Abstract
Background
Glomerulopathy is an increasingly identified complication in young patients with sickle cell disease (SCD). Hyperfiltration and albuminuria followed by declining glomerular filtration rates and eventual end-stage renal disease (ESRD) is assumed to be the typical progression of glomerular disease. There are only a few reported biomarkers to identify early-stage renal disease in SCD.
Procedures
We detail the renal profile of 101 children with SCD in Malawi and propose a novel urinary biomarker for the identification of early renal disease.
Results
Among children with sickle cell anemia, 24.8% had a urine albumin-creatinine ratio of 30 mg/g or above. In univariate analysis, only patients with higher urinary nephrin, a urinary marker of glomerular injury, had significantly greater odds of having albuminuria. In multivariable analysis, nephrin remained significantly associated with albuminuria. A nephrin-creatinine ratio (NCR) cut-point of 622 ng/mg, the 50th percentile, was associated with a 45.8 times greater odds of having albuminuria in children with nephrinuria above this value. Further analysis revealed this urinary NCR cut-point to have 96% sensitivity, 64% specificity, 47% positive predictive value and 98% negative predictive value for the presence of albuminuria.
Conclusions
These data suggest that a substantial number of children with SCD in Malawi have renal disease and could be at risk for worsening nephropathy and ESRD as they age. Our data suggest that urinary nephrin could be utilized as an early marker of glomerular disease in SCD.
Keywords: Sickle cell disease, Nephrin, Albuminuria, Glomerulopathy, D-Dimer, Sensitivity
INTRODUCTION
Sickle cell disease (SCD) is a monogenic disorder that affects millions of people worldwide. Renal disease, including glomerulopathy, is a prevalent complication among adult patients and is a prominent cause of morbidity and mortality 2. The prevalence of albuminuria is up to 58% in adults with SCD, and a proportion of these patients will progress to end-stage renal disease (ESRD) 3,4. The median time to development of ESRD is 23 years, with a 2-year life expectancy once ESRD is reached even with aggressive renal replacement therapy 5. Proteinuria appears to be an independent predictor of mortality among HbSS patients 6. Hyperfiltration and albuminuria have been observed as early as infancy, suggesting the presence of significant early glomerular disease in patients with SCD 7,8.
Given the severe consequences of progressive disease, emphasis should be placed on the early identification of patients with renal disease. However, the surveillance of sickle glomerulopathy is limited by a dearth of clinically validated biomarkers. Current guidelines and best practices recommend the use of routine urinalysis as well as evaluation of microalbuminuria in the surveillance of renal disease. However, this strategy only identifies patients with clinically evident renal disease. A previous study of renal disease in patients with SCD found that the presence of measureable albuminuria is accompanied by a loss of glomerular permselectivity despite normal glomerular filtration rate (GFR), and suggests the presence of irreversible damage9. Early detection of renal disease with use of novel biomarkers could enable earlier intervention and subsequent slowing of disease progression, potentially limiting irreversible damage to the kidney.
The current treatment strategy of albuminuria is similar to that of other proteinuric renal diseases with the mainstay approach using angiotensin converting enzyme (ACE) inhibitors, and more recently with angiotensin receptor blockers (ARBs). There are no published studies of any long-term therapies for proteinuria in SCD. However, short-term treatment with ACE inhibitors significantly decreases both microalbuminuria and macroalbuminuria 10,11,12. Hydroxyurea, approved by the US Food and Drug Administration to treat sickle cell anemia, may have renoprotective effects. Specifically, treatment of adult patients with hydroxyurea is associated with lower levels of albuminuria 13. Treatment of children with hydroxyurea also resulted in decreased glomerular hyperfiltration and glomerular hypertrophy 13. A recent prospective, open-label study reported improvement in albuminuria following 6 months of treatment with hydroxyurea 14.
Nephrin is a slit diaphragm protein that is critical for glomerular filtration by providing architectural support to podocytes. Nephrin shedding is used as a marker of glomerular-specific renal damage in a variety of settings and was observed prior to the development of albuminuria in a mouse model of proteinuric renal disease15. Increased levels of urinary nephrin are present in humanized sickle cell mice and correlate with eGFR in adults with SCD16,17. As nephrinuria develops before albuminuria, nephrin may be a suitable biomarker for early detection of glomerulopathy in SCD.
