Abstract
Introduction
Ischemic heart disease (IHD) is clinical manifestation of chronic inflammatory progressive pathological process of atherosclerosis in coronary arteries. IHD is the leading cause of morbidity and mortality in the world. The question is whether it is possible to improve and direct the therapeutic treatment of IHD patients in the treatment of the inflammatory process in the atherosclerotic leasions.
Material and Methods
A prospective, comparative, analytica,clinically applicable, open-type study was performed. The study was conducted on 80 subjects with controlled biohumoral markers: troponin, CK, CK MB, BNP; markers of atherogenesis: LDL and homocystein; inflammatory markers: CRP, amyloid, cytokines IL-2, IL-6,TNF-alpha. The experimental group of 38 respondents had in addition to the conventional IHD treatment with: ampicillin (which included organosulfur compounds), cyancobalamin, vitamin B complex (B1, B2 and B6) and folacin. A control group of 42 respondents did not have this additional treatment.
Results
Major adverse cardic events (MACE) such as postinfarctic angina pectoris and repeated infarction, need for surgical interventions of myocardial revascularization, signs of cardiac insufficiency and death were observed during the one-year period. There was no correlation between the IL-2, IL-6 and TNF-alpha, as well as CK, CKMB and troponin and MACE in one-year follow-up. There was a strong positive correlation between MACE and CRP (p = 0,0002) and amyloid (p = 0,0005) as inflamatory markers; a strong positive correlation between MACE and homocysteine as an atherogenic marker (p = 0,0002, and amoderate positive correlation between MACE and BNP (p = 0.0403) as ischemic marker and marker of cardiac insufficiency. The echocardiographically monitored systolic function showed a moderate difference in the groups with average higher values in the experimantal group (p = 0.0282).
Conclusion
The applied treatment exhibited a moderate positive effect on the systolic function of LV and significantly reduced the MACE in the work compared to the control group (p <0.0001), and demonstrated a potential anti-inflammatory effect.
Keywords: ischemic heart disease, marker, ampicillin, vitamin B complex, MACE
1. INTRODUCTION
Cardiovascular diseases are the leading cause of morbidity and mortality in the world, with 29% of total annual mortality. It is estimated that by 2030, 23.6 million people will die annually of heart disease, or every two seconds in the world will cause a death due to cardiovascular disease (1). Cardiovascular diseases are the cause of death in our country, especially in younger age population, according to data from the FBiH Public Health Institute (2). Ischemic heart disease (IHD) in cardiovascular disease amount to 42%. Myocardial infarction is the leading cause of mortality in Western Europe with an intrahospital mortality rate of 6-13%, or 30%-40% when extrahosital mortality is considered (3). The pathophysiological basis of cardiovascular
disease is atherosclerosis as an inflammatory disease in which immune mechanisms interact with metabolic risk factors in the occurrence, propagation and activation of lesions in the arterial wall. Atherosclerosis ends with occlusive disease of coronary, carotid, cerebral and peripheral arteries. Atherogenic factors in the emergence and progression of atherosclerosis are: elevated levels of LDL cholesterol, elevated homocysteine, smoking, hypertension, diabetes, viral and bacterial infections, immune complexes and insufficiently defined toxins. All stages of atherogenesis are morphological and functional changes of the endothelium known as endothelial dysfunction (ED). The essential factor of atherogenesis and ED is homocysteine which causes smooth muscle cell hyperplasia, decreases NO secretion and oxidizes LDL, and acts atherogenic and thrombogenic (4). Ultimately ED causes rupture of atherosclerotic plaque, formation of fever, ulceration and thrombosis. The clinical manifestation of this process on the coronary arteries is IHD. After occlusion of the coronary artery, myocyte necrosis occurs, and intracellular macromolecules diffuse into interstitial and microvascular structures in the area of myocardial infarction as serum cardiac markers. Significant sensitized and specific myocardial necrosis markers are: creatine kinase (CK), its isoenzyme CK MB, troponin, and a new proven