14279. doi: 10.1073/pnas.222371199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chemical senses. 2009;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Ito K. Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. Journal of Comparative Neurology. 2010;518:4147–4181. doi: 10.1002/cne.22433. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Lin TY, Ito K, Lee CH, Stopfer M. A gustatory second-order neuron that connects sucrose-sensitive primary neurons and a distinct region of the gnathal ganglion in the Drosophila brain. Journal of Neurogenetics. 2015;29(2–3):144–155. doi: 10.3109/01677063.2015.1054993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Baldwin D, Hannaford S, Palka J, Montell C. Defective proboscis extension response (DPR), a member of the Ig superfamily required for the gustatory response to salt. Journal of Neuroscience. 2002;22:3463–3472. doi: 10.1523/JNEUROSCI.22-09-03463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel D, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell and Tissue Research. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- Oswald D, Waddell S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr Opinion in Neurobiology. 2015;35:178–184. doi: 10.1016/j.conb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald D, Lin S, Waddell S. Light, heat, action: neural control of fruit fly behaviour. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences. 2015;370:20140211. doi: 10.1098/rstb.2014.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86:417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learning and Behavior. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Pool AH, Kvello P, Mann K, Cheung SK, Gordon MD, Wang L, Scott K. Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron. 2014;83:164–177. doi: 10.1016/j.neuron.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. Journal of Neurophysiology. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter S, Campillo Rodriguez C, Sun K, Stopfer M. Spatiotemporal coding of individual chemicals by the gustatory system. Journal of neuroscience. 2015;35:12309–12321. doi: 10.1523/JNEUROSCI.3802-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidner G, Robinson J, Wu M, Worden K, Masek P, Roberts S, Joiner W. Identification of neurons with a privileged role in sleep homeostasis in Drosophila melanogaster. Current Biology. 2015;25:2928–2939. doi: 10.1016/j.cub.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Donlea JM, Gottschalk L, Shaw PJ. Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep. 2011;34:137–146. doi: 10.1093/sleep/34.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Lee Y, Jeong Y, Kim Y, Lee M, Montell C, Moon S. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nature Communications. 2015;16:8867. doi: 10.1038/ncomms9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen Y-C, Sable-Smith A, Kitamoto T, Zars T. Serotonin is necessary for place memory in Drosophila. Proceedings of National Academic Sciences of United States of America. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. Journal of Neuroscience. 2012;32:14767–14774. doi: 10.1523/JNEUROSCI.1887-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Current Biology. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tootoonian S, Coen P, Kawai R, Murthy M. Neural representations of courtship song in the Drosophila brain. Journal of Neuroscience. 2012;32:787–798. doi: 10.1523/JNEUROSCI.5104-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima N, Tanimura T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. Journal of Experimental Biology. 2012;215:2827–2832. doi: 10.1242/jeb.069146. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of Comparative Physiology A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Vaughan AG, Zhou C, Manoli DS, Baker BS. Neural pathways for the detection and discrimination of conspecific song in D. melanogaster. Current Biology. 2014;24:1039–1049. doi: 10.1016/j.cub.2014.03.048. [DOI] [PubMed] [Google Scholar]
- Vaysse G, Galissie M, Corbiere M. Induced variation of serotonin in Drosophila melanogaster and its relation to learning performance. Journal of Comparative Psychology. 1988;102:225–229. doi: 10.1037/0735-7036.102.3.225. [DOI] [PubMed] [Google Scholar]
- Vogt K, Schnaitmann C, Dylla KV, Knapek S, Aso Y, Rubin GM, Tanimoto H. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. Elife. 2014;3:e02395. doi: 10.7554/eLife.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Yarali A, Tanimot H. Reversing stimulus timing in visual conditioning leads to memories with opposite valence in Drosophila. PLoS One. 2015;10:e0139797. doi: 10.1371/journal.pone.0139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annual Review of Neuroscience. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends in Neuroscience. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzl H, D’Adamo P, Lipp HP. Conditioned taste aversion as a learning and memory paradigm. Behaviour Brain Research. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Wolf R, Wittig T, Liu L, Wustmann G, Eyding D, Eyding DEyding, DHeisenberg M. Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learning Memory. 1998;5:166–178. [PMC free article] [PubMed] [Google Scholar]
- Yamagata N, Ichinose T, Aso Y, Placais P, Friedrich A, Sima R, Tanimoto H. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proceedings of National Academic Sciences of United States of America. 2015;112:578–583. doi: 10.1073/pnas.1421930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa A, Guigue AMA, Marion-Poll F. Hygienic grooming is induced by contact chemicals in Drosophila melanogaster. Frontiers in Behaviour Neuroscience. 2014;8:254. doi: 10.3389/fnbeh.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]