Abstract
The plant hormone abscisic acid (ABA) mediates many vital processes in plant growth and development, including seed dormancy, cell division, water use efficiency, and adaptation to drought, salinity, chilling, pathogen attack, and UV light. Our understanding of ABA signal transduction is fragmentary and would benefit from specific and facile probes of the process. Protoplasts from rice (Oryza sativa L. cv IR54) embryonic suspension cultures cotransformed with effector plasmids encoding the maize (Zea mays) VIVIPAROUS1 cDNA and/or the Arabidopsis dominant negative mutant (abi1-1) ABA-insensitive cDNA demonstrated genetic interactions of VIVIPAROUS1 and abi1-1 in transactivation of the ABA-inducible HVA1 promoter from barley (Hordeum vulgare), suggesting the mechanisms of these effectors are conserved among monocots and dicots. Trivalent ions have been shown to act as an effector of gene expression in plants and animals, although the mechanism of action is unknown. We show in two complementary transient ABA-inducible gene expression assays (β-glucuronidase and luciferase enzymatic activities and quantitative flow cytometry of green fluorescent protein) that trivalent ions specifically interact with an ABI1-dependent ABA-signaling pathway leading to gene expression. Trivalent ions mimic ABA effects on gene expression and may be a useful tool to study ABA signaling.
Abscisic acid (ABA) acts via multiple pathways, for example by inducing rapid closure of stomatal pores by ion efflux from guard cells and by slower changes in gene expression. Despite the complex multitude of data (physiological, molecular, genetic, biochemical, and pharmacological) that implicate ABA in stress responses, the adaptive responses of plants to ABA, stresses, and the pathways that trigger them are largely unknown (Grill and Himmelbach, 1998; Hetherington et al., 1998; Leung and Giraudat, 1998).
Rapid progress in understanding ABA signaling has been made with reverse genetic approaches to reconstruct minimal cascades leading to gene expression (for review, see Shen and Ho, 1997; Shinozaki and Yamaguchi-Shinozaki, 1997; Busk and Pagès, 1998). Sheen (1996) showed in maize (Zea mays) protoplasts that exogenous calcium and/or overexpressed protein kinases from Arabidopsis could activate the ABA- and stress-inducible barley (Hordeum vulgare) HVA1 promoter. In barley aleurone, an ABA-inducible protein kinase and phospholipase D activity have been implicated in ABA-regulated gene repression (Ritchie and Gilroy, 1998; Gómez-Cadenas et al., 1999). A cADP-Rib-dependent ABA-signaling pathway involving intracellular calcium and protein phosphorylation has been shown to exist by micro-injection experiments in tomato hypocotyl cells (Wu et al., 1997). cADP-Rib has recently also been shown to mediate rapid ABA-induced stomatal closure in Commelina communis guard cell protoplasts (Leckie et al., 1998), suggesting that rapid ABA responses at the plasma membrane and slower nuclear responses may share some common intermediates such as calcium transients.
Genetic studies in the model organisms maize and Arabidopsis have resulted in the identification of mutants altered in ABA- and environmental stress signaling (McCarty, 1995; Bonetta and McCourt, 1998; Grill and Himmelbach, 1998; Foster and Chua, 1999; Lee et al., 1999). Several ABA-signaling genes have been cloned and shown to encode protein phosphatases, transcription factors, and a subunit of farnesyl transferase (Cutler et al., 1996; Finkelstein et al., 1998; Luerssen et al., 1998). The ABA INSENSITIVE1 (ABI1) and ABI2 ABA response genes encode homologous protein Ser/Thr phosphatases that act as negative regulators of ABA sensitivity (Leung et al., 1997; Rodriguez et al., 1998; Sheen, 1998; Gosti et al., 1999). The VIVIPAROUS1 (VP1) gene of maize is orthologous to ABI3 of Arabidopsis and encodes a DNA-binding protein (Carson et al., 1997; Suzuki et al., 1997). Although the exact molecular mechanisms of these signaling effectors are not known, VP1 potentiates ABA-inducible gene expression by remodeling of chromatin architecture and forms a DNA binding complex with 14-3-3 and basic Leu zipper proteins (Schultz et al., 1998; Li et al., 1999).
Lanthanide salts inhibit ion channels in plants and animals and have been used extensively as plasma membrane calcium channel antagonists (Huang et al., 1994; Bush, 1995; Van der Meulen et al., 1996; Gelli and Blumwald, 1997; Tähtiharju et al., 1997; Clayton et al., 1999). There is recent evidence for non-specific inhibition of ion channels by lanthanum (Lewis and Spalding, 1998). We have previously observed lanthanide and trivalent ion effects on gene expression (Rock and Quatrano, 1996). Here we have used ABA-inducible and noninducible enzymatic reporter gene expression, flow cytometry of protoplasts expressing ABA-inducible green fluorescent protein (GFP), and the dominant-negative abi1-1 gene to provide evidence for specific activation by trivalent ions of ABA-inducible gene expression.