ischemic marker and marker of cardiac insufficiency–brain natriuretic peptide (BNP). Increasing the level of circulatory inflammatory markers correlates with the prognosis of acute coronary syndrome (ACS), regardless of the degree of ischemia or the extent of atherosclerotic changes. Such inflammatory markers are: CRP, serum amyloid, IL-6, TNF-α, etc. Thus, it can be assumed that inflammation is one of the possible goals of IHD treatment. The leading predictor of inflammatory marker i CRP. Synthesis and CRP production (controlled by predominantly IL-6) begins very rapidly in acute myocardial infarction (AMI), increases by 5 mg/l for 6 hours and reaches maximum within 48 h. Under the influence of IL-6 and TNF-α very rapidly in AMI, the synthesis of another inflammatory marker amyloid begins (5). During AMI serum amyloid starts to grow within 24 hours and its peak value reaches three days after the onset of pain 2000 times the third day by AMI (6). The gold standard in IHD diagnosis and coronary syndrome is coronarography. Coronary plaques with luminescent stenosis of up to 50% usually do not fall under percutaneous coronary treatment, but unfortunately these inflammatory plaques are in the large number of cases caused by AMI. In coronary heart disease, it was found that up to 70% of people with AMI plaque of less than 50% had the rupture. The so-called unstable, lipid-rich plaques are most suitable for modification, reduction of lipid content and degree of inflammation, and therefore, theoretical, and possible regression. Treatment of inflammation in the coronary artery would achieve a better cost-benefit in IHD treatment, the costs of diagnosis and treatment, absenteeism, disability, and mortality of the patients would be reduced. The question arises as to whether additional antibiotic therapy due to the potential involvement of plaque-afflicted microbes and vitamin B complex therapy due to the positive effect on homocysteine metabolism may influence the aterogenesis and inflammation of atherosclerotic plaques of coronary arteries and obtain benefit in the treatment of IHD.
Goals of the investigation are:
Determine the plasma levels of cytokines (IL-2, IL-6, TNF-α), CRP and amyloid as inflammatory markers in the investigated groups and determine the plasma levels of homocysteine and LDL, CK, CK- troponin, and BNP.
Determine the correlation between the value of the examined markers and the systolic function of LV.
Apply an additional therapeutic treatment with ampicillin, cyancobalamin, folacin and vitamin B complexes (B1, B2, B6) in subjects.
Determine the possible relationship between the values of the examined markers and the applied therapeutic treatment with MACE in IHD.
2. MATERIAL AND METHODS
A prospective, comparative, analytical, clinically applicable open type study was performed, which included 80 subjects, of both genders, divided into the experimental (38) and control group (42). The study was conducted on the basis of a common approach to a patient including anamnesis, physical examination, ECG, echocardiography and laboratory tests at the Clinic for Heart, Blood Vessel and Rheumatic Diseases, in Intensive Coronary Unit. Blood samples were analyzed by the Central Laboratory for Clinical Biochemistry and Immunology Institute CCUS. The research was conducted in 2008. Inclusion criteria were: unstable angina pectoris, vasospastic angina pectoris, acute myocardial infarction without ST elevation (NSTEMI), acute myocardial infarction with ST elevation (STEMI). The non-inclusion criteria were: previous cardiac valve disease, advanced cardiomyopathy, associated renal insufficiency, acute infection, seropositive rheumatic diseases, malignancy, pregnancy, and allergy to penicillin and vitamin B complex. The exclusion criteria were allergies during the research and the patient’s non-cooperation. STEMI patients were initially treated with fibrinolytic therapy-streptokinase, and all patients were treated with enoxaparin in intrahospital treatment and under the therapy of statin, aspirin, ACE inhibitors, beta blockers and nitro preperations. The experimental group had in th. addition ampicillin 2 gr. daily for 7 days, and vitamin B complex (Polibevit: B1 4.0 mg, B2 5.0 mg, B6 2 mg) 3 x daily with cyancobalamin 500 μg 2 daily and folic acid 5 mg 1 x daily perorally, 1 month. Laboratory control of blood on cardiac biohumoral markers: troponin, CK, CK