Abstract
We investigated risks of preeclampsia phenotypes from potential residential pesticide exposures, including 543 individual chemicals and 69 physicochemical groupings that were applied in the San Joaquin Valley of California during the study period, 1998–2011. The study population was derived from birth certificate data linked with Office of Statewide Health Planning and Development maternal and infant hospital discharge data. The following numbers of women with preeclampsia phenotypes were identified: 1045 with superimposed (pre-existing hypertension with preeclampsia) preeclampsia (265 with gestational weeks 20–31 and 780 with gestational weeks 32–36); 3471 with severe preeclampsia (824 with gestational weeks 20–31 and 2647 with gestational weeks 32–36); and 2780 with mild preeclampsia (207 with gestational weeks 20–31 and 2573 with gestational weeks 32–36). The reference population for these groups was 197,461 women who did not have diabetes (gestational or pre-existing), did not have any hypertensive disorder, and who delivered at 37 weeks or later. The frequency of any exposure was lower or about the same in each preeclampsia case group (further delineated by gestational age), and month time period, relative to the frequency in reference population controls. Nearly all odds ratios were below 1.0 for these any vs no exposure comparisons. This study showed a general lack of increased risks between a range of agriculture pesticide exposures near women’s residences and various preeclampsia phenotypes.
Keywords: pesticides, environment, hypertension, endocrine disruptors, pregnancy
1. INTRODUCTION
Preeclampsia, commonly defined as high blood pressure and proteinuria after 20 weeks of pregnancy, affects upwards of 5% of pregnancies and contributes substantially to maternal morbidity and mortality in the United States (Mol et al. 2016). Factors contributing to elevated risks of preeclampsia include nulliparity, African-American race, obesity, nonsmoking, a clinical history of preeclampsia, hypertension, diabetes, and autoimmune conditions (Jeyabalan 2013). Gene variants in selected pathways such as oxidative stress, inflammation, and angiogenesis have also been put forward as contributors to the risk profile of women who develop preeclampsia (Jebbink et al. 2012).
Environmental exposures have been rarely investigated for their potential etiologic contribution to preeclampsia. Certain pesticide exposures (e.g., organochlorines, have been suggested to elevate risk of hypertensive disorders in general (Morgan et al. 1980; Siddiqui et al. 2002; Rosenbaum et al. 2017) and in pregnancy specifically, preeclampsia (Saldana et al. 2009; Nugteren JJ et al. 2012). Despite a few studies suggesting associations, though not all (Willis et al. 1993; Nordby et al. 2006; Saunders et al. 2014), between pesticide exposures and preeclampsia, the scant literature is insufficient to draw clear inferences. In general, such studies have been nonspecific to the pesticide chemical (e.g., any pesticide exposure yes vs no), small in sample size, varied in how women’s activities may have facilitated pesticide exposure (e.g., employment or self-reported activities), or did not consider pertinent comorbidities like gestational diabetes.
To substantially extend the limited extant information, we investigated population-based data on >200,000 births and proximal residential exposures to more than 500 commercial agricultural pesticide active ingredients and adjuvants during multiple gestational time points. The study population derived from the San Joaquin Valley of California, one of the highest agricultural pesticide use areas in the US.
2. MATERIALS AND METHODS
2.1. Study population
This study was approved by the Stanford University Institutional Review Board and the California State Committee for the Protection of Human Subjects.
Data for this case-control study derive from 1998–2011 California births to women residing in the San Joaquin Valley (Fresno, Kern, Kings, Madera, Merced, San Joaquin, Stanislaus, and Tulare counties). In this region and time period there were 892,088 livebirths delivered in non-military hospitals. We restricted the study to those with gestational ages 20–41 weeks (determined by obstetric estimate for 2007–11 and by last menstrual period for 1998–2006), birth weights between 500 and 5000 grams, and singleton births – a total of 771,416 births. This analysis was an opportunistic extension of a previously conducted study specific to preterm birth (Shaw et al. 2018) whereby the 771,416 eligible births were 78,421 preterm (i.e., <37 weeks gestation) and 692,995 term (i.e., >37 weeks gestation). For analytic efficiency that study was based on a randomly selected group of 235,263 (from the 692,995) term births in a 3:1 ratio of term to preterm infants.
For each of these 313,684 (78,421 + 235,263) births, we obtained the mother’s residential address at the time of delivery from the electronic birth certificate. A REST API Geocode Service maintained by the California Department of Public Health Information Technology Services Division was used to geocode addresses. This service standardizes, verifies, and corrects addresses before matching against multiple address-attributed reference databases. Successful geocoding was achieved for 295,387 births (94%).
We further linked the 295,387 births with Office of Statewide Health and Planning (OSHPD) maternal and infant hospital discharge data. This linkage allowed for information on a range of maternal and pregnancy characteristics found on the birth certificate paired with clinical detail from the delivery hospitalization for practically all inpatient live births. The algorithm employed for this linkage is accurate and previously described (Herrchen et al. 1997; Lyndon et al. 2012). Successful linkage was achieved for 99% (n=293,044).
Our analytic goal was to investigate various preeclampsia phenotypes among pregnancies that delivered before 37 weeks gestation. To identify preeclampsia as well as other comorbidities from hospital discharge data, we employed International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. Included as comorbidities were pre-existing diabetes (Type 1 (250.x1, 250.x3) and Type 2 (250.x0, 250.x2, 648.0)) and gestational diabetes (648.8). For hypertensive disorders we identified: pre-existing hypertension (401–405, 642.0, 642.1, 642.2, 642.9); gestational hypertension (642.3); mild preeclampsia (642.4); severe preeclampsia/eclampsia (642.5, 642.6); and preeclampsia or eclampsia superimposed on preexisting hypertension (642.7). Women with multiple ICD9 codes for hypertensive disorders were reclassified to allow for mutually exclusive groups. Specifically, women with multiple codes were classified as: women with a pre-existing hypertension code and a preeclampsia or eclampsia code were classified as having preeclampsia or eclampsia superimposed on preexisting hypertension; women with pre-existing hypertension and gestational hypertension were classified as having pre-existing hypertension; and women with multiple codes for gestational hypertension and preeclampsia or eclampsia were classified as the most severe condition. Thus, for our primary analytic queries women were grouped into one of 3 “case” phenotypic groups: 1) preeclampsia or eclampsia superimposed on pre-existing hypertension; 2) severe preeclampsia/eclampsia; and 3) mild preeclampsia. The 3 preeclampsia phenotypic groups were further stratified by gestational age of delivery as 20–31 weeks or 32–36 weeks. Women who delivered in the study period who did not have diabetes (gestational or pre-existing), did not have any hypertensive disorder (including preeclampsia), and who delivered at 37 weeks or greater served as the referent population (controls).
2.2. Pesticide and adjuvant compounds studied
We assessed exposure to 543 individual chemicals used as pesticides or as adjuvants in pesticide products or application mixtures and 69 physicochemical groupings having the same chemical classification and proven or putative mechanism of action (e.g., organophosphates) that were applied at >100 lb in any of the 8 San Joaquin Valley counties in any year during the study period, 1998–2011 (California-Department-of-Pesticide-Regulation, Pesticide Use Reporting). Low-toxicity chemicals such as biopesticides (e.g., microbial pesticides, soaps, essential oils), low-toxicity inorganic compounds (e.g., sulfur, kaolin clay), and other compounds determined by US EPA to have low toxicity, as described in US EPA Risk Assessment documents for each chemical were excluded (EPA-U.S.-Environmental-Protection-Agency, Pesticide Chemical Search). In addition, compounds were flagged as having reproductive or developmental toxicity based on the California Proposition 65 list (California-Office-of-Environmental-Health-Hazard-Assessment) or as endocrine disruptors (Colborn T; European-Commission, 2012; Keith 1997). Chemicals with a US EPA-determined Reference Dose based on a toxicological study with a reproductive or developmental endpoint as described in EPA risk assessment documents were included (EPA-U.S.-Environmental-Protection-Agency. Pesticide Chemical Search).
2.3. Pesticide exposure assessment
To estimate pesticide exposures, we assigned a time window of exposure for each case or control woman from one month before conception (B1) to date of delivery by every 4 weeks of pregnancy (P1-P9).
To estimate pesticide applications, we obtained statewide Pesticide Use Reporting (PUR) records from the California Department of Pesticide Regulation describing agricultural pesticide applications occurring between 1 January 1998 and 31 December 2011 (California-Department-of-Pesticide-Regulation. Pesticide Use Reporting). These data are submitted by county agriculture commissioners and are spatially referenced to public land survey sections (PLSS). For the study period, the total number of active ingredient daily production agricultural use records with a PLSS specified, and for the 543 chemicals that were present in PUR records, exceeded 24 million. Following the method of Rull and Ritz (2003), we spatially refined PLSS polygons through overlay of matched land-use survey field polygons provided by the California Department of Water Resources. We matched each PUR record to the land-use survey conducted closest in time to the application date (surveys are conducted roughly every 5–7 years in each California county). Matching is based on PLSS and crop type as specified in records. Infrequently rotated crops, such as orchard crops and vineyards, were matched one-to-one, while frequently rotated crops, such as field and truck crops, were grouped together in a single category, and non-agricultural land-uses were subtracted from PLSS polygons when no crop types were matched to available polygons. Of the total applications (and active-ingredient poundage) recorded spanning 1998–2011 for the 543 chemicals of interest, >90% were successfully linked to polygons. For those where no field polygon was specified, no spatial refinement was possible. We determined temporal proximity by comparing recorded dates of applications, believed to be accurate within a few days, to the time window of exposure for each case or control woman.
