Abstract
Purpose
Our understanding of the temporal dynamics and age-specific mortality patterns of the 1918–1921 influenza pandemic remains scarce due to lack of detailed respiratory mortality datasets in the United States and abroad.
Methods
We manually retrieved individual death records from Arizona during 1915–1921 and applied time series models to estimate the age specific mortality burden of the 1918–1921 influenza pandemic. We estimated influenza-related excess mortality rates and mortality rate ratio increase over baseline based on Pneumonia and Influenza (P&I), respiratory, tuberculosis and all-cause death categories.
Results
Based on our analysis of 35,151 individual mortality records from Arizona, we identified three successive pandemic waves in spring 1918, fall 1918–winter 1919 and winter 1920. The pandemic associated excess mortality rates per 10,000 population in Arizona was estimated at 83 for P&I, 86 for respiratory causes, 84 for all-causes and 9 for tuberculosis. Age-specific P&I and tuberculosis excess death rates were highest among 25-44-year-olds and individuals ≥65 years, respectively. The 25-44-year-olds and 5-14-year-olds had highest P&I and tuberculosis mortality impact respectively when considering the ratio over background mortality.
Conclusions
The 1918–1921 influenza pandemic killed an estimated 0.8% of the Arizona population in three closely spaced consecutive waves. The mortality impact of the fall 1918 wave in Arizona lies in the upper range of previous estimates reported for other US settings and Europe, with a telltale age distribution of deaths concentrated among young adults. We identified a significant rise in tuberculosis-related mortality during the pandemic, lending support to the hypothesis that tuberculosis was a risk factor for severe pandemic infection. Our findings add to our current understanding of the mortality impact of this pandemic in the US and globally.
Keywords: Arizona, Cause of death, Excess mortality, Influenza pandemic, 1918–1919, Tuberculosis, Age pattern, Timeseries
Introduction
Pandemic preparedness may be enhanced through a detailed understanding of past pandemics. In particular, the 1918–20 influenza pandemic, commonly referred to as the “Spanish” flu, is the most devastating influenza pandemic on record [1]. It caused an estimated 50-100 million deaths globally, with a mortality rate of 2.5-5 per 1,000 and approximately 675,000 deaths in the United States alone [1]. In contrast to seasonal influenza epidemics that primarily affect the very young and elderly [2]; the 1918–20 pandemic was characterized by an atypical mortality elevation among young adults [3, 4]. It has been estimated that half of the influenza-related deaths associated with this pandemic occurred among young adults 20-40 years [2, 3]. In parallel, several studies in Europe [5, 6], and the US [7] reported low or negative excess mortality among senior populations, suggesting a substantial clinical protection in this age group. Another unusual feature of this pandemic is the rapid succession of pandemic waves over a period of 9-12 months [2, 3, 8].
Up to two distinct pandemic waves have been identified in 1918 in a number of areas of the world including US cities [7, 9, 10]. The first “herald” wave likely started between February [7] and March 1918 [2] followed by a major pandemic wave in September of the same year [2, 7, 9]. Estimates of the mortality impact of this pandemic relying on mortality data from 24 US states with vital registration systems in place during the pandemic range from 0.25% in Wisconsin to 1% in Colorado [11]. These estimates are imprecise however, as they rely on analyzing annual all-cause mortality data, an approach that poorly controls for background deaths unrelated to influenza. More refined quantitative mortality studies based on daily, weekly or monthly respiratory and all-cause mortality data have estimated the excess pneumonia and influenza (P&I) mortality rate at 51.8 and 42.9 per 10,000 populations in New York City [7] and Kentucky [10] during the fall pandemic wave, respectively. Further studies are needed for a more comprehensive account of the pandemic impact in the US; however intensive efforts to retrieve historical individual-level mortality data make such detailed studies prohibitive [12].
A long-standing debate surrounding the 1918 pandemic is the potential role of tuberculosis in driving the unusually high impact of this outbreak, as tuberculosis was predominant among adults in the early 20th century [13]. It has been observed that tuberculosis mortality in the United States increased sharply during the 1918–1919 pandemic period, followed by a significant decline in tuberculosis mortality rates in the subsequent 2 years, compared to rates during the pre-pandemic period [14, 15]. However, it is unknown whether such patterns are consistent at different spatial scales.
A better understanding of the factors that shaped the mortality patterns during the 1918–1920 influenza pandemic in diverse geographic settings can lead to improved pandemic preparedness plans [16]. Here we set out to comprehensively analyze the age-specific absolute and relative mortality impact of the pandemic and the role of tuberculosis in the state of Arizona using 35,151 mortality records manually retrieved from the Arizona Genealogy Database in the years surrounding the pandemic.
Methodology
Study setting
The state of Arizona is located in the southwest United States, bordering Mexico. It became a state of the US only a few years prior to the 1918 influenza pandemic. Arizona’s climate is arid with a landscape ranging from low elevation deserts in the south to mountains and forests in the north. Arizona’s population increased by 64% in 10 years, from 204,354 in 1910 to 334,162 in 1920 [17].
During late 1800s and early to mid-1900, the state of Arizona was a popular destination for individuals seeking a cure for diseases such as tuberculosis [18, 19]. It was believed that the arid climate of Arizona would facilitate recovery of individuals afflicted by tuberculosis [18, 20]. During the early 1900s, Arizona had already established health institutions like the Pamsetgaaf sanatorium (established 1903, for cases of pulmonary and laryngeal tuberculosis), St. Luke’s Home (established 1907 for incipient cases), East Farm Sanatorium (established 1909 for American Indian tuberculosis patients) and Maricopa hospital (established 1909 for advanced cases) [21, 22]. As a result, Arizona was one of the states with the highest tuberculosis prevalence in the country [19] and hence it is a particular interesting area to study the interaction between the 1918 pandemic influenza virus and tuberculosis.