We have recently introduced hemoglobin electrophoresis testing and clinically characterized a cohort of children in Lilongwe, Malawi 18. Malawi is a country of 18 million people in Southeastern Africa and has a substantial SCD burden, but only scarce resources for its diagnosis and treatment.19,20 In this study, we describe the renal profile of children with SCD followed at Kamuzu Central Hospital, a tertiary referral hospital, in Lilongwe, Malawi. We propose that nephrin, an early marker of glomerular injury, is associated with albuminuria and may represent a potential novel urinary biomarker for the identification of children with early renal disease.
METHODS
Consecutive patients seen at a pediatric chronic care clinic at Kamuzu Central Hospital in Lilongwe, Malawi, between January and May 2015 were recruited. The University of North Carolina at Chapel Hill (UNC) Institutional Review Board (IRB) and Malawi National Health Science Review Committee approved the study. Written informed consent was obtained from the parents of 119 enrolled children. One patient declined participation in the study. Children aged 7–17 years also provided informed assent prior to study participation.
Laboratory Analyses
Routine laboratory studies, including complete blood counts, chemistries, hemoglobin electrophoresis and urinalysis were performed by the UNC Project laboratory in Lilongwe, Malawi. The normal ranges for clinical laboratory variables are provided in supplemental table S1. Plasma and urine samples were aliquoted and frozen immediately at −80°C for subsequent analysis. Urine albumin-creatinine ratio (UACR) measurements were performed by the McClendon Clinical Laboratory at UNC Hospitals. Plasma hemoglobin was quantified by using a laboratory developed test (LDT, Core Laboratory, McLendon Clinical Laboratories, UNC, Chapel Hill) adapted to the Vitros 5600 chemistry platform (Ortho Clinical Diagnostics, Raritan NJ). Plasma levels of vascular endothelial growth factor (VEGF) (R&D Systems, Minneapolis, MN), soluble fms-like tyrosine kinase-1 (sFLT-1, also referred to as vascular endothelial growth factor receptor-1 [VEGFR-1]) (R&D Systems, Minneapolis, MN), D-dimer (RayBiotech, Norcross, GA), and urinary nephrin (Exocell, Philadelphia, PA) were measured using commercially available ELISA kits. Nephrin concentration in the urine was corrected by urine creatinine concentration, and expressed as nephrin-creatinine ratio (NCR; ng/mg). Estimated GFR was derived using the Schwartz formula for children21.
Quality control at UNC Project Malawi was assured by storing the plasma and urine samples immediately after collection at −80°C in freezers with Sensaphone® temperature monitoring, with back-up generators in the event of power outage. The samples were shipped on dry ice to UNC, Chapel Hill, with replenishment of dry ice by the courier during transit. Measurements of albumin-creatinine ratio have been performed in urine samples stored at −80°C.22,23
Statistical Analysis
Demographic and laboratory variables were summarized by counts and percentages, if categorical, or by median and interquartile ranges (IQR), if continuous. Each variable was examined individually using a logistic regression model for albuminuria (UACR ≥ 30 mg/g), with adjustment for age and sex. Those variables with p-values < 0.35 in the univariate analyses were evaluated as potential predictors of albuminuria via a multivariable logistic regression model that was also adjusted for age and sex. Variable selection was conducted at the 0.05 level. To further assess the predictive capacity of urinary NCR for albuminuria, a receiver operating characteristic (ROC) curve was obtained, and area under the curve was estimated, with associated confidence interval. Sensitivities, specificities, positive predictive values, and negative predictive values were calculated for selected cut-points of urinary NCR in predicting albuminuria. Logistic regression analysis using a urinary NCR cut-point of 622 ng/mg (the median value in the cohort) was performed and the odds ratio for albuminuria with urine NCR above and below 622 ng/mg was estimated. Finally, Spearman correlation coefficients evaluating the associations of urine NCR with other laboratory variables were calculated. Reported p-values are for individual tests, unadjusted for multiple comparisons because of the exploratory nature of this study. The ROC curve for urinary NCR was generated using R (version 3.3.2), and other analyses were conducted using SAS University Edition (SAS Institute, Cary, NC).