MB, BNP; and inflammatory markers: CRP, amyloid, cytokine IL-2, IL-6, TNF-α; with homocysteine and LDL as atherogenic markers. On the tenth day of hospitalization, laboratory blood tests on the same markers and echocardiography were performed. One year later, a control echocardiographic examination was performed and adverse cardiovascular events were followed: postinfarctic angina pectoris and myocardial reinfarction, need for surgical revascularization, heart failure (NYHA II-IV), and death outcome. IL-2, IL-6 and TNF-α were determined by Elisa assays that are enzymatically mediated immunoassays for quantitative determination of human cytokines in serum. Concentration of CRP was determined by laser nefelometry (BM II analyzer), with a reference value of 0-5 mg/L. Determination of the serum amyloid was performed by a non-ephemeral method. The reference serum amyloid value determined by this method is up to 6.4 mg/L. Homocysteine in serum was determined from blood samples in gel tubes that were transported on ice to the Central Laboratory. The concentration of homocysteine is quantitatively determined by the method of fluorescence polarization immunoassays on AxSYM system, with a normal value of 5-15 μmol/L, and preferably 9-10 μmol/L. LDL concentration is determined according to the Friedvald formula: LDLC = UH–HDLC–VLDLC, and the concentration of total cholesterol, triglyceride and HDL cholesterol for its conversion is determined by the enzymatic, colorimetric method on the Dade Dimension AR analyzer. Troponin I concentration was determined quantitatively by hemiluminescence immunoassay with STAT Troponin-I reagent Abbot architect system. The upper reference value of troponin concentration by this method is 0.4 ng / mL. CK was determined by quantitative kinetic UV method, while CK-MB activity was determined by quantitative immunoinhibitory kinetic UV test. For laboratory measurement of BNP, a microparticulate enzyme immunoassase for quantitative determination of human BNP in EDTA plasma was used, on the AXSYM system. Transthoracic echocardiography was performed on the TOSHIBA POWER VISION 7000, and systolic function LV was estimated through ejection fraction (EF) by Simpson method. Statistical analysis was performed using MedCalc for Windows version 11.2.0.0. Mann Whitney’s test and Spearman’s coefficient rank correlation ρ (rho) were applied. The correlations between the outcomes were calculated using the chi-square test. Values for which p<0.05 were accepted as statistically significant.
3. RESULTS
Results are presented by tables and graphs. There was no statistically significant difference in age and gender between the analyzed groups. Of the tested CRP markers, amyloid, homocysteine and BNP were in positive correlation with MACE. In the experimental group with the applied additional treatment there was a significant decrease in these markers and significantly less frequent MACE. Student’s T-test for independent samples compared the LVEF findings. There was a significant difference in the results between the experimental (M = 51.5,000, SD = 6.9038) compared to the control (M = 48.1905, SD = 6.3370) group t (78) = -2.236, p = 0.0282 (two-sided). The difference between mean values per group (mean difference = -3.3095, 95%, CI: -6.2566 to -0.3624) was moderate. MACE by groups (PA–postinfarctic angina, RMI–Repeated myocardial infarction, SRM–surgical myocardial revascularization, CI–cardiac insufficiency, D–death)
OR = ad / bc = 16 * 5/37 * 22 = 80/814 = 0.0983
In the experimental group, MACE was observed in 16 subjects within 12 months and in the control group in 37 subjects.
There was no a statistically significant imbalance by age and gender groups tested in this research.
Since the tested markers CRP, amyloid, homocysteine and BNP were positively correlated to MACE by the reference. In the experimental group with the applied additional treatment was a significant drop in these markers and significantly less frequent adverse cardiac parent events.
Student t-test for independent samples were compared to the results of the LVEF at baseline. A significant difference in the results between the experimental (M = 51.5000, SD = 6.9038) compared to control (M = 48.1905, SD = 6.3370) group t (78) = -2.236, p = 0, 0282 (both sides). The difference between means of the groups (mean difference = -3.3095, 95% CI: -6.2566 to -0.3624) was moderate.