RESULTS AND DISCUSSION
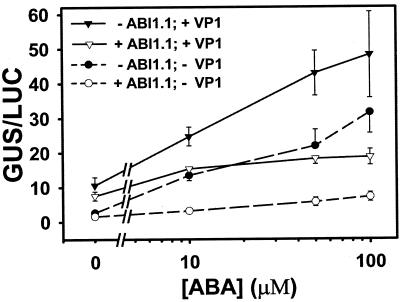
The ABA response genes ABI3 (orthologous to maize VP1) and ABI1 have been shown to genetically interact in planta (Finkelstein and Somerville 1990; Parcy et al., 1997), although their mechanisms of action are not known. Because we use a heterologous, artificial system of transiently expressed promoters and effector cDNAs in protoplasts to assay ABA signaling, we sought to validate the fidelity of the system by testing whether the Arabidopsis abi1-1 cDNA could function as a bona fide effector of ABA responses in rice (Oryza sativa L. cv IR54) protoplasts. Figure 1 shows the results of transient gene co-expression assays testing the interactions of ABA and overexpressed maize VP1 cDNA and/or the dominant negative Arabidopsis abi1-1 cDNA on transactivation of the barley HVA1 promoter. There is an antagonistic effect of overexpressed abi1-1 on both ABA-dependent and VP1-dependent HVA1 activation. This result is consistent with abi1-1 dominant negative effects observed in transgenic tomato, tobacco, and maize (Grabov et al., 1997; Parcy and Giraudat, 1997; Carrera and Prat, 1998; Sheen, 1998) and a model whereby abi1-1 negatively affects ABA-signaling amplitude and VP1 indirectly potentiates it (Gosti et al., 1999; Li et al., 1999).
Figure 1.
Single and combined effects of VP1 and abi1-1 overexpression on HVA1 promoter-β-glucuronidase (GUS) expression in transiently transformed rice protoplasts. The non-ABA-inducible ubiquitin promoter-luciferase cDNA reporter construct (Christensen and Quail, 1996) was cotransformed as an internal control for transcription activity. Treatments were performed in triplicate; variance bars are ±se.
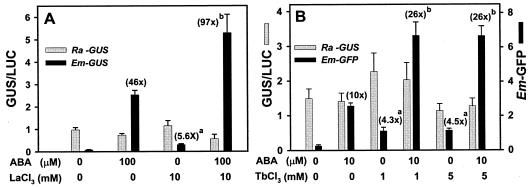
We showed previously that lanthanum ions had a specific agonist effect on ABA-inducible endogenous Em expression in rice suspension cells (Rock and Quatrano, 1996). We further investigated the lanthanum specificity toward ABA-inducible gene expression in transient co-expression assays using reference promoter-reporter constructs as internal controls for transformation and transcription. The maize ubiquitin promoter is not inducible by ABA (Shen and Ho, 1997). We tested the effects of ABA and lanthanum chloride on activation of the wheat (Triticum aesitivum) Em, barley HVA1 and HVA22, rice actin, and cauliflower mosaic virus 35S promoters. Figure 2A shows that the Em promoter was specifically activated (46-fold) by a saturating concentration of ABA (100 μm; Desikan et al., 1999), almost 6-fold by lanthanum chloride (10 mm), and exogenous ABA and lanthanum together acted in a synergistic manner relative to expression of the reference ubiquitin-firefly luciferase (LUC) reporter (Fig. 2A). Similar results were obtained when sub-saturating concentrations of ABA (10 μm) and the lanthanide terbium were used to activate the Em promoter (Fig. 2B); however, the rice actin promoter was not significantly affected by ABA or lanthanide treatments (Fig. 2). Likewise, the lanthanide agonist and synergistic effects were significant and specific for the ABA-inducible HVA1 promoter (Table I) and the ABA-inducible HVA22 promoter (D. Hagenbeek and C.D. Rock, unpublished data) but not the control cauliflower mosaic virus 35S promoter (Table I).
Figure 2.
Specific synergistic effect of lanthanide ions on the ABA-inducible Em promoter, but not the non-ABA-inducible rice actin (Ra) promoter. Numbers in parentheses show fold induction over untreated controls. A, Effects of lanthanum with or without ABA cotreatment on the actin and Em promoters, measured by GUS/LUC reporter enzyme assays. B, Effects of terbium on the actin and Em promoters, cotransformed and measured in the same samples by reporter enzyme activities and GFP flow cytometry, respectively. Results are the average of three to nine replicates (±se). a, Significantly higher than control treatment (paired t test, P < 0.02). b, Significantly higher than ABA (P < 0.05).