To assign exposure, we utilized the CEHTP Pesticide Linkage Tool, a custom-developed Java (Oracle, Redwood Shores, CA) application that incorporates the PostGIS spatial and geographic objects library for PostgreSQL (http://www.postgis.net/) and the GeoTools Java GIS Toolkit, version Release 12 (open source, http://www.geotools.org/) for Geographic information system data management and spatial analysis (California-Environmental-Health-Tracking-Program. Geocoding Service; California-Environmental-Health-Tracking-Program. Agricultural Pesticide Mapping Tool). We characterized pesticides used during each monthly time window (B1-P9) within a 500 m radius of a geocoded point (Roberts et al. 2007), intersecting polygons with the buffer, and assuming homogeneous distribution of pesticides within each polygon.
2.4. Statistical analysis
Risks associated with residential pesticide exposures were estimated using logistic regression. Univariate analyses were conducted to estimate crude odds ratios and 95% confidence intervals (CIs) reflecting associations between pesticide exposures and each of the three preeclampsia phenotypic groups. We performed multivariable analyses adjusting for maternal age (years), race/ethnicity (non-Hispanic white, U.S.-born Hispanic, foreign-born Hispanic, other), education (less than high school, high school, more than high school), parity (1 or ≥2), prenatal care initiated by fifth month (yes vs no), and payer source for care (Medi-Cal, private, or other). Additional analyses based on availability of data beginning with 2007 births were performed adjusting for pre-pregnancy body mass index (BMI in kg/m2, continuous) and neighborhood poverty derived from US Census data for census tracts. Given that the source of potential covariate information was derived from the birth certificate we determined that women’s cigarette smoking was too incomplete to include in analyses.
Comparisons were performed based on the following. For pesticides that had ≥5 exposed case and control women, risks were estimated that compared any versus no exposure. Risks were not estimated for pesticides that had fewer than 5 exposed case and control women owing to a lack of statistical precision. We also created exposure groupings by flagging studied chemicals as having reproductive or developmental toxicity based on the California Proposition 65 list (California-Office-of-Environmental-Health-Hazard-Assessment) or as endocrine disruptors (Colborn T; European-Commission, 2012; Keith 1997). Chemicals with an EPA-determined reference dose based on an acute toxicological study with a reproductive or developmental endpoint as described in EPA risk assessment documents were also included (EPA-(U.S.-Environmental-Protection-Agency. Pesticide Chemical Search). We created exposure groups by summing total number of chemicals designated as endocrine disruptors, Proposition 65 chemicals, or chemicals in EPA lists. For each group, we explored associations of preeclampsia phenotypes with group sums of chemicals as categorical variables; i.e., exposed subjects were divided into tertiles based on the control distribution sums. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, 2016–2017).
An elastic net (EN) algorithm was used for agnostic multivariate analysis (Zou and Hastie 2005). 10% of the data was randomly and uniformly selected for training purposes. The remaining 90% was used as a blinded test-set. For a matrix X of all exposure levels, and a vector of diagnosis results Y, a multivariate model was developed to calculate the coefficients β for each entity in X to minimize the overall differences from Y: L(β)=| Y − Xβ |2. A L1 regularization was applied on the β coefficients to reduce the model complexity, such that L(β)=| Y − Xβ |2 + λ1 | β |1 where λ1 is selected by cross-validation (Tibshirani 1996). This produces a sparse model in which only a limited number of features is utilized. However, this approach is not ideal for the analysis of highly interrelated pesticides, as it would select only representatives of correlated features, while disregarding highly correlated but potentially biologically relevant features. This limitation is addressed by using an additional L2 regularization to allow the inclusion of highly correlated measurements: L(β)=| Y − Xβ |2 + λ1 | β |1 + λ2 | β |2 where λ1 and λ2 are selected by cross-validation (Zou and Hastie 2005).
3. RESULTS
The following numbers of women with preeclampsia phenotypes were identified: 1045 with superimposed preeclampsia (265 with gestational weeks 20–31 and 780 with gestational weeks 32–36); 3471 with severe preeclampsia or eclampsia (824 with gestational weeks 20–31 and 2647 with gestational weeks 32–36); and 2780 with mild preeclampsia (207 with gestational weeks 20–31 and 2573 with gestational weeks 32–36). The reference (controls) population for these groups was 197,461 women who delivered in the study period, did not have diabetes (gestational or pre-existing), did not have any hypertensive disorder, and delivered at 37 weeks or greater. Characteristics of case women and controls are displayed in Table 1.
Table 1.
Descriptive characteristics (percentages)1 of preeclampsia cases and their referent population (controls), California, 1998–2011
| Characteristic | Preeclampsia Phenotypes
|
|||
|---|---|---|---|---|
| CASES | CONTROLS2 | |||
|
|
||||
| Superimposed n=1045 |
Severe n=3471 |
Mild n=2780 |
37–41 weeks n=197,461 |
|
| Maternal age (years) | ||||
| <20 | 2.8 | 16.9 | 17.2 | 14.2 |
| 20–24 | 16.5 | 27.4 | 26.5 | 29.9 |
| 25–29 | 23.4 | 23.7 | 24.0 | 28.0 |
| 30–34 | 29.5 | 17.8 | 18.0 | 18.4 |
| >35 | 27.9 | 14.2 | 14.2 | 9.5 |
| Missing | 0 | 0 | <0.1 | <0.1 |
| Maternal race/ethnicity | ||||
| White, nonHispanic | 25.2 | 25.0 | 28.6 | 29.4 |
| White, Hispanic | 51.9 | 59.7 | 56.7 | 57.0 |
| Black | 12.3 | 5.9 | 6.4 | 4.5 |
| Asian | 7.2 | 6.2 | 6.0 | 7.1 |
| Other | 2.4 | 2.4 | 1.6 | 1.5 |
| Missing | 1.1 | 0.8 | 0.8 | 0.6 |
| Maternal education | ||||
| Less than high school | 24.3 | 28.9 | 28.6 | 32.6 |
| High school | 29.8 | 31.7 | 33.0 | 31.8 |
| More than high school | 43.8 | 37.0 | 36.4 | 34.0 |
| Missing | 2.1 | 2.4 | 2.0 | 1.6 |
| Prenatal care initiation by fifth month of gestation | ||||
| Yes | 89.8 | 89.6 | 90.5 | 91.8 |
| No | 7.5 | 7.1 | 6.5 | 6.4 |
| Missing | 2.8 | 3.3 | 3.0 | 1.8 |
| Parity | ||||
| 1 | 32.3 | 56.4 | 52.5 | 34.8 |
| ≥2 | 67.7 | 43.4 | 47.4 | 65.2 |
| Missing | 0.1 | 0.1 | 0.1 | 0.1 |
| Payer type for delivery | ||||
| Medi-Cal | 55.1 | 56.9 | 58.4 | 56.9 |
| Private | 41.2 | 38.7 | 38.3 | 39.7 |
| Uninsured | 2.9 | 3.1 | 1.9 | 1.7 |
| Other | 0.7 | 1.1 | 1.1 | 1.5 |
| Missing | 0.2 | 0.2 | 0.3 | 0.2 |
| Infant sex | ||||
| Male | 48.9 | 51.8 | 53.1 | 50.6 |
| Female | 51.1 | 48.2 | 46.8 | 49.4 |
| Missing | 0 | 0 | <0.1 | <0.1 |
| Infant Birth Year | ||||
| 1998 | 3.1 | 4.9 | 6.9 | 6.7 |
| 1999 | 3.2 | 4.7 | 6.2 | 6.7 |
| 2000 | 2.6 | 5.5 | 5.8 | 6.9 |
| 2001 | 2.6 | 5.3 | 5.2 | 6.9 |
| 2002 | 5.0 | 5.5 | 6.5 | 7.1 |
| 2003 | 5.2 | 6.8 | 5.9 | 7.4 |
| 2004 | 7.0 | 6.5 | 7.7 | 7.9 |
| 2005 | 6.8 | 7.1 | 8.4 | 9.1 |
| 2006 | 7.0 | 7.4 | 8.2 | 9.5 |
| 2007 | 10.7 | 9.0 | 9.1 | 6.8 |
| 2008 | 10.2 | 8.4 | 8.0 | 6.7 |
| 2009 | 10.8 | 9.6 | 7.8 | 6.3 |
| 2010 | 12.7 | 10.3 | 7.5 | 6.1 |
| 2011 | 13.2 | 9.0 | 6.7 | 6.0 |
| Years 2007–2011 | 603 | 1609 | 1086 | 63021 |
| Prepregnancy body mass index (kg/m2) (2007–2011) | ||||
| Underweight (BMI<18.5) | 0.8 | 1.3 | 2.1 | 3.1 |
| Normal (18.5≤BMI<25) | 14.6 | 30.5 | 27.0 | 40.2 |
| Overweight (25≤BMI<30) | 23.6 | 24.6 | 22.5 | 24.7 |
| Obese (BMI≥30) | 48.8 | 31.5 | 32.7 | 20.3 |
| Missing | 12.3 | 12.1 | 15.8 | 11.8 |
| Poverty (2007–2011) 3 | ||||
| ≤107.25 | 20.9 | 20.2 | 16.0 | 19.4 |
| 107.26 – ≤180.14 | 17.9 | 19.5 | 20.2 | 19.4 |
| 180.15 – ≤260.29 | 19.6 | 17.6 | 17.9 | 19.5 |
| 260.30 – ≤365.66 | 19.1 | 20.9 | 23.3 | 19.6 |
| >365.66 | 20.4 | 19.3 | 20.4 | 19.3 |
| Missing | 2.2 | 2.5 | 2.3 | 2.7 |
Percentages may not equal 100 owing to rounding
Defined as women who delivered in the study period who did not have diabetes (gestational or pre-existing), did not have any hypertensive disorder, and delivered at 37 weeks or greater.
Quintile cutoffs were determined among term births. The highest quintile reflects the highest degree of poverty.