Major adverse cardiac events * (PA–postinfarction angina, RMI–reinfarction, SR–surgical revascularization, SI–cardiac insufficiency, D- death)
OR = ad / bc = 16 * 5/37 * 22 = 80/814 = 0.0983 in the experimental group in 16 followed were recorded by major adverse cardiac events root (Nkido) for 12 months in the control group 37 subjects. Based on the result obtained by the OR these results OR = 0.0983, 95% CI = 0.0316 to 0.3056, z = 4.008, p = 0.0001.
Chi-square test showed a significant difference between the applied treatment and outcome. p <0.0001, r = 0.417. Chart for Chi-square test based on the frequency of MACE originating in both groups. MACE are significantly more frequent in the control compared to a experimental group of respondents.
4. DISCUSSION
It is known that 50% of patients with IHD have no known risk factors. It is assumed that in such cases inflammatory reactions have a significant role in the pathogenesis of ischemic heart disease (7). The associationof IHD and some infectious agents such as Chlamydia pneumoniae, Helicobacter pylori, Herpes Simplex Virus, CytoMegaloVirus and Hepatitis A, respiratory tract infections and dental infections are mentioned in earlier epidemiological studies (8, 9). The Helsinki Heart Study suggests that chronic infection with Chlamydia pneumoniae can be a significant risk factor for IHD (10). The presence of Helicobacter pylori DNA was demonstrated in the aortic wall in atherosclerotic plaques of most patients with IHD. Jaffarzadeh et al.
Studied anti-Helicobacter pylori IgG seroprevalence and anti-Helicobacter pylori antibody titre titer, and finds that it is significantly higher in IHD patients than in the control group (11). In the CLARIFY study of 148 subjects with unstable angina and non-Q MI, treated with 500 mg of clarithromycin daily 3 months, AMI and heart failure were observed, and better outcome was observed in placebo vs. p = 0.015. (12) The AZACS study with 1450 subjects in the 5-day period did not demonstrate the benefit of such short-term antibiotic therapy (13). In 12 studies vitamin B12 in the 5-year period (16), while NORVIT study lasting 40 months showed a decrease in homocysteine, but a paradoxical increase in vascular risk in patients with combined folacin and vitamin B6 therapy (17). The SEARCH study, conducted on 12,000 subjects divided into two groups according to the dose of statin simvastatin (80mg: 20mg + both groups of folic acid 2mg and B12 1mg, 6.7 years), had more frequents myopathy (18). In most studies conducted with antibiotics, macrolide antibiotics were used, ampicillin was used in this paper for its good tolerability, broad spectrum of activity, and the cost of the cost. Ampicillin is a molecular formula C16H19N3O4S, and belongs to organosulfuron compounds including alicycline, cysteine, methionine and alpha lipoic acid. More recently, the current theory of sulphate deficiency is the cause of atherosclerosis, that is, of the deficiency of cholesterol sulphate (19,20). There is growing discussion about the positive role of sulphates in metabolism and prevention of oxidative stress, and their cardioprotective action. It is possible that the choice of ampicillin in the work, not some macrolide antibiotics, in combination with folacin, cyancobalamin and vitamin B6, which are necessary co-factors in the metabolism of homocysteine, was the key to success and more favorable outcome in the treatment of the study group. Namely, the American Food and Drug Administration (FDA) issued a 2013 warning that the macrolide antibiotic azithromycin could potentially cause fatal heart rhythm disorders due to the risk of prolongation of the QT interval (22, 23, 24), although it was apparent that its proaritmogenic effect was lower in compared to other macrolides (25). In the 2014 meta-analysis, the use of azithromycin was analyzed for the purpose of secondary prevention of MACEs and there was no proven benefit of its use compared to placebo (26). Ampicillin has been used for the prevention of endocarditis in valve patients for years and, as far as it is known, there have been no warnings about the detriment of its effect on cardiac patients. In our research, no respondent was excluded from the experimental group due to the intolerability of the treatment. Furthermore, no correlation or statistically significant correlation between the value of the investigated cytokines: IL-2, IL-6 and TNF-α, as well as LDL, CK, CKMB and troponin and MACE, was not demonstrated in oneyear follow-up. There was a strong positive correlation between MACE and CRP (p = 0,0002), amyloid (p = 0,0005), homocysteine (p = 0,0002), and moderate for BNP (p = 0,0403), so the applied treatment in the experimantal group led to a significant fall in their values, and these markers proved to be prognostic because the number of subjects in the experimental group was significantly lower in the MACE. The echocardiographically monitored systolic function, by measuring the EF LV method by Simpson, showed a moderate difference in the groups with average higher values in the experimantal group (p = 0.0282). The additional therapeutic treatment with ampicillin, vitamin B complex, folacin and cyancobalamin in the study team significantly reduced MACE and demonstrated potential antiatherogenic anti-inflammatory effects. The proportion of patients with MACE was significantly lower in the working group, Χ2 = 16,871, p <0,0001, K = 0,417 (Yates’ Correction for Continuity) was applied.