Table I.
Specificity of the lanthanide effect on the ABA-inducible Em and HVA1 promoters in transiently transformed protoplasts
| Treatments
|
Promoter Fold-Induction (±se)
|
|||
|---|---|---|---|---|
| ABA | Salt | 35S | Em | HVA1 |
| μm | mm | |||
| 0 | 0 | 1.0 | 1.0 | 1.0 |
| 100 | 0 | 0.8 (0.1) | 16.6 (2.0) | 13.7 (1.0) |
| 0 | 1 LaCl3 | 1.1 (0.0) | 2.2 (0.6) | 3.2a (0.5) |
| 0 | 2 TbCl3 | 1.1 (0.1) | 4.6a (0.2) | n/ac |
| 0 | 5 LaCl3 | 1.4 (0.3) | 2.7 (0.8) | 3.8a (0.4) |
| 100 | 1 LaCl3 | 1.1 (0.1) | 32.6b (2.9) | 29.6b (5.6) |
| 100 | 2 TbCl3 | 1.2 (0.1) | 31.9b (1.5) | n/ac |
| 100 | 5 LaCl3 | 1.1 (0.4) | 38.8b (9.9) | 24.1 (5.5) |
Induction was calculated as the average ratio of relative reporter gene activities (promoter-GUS/Ubi-LUC) between treatment and control (set to unity) from three- or four-paired replicates in two independent experiments.
Significantly higher than control, P < 0.03 (one-sided paired t test).
Significantly higher than ABA treatment, P < 0.06 (one-sided paired t test).
n/a, Not analyzed.
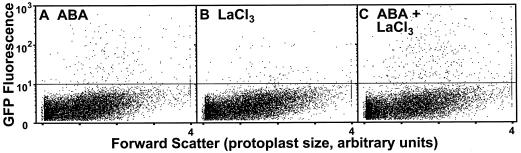
Because protoplasts are a heterogeneous population with potentially different characteristics that might complicate analysis of signaling pathways, we have developed a novel, quantitative, ABA-inducible reporter gene assay based on GFP and flow cytometry (Desikan et al., 1999). This method allows quantitation of thousands of gene expression events (and other correlative cell biology parameters) in potentially complex populations on a per cell basis. We further tested the specificity and synergy of trivalent ion action on ABA signaling by observing the effects of overexpressed abi1-1 cDNA on ABA- and lanthanide-inducible Em-GFP reporter gene expression. Figure 3 shows representative scatter plots (cell size versus GFP fluorescence) of 10,000 protoplasts expressing Em-GFP after 20-h treatments of 10 μm ABA, 5 mm lanthanum chloride, or both. The transformation efficiency was observed to be approximately 4% by this method. On the basis of scoring a fixed number of cells, it is apparent that the cells expressing, Em-GFP generally respond to treatments uniformly as a population, both in terms of cell numbers and fluorescence intensities (Fig. 3). For reference, Em-GFP-transformed control samples that were not treated with ABA or lanthanides resulted in 18 protoplasts above the arbitrary background threshold per 10,000 (D. Hagenbeek and C.D. Rock, unpublished data), whereas the number of Em-GFP expressing protoplasts in Figure 3C is 340. The quantitative measurement of a large number of GFP-expressing cells by flow cytometry yields results comparable with those obtained with enzymatic reporter assays (Fig. 2) with the advantage of being able to observe population dynamics. Expression of Em-GFP was positively correlated with exogenous ABA concentration (Desikan et al., 1999; D. Hagenbeek and C.D. Rock, unpublished data). From these results we conclude that heterogeneity of ABA and/or lanthanum sensitivity/responses by sub-populations of rice protoplasts does not play a major role in ABA- and lanthanide-signaling pathways, in contrast to reports of heterogeneity of gibberellin sensitivity in aleurone protoplasts (Ritchie et al., 1999).
Figure 3.
Scatter plot (cell size versus GFP fluorescence) of Em-GFP-transformed protoplasts treated for 20 h with 10 μm ABA (A), 5 mm LaCl3 (B), or both ABA plus LaCl3 (C). Ten-thousand protoplasts were measured by flow cytometry, and those with fluorescence above an arbitrary background (horizontal line) were gated for quantitation of fluorescence intensity.