Frequencies of preeclampsia cases and their controls with any vs no pesticide exposure assignments for the B1-P9 month periods are shown in Table 2. The frequency of any exposure was lower or about the same in each preeclampsia case group (further delineated by gestational age), and month time period, relative to the frequency in controls. The corresponding odds ratios (crude and adjusted) are shown in Table 3. Nearly all odds ratios were below 1.0 for these any vs no exposure comparisons.
Table 2.
Any (as percentage of total) gestational pesticide exposure per month among women with preeclampsia and their controls
| Count and percentage of any exposure (yes) per month1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Preeclampsia Phenotype |
B1 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | |
| Cases | Superimposed (gestational weeks 20–31) n=265 | 60 (22.6) | 66 (24.9) | 63 (23.8) | 66 (24.9) | 70 (26.4) | 66 (24.9) | 61 (23.0) | |||
| Superimposed (gestational weeks 32–36) n=780 | 198 (25.4) | 204 (26.2) | 202 (25.9) | 203 (26.0) | 204 (26.2) | 196 (25.1) | 207 (26.5) | 197 (25.3) | 207 (26.5) | ||
| Severe (gestational weeks 20–31) n=824 | 207 (25.1) | 213 (25.8) | 212 (25.7) | 212 (25.7) | 214 (26.0) | 215 (26.1) | 226 (27.4) | ||||
| Severe (gestational weeks 32–36) n=2647 | 715 (27.0) | 691 (26.1) | 722 (27.3) | 699 (26.4) | 720 (27.2) | 712 (26.9) | 723 (27.3) | 742 (28.0) | 714 (27.0) | ||
| Mild (gestational weeks 20–31) n=207 | 58 (28.0) | 58 (28.0) | 49 (23.7) | 58 (28.0) | 49 (23.7) | 57 (27.5) | 57 (27.5) | ||||
| Mild (gestational weeks 32–36) n=2573 | 717 (27.9) | 706 (27.4) | 677 (26.3) | 698 (27.1) | 693 (26.9) | 703 (27.3) | 698 (27.1) | 693 (26.9) | 673 (26.2) | ||
| Controls (n=197,461) | 55,136 (27.9) | 55,507 (28.1) | 55,770 (28.2) | 56,019(28.4) | 55,834 (28.3) | 56,029 (28.4) | 56,079 (28.4) | 56,000 (28.4) | 55,662 (28.2) | 54,960 (27.8) | |
B1=month before conception, P1-P9=each successive month from first to ninth month of pregnancy.
Table 3.
Odds ratios (ORs) for any vs no gestational pesticide exposure (per month) among women with preeclampsia and their controls
| Preeclampsia Phenotype |
Month of Exposure1 |
Cases (exposed/not- exposed) |
Controls (exposed/not- exposed) |
Crude OR (95%CI) |
Adjusted2 OR (95%CI) |
|---|---|---|---|---|---|
| Superimposed (gestational weeks 20–31) n=265 | B1 | 60/205 | 55136/142325 | 0.76 (0.57–1.01) | 0.69 (0.51–0.94) |
| P1 | 66/199 | 55507/141954 | 0.85 (0.64–1.12) | 0.78 (0.58–1.05) | |
| P2 | 63/202 | 55770/141691 | 0.79 (0.60–1.05) | 0.74 (0.55–0.99) | |
| P3 | 66/199 | 56019/141442 | 0.84 (0.63–1.11) | 0.80 (0.60–1.08) | |
| P4 | 70/195 | 55834/141627 | 0.91 (0.69–1.20) | 0.86 (0.64–1.15) | |
| P5 | 66/199 | 56029/141432 | 0.84 (0.63–1.11) | 0.79 (0.59–1.06) | |
| P6 | 61/204 | 56079/141382 | 0.75 (0.57–1.00) | 0.72 (0.53–0.97) | |
| Superimposed (gestational weeks 32–36) n=780 | B1 | 198/582 | 55136/142325 | 0.88 (0.75–1.03) | 0.88 (0.75–1.05) |
| P1 | 204/576 | 55507/141954 | 0.91 (0.77–1.06) | 0.92 (0.78–1.09) | |
| P2 | 202/578 | 55770/141691 | 0.89 (0.76–1.04) | 0.91 (0.77–1.07) | |
| P3 | 203/577 | 56019/141442 | 0.89 (0.76–1.04) | 0.92 (0.78–1.08) | |
| P4 | 204/576 | 55834/141627 | 0.90 (0.77–1.05) | 0.94 (0.79–1.10) | |
| P5 | 196/584 | 56029/141432 | 0.85 (0.72–1.00) | 0.88 (0.75–1.04) | |
| P6 | 207/573 | 56079/141382 | 0.91 (0.78–1.07) | 0.94 (0.80–1.11) | |
| P7 | 197/583 | 56000/141461 | 0.85 (0.73–1.00) | 0.88 (0.75–1.04) | |
| P8 | 207/573 | 55662/141799 | 0.92 (0.78–1.08) | 0.94 (0.79–1.10) | |
| Severe (gestational weeks 20–31) n=824 | B1 | 207/617 | 55136/142325 | 0.87 (0.74–1.01) | 0.89 (0.76–1.05) |
| P1 | 213/611 | 55507/141954 | 0.89 (0.76–1.04) | 0.91 (0.77–1.07) | |
| P2 | 212/612 | 55770/141691 | 0.88 (0.75–1.03) | 0.89 (0.76–1.05) | |
| P3 | 212/612 | 56019/141442 | 0.87 (0.75–1.02) | 0.89 (0.76–1.05) | |
| P4 | 214/610 | 55834/141627 | 0.89 (0.76–1.04) | 0.92 (0.78–1.08) | |
| P5 | 215/609 | 56029/141432 | 0.89 (0.76–1.04) | 0.92 (0.78–1.08) | |
| P6 | 226/598 | 56079/141382 | 0.95 (0.82–1.11) | 0.99 (0.85–1.16) | |
| Severe (gestational weeks 32–36) n=2647 | B1 | 715/1932 | 55136/142325 | 0.96 (0.88–1.04) | 0.95 (0.87–1.04) |
| P1 | 691/1956 | 55507/141954 | 0.90 (0.83–0.99) | 0.90 (0.82–0.98) | |
| P2 | 722/1925 | 55770/141691 | 0.95 (0.87–1.04) | 0.95 (0.87–1.04) | |
| P3 | 699/1948 | 56019/141442 | 0.91 (0.83–0.99) | 0.90 (0.83–0.99) | |
| P4 | 720/1927 | 55834/141627 | 0.95 (0.87–1.03) | 0.93 (0.85–1.02) | |
| P5 | 712/1935 | 56029/141432 | 0.93 (0.85–1.01) | 0.91 (0.84–1.00) | |
| P6 | 723/1924 | 56079/141382 | 0.95 (0.87–1.03) | 0.93 (0.85–1.02) | |
| P7 | 742/1905 | 56000/141461 | 0.98 (0.90–1.07) | 0.98 (0.89–1.07) | |
| P8 | 714/1933 | 55662/141799 | 0.94 (0.86–1.03) | 0.94 (0.86–1.03) | |
| Mild (gestational weeks 20–31) n=207 | B1 | 58/149 | 55136/142325 | 1.00 (0.74–1.36) | 1.07 (0.78–1.46) |
| P1 | 58/149 | 55507/141954 | 1.00 (0.73–1.35) | 1.08 (0.79–1.48) | |
| P2 | 49/158 | 55770/141691 | 0.79 (0.57–1.09) | 0.84 (0.61–1.17) | |
| P3 | 58/149 | 56019/141442 | 0.98 (0.73–1.33) | 1.01 (0.74–1.39) | |
| P4 | 49/158 | 55834/141627 | 0.79 (0.57–1.08) | 0.84 (0.60–1.17) | |
| P5 | 57/150 | 56029/141432 | 0.96 (0.71–1.30) | 0.99 (0.72–1.36) | |
| P6 | 57/150 | 56079/141382 | 0.96 (0.71–1.30) | 1.04 (0.76–1.43) | |
| Mild (gestational weeks 32–36) n=2573 | B1 | 717/1856 | 55136/142325 | 1.00 (0.91–1.09) | 1.00 (0.91–1.09) |
| P1 | 706/1867 | 55507/141954 | 0.97 (0.89–1.06) | 0.96 (0.88–1.06) | |
| P2 | 677/1896 | 55770/141691 | 0.91 (0.83–0.99) | 0.91 (0.83–1.00) | |
| P3 | 698/1875 | 56019/141442 | 0.94 (0.86–1.03) | 0.94 (0.86–1.02) | |
| P4 | 693/1880 | 55834/141627 | 0.94 (0.86–1.02) | 0.92 (0.84–1.01) | |
| P5 | 703/1870 | 56029/141432 | 0.95 (0.87–1.04) | 0.95 (0.87–1.04) | |
| P6 | 698/1875 | 56079/141382 | 0.94 (0.86–1.02) | 0.94 (0.86–1.03) | |
| P7 | 693/1880 | 56000/141461 | 0.93 (0.85–1.02) | 0.93 (0.85–1.02) | |
| P8 | 673/1900 | 55662/141799 | 0.90 (0.83–0.99) | 0.90 (0.82–0.99) |
B1=month before conception, P1-P8=each successive month from first to ninth month of pregnancy.
Odds ratio adjusted for maternal age (years), race/ethnicity (non-Hispanic white, U.S.-born Hispanic, foreign-born Hispanic, other), education (less than high school, high school, more than high school), parity (1 or >2), prenatal care initiated by fifth month (yes vs no), payer source for care (Medi-Cal, private, or other).