5. CONCLUSION
Examined additional treatment in IHD, with ampicillin and vitamin B complex, folacin and cyancobalamin showed potential anti-atherogenic and anti-inflammatory effects and statistically significantly reduced the risk of MACE. Such therapeutic treatment should be investigated on a large number of subjects and considered such a therapeutic approach to the prevention of adverse outcomes in ischemic heart disease.
Figure 1. Chart based on MACE frequency in both groups.
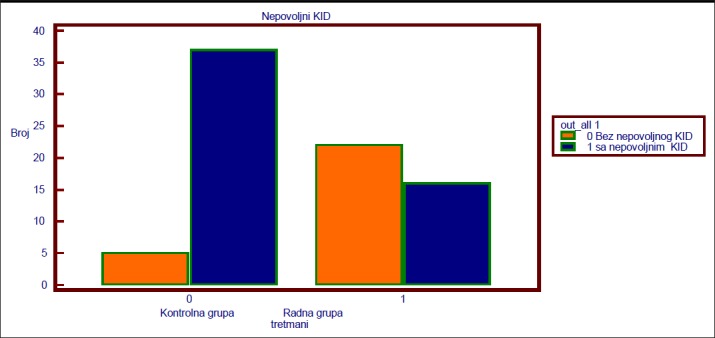
Table 1. Basic demographic characteristics of respondents.
| Demographic characteristics of respondents | Experimental group N=38 | Control group N=42 | P |
|---|---|---|---|
| Age | |||
| Mean (SD) | 55.42 (6.98) | 55.71 (9.60) | P = 0.8773 |
| Median | 54 | 56 | |
| Rank | 43 – 72 | 33 – 78 | |
| Gender | |||
| Female (%) | 13 (34%) | 13 (31%) | P = 0.9624 |
| Male (%) | 25 (66%) | 29 (69%) | |
Table 2. The correlation of the tested markers between the experimental and control groups.
| Marker | Rho (rank correlation) | N | p |
|---|---|---|---|
| IL-2 | Rho=0.0460 | 80 | p=0.7778 |
| IL-6 | Rho=0.234 | 80 | p=0.1463 |
| TNF-α | Rho=0.0447 | 80 | p=0.694 |
| CRP | Rho=0.407 | 80 | p=0.0002 |
| Amyloid | Rho=0.381 | 80 | p=0.0005 |
| Homocysteine | Rho=0.403 | 80 | p=0.0002 |
| LDL | Rho=0.102 | 80 | p=0.3659 |
| CK | Rho=0.0515 | 80 | p=0.6500 |
| CK MB | Rho=0.0784 | 80 | p=0.4896 |
| Troponin I | Rho=0.145 | 80 | p=0.1982 |
| BNP | Rho=0.230 | 80 | p=0.0403 |
Table 3. Statistical analysis of heart ECHO for LVEF–control measurement.
| Experimental group EF (%) | Control group EF (%) | |
|---|---|---|
| Sample | 38 | 42 |
| Mean | 51.5000 | 48.1905 |
| 95% CI of mean | 49.2308 to 53.7692 | 46.2157 to 50.1652 |
| Variance | 47.6622 | 40.1580 |
| SD | 6.9038 | 6.3370 |
| SEM | 1.1199 | 0.9778 |
Table 4. Statistical analysis of the relationship of probable linkage (ODS).