Data sources
Historical death records
The Arizona Genealogy Database is freely available online and contains mortality records during the years 1870–1996 in the state of Arizona. For our study, a total of 35,151 individual death certificates from January 1915 to December 1921 were manually retrieved from this database. For each death record, we compiled date of death, cause of death and age. We then created weekly and monthly mortality time series for the following death categories: pneumonia and influenza (P&I), respiratory causes, tuberculosis and all-causes stratified by 6 age groups: <5, 5–14, 15–24, 25–44, 45–64 and ≥ 65. Deaths due to respiratory causes included influenza, pneumonia, bronco-pneumonia, bronchitis and lung congestion as in previous studies [23, 24]. Information about age or cause of death was missing or could not be identified for only 0.34% (121 out of 35,151) of all the records.
We also derived the overall population size and age-specific population size estimates from 1915 to 1921 in the state of Arizona by the method of linear interpolation of population size from the 1910, 1920 and 1930 decennial censuses [17,25].
Statistical analysis
To quantify the mortality pattern associated with the 1918–21 influenza pandemic in the state of Arizona, we estimated excess mortality rates per 10,000 population across age groups by computing the mortality rate above a seasonal baseline of expected mortality rates in the absence of influenza activity, as in prior studies (e.g., [10, 26]).
Definition of pandemic periods
First, we determined the most likely period of pandemic influenza activity from the time-series of weekly P&I death rates (the most specific mortality outcome). Weeks associated with influenza activity were excluded from further modeling of baseline non-influenza mortality. Baseline mortality level was estimated by fitting cyclical Serfling regression models to P&I deaths in non-influenza weeks. Once a weekly baseline and 95% CI were obtained, periods of pandemic influenza circulation were defined as the weeks in 1918–1921 where observed total P&I mortality rate exceeded the upper 95% confidence limit of the baseline. The same pandemic waves were used for estimation of the total, age specific and cause specific excess mortality rates in line with prior work [10, 26].
Excess mortality estimation
Separate seasonal baseline models were fitted to age- and cause-specific weekly mortality time series after exclusion of pandemic weeks. Excess mortality was defined as the difference between the observed and model adjusted baseline mortality rates for each week constituting a pandemic wave. Negative excess mortality estimates were replaced by zero in our analyses. Overall pandemic excess mortality attributed to each cause for each age group and total population was calculated by summing excess death rates across pandemic waves in 1918–1921 [26]. We also calculated the ratio of observed mortality rate during each pandemic wave to the model predicted baseline mortality level in the absence of influenza for the given age category (RR). This ratio has been shown to standardize differences in background mortality across age groups (or countries).
Results
P&I and respiratory deaths accounted for 37% and 42% of the total recorded mortality in 1918, respectively, while these causes of death represented only an average of 10% and 16% of total mortality in pre-pandemic years (1915–1917). This indicates a 2 to 4-fold increase in P&I and respiratory deaths in 1918 compared to non-pandemic years. In the years following 1918, deaths due to P&I and respiratory deaths started to decline compared to 1918. Unlike P&I and respiratory deaths, tuberculosis deaths displayed a slight decline in 1918 compared to the pre-pandemic period and then subsequently increased to similar levels observed during the pre-pandemic period (17% in 1918, vs 19-25% in other study years) (Table 1).