RESULTS
One hundred and nineteen children were evaluated, with 101 confirmed to have sickle cell anemia (HbSS) (median age: 9 years [IQR: 4–11], 51% male), seven with sickle cell trait (HbAS), and nine with HbAA as determined by hemoglobin electrophoresis. Two patients had indeterminate results. Baseline demographic and laboratory data are shown in Table 1. As expected, HbSS patients had lower median hemoglobin levels, and higher WBC counts, as well as levels of lactate dehydrogenase, total bilirubin and indirect bilirubin than individuals with HbAA (See reference values for children in Malawi in Supplemental Table S1). Median urinary NCR was also higher in HbSS patients compared with individuals with HbAA. Albuminuria, defined as UACR of 30 mg/g or higher, was present in 24.8% of HbSS patients. The median UACR in the albuminuria subgroup of HbSS patients was 58.8 mg/g (IQR: 52.7 – 125.4) vs. 0.0 mg/g (IQR: 0.0 – 9.9) in patients without albuminuria. In univariate analyses, patients with higher urinary NCR had significantly higher odds of albuminuria (p=0.0003). However, no other significant associations were seen between laboratory variables and albuminuria (Table 2).
Table 1.
Demographics and Laboratory Variables
| Variable | Number | Median/Number | InterQuartile Range/Percentage |
|---|---|---|---|
|
| |||
| Age (years) | 119 | 9 | 5 – 11 |
|
| |||
| Sex | 119 | ||
| Female (%) | 57 | 48 | |
| Male (%) | 62 | 52 | |
|
| |||
| Weight (kg) | 114 | 20.5 | 14.5 – 27 |
|
| |||
| Systolic Blood Pressure (mm Hg) | 97 | 103 | 95 – 109 |
|
| |||
| Diastolic Blood Pressure (mm Hg) | 97 | 60 | 55 – 64 |
|
| |||
| Oxygen Saturation (%) | 114 | 93 | 88 – 97 |
|
| |||
| White Blood Cell Count (× 109/L) | 112 | 13.95 | 10.95 – 18.3 |
|
| |||
| Hemoglobin (g/dL) | 112 | 7.4 | 6.9 – 8.1 |
|
| |||
| Platelet Count (× 109/L) | 112 | 438.5 | 349 – 571 |
|
| |||
| Lactate Dehydrogenase (U/L) | 113 | 606 | 489 – 752 |
|
| |||
| Total Bilirubin (mg/dL) | 114 | 1.6 | 0.9 – 2.5 |
|
| |||
| Direct Bilirubin (mg/dL) | 114 | 0.6 | 0.4 – 0.8 |
|
| |||
| Indirect Bilirubin (mg/dL) | 114 | 0.95 | 0.5 – 1.8 |
|
| |||
| Creatinine (mg/dL) | 113 | 0.3 | 0.2 – 0.4 |
|
| |||
| Urine Albumin-Creatinine Ratio (mg/g) | 119 | 0.0 | 0.0 – 23.6 |
|
| |||
| Estimated Glomerular filtration rate (mL/min per 1.73 m2) | 76 | 199.6 | 113.7 – 240.2 |
|
| |||
| Vascular Endothelial Growth Factor (pg/mL) | 119 | 96.74 | 34.5 – 210.6 |
|
| |||
| D-Dimer (ng/mL) | 119 | 1264.4 | 1034.7 – 1569.2 |
|
| |||
| Urinary Nephrin-Creatinine Ratio (ng/mg) | 119 | 620 | 301 – 1186 |
Table 2.