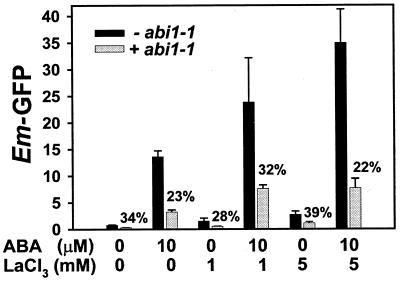
A quantitative, flow-cytometric analysis of the interaction of abi1-1 gene, ABA, and lanthanum ions on Em-GFP expression is shown in Figure 4. ABA (10 μm) gave a 21-fold activation of the Em promoter, whereas lanthanum gave a dose-dependent 2- to 4-fold activation that was synergistic with the ABA induction. Overexpression of the dominant negative abi1-1 cDNA resulted in a similar (60%–75%) inhibition of Em-GFP expression in untreated controls, ABA, lanthanum, and ABA plus lanthanum treatments (Fig. 4). This result supports the hypothesis that lanthanum acts on an ABA signal transduction pathway upstream from ABI1 and VP1. Furthermore, quantitative flow cytometry of Em-GFP was sensitive enough to reveal that Arabidopsis abi1-1 significantly inhibits (P < 0.03) Em promoter activity in the absence of exogenous ABA (Fig. 4), which was not obvious in reporter enzyme assays (Fig. 1). This observation underscores the conservation of ABA-signaling mechanisms among various species including rice.
Figure 4.
Overexpression of abi1-1 inhibits lanthanum-induced Em expression to a similar extent as ABA-induced Em expression. Rice protoplasts were transformed with Em-GFP in the presence of overexpressed abi1-1 (pG2) or a null ABI1 control (pG1; Sheen, 1998). Numbers above the abi1-1-treated samples (white bars) indicate the percentage Em expression relative to control. Transformations were performed in triplicate and flow cytometry measurements in duplicate; variance bars are ±se.
The viability of protoplasts has been shown to be negatively correlated with ABA concentration (Desikan et al., 1999); therefore, the effect of lanthanides on protoplast viability was tested. Results of several experiments (Table II) demonstrated that day-to-day variation in protoplast viability was substantial and not strictly correlated with ABA or lanthanide treatments. The significance of this environmentally induced cell death is not known, but the observation clearly points out that the inductive effects of ABA and lanthanum on individual cells (Fig. 3) are stronger than measured by reporter enzyme assays, which are often normalized to total protein concentration. Our previous results showing lack of specificity of lanthanide effects in protoplasts may have been confounded by the effects of cell death on protein concentrations (Rock and Quatrano, 1996). Flow cytometry permits quantitative and qualitative analysis of the effects of ABA and other signaling factors on heterogeneous cell populations as well as simultaneous correlation of multiple cell biological parameters in single cells and populations.
Table II.
ABA and lanthanide salt effects on viability of transformed protoplasts after an 18- to 20-h treatment
| Treatment
|
Viability Percentage (±se)
|
|||
|---|---|---|---|---|
| ABAa | Saltb | Experiment I | Experiment II | Experiment III |
| μm | mm | |||
| 0 | 0 | 36.2 (6.7) | 15.0 (0.6) | 45.4 (0.6) |
| + | 0 | 31.7 (4.0) | 15.5 (0.7) | 43.8 (0.6) |
| 0 | 1 | 14.9 (1.2) | 15.5 (0.6) | 49.6 (1.7) |
| + | 1 | 9.4 (4.6) | 17.7 (0.7) | 48.5 (0.7) |
| 0 | 5 | 12.8 (11.0) | 17.4 (0.3) | 48.5 (1.4) |
| + | 5 | 3.4 (1.8) | 16.5 (0.4) | 47.5 (1.3) |
Samples were assayed by flow cytometry of protoplasts treated with 0.01% (w/v) fluorescein diacetate for 5 min.
Ten micromolar ABA in experiments I and II; 100 μm ABA in experiment III.
Experiment I, LaCl3 (two independent transformations); experiment II, TbCl3 (five–nine independent transformations); experiment III, average of parallel LaCl3 and TbCl3 treatments (three–nine independent transformations).
We reported previously that lanthanide and trivalent aluminum ions had inductive effects on transient gene expression in protoplasts, whereas monovalent salts such as potassium, sodium, and lithium had no such effect (Rock and Quatrano, 1996). We further analyzed the lower limits of the lanthanide and the trivalent aluminum ion effects on Em promoter activity and extended the analysis of possible salt effects to the divalent ions magnesium and manganese. Representative results of experiments performed with different concentrations of salts are shown in Table III. Lithium, manganese, and magnesium salts had no effect on Em promoter activity at more than 10-fold higher concentrations than those where lanthanides and the trivalent ion aluminum had significant effects (Table III). These results indicate that the trivalent ion effect is not a general stress or salt response.
Table III.