As noted in the Methods, we employed a minimum sample size criterion for risk estimation, i.e., pesticides (groups or specific chemicals) that had 5 or more exposed cases and controls for each preeclampsia phenotype. This produced >40,000 comparisons based on 6 preeclampsia case groups (superimposed, severe and mild each for the gestational weeks of 20–31 and 32–36), as many as 9 exposure months (i.e., B1-P9), 313 chemical groups with exposure, 61 chemical classes of exposure, and crude and adjusted odds ratios. Owing to this large number of comparisons, we have limited our presentation of results as follows, but summarize in text the general pattern of findings not specifically shown. We show adjusted odds ratios for chemical groups and specific chemicals for which 1) there were >5 cases exposed (this criterion biases toward identifying elevated risks) and 2) only for the exposure month closest to the time of delivery (e.g., for preeclampsia cases delivered at 20–31 weeks, odds ratios are shown for month P6). These results are displayed in Table 4 for chemical groups, and supplementary Table 1 for specific chemicals.
Table 4.
Odds ratios (ORs) for any vs no gestational exposures (per month) for specific pesticide chemical groups among women with preeclampsia and their controls. Shown are adjusted odds ratios for chemical groups where there were >5 cases exposed and for the exposure month closest to the time of delivery (e.g., for preeclampsia cases 32–36 weeks at delivery the odds ratios shown are for month P8).
| Chemical Class | Preeclampsia Phenotype |
Gestation Weeks at Delivery |
Month of Exposure1 |
Cases (exposed/not -exposed) |
Control (exposed /not- exposed) |
Adjusted OR2 ( 95%CI) |
|---|---|---|---|---|---|---|
| 2,4 - Dichlorophenoxy acid or ester | superimposed | 32–36 | P8 | 13/767 | 5710/191751 | 0.61 (0.35–1.06) |
| 2,4 - Dichlorophenoxy acid or ester | severe | 20–31 | P6 | 26/798 | 5754/191707 | 1.17 (0.79–1.73) |
| 2,4 - Dichlorophenoxy acid or ester | severe | 32–36 | P8 | 54/2593 | 5710/191751 | 0.72 (0.54–0.94) |
| 2,4 - Dichlorophenoxy acid or ester | mild | 20–31 | P6 | 10/197 | 5754/191707 | 1.70 (0.87–3.32) |
| 2,4 - Dichlorophenoxy acid or ester | mild | 32–36 | P8 | 69/2504 | 5710/191751 | 0.90 (0.70–1.15) |
| 2,6-Dinitroaniline | superimposed | 20–31 | P6 | 10/255 | 8657/188804 | 0.93 (0.50–1.76) |
| 2,6-Dinitroaniline | superimposed | 32–36 | P8 | 31/749 | 8442/189019 | 0.99 (0.69–1.42) |
| 2,6-Dinitroaniline | severe | 20–31 | P6 | 34/790 | 8657/188804 | 0.98 (0.69–1.39) |
| 2,6-Dinitroaniline | severe | 32–36 | P8 | 100/2547 | 8442/189019 | 0.92 (0.75–1.13) |
| 2,6-Dinitroaniline | mild | 20–31 | P6 | 7/200 | 8657/188804 | 0.86 (0.40–1.83) |
| 2,6-Dinitroaniline | mild | 32–36 | P8 | 116/2457 | 8442/189019 | 1.07 (0.88–1.29) |
| Alcohol/Ether | superimposed | 32–36 | P8 | 8/772 | 6273/191188 | 0.35 (0.18–0.71) |
| Alcohol/Ether | severe | 20–31 | P6 | 24/800 | 6340/191121 | 0.97 (0.64–1.46) |
| Alcohol/Ether | severe | 32–36 | P8 | 67/2580 | 6273/191188 | 0.80 (0.62–1.03) |
| Alcohol/Ether | mild | 20–31 | P6 | 8/199 | 6340/191121 | 1.35 (0.66–2.74) |
| Alcohol/Ether | mild | 32–36 | P8 | 61/2512 | 6273/191188 | 0.77 (0.60–1.00) |
| Amide | severe | 20–31 | P6 | 6/818 | 1214/196247 | 1.03 (0.43–2.50) |
| Amide | severe | 32–36 | P8 | 16/2631 | 1232/196229 | 1.03 (0.63–1.70) |
| Amide | mild | 32–36 | P8 | 18/2555 | 1232/196229 | 1.11 (0.69–1.80) |
| Amine | severe | 32–36 | P8 | 12/2635 | 679/196782 | 1.03 (0.53–2.00) |
| Amine | mild | 32–36 | P8 | 13/2560 | 679/196782 | 1.55 (0.89–2.69) |
| Anthranilic diamide | superimposed | 32–36 | P8 | 6/774 | 675/196786 | 2.04 (0.84–4.95) |
| Anthranilic diamide | severe | 20–31 | P6 | 6/818 | 547/196914 | 2.25 (0.93–5.47) |
| Anthranilic diamide | severe | 32–36 | P8 | 14/2633 | 675/196786 | 1.59 (0.93–2.70) |
| Anthranilic diamide | mild | 32–36 | P8 | 17/2556 | 675/196786 | 1.53 (0.88–2.66) |
| Aryloxyphenoxy propionic acid | severe | 32–36 | P8 | 5/2642 | 296/197165 | 1.32 (0.54–3.21) |
| Avermectin | superimposed | 32–36 | P8 | 16/764 | 5232/192229 | 0.77 (0.46–1.28) |
| Avermectin | severe | 20–31 | P6 | 16/808 | 5249/192212 | 0.72 (0.43–1.21) |
| Avermectin | severe | 32–36 | P8 | 66/2581 | 5232/192229 | 0.96 (0.74–1.23) |
| Avermectin | mild | 20–31 | P6 | 5/202 | 5249/192212 | 0.79 (0.29–2.13) |
| Avermectin | mild | 32–36 | P8 | 70/2503 | 5232/192229 | 1.03 (0.81–1.31) |
| Azole | superimposed | 20–31 | P6 | 8/257 | 8914/188547 | 0.62 (0.29–1.31) |
| Azole | superimposed | 32–36 | P8 | 28/752 | 8588/188873 | 0.82 (0.55–1.21) |
| Azole | severe | 20–31 | P6 | 45/779 | 8914/188547 | 1.27 (0.94–1.73) |
| Azole | severe | 32–36 | P8 | 106/2541 | 8588/188873 | 0.90 (0.74–1.11) |
| Azole | mild | 20–31 | P6 | 10/197 | 8914/188547 | 1.19 (0.63–2.25) |
| Azole | mild | 32–36 | P8 | 105/2468 | 8588/188873 | 0.92 (0.75–1.12) |
| Benzoic acid | superimposed | 32–36 | P8 | 9/771 | 1425/196036 | 1.56 (0.77–3.15) |
| Benzoic acid | severe | 20–31 | P6 | 5/819 | 1453/196008 | 0.88 (0.37–2.13) |
| Benzoic acid | severe | 32–36 | P8 | 17/2630 | 1425/196036 | 0.83 (0.50–1.38) |
| Benzoic acid | mild | 32–36 | P8 | 17/2556 | 1425/196036 | 0.91 (0.55–1.49) |
| Bipyridylium | superimposed | 20–31 | P6 | 10/255 | 9839/187622 | 0.73 (0.38–1.43) |
| Bipyridylium | superimposed | 32–36 | P8 | 24/756 | 9717/187744 | 0.60 (0.39–0.91) |
| Bipyridylium | severe | 20–31 | P6 | 41/783 | 9839/187622 | 0.98 (0.71–1.37) |
| Bipyridylium | severe | 32–36 | P8 | 99/2548 | 9717/187744 | 0.76 (0.62–0.93) |
| Bipyridylium | mild | 20–31 | P6 | 10/197 | 9839/187622 | 1.08 (0.57–2.04) |
| Bipyridylium | mild | 32–36 | P8 | 113/2460 | 9717/187744 | 0.91 (0.75–1.10) |
| Botanical | superimposed | 32–36 | P8 | 12/768 | 3304/194157 | 1.02 (0.58–1.82) |
| Botanical | severe | 20–31 | P6 | 14/810 | 3393/194068 | 0.99 (0.57–1.72) |
| Botanical | severe | 32–36 | P8 | 36/2611 | 3304/194157 | 0.77 (0.55–1.09) |
| Botanical | mild | 20–31 | P6 | 6/201 | 3393/194068 | 1.92 (0.85–4.35) |
| Botanical | mild | 32–36 | P8 | 39/2534 | 3304/194157 | 0.90 (0.65–1.25) |
| Chloroacetanilide | superimposed | 32–36 | P8 | 5/775 | 890/196571 | 1.50 (0.62–3.63) |
| Chloroacetanilide | severe | 32–36 | P8 | 15/2632 | 890/196571 | 1.24 (0.73–2.10) |
| Chloroacetanilide | mild | 32–36 | P8 | 11/2562 | 890/196571 | 0.91 (0.49–1.70) |
| Copper compound | superimposed | 20–31 | P6 | 11/254 | 11537/185924 | 0.70 (0.37–1.31) |
| Copper compound | superimposed | 32–36 | P8 | 29/751 | 11486/185975 | 0.59 (0.40–0.88) |
| Copper compound | severe | 20–31 | P6 | 55/769 | 11537/185924 | 1.23 (0.93–1.62) |