Table 1.
Causes of deaths in Arizona from 1915–1921*
| Cause of death | 1915 n (%) | 1916 n (%) | 1917 n (%) | 1918 n (%) | 1919 n (%) | 1920 n (%) | 1921 n (%) |
|---|---|---|---|---|---|---|---|
| P&I** | 318 (8.83) | 424 (9.86) | 512 (10.94) | 2566 (37.23) | 957 (19.07) | 775 (13.93) | 441 (8.66) |
| Respiratory causes | 516 (14.32) | 672 (15.62) | 784 (16.74) | 2890 (41.93) | 1213 (24.17) | 1172 (21.06) | 736 (14.46) |
| Tuberculosis | 842 (23.38) | 819 (19.04) | 942 (20.12) | 1184 (17.18) | 1091 (21.74) | 1281 (23.02) | 1259 (24.73) |
| Others | 2264 62.85) | 2817 (65.48) | 3000 64.08) | 2926 (42.46) | 2805 (58.89) | 3189 (57.31) | 3151 (61.91) |
| Total number of deaths in the given year | 3602 | 4302 | 4682 | 6892 | 5019 | 5564 | 5090 |
Percentage is calculated out of total number of deaths in that year
Respiratory causes includes death due to influenza or pneumonia or bronco-pneumonia or bronchitis or lung congestion,
Death due to P&I is a part of respiratory causes that includes only pneumonia and influenza, multiple causes of deaths were recorded for only some cases
Timing of pandemic waves and excess mortality patterns
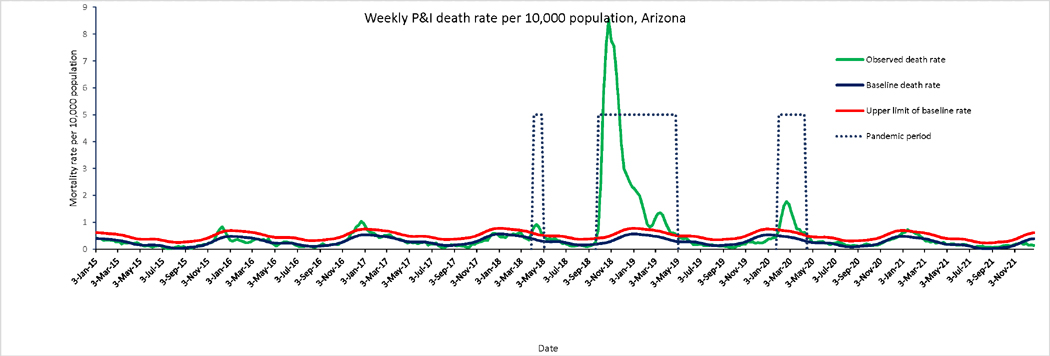
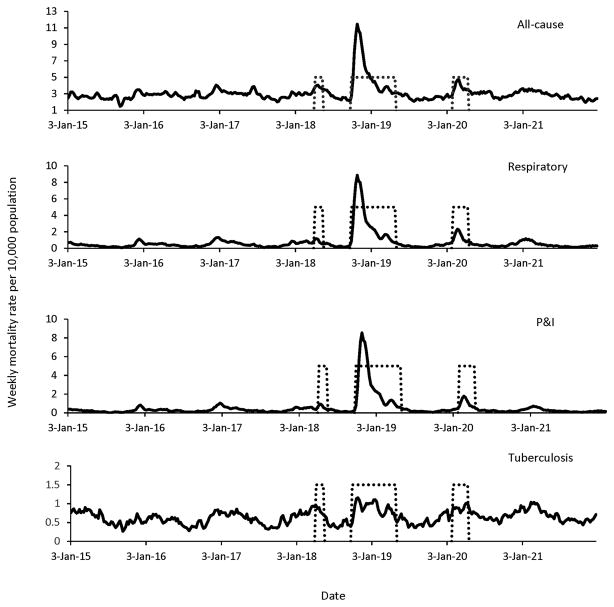
The time series of P&I mortality in Arizona (Figure 1) displays three successive waves of increased mortality, a brief episode in spring 1918 (April), a prolonged and intense wave in fall 1918–winter 1919 (October 1918–April 1919), and a wave of intermediate density in winter 1920 (February–April). We have described the data from the long second wave in two periods: Fall 1918 (October– December) and winter 1919 (January–April). Weekly time series for other causes of deaths are shown in Figure 2, overlaid with the pandemic periods. Peaks in respiratory and all-cause mortality aligned those in with P&I mortality, while tuberculosis mortality showed only weak departure from typical seasonal patterns in periods of pandemic activity.
Figure 1.
Weekly time series of P&I death rates per 10,000 population in Arizona, 1915–1921. The green line is the weekly P&I death rates. Dotted lines highlight pandemic waves. The Serfling seasonal regression model baseline (blue curve) and corresponding upper limit of the 95% confidence interval of the baseline (red curve) are also shown.
Figure 2.
Weekly time series of all the studied causes of death per 10,000 population in Arizona, 1915–1921. The dotted lines represent the time period of high mortality associated with pandemic waves occurring in spring of 1918 (April), fall 1918 (October–December), winter 1919 (January–April) and winter 1920 (February–April)
In the spring 1918 wave, the rate of P&I mortality was between 1.5 to 4.2 times the baseline mortality depending upon the age group. For the fall 1918 wave, the P&I mortality was more pronounced, ranging from 2.5 to 26.1 times. Similarly, this rate ranged from 1.7 to 5.2 times the baseline in winter 1919 and from 1.6 to 4.6 in winter 1920. There was a similar pattern for respiratory mortality rates. Tuberculosis mortality rate increase ranged from 1.2 to 2.2 times the baseline levels in spring 1918 wave, 1.4 to 5.5 times in fall wave, 1.3 to 4.9 times in winter 1919 and 1.2 to 4.1 times in winter 1920 wave. Total pandemic attributed excess mortality rate per 10,000 population in Arizona was estimated at 82.8 for P&I, 86.1 for respiratory causes, 84.1 for all-causes and 8.6 for tuberculosis (Table 2).
Table 2.