Trivalent, but not divalent, ions stimulate Em promoter activity in transiently transformed protoplasts
| Treatment
|
Em Promoter Fold-Induction (±se) | P Valuea | |
|---|---|---|---|
| Concentration | Salt | ||
| mm | |||
| 5.0 | LiCl | 0.9 (0.1) | 0.29 |
| 5.0 | MgCl2 | 1.0 (0.1) | 0.44 |
| 5.0 | MgSO4 | 1.0 (0.1) | 0.48 |
| 10.0 | MnCl2 | 1.0 (0.3) | 0.44 |
| 0.13 | AlCl3 | 3.7 (0.6) | 0.03 |
| 0.13 | LaCl3 | 2.4 (0.4) | 0.05 |
| 0.13 | TbCl3 | 1.6 (0.2) | 0.09 |
Induction was calculated as the average ratio of relative reporter gene activities (Em-GUS/Ubi-LUC) between treatment and control (set to unity) from three paired replicates for the LiCl, MgCl2, MgSO4, and MnCl2 treatments. For the AlCl3, LaCl3, and TbCl3 treatments, induction was calculated as the average ratio of relative weighted Em-GFP expression between treatment and control from three paired replicates.
One-sided paired t test.
We have demonstrated, using various reporter genes and salts that the synergistic effect of trivalent ions on ABA-inducible promoters in transiently transformed rice protoplasts is specific and dependent upon the activity of ABI1. In previous transient gene expression experiments with trivalent ions (Rock and Quatrano, 1996), no internal reference reporter was used to normalize gene transcription against environmental factors such as transformation efficiency or cell viability, and specificity for ABA signaling was not established. Here we have used the dominant negative ABA regulatory gene abi1-1 and two independent reporter assays (a ubiquitin promoter-LUC internal reference reporter gene construct ]Christensen and Quail, 1996[ and flow cytometry of large populations of GFP-expressing protoplasts) to demonstrate specificity and homogeneity of the trivalent ion effect on ABA-inducible promoters.
The mechanism of trivalent ion activity on gene expression is not known. Aluminum ions have also been reported to activate c-fos gene expression in fibroblasts (Hughes and Pennington, 1993). It is unlikely that lanthanides increase the sensitivity of cells to ABA, since the synergistic effect of lanthanum is observed at saturating concentrations of ABA (Fig. 2A; Desikan et al., 1999). Because lanthanum binds with high affinity to the plasma membrane and intracellular vesicles (van Steveninck et al., 1976), we speculate that it may exert effects on ABA-inducible gene expression via ion channels (Lewis and Spalding, 1998) or other membrane-associated proteins. Lanthanum is routinely used in plants as a calcium channel blocker and alters gene expression (Tähtiharju et al., 1997; Clayton et al., 1999). Calcium is required for ABA-inducible gene expression (Sheen, 1996; Van der Meulen et al., 1996), and lanthanum can substitute for the calcium requirement for Em gene expression (Rock and Quatrano, 1996). However, the role of extracellular calcium in ABA signaling is not known, and lanthanides are not specific for calcium channels (Lewis and Spalding, 1998). The calcium channel blockers bepridil, nifedipine, and verapamil had no effect on Em promoter activity, although bepridil did block calcium uptake in rice protoplasts (C.D. Rock, unpublished data). Relatively high concentrations of lanthanides were required for maximum agonist activity in these experiments, suggesting a possible intracellular site of lanthanide action. In this context it is interesting to note that lanthanum has been shown to inhibit calcium-dependent protein kinases (Polya et al., 1987), which have been implicated in ABA signaling (Sheen, 1996). It is plausible that an integrin-like molecule in plants (Zhang et al., 1996; Faik et al., 1998; Nagpal and Quatrano, 1999) could be activated by trivalent ion binding (D'Souza et al., 1994; Obsil et al., 1999). Lanthanide ions act as agonists of integrin expression in animal cells (Ahmad et al., 1999). Recent characterization of a glycoprotein in rice plasma membranes that may undergo conformational changes (both are properties of integrins) and is involved in ABA signaling (Desikan et al., 1999) is consistent with a hypothetical role for integrins in ABA responses. Multidisciplinary and integrative approaches such as flow cytometry, patch-clamping of rice protoplasts, and an in vitro biosensor assay for ABA interaction with plasma membrane vesicles (Desikan et al., 1999) may provide insights into the cell biology of ABA signaling and the mechanism of action of trivalent ions.
MATERIALS AND METHODS
Plant Materials
Rice (Oryza sativa L. cv IR54) suspension cultures from the International Rice Research Institute, Los Baños, Phillipines), initiated from germinating embryos, were propagated and digested for making protoplasts as previously described (Marcotte et al., 1988; Desikan et al., 1999). Protoplasts were transiently transformed with polyethylene glycol as described by Maas et al. (1995) with modifications (Desikan et al., 1999). It was typical that 70 μg of reporter plasmid and 20 μg of effector plasmids were mixed with 2.5 × 106 rice protoplasts per transformation. Transformations were split into four- or six-paired samples and treated for 20 h in a final volume of 0.8 mL of Krens solution.