| Copper compound | severe | 32–36 | P8 | 148/2499 | 11486/185975 | 0.98 (0.83–1.17) |
| Copper compound | mild | 20–31 | P6 | 12/195 | 11537/185924 | 1.11 (0.62–2.00) |
| Copper compound | mild | 32–36 | P8 | 143/2430 | 11486/185975 | 0.95 (0.80–1.13) |
| Cyclohexenone derivative | superimposed | 32–36 | P8 | 5/775 | 1375/196086 | 0.93 (0.39–2.26) |
| Cyclohexenone derivative | severe | 32–36 | P8 | 20/2627 | 1375/196086 | 1.09 (0.69–1.71) |
| Cyclohexenone derivative | mild | 32–36 | P8 | 18/2555 | 1375/196086 | 0.94 (0.57–1.54) |
| Diacylhydrazine | superimposed | 20–31 | P6 | 5/260 | 2906/194555 | 1.11 (0.41–2.98) |
| Diacylhydrazine | superimposed | 32–36 | P8 | 10/770 | 2907/194554 | 0.82 (0.42–1.58) |
| Diacylhydrazine | severe | 20–31 | P6 | 13/811 | 2906/194555 | 0.97 (0.53–1.76) |
| Diacylhydrazine | severe | 32–36 | P8 | 36/2611 | 2907/194554 | 0.90 (0.64–1.27) |
| Diacylhydrazine | mild | 32–36 | P8 | 36/2537 | 2907/194554 | 0.96 (0.68–1.34) |
| Dicarboximide | superimposed | 32–36 | P8 | 13/767 | 3825/193636 | 0.84 (0.47–1.49) |
| Dicarboximide | severe | 20–31 | P6 | 18/806 | 4085/193376 | 1.12 (0.70–1.79) |
| Dicarboximide | severe | 32–36 | P8 | 51/2596 | 3825/193636 | 1.01 (0.76–1.35) |
| Dicarboximide | mild | 32–36 | P8 | 53/2520 | 3825/193636 | 1.05 (0.79–1.40) |
| Dithiocarbamate–ETU | superimposed | 32–36 | P8 | 10/770 | 4290/193171 | 0.57 (0.29–1.10) |
| Dithiocarbamate-ETU | severe | 20–31 | P6 | 25/799 | 4364/193097 | 1.44 (0.96–2.16) |
| Dithiocarbamate-ETU | severe | 32–36 | P8 | 54/2593 | 4290/193171 | 1.00 (0.76–1.31) |
| Dithiocarbamate-ETU | mild | 32–36 | P8 | 60/2513 | 4290/193171 | 1.04 (0.80–1.37) |
| Dithiocarbamate-MITC | severe | 32–36 | P8 | 16/2631 | 1142/196319 | 1.05 (0.63–1.76) |
| Dithiocarbamate-MITC | mild | 32–36 | P8 | 18/2555 | 1142/196319 | 1.22 (0.75–1.98) |
| Glycol | severe | 20–31 | P6 | 13/811 | 3201/194260 | 1.06 (0.61–1.84) |
| Glycol | severe | 32–36 | P8 | 32/2615 | 3308/194153 | 0.73 (0.51–1.05) |
| Glycol | mild | 32–36 | P8 | 31/2542 | 3308/194153 | 0.75 (0.53–1.07) |
| Halogenated organic | severe | 20–31 | P6 | 8/816 | 2054/195407 | 0.99 (0.49–2.00) |
| Halogenated organic | severe | 32–36 | P8 | 20/2627 | 1939/195522 | 0.76 (0.49–1.20) |
| Halogenated organic | mild | 32–36 | P8 | 20/2553 | 1939/195522 | 0.83 (0.53–1.29) |
| Hydroxybenzonitrile | severe | 32–36 | P8 | 16/2631 | 1117/196344 | 1.14 (0.69–1.88) |
| Hydroxybenzonitrile | mild | 32–36 | P8 | 10/2563 | 1117/196344 | 0.73 (0.39–1.36) |
| Imidazolinone | severe | 32–36 | P8 | 9/2638 | 664/196797 | 1.08 (0.56–2.09) |
| Insect growth regulator (Chitin) | superimposed | 32–36 | P8 | 7/773 | 1496/195965 | 1.06 (0.47–2.38) |
| Insect growth regulator (Chitin) | severe | 20–31 | P6 | 9/815 | 1444/196017 | 1.45 (0.72–2.91) |
| Insect growth regulator (Chitin) | severe | 32–36 | P8 | 16/2631 | 1496/195965 | 0.71 (0.42–1.20) |
| Insect growth regulator (Chitin) | mild | 32–36 | P8 | 23/2550 | 1496/195965 | 1.16 (0.76–1.77) |
| Monochlorophenoxy acid or ester | severe | 20–31 | P6 | 5/819 | 1518/195943 | 0.85 (0.35–2.06) |
| Monochlorophenoxy acid or ester | severe | 32–36 | P8 | 16/2631 | 1506/195955 | 0.73 (0.43–1.23) |
| Monochlorophenoxy acid or ester | mild | 32–36 | P8 | 24/2549 | 1506/195955 | 1.28 (0.86–1.93) |
| N-Methyl Carbamate | superimposed | 32–36 | P8 | 13/767 | 5382/192079 | 0.60 (0.34–1.07) |
| N-Methyl Carbamate | severe | 20–31 | P6 | 17/807 | 5436/192025 | 0.79 (0.49–1.28) |
| N-Methyl Carbamate | severe | 32–36 | P8 | 68/2579 | 5382/192079 | 0.92 (0.71–1.18) |
| N-Methyl Carbamate | mild | 20–31 | P6 | 7/200 | 5436/192025 | 1.16 (0.51–2.61) |
| N-Methyl Carbamate | mild | 32–36 | P8 | 56/2517 | 5382/192079 | 0.80 (0.61–1.05) |
| Neonicotinoid | superimposed | 32–36 | P8 | 19/761 | 5317/192144 | 0.77 (0.46–1.28) |
| Neonicotinoid | severe | 20–31 | P6 | 24/800 | 5197/192264 | 1.15 (0.75–1.74) |
| Neonicotinoid | severe | 32–36 | P8 | 65/2582 | 5317/192144 | 0.88 (0.68–1.14) |
| Neonicotinoid | mild | 20–31 | P6 | 5/202 | 5197/192264 | 0.81 (0.30–2.19) |
| Neonicotinoid | mild | 32–36 | P8 | 58/2515 | 5317/192144 | 0.84 (0.65–1.10) |
| Organoarsenic | severe | 32–36 | P8 | 6/2641 | 310/197151 | 1.49 (0.66–3.35) |
| Organoarsenic | mild | 32–36 | P8 | 7/2566 | 310/197151 | 1.82 (0.86–3.87) |
| Organochlorine | severe | 20–31 | P6 | 5/819 | 1326/196135 | 0.99 (0.41–2.39) |
| Organochlorine | severe | 32–36 | P8 | 17/2630 | 1309/196152 | 1.02 (0.63–1.64) |
| Organochlorine | mild | 32–36 | P8 | 10/2563 | 1309/196152 | 0.62 (0.33–1.15) |
| Organophosphate | superimposed | 20–31 | P6 | 14/251 | 16763/180698 | 0.55 (0.31–0.98) |
| Organophosphate | superimposed | 32–36 | P8 | 55/725 | 17033/180428 | 0.84 (0.63–1.10) |
| Organophosphate | severe | 20–31 | P6 | 69/755 | 16763/180698 | 1.03 (0.80–1.32) |
| Organophosphate | severe | 32–36 | P8 | 215/2432 | 17033/180428 | 0.95 (0.82–1.09) |
| Organophosphate | mild | 20–31 | P6 | 17/190 | 16763/180698 | 1.01 (0.60–1.69) |
| Organophosphate | mild | 32–36 | P8 | 171/2402 | 17033/180428 | 0.74 (0.63–0.87) |
| Petroleum derivative-Aromatic | superimposed | 20–31 | P6 | 7/258 | 11398/186063 | 0.42 (0.19–0.94) |
| Petroleum derivative-Aromatic | superimposed | 32–36 | P8 | 32/748 | 11470/185991 | 0.73 (0.51–1.05) |
| Petroleum derivative-Aromatic | severe | 20–31 | P6 | 45/779 | 11398/186063 | 0.95 (0.69–1.29) |
| Petroleum derivative-Aromatic | severe | 32–36 | P8 | 132/2515 | 11470/185991 | 0.87 (0.73–1.04) |
| Petroleum derivative-Aromatic | mild | 20–31 | P6 | 15/192 | 11398/186063 | 1.33 (0.77–2.30) |
| Petroleum derivative-Aromatic | mild | 32–36 | P8 | 132/2441 | 11470/185991 | 0.86 (0.71–1.03) |
| Phosphine | severe | 32–36 | P8 | 13/2634 | 974/196487 | 1.06 (0.61–1.84) |
| Phosphine | mild | 32–36 | P8 | 8/2565 | 974/196487 | 0.65 (0.32–1.31) |
| Phosphonoglycine | superimposed | 20–31 | P6 | 22/243 | 25816/171645 | 0.62 (0.40–0.98) |
| Phosphonoglycine | superimposed | 32–36 | P8 | 101/679 | 25416/172045 | 1.05 (0.85–1.31) |
| Phosphonoglycine | severe | 20–31 | P6 | 105/719 | 25816/171645 | 1.02 (0.82–1.25) |
| Phosphonoglycine | severe | 32–36 | P8 | 295/2352 | 25416/172045 | 0.86 (0.76–0.98) |
| Phosphonoglycine | mild | 20–31 | P6 | 29/178 | 25816/171645 | 1.17 (0.78–1.76) |
| Phosphonoglycine | mild | 32–36 | P8 | 288/2285 | 25416/172045 | 0.85 (0.75–0.96) |
| Piperonyl | severe | 32–36 | P8 | 5/2642 | 465/196996 | 0.86 (0.36–2.09) |
| Piperonyl | mild | 32–36 | P8 | 6/2567 | 465/196996 | 1.04 (0.46–2.33) |
| Polyalkyloxy Compound | superimposed | 32–36 | P8 | 18/762 | 11641/185820 | 0.37 (0.22–0.61) |
| Polyalkyloxy Compound | severe | 20–31 | P6 | 45/779 | 11826/185635 | 0.94 (0.69–1.28) |