Estimates of excess mortality rate per 10,000 population and rate ratio (RR) attributable to pandemic influenza, by time of pandemic wave, age and cause of death, Arizona, 1918–1921
| Time, Cause of death | Excess death rate per 10,000 population, by age (RR) | Excess deaths by total population (RR) | |||||
|---|---|---|---|---|---|---|---|
| <5 | 5–14 | 15–24 | 25–44 | 45–64 | ≥65 | ||
| P&I deaths | |||||||
| Spring 1918 (April, 1918) | 1.80 (1.46) | 0.24 (2.03) | 1.68 (2.78) | 3.31 (4.18) | 0.76 (1.43) | 1.48 (1.68) | 1.78 (2.32) |
| Fall 1918 (Oct to Dec, 1918) | 66.52 (6.68) | 19.51 (16.37) | 66.28 (22.30) | 93.91 (26.12) | 30.98 (5.88) | 39.49 (2.47) | 60.85 (13.13) |
| Winter 1919 (Jan to April, 1919) | 11.95 (1.70) | 3.08 (2.98) | 12.56 (3.46) | 23.93 (5.28) | 6.71 (1.75) | 7.55 (1.86) | 12.79 (2.63) |
| Winter 1920 (1st Feb to 11th April, 1920) | 16.51 (2.21) | 1.80 (3.15) | 2.91 (1.97) | 11.33 (4.60) | 3.28 (1.69) | 12.79 (1.62) | 7.42 (2.58) |
| Total pandemic period | 96.78 (3.10) | 24.63 (7.31) | 83.43 (7.86) | 132.48 (10.80) | 41.73 (2.91) | 61.32 (2.05) | 82.84 (5.38) |
| Respiratory deaths | |||||||
| Spring 1918 (April, 1918) | 1.67 (1.20) | 0.24 (1.58) | 1.72 (2.58) | 3.66 (3.83) | 0.82 (1.40) | 6.65 (2.23) | 2.02 (1.96) |
| Fall 1918 (Oct to Dec, 1918) | 71.56 (4.28) | 20.58 (12.40) | 69.18 (20.80) | 95.01 (22.22) | 32.63 (5.41) | 34.43 (2.13) | 62.56 (9.70) |
| Winter 1919 (Jan to April, 1919) | 9.13 (1.25) | 2.75 (2.17) | 13.13 (3.14) | 24.86 (4.67) | 9.97 (1.87) | 8.05 (1.62) | 13.01 (2.09) |
| Winter 1920 (1st Feb to 11th April, 1920) | 21.18 (1.80) | 1.85 (2.36) | 3.07 (1.82) | 11.83 (4.06) | 5.01 (1.81) | 15.73 (1.60) | 8.50 (2.14) |
| Total pandemic period | 103.54 (2.11) | 25.42 (5.29) | 87.10 (7.03) | 135.36 (9.25) | 48.43 (2.78) | 64.86 (1.86) | 86.09 (3.99) |
| Tuberculosis | |||||||
| Spring 1918 (April, 1918) | 0.08 (1.40) | 0.07 (1.34) | 1.17 (1.39) | 1.09 (1.21) | 0.59 (1.26) | 2.94 (2.16) | 0.73 (1.27) |
| Fall 1918 (Oct to Dec, 1918) | 0.43 (1.79) | 0.97 (5.54) | 3.46 (1.47) | 11.09 (1.84) | 3.31 (1.38) | 6.60 (1.70) | 4.99 (1.65) |
| Winter 1919 (Jan to April, 1919) | 0.73 (1.65) | 0.59 (4.90) | 2.25 (1.25) | 3.81 (1.27) | 1.83 (1.29) | 7.60 (1.83) | 1.94 (1.24) |
| Winter 1920 (1st Feb to 11th April, 1920) | 1.02 (1.99) | 0.43 (4.05) | 4.19 (1.54) | 1.16 (1.13) | 1.44 (1.42) | 4.30 (2.59) | 0.98 (1.14) |
| Total pandemic period | 2.26 (1.78) | 2.06 (3.95) | 11.07 (1.41) | 17.15 (1.41) | 7.16 (1.34) | 21.44 (1.90) | 8.64 (1.33) |
| All cause | |||||||
| Spring 1918 (April, 1918) | 4.74 (1.13) | 0.98 (1.46) | 2.30 (1.31) | 3.86 (1.37) | 4.62 (1.34) | 14.04 (1.33) | 3.54 (1.29) |
| Fall 1918 (Oct to Dec, 1918) | 62.01 (1.77) | 21.33 (4.29) | 68.64 (4.39) | 100.39 (4.32) | 35.78 (1.75) | 32.45 (1.34) | 63.41 (2.72) |
| Winter 1919 (Jan to April, 1919) | 4.75 (1.07) | 3.28 (1.64) | 8.36 (1.37) | 19.95 (1.45) | 6.91 (1.15) | 10.30 (1.15) | 8.72 (1.17) |
| Winter 1920 (1st Feb to 11th April, 1920) | 15.73 (1.23) | 1.93 (1.59) | 6.65 (1.33) | 10.81 (1.36) | 2.91 (1.09) | 28.44 (1.23) | 8.38 (1.24) |
| Total pandemic period | 87.23 (1.34) | 27.52 (2.62) | 85.95 (2.22) | 135.01 (2.18) | 50.23 (1.35) | 85.23 (1.26) | 84.05 (1.63) |
Age specific excess mortality and RR pattern
Over the total pandemic period, age-specific excess death rates were highest among 25-44-year-olds followed by young children (<5 year olds) for P&I, respiratory, and all-cause deaths. For tuberculosis, however, the highest excess death rate during the pandemic period was observed among individuals ≥65 years followed by 25-44-year olds. Among ≥65-year olds, P&I and TB excess death rates per 10,000 population were 64.9 and 21.4, respectively. Similarly, among 25-44-year-olds, P&I and TB rates were 135.4 and 17.2 respectively (Table 2).