Plasmid Constructions
The plasmid pCR559 contains the wheat (Triticum aesitivum) Em promoter driving a modified Aequoria victoria GFP (Chiu et al., 1996; Desikan et al., 1999). Plasmids pBM207 and pBM314 contain the wheat Em and cauliflower mosaic virus 35S promoters, respectively, driving uidA expression (GUS; Marcotte et al., 1988; Hill et al., 1996). Plasmids pQS264 and pLSP contain the barley (Hordeum vulgare) HVA1 and HVA22 promoters driving GUS expression, respectively (Shen and Ho, 1997). Plasmid pAct1D contains the rice actin promoter driving GUS expression (Zhang et al., 1991). Plasmid pAHC18 contains the maize (Zea mays) ubiquitin promoter driving LUC (Christensen and Quail, 1996) and was included in transformations as an internal reference for non-ABA-inducible transient transcription. Plasmid pG2 encodes the chimeric 35SC4PPDK (cauliflower mosaic virus 35S-maize C4 pyruvate orthophosphate dikinase) promoter driving the coding region of the Arabidopsis abi1-1 dominant negative G180D mutant allele (Sheen, 1996, 1998). Plasmid pG1 is a control construct that is identical to pG2 except it is wild type at aa180 (Gly), and the phosphatase active site has been mutated (G174D) to produce a null mutant (Sheen, 1998).
Functional Assays
Flow cytometry of live protoplasts expressing GFP was performed on a dual beam instrument (FACS Vantage, Becton-Dickinson, San Jose, CA) equipped with a 200-μm nozzle and Lysis II acquisition and analysis software. The excitation wavelength was 488 nm, and emission detection was with a fluorescein isothiocyanate 530/30-nm filter set. In order to take advantage of the large numbers of individual measurements inherent in flow cytometry, a quantitative method, “weighted fluorescence intensity,” was developed that proved more sensitive than previous reporter gene measurements (Desikan et al., 1999). For each sample, 10,000 protoplasts were gated, and the weighted-GFP fluorescence per 10,000 cells was calculated as the product of the average fluorescence intensity of the population expressing above background threshold and the number of individual cells. Cell viability was determined by flow cytometry of an aliquot of live protoplasts treated 5 min with 0.01% (w/v) fluorescein diacetate (Molecular Probes, Eugene, OR). Enzyme-based reporter assays were as described previously (Desikan et al., 1999).
ACKNOWLEDGMENTS
The authors thank Dr. Jen Sheen (Department of Molecular Biology, Massachusetts General Hospital, Harvard Medical School, Boston) for the pG1, pG2, and Aequoria victoria GFP clones, Prof. T.-H.D. Ho and Dr. Qingxi Shen (Biology Department, Washington University, St. Louis) for the pAHC18, pLSP, and pQS264 clones, Dr. Thomas Altmann for critical reading of the manuscript, and Regina Chak, Patrick Ng, and Frances Chan for technical assistance.
Footnotes
This work was supported by the Hong Kong Research Grants Council's Competitive Earmarked Research Grant (no. HKUST–6173/97M to C.D.R.).