Univariate Predictors of Albuminuria as Determined by Logistic Regression Analysis
| * Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Systolic Blood Pressure | 1.009 | (0.985,1.035) | 0.46 |
| Diastolic Blood Pressure | 1.013 | (0.974,1.053) | 0.52 |
| White Blood Cell Count | 0.991 | (0.893,1.100) | 0.87 |
| Hemoglobin | 0.918 | (0.522,1.614) | 0.77 |
| Estimated GFR | 1.002 | (0.996,1.007) | 0.62 |
| Lactate Dehydrogenase | 1.002 | (1.000,1.005) | 0.077 |
| Total Bilirubin | 1.240 | (0.927,1.657) | 0.15 |
| Direct Bilirubin | 1.879 | (0.405,8.728) | 0.42 |
| Indirect Bilirubin | 1.269 | (0.918,1.753) | 0.15 |
| Plasma Hemoglobin | 1.017 | (0.997,1.037) | 0.10 |
| VEGF | 1.000 | (0.999,1.001) | 0.37 |
| VEGF R1 | 1.002 | (0.994,1.010) | 0.67 |
| D-dimer | 0.999 | (0.998,1.000) | 0.11 |
| Urinary Nephrin-Creatinine Ratio | 1.002 | (1.001,1.003) | 0.0003 |
Adjusted for Age and Sex
UACR – Urine Albumin-Creatinine Ratio; VEGF – Vascular Endothelial Growth Factor; VEGF R1 - Vascular Endothelial Growth Factor Receptor -1
In the multivariable logistic regression analysis, only urinary NCR was significantly associated with albuminuria (odds ratio estimate = 1.002, 95% confidence interval [CI]: {1.001, 1.003}, p = 0.0003). Additional analysis using an NCR cut-point of 622 ng/mg, the median value for the cohort, revealed a 45.9 times greater odds of having albuminuria in children with NCR above this value. The area under the ROC curve that illustrates the ability of urinary NCR in predicting albuminuria was 0.89 (95% CI: [0.83, 0.96]) (Figure 1). An NCR of 622 ng/mg or greater, representing the 50th percentile for the population, provided a sensitivity of 96%, a specificity of 64%, a positive predictive value (PPV) of 47% and a negative predictive value (NPV) of 98% for the presence of albuminuria. An NCR of 1213 ng/mg or greater, representing the 75th percentile for the population, provided a sensitivity of 64%, a specificity of 87%, a PPV of 62% and an NPV of 88% for the presence of albuminuria. An NCR of 2107 ng/mg or greater, representing the 90th percentile for the population, provided a sensitivity of 28%, a specificity of 96%, a PPV of 70% and an NPV of 80% for the presence of albuminuria.
Figure 1.
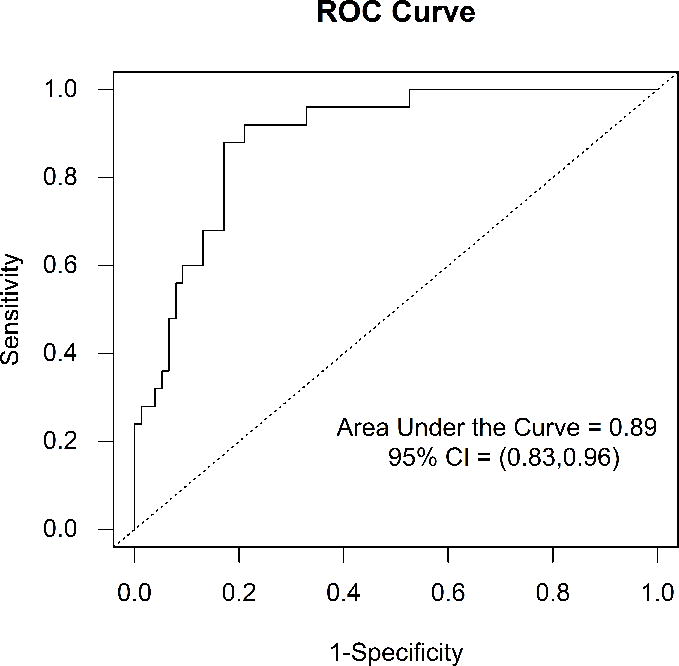
Receiver Operating Characteristic Curve for Urinary Nephrin-Creatinine Ratio for the Prediction of Albuminuria in Children with Sickle Cell Anemia. The area under the receiver operating characteristic curve was 0.89 (95% CI: [0.83, 0.96])
Urinary NCR was positively correlated with total and indirect bilirubin levels while negatively correlated with D-dimer levels (p<0.05). However, no significant correlations were seen between urinary NCR and hemoglobin or serum creatinine (Table 3). In addition, a significant correlation was not observed between NCR and eGFR (r = 0.03, p = 0.84).
Table 3.