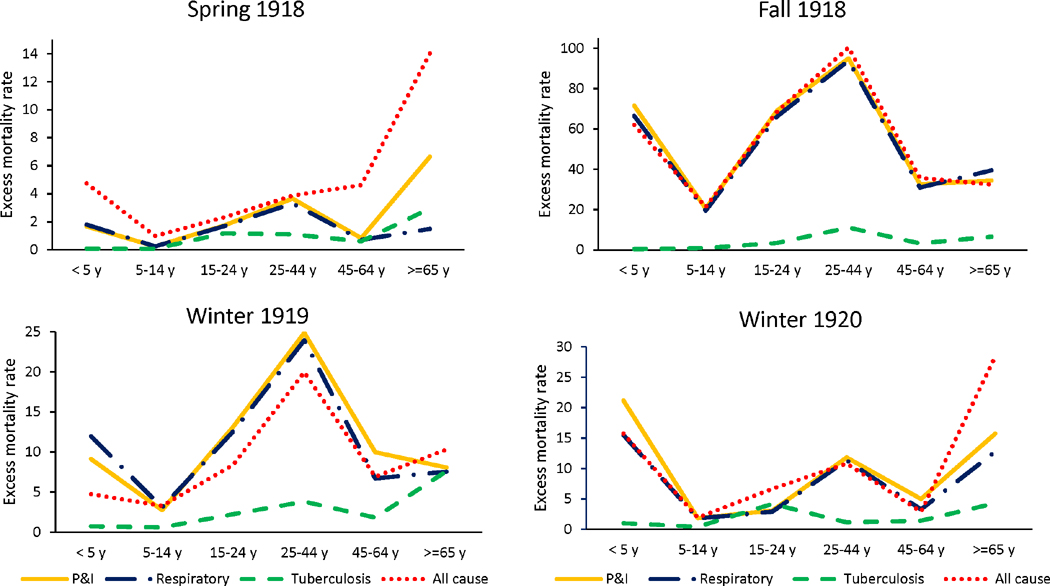
Figure 3 represents the curve of excess mortality rate, for P&I, respiratory and all-causes, by age group and pandemic wave. In the most intense pandemic wave in fall 1918–winter 1919, the influenza age mortality profile displayed a “broken W” like shape, with peak mortality in youngest children and young adults, and reduced excess mortality among the elderly compared to other age groups. In this lethal pandemic wave, excess mortality estimates from P&I, respiratory and all-cause mortality aligned particularly well. The herald wave in spring 1918 was characterized by a W-mortality profile, indicating that young adults were at high risk of mortality, but so were seniors. A similar profile of excess mortality was observed in the recrudescent wave in 1920. Of all 3 waves, individuals 5-64-years experienced highest P&I excess death rates in fall 1918–winter 1919.
Figure 3.
Age-specific excess mortality rate per 10,000 population for each pandemic wave.
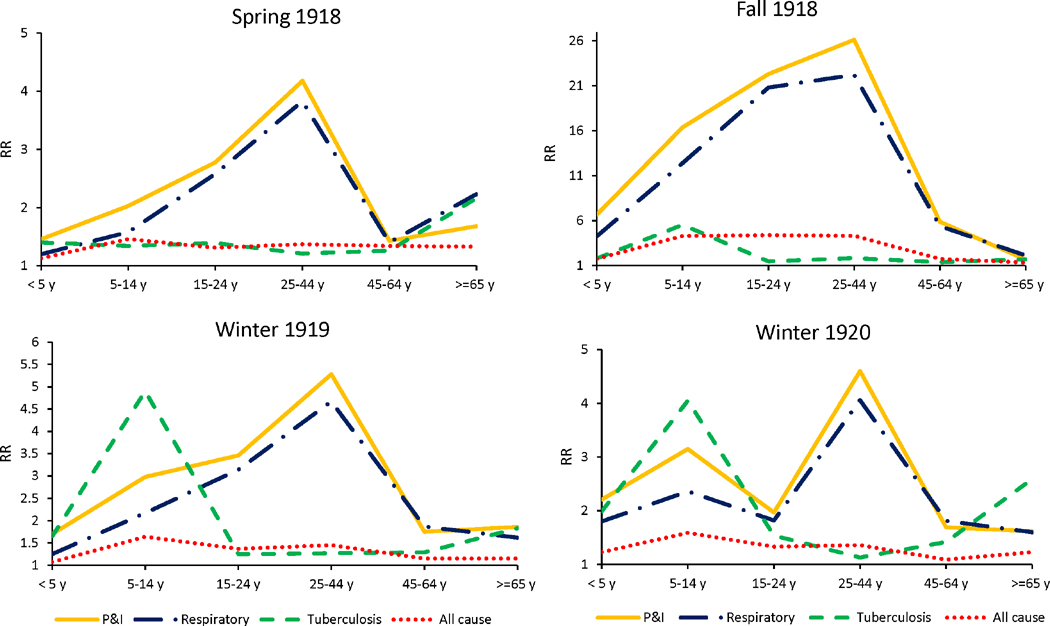
Interestingly, absolute and relative mortality rates showed different age patterns. The rate ratio (RR) for P&I and respiratory causes was highest among 25-44-year-olds across all pandemic waves (Figure 4). For both P&I and respiratory causes, excess death rates were lowest among 5-14-year-olds across pandemic waves but this was not the case for RR estimates. In most of the waves, P&I and respiratory RR was lowest among <5 years and ≥ 65 years age groups (Table 2). In addition, in the winter 1920 wave, two RR peaks were observed: one among individuals aged 25-44-years and another among individuals 5-14-years for both P&I and respiratory causes. As regards tuberculosis, the age group with highest excess mortality was individuals ≥ 65 years followed by adults 25-44 years. In contrast, the RR peaked among children 5-14 years across pandemic waves (except for the herald wave). The rise in TB excess mortality was balanced between the fall 1918 and winter 1919 waves, while most of the P&I and respiratory excess deaths were concentrated in the fall.
Figure 4.
Age-specific rate ratio of death (RR) for each pandemic wave.