LITERATURE CITED
- Ahmad N, Gardner CR, Yurkow EJ, Laskin DL. Inhibition of macrophages with gadolinium chloride alters intercellular adhesion molecule-1 expression in the liver during acute endotoxemia in rats. Hepatology. 1999;29:728–736. doi: 10.1002/hep.510290324. [DOI] [PubMed] [Google Scholar]
- Bonetta D, McCourt P. Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 1998;3:231–235. [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Busk PK, Pagès M. Regulation of abscisic acid-induced transcription. Plant Mol Biol. 1998;37:425–435. doi: 10.1023/a:1006058700720. [DOI] [PubMed] [Google Scholar]
- Carrera E, Prat S. Expression of the Arabidopsis Abi1-1 mutant allele inhibits proteinase inhibitor wound induction in tomato. Plant J. 1998;15:765–771. doi: 10.1046/j.1365-313x.1998.00261.x. [DOI] [PubMed] [Google Scholar]
- Carson CB, Hattori T, Rosenkrans L, Vasil V, Vasil IK, Peterson PA, McCarty DR. The quiescent/colorless alleles of Viviparous1 show that the conserved B3 domain of Vp1 is not essential for ABA-regulated gene-expression in the seed. Plant J. 1997;12:1231–1240. doi: 10.1046/j.1365-313x.1997.12061231.x. [DOI] [PubMed] [Google Scholar]
- Chiu WL, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Curr Biol. 1996;6:325–333. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- Clayton H, Knight MR, Knight H, McAinsh MR, Hetherington AM. Dissection of the ozone-induced calcium signature. Plant J. 1999;17:575–579. doi: 10.1046/j.1365-313x.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hagenbeek D, Neill SJ, Rock CD. Flow cytometry and surface plasmon resonance analyses demonstrate that the monoclonal antibody JIM19 interacts with a rice cell surface component involved in abscisic acid signalling in protoplasts. FEBS Lett. 1999;456:257–262. doi: 10.1016/s0014-5793(99)00972-2. [DOI] [PubMed] [Google Scholar]
- D'Souza SE, Haas TA, Piotrowicz RS, Byers-Ward V, McGrath DE, Soule HR, Cierniewski C, Plow EF, Smith JW. Ligand and cation binding are dual functions of a discrete segment of the integrin beta 3 subunit: cation displacement is involved in ligand binding. Cell. 1994;79:659–667. doi: 10.1016/0092-8674(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Faik A, Laboré AM, Gulino D, Mandaron P, Falconet D. A plant surface protein sharing structural properties with animal integrins. Eur J Biochem. 1998;253:552–559. doi: 10.1046/j.1432-1327.1998.2530552.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Somerville CR. Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 1990;94:1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998;10:1043–1054. doi: 10.1105/tpc.10.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R, Chua NH. An Arabidopsis mutant with deregulated ABA gene expression: implications for negative regulator function. Plant J. 1999;17:363–372. doi: 10.1046/j.1365-313x.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Gelli A, Blumwald E. Hyperpolarization-activated Ca2+-permeable channels in the plasma-membrane of tomato cells. J Membr Biol. 1997;155:35–45. doi: 10.1007/s002329900156. [DOI] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho THD, Walker-Simmons MK. An abscisic acid-induced PKABA1 mediates abscisic acid-supressed gene expression in aleurone layers. Proc Natl Acad Sci USA. 1999;96:1767–1772. doi: 10.1073/pnas.96.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J. The ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell. 1999;11:1897–1909. doi: 10.1105/tpc.11.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Leung J, Giraudat J, Blatt MR. Alteration of anion channel kinetics in wild-type and abi1-1 transgenic Nicotiana benthamiana guard cells by abscisic acid. Plant J. 1997;12:203–213. doi: 10.1046/j.1365-313x.1997.12010203.x. [DOI] [PubMed] [Google Scholar]
- Grill E, Himmelbach A. ABA signal transduction. Curr Opin Plant Biol. 1998;1:412–418. doi: 10.1016/s1369-5266(98)80265-3. [DOI] [PubMed] [Google Scholar]
- Hetherington A, Gray JE, Leckie CP, McAinsh MR, Ng C, Pical C, Priestly AJ, Saxén I, Webb AAR. The control of specificity in guard cell signal transduction. Philos Trans R Soc Lond B. 1998;353:1489–1494. [Google Scholar]
- Hill A, Nantel A, Rock CD, Quatrano RS. A conserved domain of the viviparous-1 gene product enhances the DNA binding activity of the bZIP protein EmBP-1 and other transcription factors. J Biol Chem. 1996;271:3366–3374. doi: 10.1074/jbc.271.7.3366. [DOI] [PubMed] [Google Scholar]
- Huang JW, Grunes DL, Kochian LV. Voltage-dependent Ca2+ influx into the right-side-out plasma membrane vesicles isolated from wheat roots: characterization of a putative Ca2+ channel. Proc Natl Acad Sci USA. 1994;91:3473–3477. doi: 10.1073/pnas.91.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PJ, Pennington SR. Aluminum stimulated c-fos gene expression in Swiss 3T3 fibroblasts. Biochem Soc Trans. 1993;21:368S. doi: 10.1042/bst021368s. [DOI] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Xiong L, Ishitani M, Stevenson B, Zhu JK. Cold-regulated gene expression and freezing tolerance in an Arabidopsis thaliana mutant. Plant J. 1999;17:301–308. doi: 10.1046/j.1365-313x.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BD, Spalding EP. Nonselective block by La3+ of Arabidopsis ion channels involved in signal-transduction. J Membr Biol. 1998;162:81–90. doi: 10.1007/s002329900344. [DOI] [PubMed] [Google Scholar]