Spearman Correlation Coefficients of Laboratory Variables with Urinary Nephrin-Creatinine Ratio
| Variable | Spearman Correlation Coefficient | P-value |
|---|---|---|
| WBC count | −0.072 | 0.49 |
| Hemoglobin | −0.023 | 0.82 |
| Platelet count | −0.014 | 0.90 |
| Lactate dehydrogenase | 0.19 | 0.063 |
| Total bilirubin | 0.26 | 0.011 |
| Direct bilirubin | 0.17 | 0.10 |
| Indirect bilirubin | 0.27 | 0.0086 |
| VEGF | −0.05 | 0.62 |
| D-Dimer | −0.25 | 0.012 |
| Estimated GFR | 0.027 | 0.84 |
| Creatinine | 0.089 | 0.39 |
WBC – White Blood Cell; VEGF – Vascular Endothelial Growth factor; Estimated GFR – Estimated Glomerular Filtration Rate
DISCUSSION
Albuminuria is a common complication in SCD, with an increasing recognition of its high prevalence in young patients4. ACE inhibitors have become the “standard of care” for the treatment of albuminuria in SCD but adequately powered controlled studies of their effect on the progression of sickle glomerulopathy and subsequent chronic kidney disease are lacking. Despite the recommendation by an NHLBI expert panel that adults with moderately increased albuminuria (previously called microalbuminuria), without other apparent cause, be started on ACE inhibitor therapy, the natural history of albuminuria in patients with sickle cell anemia remains inadequately defined24. However, multiple studies suggest that kidney function declines over time in patients with SCD25–27. Indeed, we have recently reported a rate of decline in eGFR of 2.15 mL/min per 1.73 m2 per year, much greater than that expected for healthy individuals 28,29.
The high prevalence of albuminuria in our patient cohort in Malawi suggests early, severe glomerular disease in young patients with SCD. This is similar to the results from a large, multicenter study in West Africa that reported a prevalence of 27% in children who were less than 10 years old30. A recent study of patients from Cameroon reported a prevalence of albuminuria of 60%, although this cohort was older than our patient cohort, with an average patient age of 15 years31.
In the multivariable logistic regression analysis, only NCR was significantly associated with albuminuria. Urinary nephrin has been reported to correlate with urine albumin concentration in a small cohort of adult patients with SCD17. However, our study is the first to report the association of NCR with albuminuria in young children with SCD. Although the specificity and positive predictive value of NCR (with a cut-point at the 50th percentile) for albuminuria in our study are modest, the sensitivity and negative predictive value are high. As nephrin is a glomerular-specific protein, monitoring of this biomarker might be more indicative of glomerular injury than other putative biomarkers in the current literature 32. Furthermore, evaluation of a urine biomarker is more convenient in a pediatric population than a plasma-based biomarker. With its high sensitivity and negative predictive value, nephrin represents a plausible and easily accessible biomarker for the early diagnosis of glomerulopathy associated with SCD.
In our patient cohort, D-dimer was negatively correlated with NCR. In addition, we observed that D-dimer appeared to be inversely associated with albuminuria, although this was not statistically significant. D-dimer is an assayable product of fibrin degradation that is elevated in the plasma during clotting and thrombosis. There is increasing evidence that SCD is a hypercoagulable state33. In addition to an increased risk of thrombotic complications, patients with SCD have evidence of coagulation activation, with increased levels of markers of thrombin generation, even in the non-crisis, “steady states.” High concentrations of thrombin may contribute to podocyte injury 34. It is suggested that thrombin may contribute to progression of proteinuria in animal models of nephrotic syndrome35. The role of coagulation activation in SCD pathophysiology is highlighted by a recent study which showed that genetic reduction in prothrombin levels in sickle mice, not only resulted in decreased levels of thrombin-antithrombin complexes and D-dimer, but also produced significant reductions in renal complications, including albuminuria, compared with wild type sickle mice36. In light of these previous reports, the negative association between D-dimer and both NCR and UACR was surprising and unexpected. Additional studies in patients with SCD are required to clarify the relationship between coagulation activation and glomerulopathy.