Discussion
In this study, we characterized the mortality impact of three influenza pandemic waves during the 1918–1920 by age and cause of death in the state of Arizona. The pandemic was associated with an excess mortality rate per 100,000 of 82.8 for P&I, 86.1 for respiratory causes, 84.1 for all causes and 8.6 for tuberculosis. Our estimates fall in the upper range of estimates previously reported for several US settings [7, 10, 11]. While P&I, respiratory and all-cause excess mortality rates were higher among 25-44-year-olds, excess mortality from tuberculosis peaked among individuals ≥ 65 years followed by individuals 25-44 years.
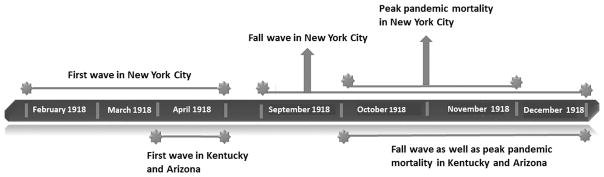
While a herald pandemic wave has been documented in February 1918 in New York City [7] and in April 1918 in Kentucky [10], our data suggest that the herald wave started the first week of April 1918 in Arizona. This information suggests that the pandemic likely had an early start in northeastern US [27]. The second and most lethal wave started in October in both Kentucky [10] and in Arizona, and in September in New York City [7]. This aligns with studies outside of the US, reporting waves of intense pandemic mortality from September to November 1918 [2]. The timing of peak pandemic activity in the US also suggests a westward travelling wave, starting in October–November 1918 in New York City [7] to October–December 1918 in Kentucky [10] and Arizona. Figure 5 compares the timing of the pandemic waves in 1918 for Kentucky, New York City and Arizona.
Figure 5.
Timeline of different pandemic events in 1918 in New York City, Kentucky and Arizona. Note: In New York City pandemic was defined based on monthly P&I mortality rate, in Kentucky it was based on daily all-cause mortality rate, and in Arizona it was based on weekly P&I mortality rates
Overall, we estimated the all-cause excess mortality rate of 0.84% associated with the 1918–1920 influenza pandemic in Arizona (Table 2). For comparison, a previous study based on all-cause annual mortality data for 24 US states reported an excess mortality rate of 0.84% for the state of Arizona [11], nicely in line with the results of our study. Among those 24 states, the excess mortality ranged from 0.25% in Wisconsin to 1% in Colorado, and Arizona ranked second in terms of excess mortality[11]. It is also noteworthy that these were based on annual mortality calculated from vital registration, while our study is based on weekly mortality estimated from individual death certificates and using more elaborate methods to estimate excess mortality. Compared to other parts of the world, the excess mortality rate in Arizona was found to be greater than in Madrid (0.53%) [28], Mexico City (0.7%) [12], Concepcion, Chile (0.76%) [26], Australia (0.29%) [11]; and it was lower than in Austria (1.61%), Japan (0.94%), Portugal (2.64%) [11]. Geographic variability in pandemic mortality impact is not entirely understood, but has been linked with factors such as socio-economic status, latitude, environmental conditions, climate and population density [11, 12, 26, 29].
We found that Arizona’s excess mortality rates for the fall 1918 pandemic wave based on P&I, respiratory and all-cause mortality data were higher than those reported for Kentucky [10] and New York City [7]. For example, for Arizona we estimated an all-cause excess mortality rate at 0.63% during the fall wave compared to 0.41% in Kentucky [10]. In New York City [7], this rate was 0.53% for fall 1918 and winter 1919 waves combined (September 1918 to April 1919). Similarly, excess P&I mortality rate for the fall wave in Arizona was 60.9 per 10,000 population compared to 51.8 in New York City [7] and 42.9 in Kentucky [10]. Compared to other countries, the all-cause excess mortality rate during the fall wave in Arizona (0.63%) was lower than in the city of Toluca, Mexico (1.62%) [12] and higher than in Mexico City (0.47%) [12], but comparable with the rate reported for Concepcion, Chile (0.64%) [26]. It is also remarkable that for the winter 1919 and winter 1920 waves, both all-cause and P&I excess mortality rates in Arizona were lower compared to those in Kentucky [10] and New York City[7], respectively. Hence the brunt of pandemic-related mortality was more intense and concentrated in time in Arizona than in New York City and Kentucky, perhaps due to differences in prior immunity, mixing, and the presence of vulnerable populations afflicted by tuberculosis.
Interestingly, a study from New York City [7] has shown that during early wave, individuals ≥ 65 years experienced little or no excess mortality based on all-cause deaths, and during the wave from September 1918 to April 1919, they experienced an excess mortality rate lower than that for individuals 5-14 years (see also [5]). Similarly, excess mortality rate for P&I, respiratory and all-cause was less among individuals ≥ 65 years compared to 5-18 years age group during the fall wave in Kentucky. Low excess mortality among senior populations has been explained as a result of protection provided by previous exposure to a similar virus [7, 30]. While we see some evidence of senior protection in the main pandemic wave in Arizona, fall 1918–winter 1919, consistent with these reports, we observe significant excess mortality among seniors during the herald 1918 wave and recrudescent 1920 wave. Perhaps these differences may be explained by differential circulation of respiratory viruses during each pandemic wave. Also, this age pattern is intermediate between the strong senior protection observed in New York City (based on all-cause mortality) or Copenhagen [7] and the lack of protection reported in Latin American settings including central Mexico [12], Colombia [31] and Chile [26]. Geographic differences in age mortality profiles likely reflect differences in levels of pre-existing immunity to influenza, based on circulation of antigenically-related viruses in a less connected world population in the 19th century [12, 32].