- Li GF, Bishop KJ, Chandrasekharan MB, Hall TC. Beta-phaseolin gene activation is a 2-step process-PvALF-facilitated chromatin modification followed by abscisic acid-mediated gene activation. Proc Natl Acad Sci USA. 1999;96:7104–7109. doi: 10.1073/pnas.96.12.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luerssen K, Kirik V, Herrmann P, Misera S. Fusca3 encodes a protein with a conserved Vp1/Abi3-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J. 1998;15:755–764. doi: 10.1046/j.1365-313x.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- Maas C, Reichel C, Schell J, Steinbiss HH. Preparation and transformation of monocot protoplasts. Methods Cell Biol. 1995;50:383–399. doi: 10.1016/s0091-679x(08)61045-6. [DOI] [PubMed] [Google Scholar]
- Marcotte WR, Jr, Bayley CC, Quatrano RS. Regulation of a wheat promoter by abscisic acid in rice protoplasts. Nature. 1988;335:454–457. [Google Scholar]
- McCarty DR. Genetic control and integration of maturation and germination pathways in seed development. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:71–93. [Google Scholar]
- Nagpal P, Quatrano RS. Isolation and characterization of a cDNA clone from Arabidopsis thaliana with partial sequence similarity to integrins. Gene. 1999;230:33–40. doi: 10.1016/s0378-1119(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Obsil T, Hofbauerova K, Amler E, Teisinger J. Different cation binding to the I domains of α1 and α2 integrins: implication of the binding site structure. FEBS Lett. 1999;457:311–315. doi: 10.1016/s0014-5793(99)01063-7. [DOI] [PubMed] [Google Scholar]
- Parcy F, Giraudat J. Interactions between the ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J. 1997;11:693–702. doi: 10.1046/j.1365-313x.1997.11040693.x. [DOI] [PubMed] [Google Scholar]
- Polya GM, Klucis E, Haritou M. Resolution and characterization of two soluble calcium dependent protein kinases from silver beet leaves. Biochim Biophys Acta. 1987;931:68–77. [Google Scholar]
- Ritchie S, Gilroy S. Abscisic acid signal transduction in barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1998;95:2697–2702. doi: 10.1073/pnas.95.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S, McCubbin A, Ambrose G, Kao T, Gilroy S. The sensitivity of barley aleurone tissue to gibberellin is heterogeneous and may be spatially determined. Plant Physiol. 1999;120:361–370. doi: 10.1104/pp.120.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD, Quatrano RS. Lanthanide ions are agonists of transient gene expression in rice protoplasts and act in synergy with ABA to increase Em gene expression. Plant Cell Rep. 1996;15:371–376. doi: 10.1007/BF00232374. [DOI] [PubMed] [Google Scholar]
- Rodriguez PL, Benning G, Grill E. ABI2, a second protein phosphatase 2C involved in abscisic acid signal transduction in Arabidopsis. FEBS Lett. 1998;421:185–190. doi: 10.1016/s0014-5793(97)01558-5. [DOI] [PubMed] [Google Scholar]
- Schultz TF, Medina J, Hill A, Quatrano RS. 14-3-3 proteins are part of an abscisic acid-VIVIPAROUS1 (VP1) response complex in the Em promoter and interact with VP1 and EMBP1. Plant Cell. 1998;10:837–847. doi: 10.1105/tpc.10.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Sheen J. Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA. 1998;95:975–980. doi: 10.1073/pnas.95.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ho TH. Promoter switches specific for abscisic acid (ABA)-induced gene expression in cereals. Physiol Plant. 1997;101:653–664. [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kao CY, McCarty DR. The conserved B3 domain of Viviparous1 has a cooperative DNA-binding activity. Plant Cell. 1997;9:799–807. doi: 10.1105/tpc.9.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tähtiharju S, Sangwan V, Monroy AF, Dhindsa RS, Borg M. The induction of kin genes in cold-acclimating Arabidopsis thaliana: evidence of a role for calcium. Planta. 1997;203:442–447. doi: 10.1007/s004250050212. [DOI] [PubMed] [Google Scholar]
- Van der Meulen RM, Visser K, Wang M. Effects of modulation of calcium levels and calcium fluxes on ABA-induced gene expression in barley aleurone. Plant Sci. 1996;117:75–82. [Google Scholar]
- van Steveninck RFM, van Steveninck ME, Chescoe D. Intracellular binding of lanthanum in root tips of barley (Hordeum vulgare) Protoplasma. 1976;90:89–97. [Google Scholar]
- Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua NH. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- Zhang S-D, Kassis J, Olde B, Mellerick DM, Odenwald WF. Pollux, a novel Drosophila adhesion molecule, belongs to a family of proteins expressed in plants, yeast, nematodes, and man. Genes Dev. 1996;10:1108–1119. doi: 10.1101/gad.10.9.1108. [DOI] [PubMed] [Google Scholar]
- Zhang W, McElroy D, Wu R. Analysis of rice Act1 5′ region activity in transgenic rice plants. Plant Cell. 1991;3:1155–1165. doi: 10.1105/tpc.3.11.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]