There were no associations between hemoglobin or plasma hemoglobin and other markers of hemolysis with albuminuria. The absence of significant associations between the various markers of hemolysis and albuminuria suggests that hemolysis may not play a role in the pathogenesis of glomerulopathy in this population. The contribution of hemolysis to the pathophysiology of SCD-related glomerulopathy remains uncertain. While multiple observational studies show an association of albuminuria with markers of hemolysis in patients with SCD 6,30,37–40 others have not shown such associations41–44. Additionally, despite the reported role for hyperfiltration in the pathogenesis of albuminuria in SCD 45,46, we found no association between the presence of albuminuria and estimated GFR in this study. In addition, we did not observe any association between albuminuria and either VEGF or VEGFR-1.
Our study has several limitations. All the study patients were recruited from a tertiary hospital clinic in Malawi. As with other populations in sub-Saharan Africa, the highest mortality is thought to occur in the less than 5-year age group 47. As such, there is a possible survivor bias in our population resulting in an overall healthier cohort than might otherwise exist. The gold standard for measurement of albuminuria is a 24-hour urine collection. However, 24-hour collections are particularly tedious in the setting of a developing country such as Malawi. Furthermore, we have previously reported very strong correlations between spot assessments for UACR and 24-hour urine protein collections providing support for the use of spot urine collections to evaluate albuminuria in patients with SCD 48. As with all cross-sectional studies, this analysis demonstrates associations, but cannot prove causation.
CONCLUSION
These data taken together suggests that a substantial proportion of children with SCD in Malawi exhibit renal involvement early in life and could be at risk for worsening glomerulopathy and ESRD as they grow older. Our data further indicate that urinary nephrin is associated with the presence of albuminuria and could be utilized as an early biomarker of glomerular disease in SCD. Confirmation of the ability of urinary nephrin to predict the development of albuminuria will require the conduct of longitudinal studies. However, early detection of renal disease through novel biomarkers could enable earlier intervention and possibly slow disease progression, potentially limiting irreversible damage to the kidney. Increased surveillance of children with SCD for renal complications can ultimately inform management strategies to improve outcomes and increase life expectancy in patients with SCD.
Supplementary Material
Supplemental Table S1: Normal Laboratory Reference Values for UNC Project Malawi.
Acknowledgments
We wish to acknowledge Mr. Wiza Kumwenda for assistance developing and maintaining the study database. We additionally acknowledge leadership of Kamuzu Central Hospital (Dr. Jonathan Ngoma) and UNC Project-Malawi (Profs. Irving Hoffman, Francis Martinson, Mina Hosseinipour, and Mr. Innocent Mofolo) for their support of this study, as well as pediatrics department and laboratory staff. Finally, we wish to thank participating children and their families. This project was supported by the UJMT Fogarty Global Health Fellows Program (grant #R25TW009340), The Medical College of Georgia at Augusta University, and the National Heart, Lung, Blood Institute (grant #U01HL117659). The funding sources had no involvement in any aspect of the study, decision to write, or submit this manuscript.
ABBREVIATIONS KEY
- SCD
Sickle Cell Disease
- ESRD
End-Stage Renal Disease
- NCR
Nephrin-Creatinine Ratio
- eGFR
Estimated Glomerular Filtration Rate
- ACE
Angiotensin Converting Enzyme
- ARB
Angiotensin Receptor Blocker
- IRB
Institutional Review Board
- VEGF
Vascular Endothelial Growth Factor
- VEGFR-1
Vascular Endothelial Growth Factor Receptor-1
- UACR
Urine Albumin-Creatinine Ratio
- IQR
Interquartile Range
- ROC
Receiving Operating Characteristic
- PPV
Positive Predictive Value
- NPV
Negative Predictive Value
- HbSS
Hemoglobin SS
- HbAS
Hemoglobin AS (Sickle Cell Trait)
- HbAA
Hemoglobin AA
- LDT
Laboratory Developed TestsFLT-1
- sFLT-1
Soluble FMS-like Tyrosine Kinase-1
- UNC
University of North Carolina at Chapel Hill
- NHLBI
National HeartLung and Blood Institute
- US
United States of America
Footnotes
Abstract previously presented at American Society of Hematology Annual Meeting December 2016 Abstract #97463
CONFLICTS OF INTEREST
The authors have no relevant conflicts of interest to disclose.