| Groups | Major adverse cardiac events PA (%) RIM(%) SRM (%) CI (%) D (%) | Total N | ||||
|---|---|---|---|---|---|---|
| Experimental | 6 16% | 1 2.5% | 5 13% | 3 8% | 1 2.5% | 38 |
| Total | 16 42% | |||||
| Control | 13 31% | 5 12% | 8 19% | 10 42% | 1 2.0% | 42 |
| Total | 37 88% | |||||
Table 5. Chi-square test for evaluation of treatment outcome. (MACE*-unfavorable cardiac outcome event).
| Codes X | |||
|---|---|---|---|
| Codes Y | Control | Experimental | |
| No UCOE* | 5 | 22 | 27 (33.7%) |
| UCOE | 37 | 16 | 53 (66.2%) |
| 42 (52.5%) | 38(47.5%) | 80 | |
| Chi-square (Χ2) | 16.871 | ||
| DF | 1 | ||
| Significance level | P < 0.0001 | ||
| Contingency coefficient | 0.417 | ||
Acknowledgment
The author wishes to thank for his contribution to the work, during statistical data processing to Begler Begovic.
Competitive interest
No competing interests.
Conflict of interest
The author has no conflict of interest.
REFERENCES
- 1.Masic I, Rahimic M, Dilic M, Kadribasic R, Toromanovic S. Socio medical Characteristics of Coronary Diseases in Bosnia and Herzegovina. Mater Sociomed. 2011;23(3):171–83. doi: 10.5455/msm.2011.23.171-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masic I, Dilic M, Raljevic E, Vulic D, Mott D. Trends in Cardiovascular diseases in Bosnia and Herzegovina and Perspectives with HeartScore Programme. Med Arh. 2010;64(4):260. doi: 10.5455/medarh.2010.64.260-263. [DOI] [PubMed] [Google Scholar]
- 3.Kam SW. Persual of risk stratification of acute myocardial infarction for half a century. Eur Hear J. 2009. pp. 1030–32. [DOI] [PubMed]
- 4.Ford ES, Smith SJ, Strup DF, Steinberg KK, Mueller PW, Thacker SB. Homocysteine and cardiovascular disease: a systemic review of the evidence with special emphais on case-control studies. Int J epidemiol. 2002;31(1):59–70. doi: 10.1093/ije/31.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw Pepine CJ, Sharaf B, Merz NB, Sopko G, Olson MB, Reis SE. Serum Amyloid A as a Predictor of Coronary Artery Disease and Cardiovascular Outcome in Women. Circulation. 2004;109:726–73. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 6.Katayama T, Nakashima I, Honda Y, Suzuki S, Yamamoto T, Iwasaki Y, Yano L, et al. The Relationship Between Acute Phase Serum Amyloid A (SAA) Protein Concentrations and Left Ventricular Systolic Function in Acute Myocardial Infarction Patients Treated With Primary Coronary Angioplasty. Int Heart J. Jan;200:45–55. doi: 10.1536/ihj.48.45. [DOI] [PubMed] [Google Scholar]
- 7.Hodzic E, Perla S, Iglica A, Vucijak M. Seasonal Incidence of Acute Coronary Syndrome and its Features. Mater Sociomed. 2018 Mar;30(1):10–14. doi: 10.5455/msm.2018.30.10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruijter W, Westendorp RGJ, Assendelft WJJ, Eizen WPJ, Craen AJM, Cessie S, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha HC, Prasad J, Mittal A. High immunoglobulin A seropositivity for combined Chlamydia pneumoniae, Helicobacter pylori infection, and high-sensitivity C-reactive protein in coronary artery disease patients in India can serve as atherosclerotic marker. Heart Vessels. 2008;23(6):390–6. doi: 10.1007/s00380-008-1062-9. [DOI] [PubMed] [Google Scholar]