| Polyalkyloxy Compound | severe | 32–36 | P8 | 124/2523 | 11641/185820 | 0.80 (0.67–0.97) |
| Polyalkyloxy Compound | mild | 20–31 | P6 | 17/190 | 11826/185635 | 1.58 (0.96–2.61) |
| Polyalkyloxy Compound | mild | 32–36 | P8 | 130/2443 | 11641/185820 | 0.87 (0.73–1.04) |
| Pyrethroid | superimposed | 20–31 | P6 | 9/256 | 12452/18500 | 0.49 (0.24–0.99) |
| Pyrethroid | superimposed | 32–36 | P8 | 52/728 | 12541/184920 | 1.09 (0.82–1.45) |
| Pyrethroid | severe | 20–31 | P6 | 45/779 | 12452/185009 | 0.86 (0.63–1.18) |
| Pyrethroid | severe | 32–36 | P8 | 143/2504 | 12541/184920 | 0.85 (0.72–1.01) |
| Pyrethroid | mild | 20–31 | P6 | 17/190 | 12452/185009 | 1.27 (0.75–2.15) |
| Pyrethroid | mild | 32–36 | P8 | 132/2441 | 12541/184920 | 0.79 (0.66–0.94) |
| Pyridazinone | severe | 20–31 | P6 | 8/816 | 1994/195467 | 1.03 (0.51–2.08) |
| Pyridazinone | severe | 32–36 | P8 | 17/2630 | 2029/195432 | 0.65 (0.40–1.05) |
| Pyridazinone | mild | 32–36 | P8 | 25/2548 | 2029/195432 | 0.99 (0.66–1.47) |
| Quaternary Ammonium Compound | severe | 32–36 | P8 | 20/2627 | 1570/195891 | 0.89 (0.56–1.43) |
| Quaternary Ammonium Compound | mild | 32–36 | P8 | 15/2558 | 1570/195891 | 0.77 (0.46–1.28) |
| Silicone | severe | 20–31 | P6 | 14/810 | 4322/193139 | 0.84 (0.49–1.43) |
| Silicone | severe | 32–36 | P8 | 43/2604 | 4358/193103 | 0.73 (0.53–1.00) |
| Silicone | mild | 32–36 | P8 | 46/2527 | 4358/193103 | 0.84 (0.62–1.13) |
| Soap | severe | 20–31 | P6 | 6/818 | 987/196474 | 1.57 (0.70–3.53) |
| Soap | severe | 32–36 | P8 | 13/2634 | 946/196515 | 0.84 (0.45–1.56) |
| Soap | mild | 32–36 | P8 | 17/2556 | 946/196515 | 1.46 (0.90–2.37) |
| Spinosyn | superimposed | 32–36 | P8 | 11/769 | 4287/193174 | 0.64 (0.34–1.21) |
| Spinosyn | severe | 20–31 | P6 | 20/804 | 4304/193157 | 1.19 (0.76–1.85) |
| Spinosyn | severe | 32–36 | P8 | 58/2589 | 4287/193174 | 1.02 (0.79–1.34) |
| Spinosyn | mild | 20–31 | P6 | 7/200 | 4304/193157 | 1.49 (0.66–3.37) |
| Spinosyn | mild | 32–36 | P8 | 44/2529 | 4287/193174 | 0.73 (0.53–1.00) |
| Streptomycin | severe | 32–36 | P8 | 7/2640 | 473/196988 | 1.18 (0.56–2.49) |
| Strobin | superimposed | 20–31 | P6 | 6/259 | 6165/191296 | 0.76 (0.34–1.71) |
| Strobin | superimposed | 32–36 | P8 | 22/758 | 6117/191344 | 0.87 (0.55–1.35) |
| Strobin | severe | 20–31 | P6 | 23/801 | 6165/191296 | 0.91 (0.60–1.40) |
| Strobin | severe | 32–36 | P8 | 86/2561 | 6117/191344 | 1.00 (0.80–1.26) |
| Strobin | mild | 20–31 | P6 | 5/202 | 6165/191296 | 0.85 (0.35–2.06) |
| Strobin | mild | 32–36 | P8 | 89/2484 | 6117/191344 | 1.11 (0.89–1.38) |
| Sulfonylurea | superimposed | 32–36 | P8 | 8/772 | 1346/196115 | 1.61 (0.80–3.24) |
| Sulfonylurea | severe | 32–36 | P8 | 20/2627 | 1346/196115 | 1.14 (0.72–1.79) |
| Sulfonylurea | mild | 32–36 | P8 | 17/2556 | 1346/196115 | 0.92 (0.55–1.53) |
| Thiocarbamate | severe | 32–36 | P8 | 8/2639 | 776/196685 | 0.81 (0.40–1.64) |
| Thiocarbamate | mild | 32–36 | P8 | 14/2559 | 776/196685 | 1.45 (0.85–2.47) |
| Thiophanate, benzimidazole precursor | severe | 20–31 | P6 | 8/816 | 854/196607 | 2.35 (1.16–4.74) |
| Thiophanate, benzimidazole precursor | severe | 32–36 | P8 | 6/2641 | 783/196678 | 0.60 (0.27–1.35) |
| Thiophanate, benzimidazole precursor | mild | 32–36 | P8 | 8/2565 | 783/196678 | 0.83 (0.41–1.66) |
| Thiophthalimide | severe | 20–31 | P6 | 5/819 | 1246/196215 | 1.00 (0.41–2.42) |
| Thiophthalimide | severe | 32–36 | P8 | 11/2636 | 1203/196258 | 0.72 (0.40–1.31) |
| Thiophthalimide | mild | 32–36 | P8 | 19/2554 | 1203/196258 | 1.28 (0.81–2.03) |
| Triazine | superimposed | 32–36 | P8 | 17/763 | 6626/190835 | 0.66 (0.40–1.08) |
| Triazine | severe | 20–31 | P6 | 30/794 | 6676/190785 | 1.19 (0.82–1.71) |
| Triazine | severe | 32–36 | P8 | 77/2570 | 6626/190835 | 0.89 (0.70–1.12) |
| Triazine | mild | 20–31 | P6 | 8/199 | 6676/190785 | 1.31 (0.64–2.65) |
| Triazine | mild | 32–36 | P8 | 84/2489 | 6626/190835 | 0.98 (0.79–1.23) |
| Urea | superimposed | 20–31 | P6 | 5/260 | 5243/192218 | 0.78 (0.32–1.90) |
| Urea | superimposed | 32–36 | P8 | 21/759 | 5139/192322 | 0.97 (0.61–1.56) |
| Urea | severe | 20–31 | P6 | 29/795 | 5243/192218 | 1.42 (0.97–2.08) |
| Urea | severe | 32–36 | P8 | 73/2574 | 5139/192322 | 1.10 (0.86–1.39) |
| Urea | mild | 20–31 | P6 | 6/201 | 5243/192218 | 1.26 (0.56–2.84) |
| Urea | mild | 32–36 | P8 | 60/2513 | 5139/192322 | 0.85 (0.65–1.12) |
| Xylylalanine | severe | 20–31 | P6 | 5/819 | 1664/195797 | 0.62 (0.23–1.66) |
| Xylylalanine | severe | 32–36 | P8 | 22/2625 | 1591/195870 | 1.07 (0.70–1.63) |
| Xylylalanine | mild | 32–36 | P8 | 24/2549 | 1591/195870 | 1.10 (0.72–1.68) |
| Zinc,inorganic | severe | 32–36 | P8 | 9/2638 | 971/196490 | 0.75 (0.39–1.46) |
| Zinc,inorganic | mild | 32–36 | P8 | 12/2561 | 971/196490 | 1.02 (0.58–1.81) |
B1=month before conception, P1-P8=each successive month from first to ninth month of pregnancy.
Odds ratio adjusted for maternal age (years), race/ethnicity (non-Hispanic white, U.S.-born Hispanic, foreign-born Hispanic, other), education (less than high school, high school, more than high school), parity (1 or >2), prenatal care initiated by fifth month (yes vs no), payer source for care (Medi-Cal, private, or other).
As shown in Table 4, there was only a single comparison (thiophanate) that had an odds ratio above 1.0 and a confidence interval that did not include 1.0. Indeed, many of the adjusted odds ratios were below 1.0 (crude estimates were similar). Results for the “months of exposure” not shown were not substantially different than those that are shown.
In Supplementary Table 1 we display adjusted odds ratios associated with specific chemicals. Similar to results for chemical groups, only a small number of statistically precise elevated risks was observed (crude estimates were similar). The 20 comparisons observed to have elevated odds ratios ranged in magnitude from 1.36 (copper hydroxide) to 3.57 (hydrogen cyanamide). The observed elevated odds ratios were associated with a variety of chemicals and did not appear to be associated with a specific preeclampsia phenotype.
To estimate potential risks from exposure to multiple pesticides, we summed women’s exposures to various chemical classifications, including number of chemical groups, endocrine disruptors, Proposition 65 listed reproductive toxicants, or EPA listed reproductive or developmental toxicants. Women’s increasing sum of exposures to each of these classifications was not associated with elevated risks (Table 5). Indeed, for superimposed preeclampsia a statistically significant inverse association for increasing sum of exposures was observed.
Table 5.