References
- 1.Brett Heimlich JGC, Ellis Graham, Elsherif Laila, David Emeraghi, Kamthunzi Portia, Krysiak Robert, Majawa Yacinta, Mafunga Pilirani, Zhou Qingning, Cai Jianwen, Key Nigel S, Gopal Satish, Ataga Kenneth I. Early Renal Disease in Children with Sickle Cell Disease from Malawi American Society of Hematology Annual Meeting. San Diego, CA: Dec 3–6, 2016. [Google Scholar]
- 2.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr Blood Cancer. 2013;60(9):1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 3.Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115(8):614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Derebail VK, Archer DR. The glomerulopathy of sickle cell disease. Am J Hematol. 2014;89(9):907–914. doi: 10.1002/ajh.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol. 2012;159(3):360–367. doi: 10.1111/bjh.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drawz P, Ayyappan S, Nouraie M, et al. Kidney Disease among Patients with Sickle Cell Disease, Hemoglobin SS and SC. Clin J Am Soc Nephrol. 2016;11(2):207–215. doi: 10.2215/CJN.03940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol. 2000;63(4):205–211. doi: 10.1002/(sici)1096-8652(200004)63:4<205::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Ware RE, Rees RC, Sarnaik SA, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J Pediatr. 2010;156(1):66–70e61. doi: 10.1016/j.jpeds.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guasch A, Cua M, Mitch WE. Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int. 1996;49(3):786–791. doi: 10.1038/ki.1996.109. [DOI] [PubMed] [Google Scholar]
- 10.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326(14):910–915. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]
- 11.Quinn CT, Saraf SL, Gordeuk VR, et al. Losartan for the Nephropathy of Sickle Cell Anemia: A Phase-2 Multi-Center Trial. Am J Hematol. 2017 doi: 10.1002/ajh.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foucan L, Bourhis V, Bangou J, Merault L, Etienne-Julan M, Salmi RL. A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. Am J Med. 1998;104(4):339–342. doi: 10.1016/s0002-9343(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 13.Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant. 2014;29(6):1211–1218. doi: 10.1093/ndt/gft295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartolucci P, Habibi A, Stehle T, et al. Six Months of Hydroxyurea Reduces Albuminuria in Patients with Sickle Cell Disease. J Am Soc Nephrol. 2016;27(6):1847–1853. doi: 10.1681/ASN.2014111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 16.Heimlich JB, Speed JS, O’Connor PM, et al. Endothelin-1 contributes to the progression of renal injury in sickle cell disease via reactive oxygen species. Br J Pharmacol. 2016;173(2):386–395. doi: 10.1111/bph.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez B, Shah B, Zhang X, Lash JP, Gordeuk VR, Saraf SL. Hyperfiltration is associated with the development of microalbuminuria in patients with sickle cell anemia. Am J Hematol. 2014;89(12):1156–1157. doi: 10.1002/ajh.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimlich JB, Chipoka G, Kamthunzi P, et al. Establishing sickle cell diagnostics and characterizing a paediatric sickle cell disease cohort in Malawi. Br J Haematol. 2016;174(2):325–329. doi: 10.1111/bjh.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brabin BJ, Prinsen-Geerligs PD, Verhoeff FH, et al. Haematological profiles of the people of rural southern Malawi: an overview. Ann Trop Med Parasitol. 2004;98(1):71–83. doi: 10.1179/000349804225003055. [DOI] [PubMed] [Google Scholar]
- 20.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez O, Miller ST, Wang WC, et al. Effect of hydroxyurea treatment on renal function parameters: results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer. 2012;59(4):668–674. doi: 10.1002/pbc.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev. 2011;32(2):97–102. [PMC free article] [PubMed] [Google Scholar]
- 23.Bao Y, Zuo L. Effect of repeated freeze-thaw cycles on urinary albumin-to-creatinine ratio. Scand J Clin Lab Invest. 2009;69(8):886–888. doi: 10.3109/00365510903323209. [DOI] [PubMed] [Google Scholar]
- 24.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 25.Asnani MR, Reid ME. Renal function in adult Jamaicans with homozygous sickle cell disease. Hematology. 2015;20(7):422–428. doi: 10.1179/1607845414Y.0000000213. [DOI] [PubMed] [Google Scholar]