- 10.Reszka E, et al. Detection of infectious agents by polymerase chain reaction in human aortic wall. Cardiovascular Pathology. 2008;17(5):297–302. doi: 10.1016/j.carpath.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman MR, Manninen V, Manttari M, Frick MH, Huttunen JK. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;11:273–8. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 12.Jafarzadeh A, Esmaeeli-Nadimi A, Nemati M, Tahmasbi M, Ahmadi P. Serum concentrations of Helicobacter pylori IgG and the virulence factor CagA in patients with ischaemic heart disease. East Mediterr Health J. 2010;16:1039–44. [PubMed] [Google Scholar]
- 13.Cercek B, Shah PK, Noc M, Zahger D, Zeymer U, Matetzky S, Maurer G, Mahrer P. AZACS Investigators. Effect of short-term therapy wiith azithromycin on recurrent ischaemic events in patients with acute coronary syndrome in the Azithromycin in Acute Coronary Syndrome (AZACS) trial: a randomised controlled trial. Lancet. 2003 Mar 8;361(9360):809–13. doi: 10.1016/S0140-6736(03)12706-7. [DOI] [PubMed] [Google Scholar]
- 14.Moens AL, Claeys MJ, Wuyts FL, Goovaerts I, Van Hertbruggen E, Wendelen LC, Van Hoof VO, Vrints CJ. Effect of folic acid on endothelial function following acute myocardial infarction. Am J Cardiol. 2007;99:476–81. doi: 10.1016/j.amjcard.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, Eberli FR, Meier B, Turi ZG, Hess OM. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 16.The Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354 doi: 10.1056/NEJMoa060900. Published online before print on March 12, 2006 ./ https://clinicaltrials.gov/ct2/show/NCT00106886. [DOI] [PubMed] [Google Scholar]
- 17.Bonaa KH, Njolstad I, Ueland PM, Schrimer H, Tverdal A, Steigen T, Weang H, Nordrehaug JE, Arnesen E, Rasmnussen K. NORVIT Trial Investigetors. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006 Apr 13;354(15):1578–88. doi: 10.1056/NEJMoa055227. Epub 2006 Mar 12. [DOI] [PubMed] [Google Scholar]
- 18.MacMahon M, Kirkpatrick C, Cummings CE, Clayton A, Robinson PJ, Tomiak RH, Liu M, Kush D, Tobert J. A pilot study with simvastatin and folic acid/vitamin B12 in preparation for the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Nutrition, Metabolism, and Cardiovascular Diseases : NMCD. 2000 Aug 01;10(4):195–203. [PubMed] [Google Scholar]
- 19.Hollertz O. Sulphur: the vulnerable factor X in atherosclerosis Medical Hypotheses. 2002 Jul;59(1):35–8. doi: 10.1016/s0306-9877(02)00193-7. [DOI] [PubMed] [Google Scholar]
- 20.Seneff S, Davidson RM, Lauritzen A, Samsel A, Wainwright G. A novel hypothesis for atherosclerosis as a cholesterol sulfate deficiency syndrome. Theor Biol Med Model. 2015 May 27;12:9. doi: 10.1186/s12976-015-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Tang C, Jin H, Du J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis. 2011 Apr;215(2):323–30. doi: 10.1016/j.atherosclerosis.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Juurlink David N. The cardiovascular safety of azithromycin. CMAJ. 2014 Oct 21;186(15):1127–1128. doi: 10.1503/cmaj.140572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WA Ray, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee RA, Guyton A, Kunz D, Cutter GR, Hoesley CJ. Evaluation of baseline corrected QT interval and azithromycin prescriptions in an academic medical center. J Hosp Med. 2016 Jan;11(1):15–20. doi: 10.1002/jhm.2448. [DOI] [PubMed] [Google Scholar]
- 25.Baker WL, Couch KA. Azithromycin for the secondary prevention of coronary artery disease: a meta-analysis. Am J Health Syst Pharm. 2007 Apr 15;64(8):830–6. doi: 10.2146/ajhp060539. [DOI] [PubMed] [Google Scholar]