Adjusted odds ratios (ORs) for sums of specific classifications of pesticide exposures and preeclampsia phenotypes.
| Superimposed 20–31 weeks |
Superimposed 32–36 weeks |
Severe 20–31 weeks |
Severe 32–36 weeks |
Mild 20–31 weeks |
Mild 32–36 weeks |
|
|---|---|---|---|---|---|---|
| OR (95%CI)1 | OR (95%CI)1 | OR (95%CI)1 |
OR (95%CI)1 |
OR (95%CI)1 |
OR (95%CI)1 |
|
| No. of chemical groups with any exposure2 | ||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1–2 | 0.93 (0.63–1.36) | 1.14 (0.92–1.41) | 0.90 (0.71–1.13) | 1.01 (0.90–1.15) | 0.84 (0.51–1.37) | 0.91 (0.80–1.03) |
| 3–5 | 0.53 (0.30–0.92) | 0.85 (0.65–1.12) | 1.09 (0.86–1.39) | 0.92 (0.80–1.06) | 1.37 (0.88–2.14) | 0.89 (0.77–1.03) |
| >5 | 0.43 (0.21–0.87) | 0.63 (0.44–0.91) | 1.10 (0.84–1.45) | 0.82 (0.70–0.98) | 1.10 (0.63–1.91) | 0.85 (0.71–1.00) |
| Continuous | 0.88 (0.81–0.95) | 0.95 (0.91–0.98) | 1.00 (0.98–1.03) | 0.98 (0.96–1.00) | 1.02 (0.97–1.08) | 0.98 (0.96–1.00) |
| No. of endocrine disruptors with any exposure | ||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1 | 0.85 (0.54–1.34) | 0.74 (0.55–0.99) | 1.04 (0.81–1.33) | 1.04 (0.91–1.19) | 1.33 (0.84–2.10) | 0.92 (0.80–1.06) |
| 2 | 0.31 (0.12–0.84) | 1.06 (0.77–1.47) | 1.20 (0.88–1.63) | 0.81 (0.66–0.99) | 1.06 (0.54–2.08) | 0.72 (0.58–0.90) |
| >2 | 0.40 (0.18–0.89) | 0.70 (0.49–1.02) | 1.16 (0.86–1.55) | 0.89 (0.74–1.06) | 1.30 (0.73–2.29) | 0.83 (0.69–1.00) |
| Continuous | 0.75 (0.63–0.91) | 0.91 (0.84–0.98) | 1.02 (0.96–1.09) | 0.96 (0.92–1.00) | 1.10 (0.99–1.22) | 0.93 (0.90–0.97) |
| No. of Prop. 65 reproductive toxicants with any exposure | ||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1 | 0.69 (0.38–1.23) | 0.73 (0.52–1.01) | 0.95 (0.72–1.27) | 0.93 (0.79–1.09) | 1.34 (0.81–2.21) | 0.85 (0.72–1.01) |
| >1 | N/A | N/A | 0.90 (0.53–1.54) | 0.93 (0.69–1.25) | N/A | 0.82 (0.60–1.13) |
| Continuous | 0.60 (0.37–0.96) | 0.64 (0.50–0.84) | 0.94 (0.78–1.13) | 0.95 (0.85–1.05) | 1.08 (0.78–1.50) | 0.89 (0.80–0.99) |
| No. of reproductive or developmental toxicants with any exposure | ||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1–2 | 1.02 (0.71–1.47) | 1.10 (0.89–1.36) | 0.94 (0.75–1.17) | 0.92 (0.81–1.04) | 0.94 (0.60–1.48) | 0.91 (0.80–1.03) |
| 3–4 | 0.63 (0.34–1.15) | 0.97 (0.72–1.30) | 1.17 (0.90–1.53) | 1.02 (0.87–1.18) | 1.11 (0.64–1.93) | 0.91 (0.77–1.07) |
| >4 | 0.46 (0.23–0.89) | 0.73 (0.53–1.01) | 0.95 (0.72–1.26) | 0.81 (0.69–0.96) | 1.08 (0.63–1.84) | 0.85 (0.72–1.00) |
| Continuous | 0.91 (0.84–0.98) | 0.96 (0.92–0.99) | 1.00 (0.97–1.03) | 0.98 (0.96–1.00) | 1.02 (0.96–1.08) | 0.98 (0.96–1.00) |
Odds ratio adjusted for maternal age (years), race/ethnicity (non-Hispanic white, U.S.-born Hispanic, foreign-born Hispanic, other), education (less than high school, high school, more than high school), parity (1 or >2), prenatal care initiated by fifth month (yes vs no), payer source for care (Medi-Cal, private, or other).
This categorization reflects the total number of chemical groups (total possible=67) that an individual was considered exposed to.
For a subset (2007–11) of the overall data (1998–2011) we had information about body mass index and poverty (see Table 1 for description and frequency). These additional variables were added as covariates to adjusted models. Results of these additional analyses did not show substantially different findings from those displayed in Tables 2–4 (data not shown).
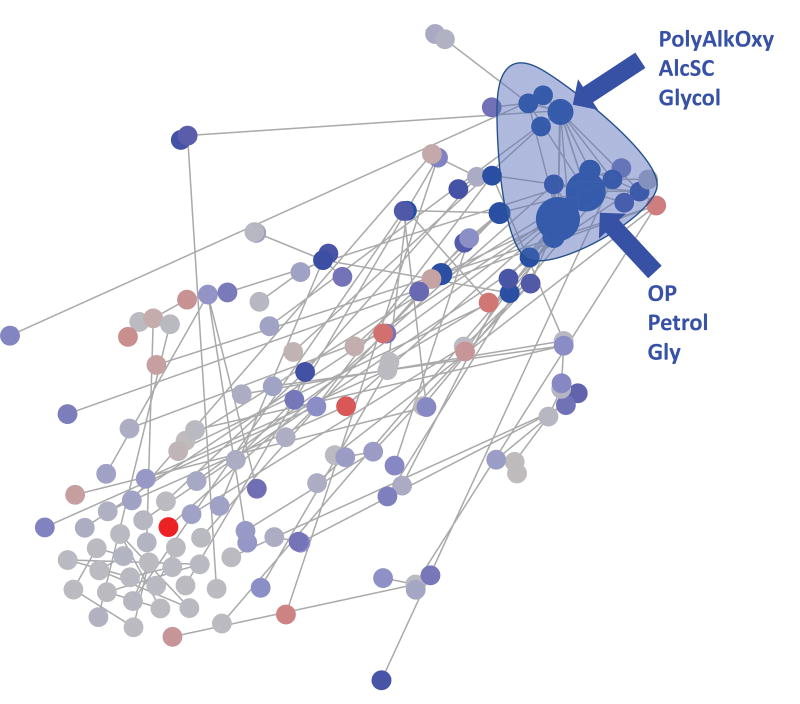
We also investigated the large amount of data employing an elastic net algorithm. This more agnostic analytic approach also revealed similar results, i.e., showing reduced risks for many of the pesticide exposures and preeclampsia phenotypes. As an example, in Figure 1 we show the results of this approach applied to exposures to chemical groups and any preeclampsia in P5 (other comparisons can be provided upon request). The elastic net model was primarily dominated by negative (in blue) coefficients for several chemical groups. These chemical groups were highly correlated across women (hence, they cluster together in Figure 1, top right). This approach confirms the inverse association with a summary score of the dataset (in this case the summary score is a weighted sum, with the weights objectively calculated using L1 and L2 penalties).
Figure 1.
(Abreviations, PolyAlkOxy=polyalkyloxy compound, AlcSC=Alcohol ether. OP=organophosphate, Petrol=petroleum derivative-aromatic, Gly=Glycol) Correlation network examining the association between any preeclampsia and pesticide chemical groups exposure during P5. An edge between two nodes indicates a significant correlation (after Bonferroni correction). Node size indicates the -log10 transformed p-value of a univariate Wilcoxon test for each feature. Node color indicates the direction of the correlation (blue and red correspond to higher and lower exposure in preeclamptic women, respectively) and the brightness of the color corresponds to the coefficient of the elastic net model (darker colors have a higher coefficient and therefore are more important).
4. DISCUSSION
We investigated population-based data on >200,000 births and proximal residential exposures to >500 commercial agricultural pesticides during multiple gestational time points for potential associations with preeclampsia. Despite a very large study population, consideration of preeclampsia into narrowly-defined phenotypes, and consideration of a variety of gestational exposure definitions such as chemical groups, specific chemicals, and number of pesticides, there was a general lack of association between pesticide exposures and elevated risks of preeclampsia. Given the large number of comparisons made, substantially more elevated risks would have been expected to emerge by chance alone.
Extant research on women’s residential proximity to pesticide applications and risks of preeclampsia is scant. To our knowledge there has been one study that investigated residential proximity to pesticides. Willis et al. (1993), in a small cohort study of 535 women, indicated that women who reported living near land used for agricultural purposes did not show a significant increased risk to have preeclampsia. Although not directly comparable to exposures in the current study, Saldana and colleagues (2009) observed an increased risk (odds ratio=1.32, 95% CI 1.02–1.60) of preeclampsia among women who reported engaging in activities related to applying pesticides in their home or garden.
Curiously, many of the analytic comparisons (including the agnostic elastic net algorithm) showed reduced risks of preeclampsia and various pesticide exposure estimations. We observed a similar enigmatic direction of findings in a recent study of spontaneous preterm birth and residential pesticide exposures (Shaw et al. 2017); that study excluded women with hypertensive disorders. In that study, as in the current one, we find it difficult to infer that such exposures would be beneficial to reducing the likelihood of either spontaneous preterm birth or preeclampsia given the manifold toxicities pesticide compounds have. Unadjusted confounding influences of cigarette smoking are unlikely to explain the direction of results either owing to 60% of the study population was Hispanic women, a population group known to have very low use of cigarettes.
As a hypothesis for the unexpected direction of some results, it is possible that unobserved early fetal loss hindered our ability to derive unbiased risk estimates. That is, pesticide exposures in pregnancy before 20 weeks, the earliest a birth would be identified in vital statistics files and before preeclampsia would be diagnosed, may selectively increase the odds of earlier loss in pregnancies destined to be preeclamptic and therefore not observable when only live birth data are the target study population. Others have described this construct as left truncation and have specifically done so to characterize some or all of the inverse association between smoking and risk for preeclampsia (Lisonkova and Joseph 2015; Kinlaw et al. 2017). Although such a bias proposition seems reasonable, the extant data investigating potential associations between miscarriage and residential pesticide exposures is too sparse to make meaningful conjectures (Shirangi et al. 2011)
Our study had several strengths, including its population-based design, large sample size, definition of specific maternal hypertensive disorders either included as cases or removed from referents (owing to there likely being different mechanisms underlying such phenotypes), and an exposure assessment that was highly detailed and spatially and temporally specific (to multiple gestational periods), and captured a broad spectrum of pesticide compounds.