In a study of 35 large cities in the United States, respiratory tuberculosis was found to be an important contributor to excess mortality during 1918–20 influenza pandemic [6]. Of all the excess deaths from causes other than P&I, respiratory tuberculosis contributed around 19% of the total excess mortality [6]; tuberculosis excess mortality during the fall wave was as high as 21.4 per 10,000 population aged ≥ 65 year and 17.1 per 10,000 population aged 25-44 years. In the late 1800’s, the state of Arizona was promoted as a place of health, in the claim that the dry climate could help alleviate or even cure those afflicted by lung diseases, including tuberculosis (TB)[18]. Due to the westward movement of TB sufferers, Arizona experienced one of the highest TB death rates of all 48 continental US states in the mid-1920’s [18, 19]. Our data revealed that in Arizona, total tuberculosis excess mortality during 1918-1920 was 8.64 per 10,000 population. This number increased to 21.4 per 10,000 population for individuals ≥ 65 years and to 17.1 per 10,000 population for those aged 25-44 years. The fall wave showed highest overall TB excess mortality rate, although those <5 years suffered highest mortality rate in the winter of 1920. RR value for those <5 years and ≥ 65 years was also highest in the winter of 1920. Relative to the typical pre-pandemic patterns of TB, those 5-14 years had highest risk of death (RR of 3.95), which coincides with the age group with highest influenza attack rates during the pandemic. It is important to note that most studies have failed to detect an elevation in TB mortality associated with the 1918 influenza pandemic [15, 33], perhaps because these studies were not set in populations affected by high TB prevalence levels.
As for the indices to measure the mortality impacts, absolute (excess mortality rate) and relative mortality (RR) displayed substantially different age patterns. Absolute excess mortality rates could be useful to guide the amount of medical resources needed to confront a similar influenza pandemic. Relative mortality (RR), on the other hand, would be practical to prioritize a vulnerable age group when available medical resources are limited.
It is worth highlighting some limitations. First, we applied Serfling regression modeling to tuberculosis mortality series although the tuberculosis mortality signal did not display a marked seasonal pattern as the other mortality signals studied here. Second, due to the lack of laboratory confirmation at the time, our excess mortality approach would not have been able to distinguish elevation in mortality rates associated with other causes including other respiratory pathogens circulating at the time and coinciding with the pandemic period. For instance, mortality due to Respiratory Syncytial Virus (RSV) cannot be ruled out and may have inflated our pandemic mortality estimates particularly for infants. Another limitation of this methodology is that estimates of excess mortality by age groups are not guaranteed to be additive as noted previously [34, 35].
In conclusion, the impact of 1918 influenza pandemic was substantial in Arizona. The pandemic-associated excess mortality rate was higher than most of those reported elsewhere in the US, and comparable with settings in South America. Our data suggests an intermediate profile of clinical protection in senior population, relative to other locations in the US and Europe (pronounced senior sparing) and Latin America (low or no sparing) but the pattern is likely marred by co-circulation of pandemic influenza and other respiratory viruses. A moderate rise in tuberculosis mortality during the fall 1918 and winter 1919, with a telltale increase among young adults during the intense fall pandemic wave, brings support to the hypothesis that TB may have been a risk factor for influenza-related mortality. On the flipside, TB cannot be the sole reason for the intriguing rise in young adult mortality, which is unique to the 1918 pandemic, given the magnitude of RR estimates and age patterns identified here. Our data also suggests that interventions directed towards influenza of similar nature in the future should be based on assessment of the expected absolute and relative mortality patterns. Further influenza historical studies in settings of high and low tuberculosis prevalence, and remote locations, will bring more light on the mechanism of prior immunity and underlying risk factors for pandemic-related mortality. As the centennial of the 1918 pandemic approaches, it gives us pause to realize how little we understand about this major epidemic event.
Acknowledgments
We thank Nirmal Vijayavel, April Cobos, Justin Cheung, Indira Harahap, Shane Dwyer, and Andrew Soule for supporting early data collection efforts. SD was a Fulbright fellow during her MPH studies at the School of Public Health at Georgia State University. LD is a 2CI doctoral fellow at Georgia State University. KM acknowledges support from the Japanese Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K20936 and 26893048 and from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers Grant Number G2801. CV and GC acknowledge support from the Multinational Influenza Seasonal Mortality Study (MISMS), an on-going international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918-1920" Spanish" influenza pandemic. Bull Hist Med. 2002;76(1):105–15. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 2.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Rev Biomed. 2006;17:69–79. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018–28. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 4.Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med. 2009;360(25):2595–8. doi: 10.1056/NEJMp0903906. [DOI] [PubMed] [Google Scholar]
- 5.Luk J, Gross P, Thompson WW. Observations on mortality during the 1918 influenza pandemic. Clin Infect Dis. 2001;33(8):1375–8. doi: 10.1086/322662. [DOI] [PubMed] [Google Scholar]