- 26.Gosmanova EO, Zaidi S, Wan JY, Adams-Graves PE. Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J Investig Med. 2014;62(5):804–807. doi: 10.1097/01.JIM.0000446836.75352.72. [DOI] [PubMed] [Google Scholar]
- 27.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 28.Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl. 2011;3(1):63–72. doi: 10.1038/kisup.2012.65. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciccone EKR, Zhou Q, Cai J, Derebail VK, Ataga KI. Progression of Chronic Kidney Disease in Sickle Cell Disease. Annual meeting of the American Society of Hematology. 2016 [Google Scholar]
- 30.Ranque B, Menet A, Diop IB, et al. Early renal damage in patients with sickle cell disease in sub-Saharan Africa: a multinational, prospective, cross-sectional study. Lancet Haematol. 2014;1(2):e64–73. doi: 10.1016/S2352-3026(14)00007-6. [DOI] [PubMed] [Google Scholar]
- 31.Geard A, Pule GD, Chetcha Chemegni B, et al. Clinical and genetic predictors of renal dysfunctions in sickle cell anaemia in Cameroon. Br J Haematol. 2017 doi: 10.1111/bjh.14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundaram N, Bennett M, Wilhelm J, et al. Biomarkers for early detection of sickle nephropathy. Am J Hematol. 2011;86(7):559–566. doi: 10.1002/ajh.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: Relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2016;30(4):245–256. doi: 10.1016/j.blre.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae JS, Kim YU, Park MK, Rezaie AR. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J Cell Physiol. 2009;219(3):744–751. doi: 10.1002/jcp.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma R, Waller AP, Agrawal S, et al. Thrombin-Induced Podocyte Injury Is Protease-Activated Receptor Dependent. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arumugam PI, Mullins ES, Shanmukhappa SK, et al. Genetic diminution of circulating prothrombin ameliorates multiorgan pathologies in sickle cell disease mice. Blood. 2015;126(15):1844–1855. doi: 10.1182/blood-2015-01-625707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becton LJ, Kalpatthi RV, Rackoff E, et al. Prevalence and clinical correlates of microalbuminuria in children with sickle cell disease. Pediatr Nephrol. 2010;25(8):1505–1511. doi: 10.1007/s00467-010-1536-8. [DOI] [PubMed] [Google Scholar]
- 38.Day TG, Drasar ER, Fulford T, Sharpe CC, Thein SL. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica. 2012;97(2):201–205. doi: 10.3324/haematol.2011.050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol. 2010;25(10):2123–2127. doi: 10.1007/s00467-010-1560-8. [DOI] [PubMed] [Google Scholar]
- 40.Maier-Redelsperger M, Levy P, Lionnet F, et al. Strong association between a new marker of hemolysis and glomerulopathy in sickle cell anemia. Blood Cells Mol Dis. 2010;45(4):289–292. doi: 10.1016/j.bcmd.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Asnani MR, Fraser RA, Reid ME. Higher rates of hemolysis are not associated with albuminuria in Jamaicans with sickle cell disease. PLoS One. 2011;6(4):e18863. doi: 10.1371/journal.pone.0018863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ataga KI, Brittain JE, Moore D, et al. Urinary albumin excretion is associated with pulmonary hypertension in sickle cell disease: potential role of soluble fms-like tyrosine kinase-1. Eur J Haematol. 2010;85(3):257–263. doi: 10.1111/j.1600-0609.2010.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17(8):2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 44.King L, MooSang M, Miller M, Reid M. Prevalence and predictors of microalbuminuria in Jamaican children with sickle cell disease. Arch Dis Child. 2011;96(12):1135–1139. doi: 10.1136/archdischild-2011-300628. [DOI] [PubMed] [Google Scholar]
- 45.Thompson J, Reid M, Hambleton I, Serjeant GR. Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med. 2007;167(7):701–708. doi: 10.1001/archinte.167.7.701. [DOI] [PubMed] [Google Scholar]
- 46.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE. Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol. 2011;26(8):1285–1290. doi: 10.1007/s00467-011-1857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makani J, Cox SE, Soka D, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2):e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ataga KI, Derebail VK, Caughey M, et al. Albuminuria Is Associated with Endothelial Dysfunction and Elevated Plasma Endothelin-1 in Sickle Cell Anemia. PLoS One. 2016;11(9):e0162652. doi: 10.1371/journal.pone.0162652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Normal Laboratory Reference Values for UNC Project Malawi.