Our study also had challenges. Our assessment of residential proximity to agricultural pesticide applications was extensive, but it did not take into account factors such as amounts of pesticides applied or qualities of the pesticides and individuals’ behaviors that could affect actual exposures (e.g., chemical half-lives and vapor pressure, wind patterns, accumulative exposures over time a woman may have had before pregnancy, and other sources of pesticide exposure such as occupation or home use). Exposure assignment relied on residence address at delivery rather than at earlier times in pregnancy. Misclassification of exposure could have occurred for women who moved their residence during gestation. If moving was unrelated to the development of preeclampsia, results would be biased toward the null; if not, the direction cannot be predicted. Further, duration of time spent at the given address is unknown and likely reflective of only a portion of what a woman may encounter in her broader exposome. Although many pesticides are prone to drift and detectable in air samples at locations beyond the application site (Kegley et al. 2012), and residential proximity to pesticide-treated fields has been associated with household dust and urine levels (Fenske et al. 2000; Simcox et al. 1995), there are certainly other exposure sources such as in food or water that were not considered here, whereby individuals could be exposed. These various sources of misclassification would be expected to be non-differential, reducing our precision to estimate potential associations.
Our study rigorously adds to the scant literature on this topic, particularly in its effort to investigate narrower phenotypic groups of preeclampsia as well as numerous pesticide compounds.
Supplementary Material
Highlights.
Environmental exposures have been rarely investigated for their potential etiologic contribution to preeclampsia, a condition that contributes substantially to maternal morbidity and mortality.
This study investigated population-based data on >200,000 births and proximal residential exposures to more than 500 commercial agricultural pesticides during multiple gestational time points in one of the highest agricultural pesticide use areas in the US.
Despite a very large study population, consideration of preeclampsia into narrowly-defined phenotypes, and consideration of a variety of gestational exposure definitions such as chemical groups, specific chemicals, and number of pesticides, there was a general lack of association between pesticide exposures and elevated risks of preeclampsia.
Nearly all odds ratios were below 1.0 for these any vs no exposure comparisons. As a hypothesis for the unexpected direction of some results, it is possible that unobserved early fetal loss hindered our ability to derive unbiased risk estimates.
Acknowledgments
Sources of Funding:
This project was supported by NIH (R01HD075761) with additional support from the March of Dimes Prematurity Research Center at Stanford University (MOD PR625253).
This study was approved by the Stanford University Institutional Review Board and the California State Committee for the Protection of Human Subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- California-Department-of-Pesticide-Regulation. Pesticide Use Reporting. http://www.cdpr.ca.gov/docs/pur/purmain.htm.
- California-Environmental-Health-Tracking-Program. [Accessed October, 2012];Agricultural Pesticide Mapping Tool. http://www.cehtp.org/page/pesticides/agricultural_pesticide_use_in_california.
- EPA-(U.S.-Environmental-Protection-Agency) Pesticide Chemical Search. http://www.epa.gov/pesticides/chemicalsearch.
- California-Office-of-Environmental-Health-Hazard-Assessment. Proposition 65. http://www.oehha.ca.gov/prop65.html.
- Colborn T. Our Stolen Future: Widespread pollutants with reproductive and endocrine-disrupting effects. http://www.ourstolenfuture.org/basics/chemlist.htm.
- European-Commission. [Accessed October 2012];Towards the Establishment of a Priority List of Substances for Further Evaluation of Their Role in Endocrine Disruption, Appendix 1. http://ec.europa.eu/environment/endocrine/strategy/substances_en.htm.
- Keith LH. Environmental endocrine disruptors : a handbook of property data. New York: Wiley; 1997. [Google Scholar]
- Herrchen B, Gould JB, Nesbitt TS. Vital statistics linked birth/infant death and hospital discharge record linkage for epidemiological studies. Comput Biomed Res. 1997;30:290–305. doi: 10.1006/cbmr.1997.1448. [DOI] [PubMed] [Google Scholar]
- Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. J Matern Fetal Neonatal Med. 2012;25:2529–35. doi: 10.3109/14767058.2012.710280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Sokooti M, Galli CL, Moretto A, Colosio C. Pesticide induced immunotoxicity in humans: a comprehensive review of the existing evidence. Toxicology. 2013;307:123–35. doi: 10.1016/j.tox.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Kissel JC, Lu C, et al. Biologically based pesticide dose estimates for children in an agricultural community. Environ Health Perspect. 2000;108(6):515–20. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013;71(Suppl 1):S18–25. doi: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebbink J, Wolters A, Fernando F, Afink G, van der Post J, Ris-Stalpers C. Molecular genetics of preeclampsia and HELLP syndrome - a review. Biochim Biophys Acta. 2012;1822:1960–9. doi: 10.1016/j.bbadis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Kegley SEHB, Orme S, Choi AH. [Accessed December 3, 2012];PAN Pesticide Database. http://www.pesticideinfo.org/
- Kinlaw AC, Buckley JP, Engel SM, Poole C, Brookhart MA, Keil AP. Left Truncation Bias to Explain the Protective Effect of Smoking on Preeclampsia: Potential, But How Plausible? Epidemiology. 2017;28:428–434. doi: 10.1097/EDE.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda C, Fiore M, Santarelli L, Bracci M, Mascali G, D’Agati MG, Busà A, Ferrante M, Rapisarda V. Gestational Hypertension and Organophosphorus Pesticide Exposure: A Cross-Sectional Study. Biomed Res Int. 2015;2015:280891. doi: 10.1155/2015/280891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisonkova S, Joseph KS. Left truncation bias as a potential explanation for the protective effect of smoking on preeclampsia. Epidemiology. 2015;263:436–40. doi: 10.1097/EDE.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol BW, Roberts CT, Thangaratinam S, Magee LA, de Groot CJ, Hofmeyr GJ. Preeclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- Morgan DP, Lin LI, Saikaly HH. Morbidity and mortality in workers occupationally exposed to pesticides. Arch Environ Contam Toxicol. 1980;9(3):349–82. doi: 10.1007/BF01057414. [DOI] [PubMed] [Google Scholar]
- Nordby KC, Irgens LM, Kristensen P. Immunological exposures in Norwegian agriculture and pre-eclampsia. Paediatr Perinat Epidemiol. 2006;20:462–70. doi: 10.1111/j.1365-3016.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- Nugteren JJ, Snijder CA, Hofman A, Jaddoe VW, Steegers EA, Burdorf A. Work-related maternal risk factors and the risk of pregnancy induced hypertension and preeclampsia during pregnancy.The Generation R Study. PLoS One. 2012;7:e39263. doi: 10.1371/journal.pone.0039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–9. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum PF, Weinstock RS, Silverstone AE, Sjödin A, Pavuk M. Metabolic syndrome is associated with exposure to organochlorine pesticides in Anniston, AL, United States. Environ Int. 2017;108:11–21. doi: 10.1016/j.envint.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull RP, Ritz B. Historical pesticide exposure in California using pesticide use reports and land-use surveys: an assessment of misclassification error and bias. Environ Health Perspect. 2003;111(13):1582–9. doi: 10.1289/ehp.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders L, Kadhel P, Costet N, Rouget F, Monfort C, Thomé JP, Guldner L, Cordier S, Multigner L. Hypertensive disorders of pregnancy and gestational diabetes mellitus among French Caribbean women chronically exposed to chlordecone. Environ Int. 2014;68:171–6. doi: 10.1016/j.envint.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Yang W, Roberts EM, Kegley SE, Stevenson DK, Carmichael SL, English PB. Residential agricultural pesticide exposures and risks of spontaneous preterm birth. Epidemiol. 2018;29:8–21. doi: 10.1097/EDE.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldana TM, Basso O, Baird DD, Hoppin JA, Weinberg CR, Blair A, Alavanja MCR, Sandler DP. Pesticide Exposure and Hypertensive Disorders During Pregnancy. Environ Health Perspect. 2009;117:1393–1396. doi: 10.1289/ehp.0900672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi A, Nieuwenhuijsen M, Vienneau D, Holman CD. Living near agricultural pesticide applications and the risk of adverse reproductive outcomes: a review of the literature. Paediatr Perinat Epidemiol. 2011;25(2):172–91. doi: 10.1111/j.1365-3016.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui MK, Nigam U, Srivastava S, Tejeshwar DS, Chandrawati Association of maternal blood pressure and hemoglobin level with organochlorines in human milk. Hum Exp Toxicol. 2002;21:1–6. doi: 10.1191/0960327102ht198oa. [DOI] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ Health Perspect. 1995;103(12):1126–34. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression Shrinkage and Selection via the Lasso. J Royal Stat Soc. Series B (Methodological) 1996;58:267–288. [Google Scholar]
- Wang K, Zhou Q, He Q, Tong G, Zhao Z, Duan T. The possible role of AhR in the protective effects of cigarette smoke on preeclampsia. Med. Hypotheses. 2011;77:872–874. doi: 10.1016/j.mehy.2011.07.061. [DOI] [PubMed] [Google Scholar]
- Willis WO, de Peyster A, Molgaard CA, Walker C, MacKendrick T. Pregnancy outcome among women exposed to pesticides through work or residence in an agricultural area. J Occup Med. 1993;35:943–9. doi: 10.1097/00043764-199309000-00019. [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T. Regularization and Variable Selecton via the Elastic Net. Journal of the Royal Statistical Society Series B (Methodological) 2005;67:301–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.