- 6.Collins SD. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep (1896-1970) 1932:2159–79. [Google Scholar]
- 7.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci USA. 2005;102(31):11059–63. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowell G, Simonsen L, Viboud C. Death March of 1918. Natural History. 2017;125(9):11–3. [Google Scholar]
- 9.Reid AH, Taubenberger JK, Fanning TG. The 1918 Spanish influenza: integrating history and biology. Microbes infect. 2001;3(1):81–7. doi: 10.1016/s1286-4579(00)01351-4. [DOI] [PubMed] [Google Scholar]
- 10.Viboud C, Eisenstein J, Reid AH, Janczewski TA, Morens DM, Taubenberger JK. Age-and sex-specific mortality associated with the 1918–1919 influenza pandemic in Kentucky. J Infect Dis. 2013;207(5):721–9. doi: 10.1093/infdis/jis745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2007;368(9554):2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 12.Chowell G, Viboud C, Simonsen L, Miller MA, Acuna-Soto R. Mortality patterns associated with the 1918 influenza pandemic in Mexico: evidence for a spring herald wave and lack of preexisting immunity in older populations. J Infect Dis. 2010;202(4):567–75. doi: 10.1086/654897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noymer A. Testing the influenza–tuberculosis selective mortality hypothesis with Union Army data. Soc Sci Med. 2009;68(9):1599–608. doi: 10.1016/j.socscimed.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott A. The death rate from tuberculosis. Science. 1922;56(1449):387–8. doi: 10.1126/science.56.1449.387-a. [DOI] [PubMed] [Google Scholar]
- 15.Noymer A. The 1918 influenza pandemic hastened the decline of tuberculosis in the United States: an age, period, cohort analysis. Vaccine. 2011;29:B38–B41. doi: 10.1016/j.vaccine.2011.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowell G, Viboud C. Pandemic influenza and socioeconomic disparities: Lessons from 1918 Chicago. Proc Natl Acad Sci USA. 2016;113(48):13557–9. doi: 10.1073/pnas.1616537113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forstall RL. Population of states and counties of the United States: 1790-1990. U.S Department of Commerce and Bureau of the Census; 1996. [Google Scholar]
- 18.Grineski SE, Bolin B, Agadjanian V. Tuberculosis and urban growth: class, race and disease in early Phoenix, Arizona, USA. Health Place. 2006;12(4):603–16. doi: 10.1016/j.healthplace.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Rak MK. A Social Survey of Arizona. University Extension Division; 1921. [Google Scholar]
- 20.Waxham F. The outdoor treatment of Tuberculosis. JAMA. 1902;39:1392–3. [Google Scholar]
- 21.Jacobs PP. A tuberculosis directory : containing a list of institutions, associations, and other agencies dealing with tuberculosis in the United States. New York: National Association for the Prevention of Tuberculosis; 1911. [Google Scholar]
- 22.Trennert RA. The Federal Government and Indian Health in the Southwest: Tuberculosis and the Phoenix East Farm Sanatorium, 1909-1955. Pac Hist Rev. 1996;65(1):61–84. [Google Scholar]
- 23.Cobos AJ, Nelson CG, Jehn M, Viboud C, Chowell G. Mortality and transmissibility patterns of the 1957 influenza pandemic in Maricopa County, Arizona. BMC Infect Dis. 2016;16(1):405. doi: 10.1186/s12879-016-1716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowell G, Erkoreka A, Viboud C, Echeverri-Dávila B. Spatial-temporal excess mortality patterns of the 1918–1919 influenza pandemic in Spain. BMC Infect Dis. 2014;14(1):371. doi: 10.1186/1471-2334-14-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bureau USC. Census of population and housing. Available from: http://www.census.gov/prod/www/decennial.html.
- 26.Chowell G, Simonsen L, Flores J, Miller MA, Viboud C. Death patterns during the 1918 influenza pandemic in Chile. Emerg Infect Dis. 2014;20(11):1803. doi: 10.3201/eid2011.130632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsen L, Chowell G, Andreasen V, Gaffey R, Barry J, Olson D, et al. Herald Pandemic Waves in 1918: Importance for Contemporary Pandemic Response Strategies. Ann Epidemiol. doi: 10.1016/j.annepidem.2018.02.013. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Erkoreka A. The Spanish influenza pandemic in occidental Europe (1918–1920) and victim age. Influenza Other Respir Viruses. 2010;4(2):81–9. doi: 10.1111/j.1750-2659.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grantz KH, Rane MS, Salje H, Glass GE, Schachterle SE, Cummings DA. Disparities in influenza mortality and transmission related to sociodemographic factors within Chicago in the pandemic of 1918. Proc Natl Acad Sci USA. 2016;113(48):13839–44. doi: 10.1073/pnas.1612838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenbaum SC, Coleman MT, Dowdle WR, Mostow SR. Epidemiology of influenza in the elderly: evidence of virus recycling. Am J Epidemiol. 1976;103(2):166–73. doi: 10.1093/oxfordjournals.aje.a112214. [DOI] [PubMed] [Google Scholar]
- 31.Chowell G, Viboud C, Simonsen L, Miller MA, Acuna-Soto R, Ospina Díaz JM, et al. The 1918–19 influenza pandemic in Boyaca, Colombia. 2012 doi: 10.3201/eid1801.101969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamelund S-E. Geography may explain adult mortality from the 1918–20 influenza pandemic. Epidemics. 2011;3(1):46–60. doi: 10.1016/j.epidem.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Oei W, Nishiura H. The relationship between tuberculosis and influenza death during the influenza (H1N1) pandemic from 1918-19. Comput Math Methods Med. 2012;2012 doi: 10.1155/2012/124861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowell G, Simonsen L, Fuentes R, Flores J, Miller MA, Viboud C. Severe mortality impact of the 1957 influenza pandemic in Chile. Influenza Other Respir Viruses. 2017;11(3):230–9. doi: 10.1111/irv.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichert TA, Christensen RA. It’s not about smoldering or neuraminidase: there were 2 variants of the A(H3N2) pandemic virus differing in internal genes. J Infect Dis. 2005;192(10):1858–60. doi: 10.1086/497153. [DOI] [PubMed] [Google Scholar]